Using Flavonoid Substitution Status to Predict Anticancer Effects in Human Melanoma Cancers: An In Vitro Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and General Experimental Procedures
2.2. Cell Viability Assays
2.2.1. 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium Bromide (MTT) Assay
2.2.2. Neutral Red Uptake (NRU) Assay
2.3. Proliferation Assay
2.4. Apoptosis Assay
2.5. Western Immunoblot
2.6. Immunofluorescence Microscopy
2.7. Statistical Analysis
3. Results
3.1. Cell Viability Assays
3.2. Proliferation Assay
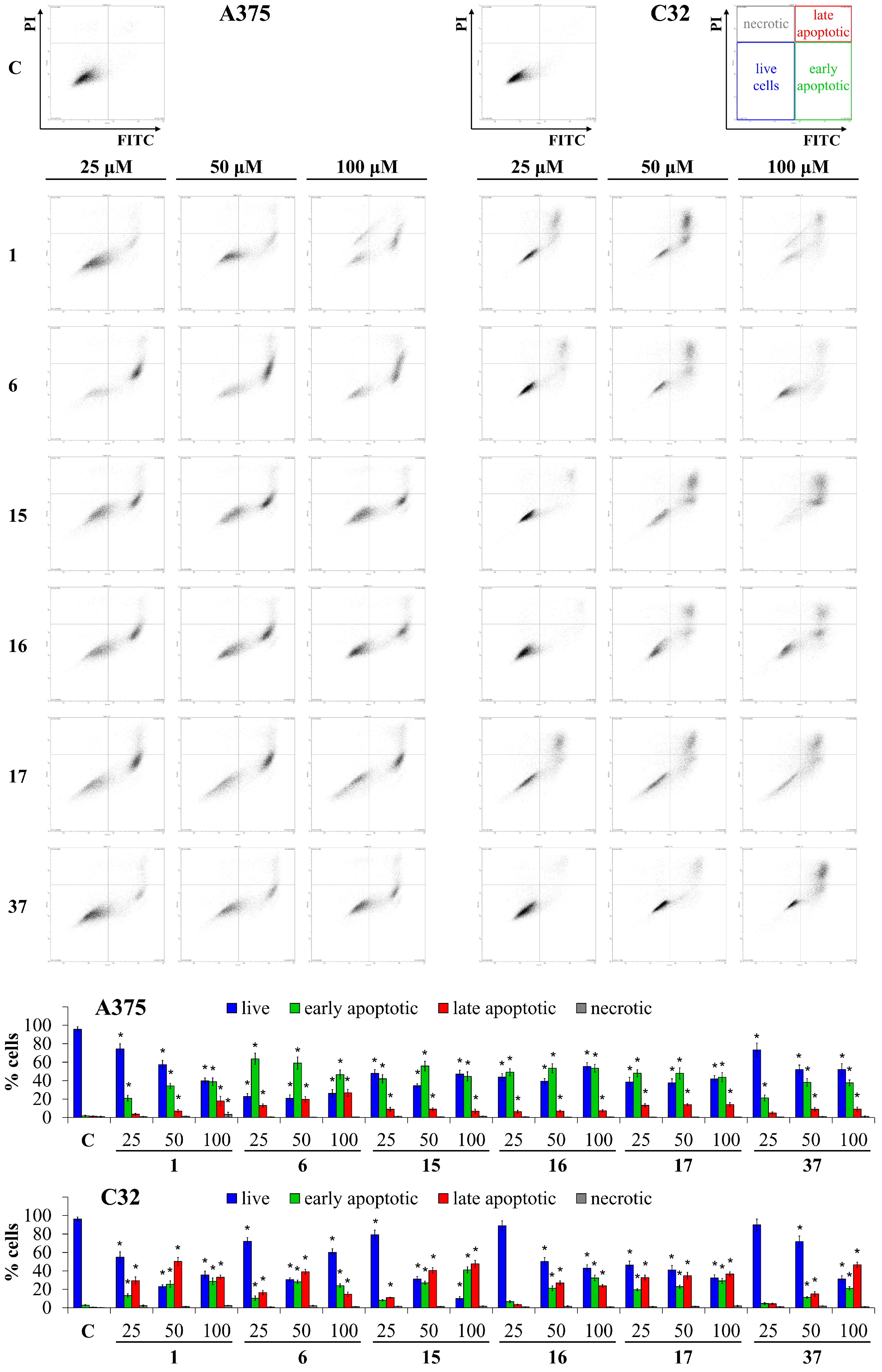
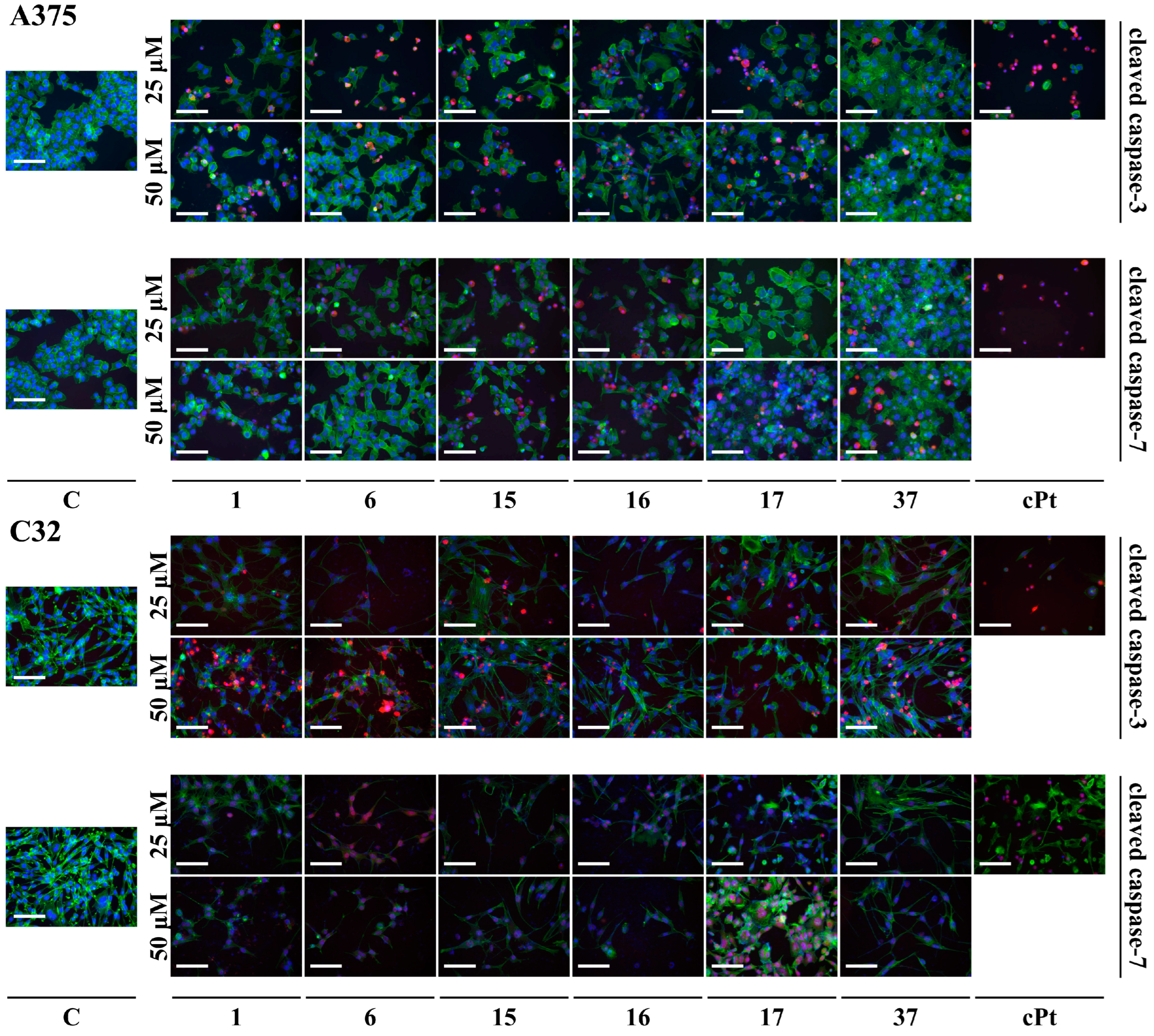
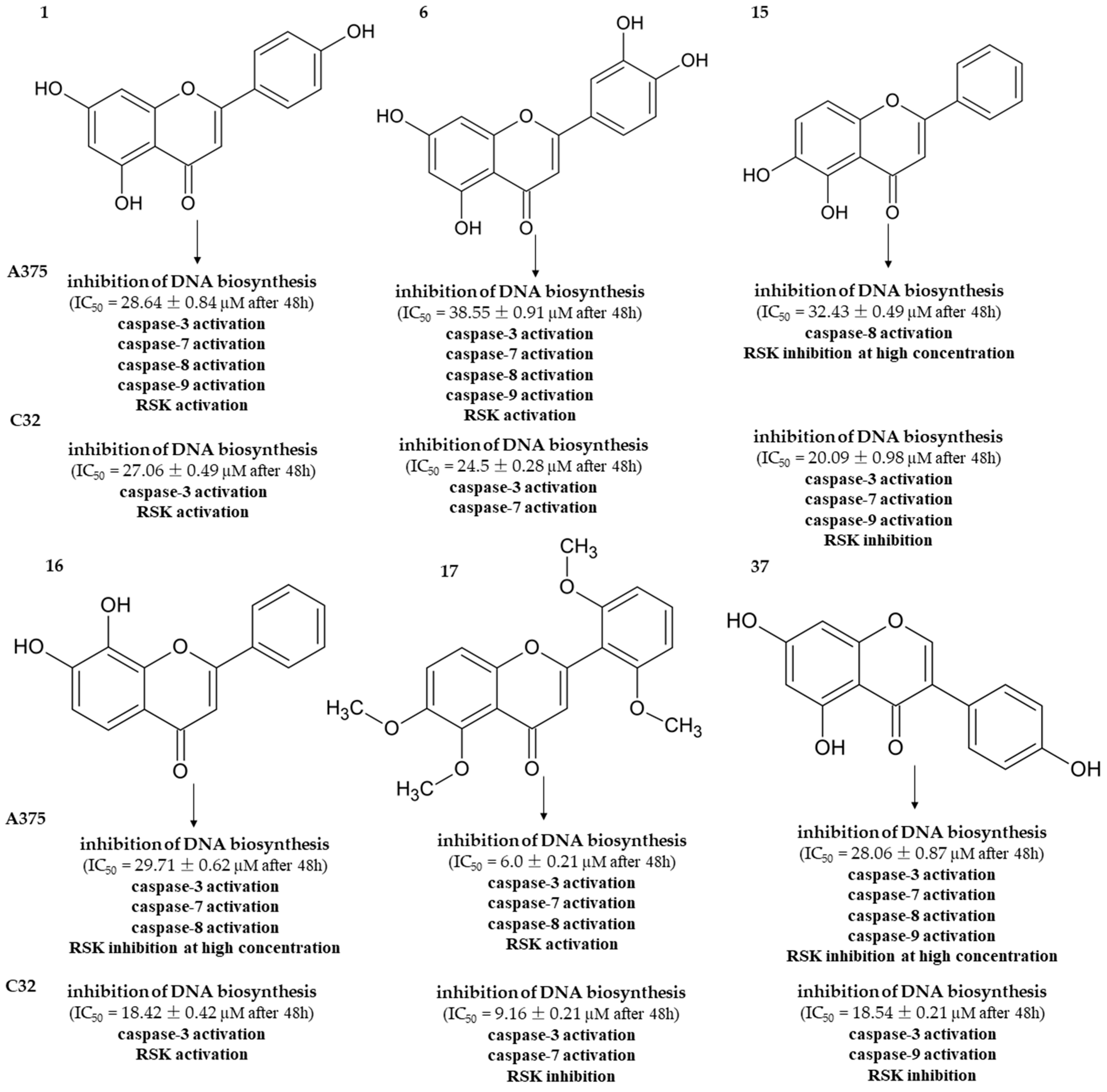
3.3. Apoptosis Assay
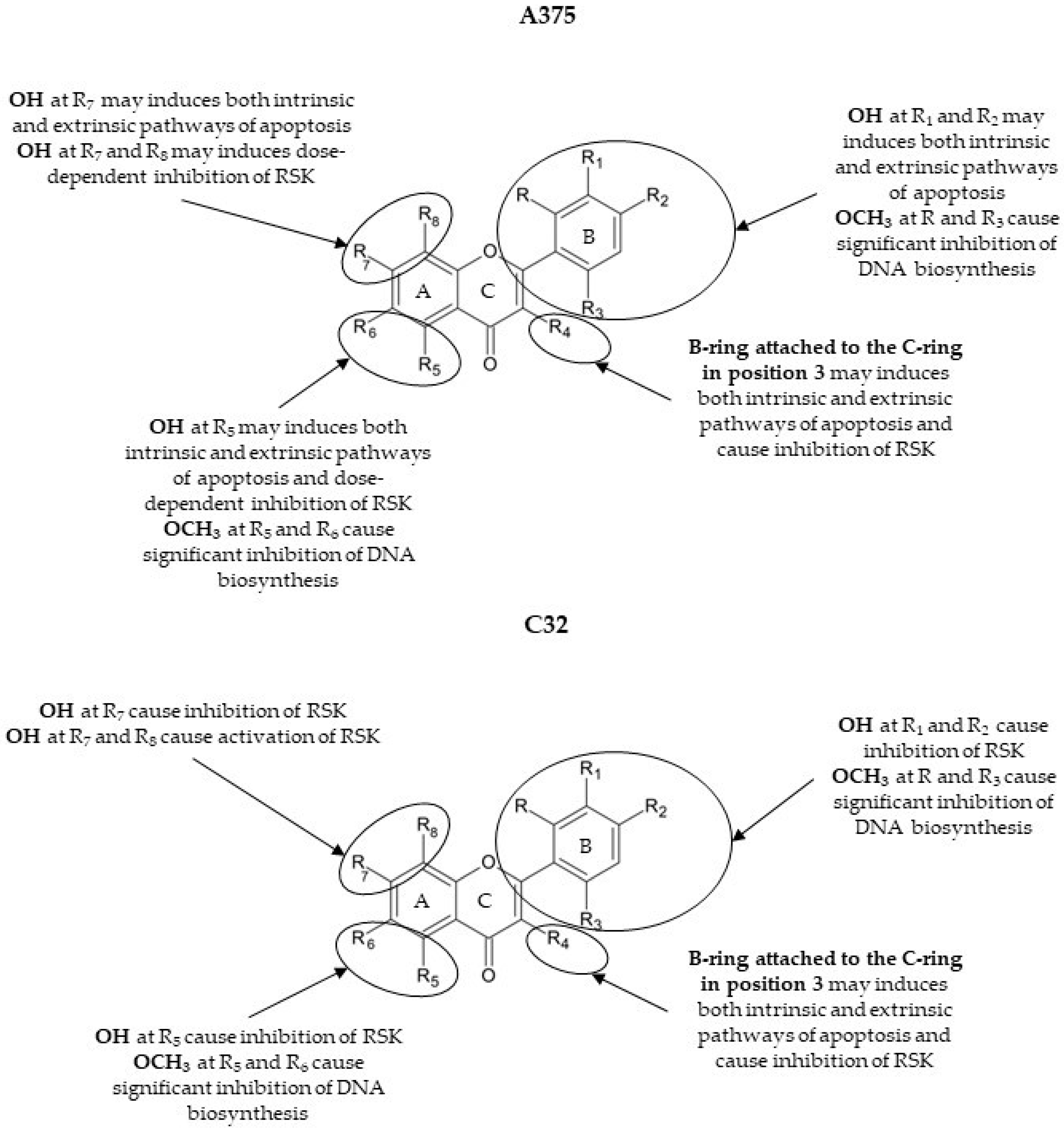
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orthaber, K.; Pristovnik, M.; Skok, K.; Perić, B.; Maver, U. Skin Cancer and Its Treatment: Novel Treatment Approaches with Emphasis on Nanotechnology. J. Nanomater. 2017, 2017, 1–20. [Google Scholar] [CrossRef]
- Liu, X.Y.; Fang, H.; Yang, Z.G.; Wang, X.Y.; Ruan, L.M.; Fang, D.R.; Ding, Y.G.; Wang, Y.N.; Zhang, Y.; Jiang, X.L.; et al. Matrine inhibits invasiveness and metastasis of human malignant melanoma cell line A375 in vitro. Int. J. Dermatol. 2008, 47, 448–456. [Google Scholar] [CrossRef]
- Atkinson, V. Recent advances in malignant melanoma. Intern. Med. J. 2017, 47, 1114–1121. [Google Scholar] [CrossRef]
- Yuan, R.; Hou, Y.; Sun, W.; Yu, J.; Liu, X.; Niu, Y.; Lu, J.J.; Chen, X. Natural products to prevent drug resistance in cancer chemotherapy: A review. Ann. N. Y. Acad. Sci. 2017, 1401, 19–27. [Google Scholar] [CrossRef]
- Szlasa, W.; Supplitt, S.; Drąg-Zalesińska, M.; Przystupski, D.; Kotowski, K.; Szewczyk, A.; Kasperkiewicz, P.; Saczko, J.; Kulbacka, J. Effects of curcumin based PDT on the viability and the organization of actin in melanotic (A375) and amelanotic melanoma (C32)– in vitro studies. Biomed. Pharmacother. 2020, 132, 12. [Google Scholar] [CrossRef]
- Mouawad, R.; Sebert, M.; Michels, J.; Bloch, J.; Spano, J.P.; Khayat, D. Treatment for metastatic malignant melanoma: Old drugs and new strategies. Crit. Rev. Oncol. Hematol. 2010, 74, 27–39. [Google Scholar] [CrossRef]
- Ijaz, S.; Akhtar, N.; Khan, M.S.; Hameed, A.; Irfan, M.; Arshad, M.A.; Ali, S.; Asrar, M. Plant derived anticancer agents: A green approach towards skin cancers. Biomed. Pharmacother. 2018, 103, 1643–1651. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Gutiérrez-Venegas, G.; Sánchez-Carballido, M.A.; Delmas Suárez, C.; Gómez-Mora, J.A.; Bonneau, N. Effects of flavonoids on tongue squamous cell carcinoma. Cell Biol. Int. 2020, 44, 686–720. [Google Scholar] [CrossRef]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic Potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Malešev, D.; Kuntić, V. Investigation of metal-flavonoid chelates and the determination of flavonoids via metal-flavonoid complexing reactions. J. Serbian Chem. Soc. 2007, 72, 921–939. [Google Scholar] [CrossRef]
- Nazaruk, J.; Jakoniuk, P. Flavonoid composition and antimicrobial activity of Cirsium rivulare (Jacq.) all. flowers. J. Ethnopharmacol. 2005, 102, 208–212. [Google Scholar] [CrossRef]
- Strawa, J.; Wajs-Bonikowska, A.; Jakimiuk, K.; Waluk, M.; Poslednik, M.; Nazaruk, J.; Tomczyk, M. Phytochemical examination of woolly burdock Arctium tomentosum leaves and flower heads. Chem. Nat. Compd. 2020, 56, 345–347. [Google Scholar] [CrossRef]
- Juszczak, A.M.; Czarnomysy, R.; Strawa, J.W.; Končić, M.Z.; Bielawski, K.; Tomczyk, M. In vitro anticancer potential of Jasione montana and its main components against human amelanotic melanoma cells. Int. J. Mol. Sci. 2021, 22, 3345. [Google Scholar] [CrossRef]
- Jakimiuk, K.; Strawa, J.W.; Granica, S.; Tomczyk, M. New flavone C-glycosides from Scleranthus perennis and their anti-collagenase activity. Molecules 2021, 26, 5631. [Google Scholar] [CrossRef]
- Jakimiuk, K.; Strawa, J.W.; Granica, S.; Locatelli, M.; Tartaglia, A.; Tomczyk, M. Determination of flavonoids in selected Scleranthus species and their anti-collagenase and antioxidant potential. Molecules 2022, 27, 2015. [Google Scholar] [CrossRef]
- Tomczyk, M. Secondary metabolites from Potentilla recta L. and Drymocallis rupestris (L.) Soják (syn. Potentilla rupestris L.) (Rosaceae). Biochem. Syst. Ecol. 2011, 39, 893–896. [Google Scholar] [CrossRef]
- Gudej, J.; Tomczyk, M. Polyphenolic compounds from flowers of Ficaria verna Huds. Acta Pol. Pharm. Drug Res. 1999, 56, 475–476. [Google Scholar]
- Tomczyk, M.; Gudej, J. Quercetin and kaempferol glycosides from Ficaria verna flowers and their structure studied by 2D NMR spectroscopy. Pol. J. Chem. 2002, 76, 1601–1605. [Google Scholar]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
- Rodgers, E.H.; Grant, M.H. The effect of the flavonoids, quercetin, myricetin and epicatechin on the growth and enzyme activities of MCF7 human breast cancer cells. Chem. Biol. Interact. 1998, 116, 213–228. [Google Scholar] [CrossRef]
- Sanders, K.; Moran, Z.; Shi, Z.; Paul, R.; Greenlee, H. Natural products for cancer prevention: Clinical update 2016. Semin. Oncol. Nurs. 2016, 32, 215–240. [Google Scholar] [CrossRef]
- Zhao, G.; Han, X.; Cheng, W.; Ni, J.; Zhang, Y.; Lin, J.; Song, Z. Apigenin inhibits proliferation and invasion, and induces apoptosis and cell cycle arrest in human melanoma cells. Oncol. Rep. 2017, 37, 2277–2285. [Google Scholar] [CrossRef]
- Cao, H.H.; Chu, J.H.; Kwan, H.Y.; Su, T.; Yu, H.; Cheng, C.Y.; Fu, X.Q.; Guo, H.; Li, T.; Tse, A.K.W.; et al. Inhibition of the STAT3 signaling pathway contributes to apigenin-mediated anti-metastatic effect in melanoma. Sci. Rep. 2016, 6, 21731. [Google Scholar] [CrossRef]
- Said, A.; Tundis, R.; Hawas, U.W.; El-kousy, S.M. In vitro antioxidant and antiproliferative activities of flavonoids from Ailanthus excelsa (Roxb.) (Simaroubaceae) leaves. Z. Naturforsh. C 2010, 65c, 180–186. [Google Scholar] [CrossRef]
- Ju, W.S.; Seo, S.Y.; Mun, S.-E.; Kim, K.; Yu, J.O.; Ryu, J.-S.; Kim, J.-S.; Choo, Y.-K. 7,8-Dihydroxyflavone induces mitochondrial apoptosis and down-regulates the expression of ganglioside GD3 in malignant melanoma cells. Discov. Oncol. 2023, 14, 13. [Google Scholar] [CrossRef]
- Toton, E.; Romaniuk, A.; Budzianowski, J.; Hofmann, J.; Rybczynska, M. Zapotin (5,6,2′,6′-tetramethoxyflavone) modulates the crosstalk between autophagy and apoptosis pathways in cancer cells with overexpressed constitutively active PKCϵ. Nutr. Cancer 2016, 68, 290–304. [Google Scholar] [CrossRef]
- Ya-Ming, X.U.; Ramirez-Ahumada, M.D.C.; Valeriote, F.A.; Gunatilaka, A.L. Solid tumor inhibitory and other constituents of Casimiroa tetrameria. Chin. J. Nat. Med. 2011, 9, 334–337. [Google Scholar] [CrossRef]
- Strawa, J.W.; Jakimiuk, K.; Tomczyk, M. Zapotin, a polymethoxyflavone, with potential therapeutic attributes. Int. J. Mol. Sci. 2021, 22, 13227. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Record, I.R.; Broadbent, J.L.; King, R.A.; Dreosti, I.E.; Head, R.J.; Tonkin, A.L. Genistein inhibits growth of B16 melanoma cells in vivo and in vitro and promotes differentiation in vitro. Int. J. Cancer 1997, 72, 860–864. [Google Scholar] [CrossRef]
- Casagrande, F.; Darbon, J.M. p21(CIP1) is dispensable for the G2 arrest caused by genistein in human melanoma cells. Exp. Cell Res. 2000, 258, 101–108. [Google Scholar] [CrossRef]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef]
- Nile, A.; Gansukh, E.; Park, G.S.; Kim, D.H.; Hariram Nile, S. Novel insights on the multi-functional properties of flavonol glucosides from red onion (Allium cepa L.) solid waste—In vitro and in silico approach. Food Chem. 2021, 335, 127650. [Google Scholar] [CrossRef]
- Plochmann, K.; Korte, G.; Koutsilieri, E.; Richling, E.; Riederer, P.; Rethwilm, A.; Schreier, P.; Scheller, C. Structure-activity relationships of flavonoid-induced cytotoxicity on human leukemia cells. Arch. Biochem. Biophys. 2007, 460, 1–9. [Google Scholar] [CrossRef]
- Wilson, P.M.; Danenberg, P.V.; Johnston, P.G.; Lenz, H.J.; Ladner, R.D. Standing the test of time: Targeting thymidylate biosynthesis in cancer therapy. Nat. Rev. Clin. Oncol. 2014, 11, 282–298. [Google Scholar] [CrossRef]
- Chu, E.; Callender, M.A.; Farrell, M.P.; Schmitz, J.C. Thymidylate synthase inhibitors as anticancer agents: From bench to bedside. Cancer Chemother. Pharmacol. Suppl. 2003, 52, 80–89. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Singh, S.K.; Gulati, M.; Chellappan, D.K.; Zacconi, F.; De Rubis, G.; Gupta, G.; Sharifi-Rad, J.; Cho, W.C. Luteolin: A flavonoid with a multifaceted anticancer potential. Cancer Cell Int. 2022, 22, 386. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in cancer and apoptosis. Cancers 2019, 11, 28. [Google Scholar] [CrossRef]
- Bo, W.; Zhao, X.H. Apigenin induces both intrinsic and extrinsic pathways of apoptosis in human colon carcinoma HCT-116 cells. Oncol. Rep. 2017, 37, 1132–1140. [Google Scholar] [CrossRef]
- Xifró, X.; Anglada-Huguet, M.; Rué, L.; Saavedra, A.; Pérez-Navarro, E.; Alberch, J. Increased 90-kDa ribosomal S6 kinase (RSK) activity is protective against mutant huntingtin toxicity. Mol. Neurodegener. 2011, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Houles, T.; Roux, P.P. Defining the role of the RSK isoforms in cancer. Semin. Cancer Biol. 2018, 48, 53–61. [Google Scholar] [CrossRef]
- Cai, X.; Ye, T.; Liu, C.; Lu, W.; Lu, M.; Zhang, J.; Wang, M.; Cao, P. Luteolin induced G2 phase cell cycle arrest and apoptosis on non-small cell lung cancer cells. Toxicol. Vitr. 2011, 25, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Park, H.S.; Lee, J.H.; Chi, G.Y.; Kim, G.Y.; Moon, S.K.; Chang, Y.C.; Hyun, J.W.; Kim, W.J.; Choi, Y.H. Induction of endoplasmic reticulum stress-mediated apoptosis and non-canonical autophagy by luteolin in NCI-H460 lung carcinoma cells. Food Chem. Toxicol. 2013, 56, 100–109. [Google Scholar] [CrossRef]
- Yang, S.-F.; Yang, W.-E.; Chang, H.-R.; Chu, S.-C.; Hsieh, Y.-S. Luteolin induces apoptosis in oral squamous cancer cells. J. Dent. Res. 2008, 87, 401–406. [Google Scholar] [CrossRef]
- Toton, E.; Lisiak, N.; Rubis, B.; Budzianowski, J.; Gruber, P.; Hofmann, J.; Rybczynska, M. The tetramethoxyflavone zapotin selectively activates protein kinase C epsilon, leading to its down-modulation accompanied by Bcl-2, c-Jun and c-Fos decrease. Eur. J. Pharmacol. 2012, 682, 21–28. [Google Scholar] [CrossRef]
- Ullah, M.F.; Ahmad, A.; Zubair, H.; Khan, H.Y.; Wang, Z.; Sarkar, F.H.; Hadi, S.M. Soy isoflavone genistein induces cell death in breast cancer cells through mobilization of endogenous copper ions and generation of reactive oxygen species. Mol. Nutr. Food Res. 2011, 55, 553–559. [Google Scholar] [CrossRef]
- Szliszka, E.; Krol, W. Soy isoflavones augment the effect of TRAIL-mediated apoptotic death in prostate cancer cells. Oncol. Rep. 2011, 26, 533–541. [Google Scholar] [CrossRef]
- Solomon, L.A.; Ali, S.; Banerjee, S.; Munkarah, A.R.; Morris, R.T.; Sarkar, F.H. Sensitization of ovarian cancer cells to cisplatin by genistein: The role of NF-kappaB. J. Ovarian Res. 2008, 1, 9–11. [Google Scholar] [CrossRef]
- Qin, J.; Teng, J.; Zhu, Z.; Chen, J.; Huang, W.J. Genistein induces activation of the mitochondrial apoptosis pathway by inhibiting phosphorylation of Akt in colorectal cancer cells. Pharm. Biol. 2016, 54, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Tophkhane, C.; Yang, S.; Bales, W.; Archer, L.; Osunkoya, A.; Thor, A.D.; Yang, X. Bcl-2 overexpression sensitizes MCF-7 cells to genistein by multiple mechanisms. Int. J. Oncol. 2007, 31, 867–874. [Google Scholar] [CrossRef]
- Krych, J.; Gebicka, L. Catalase is inhibited by flavonoids. Int. J. Biol. Macromol. 2013, 58, 148–153. [Google Scholar] [CrossRef]
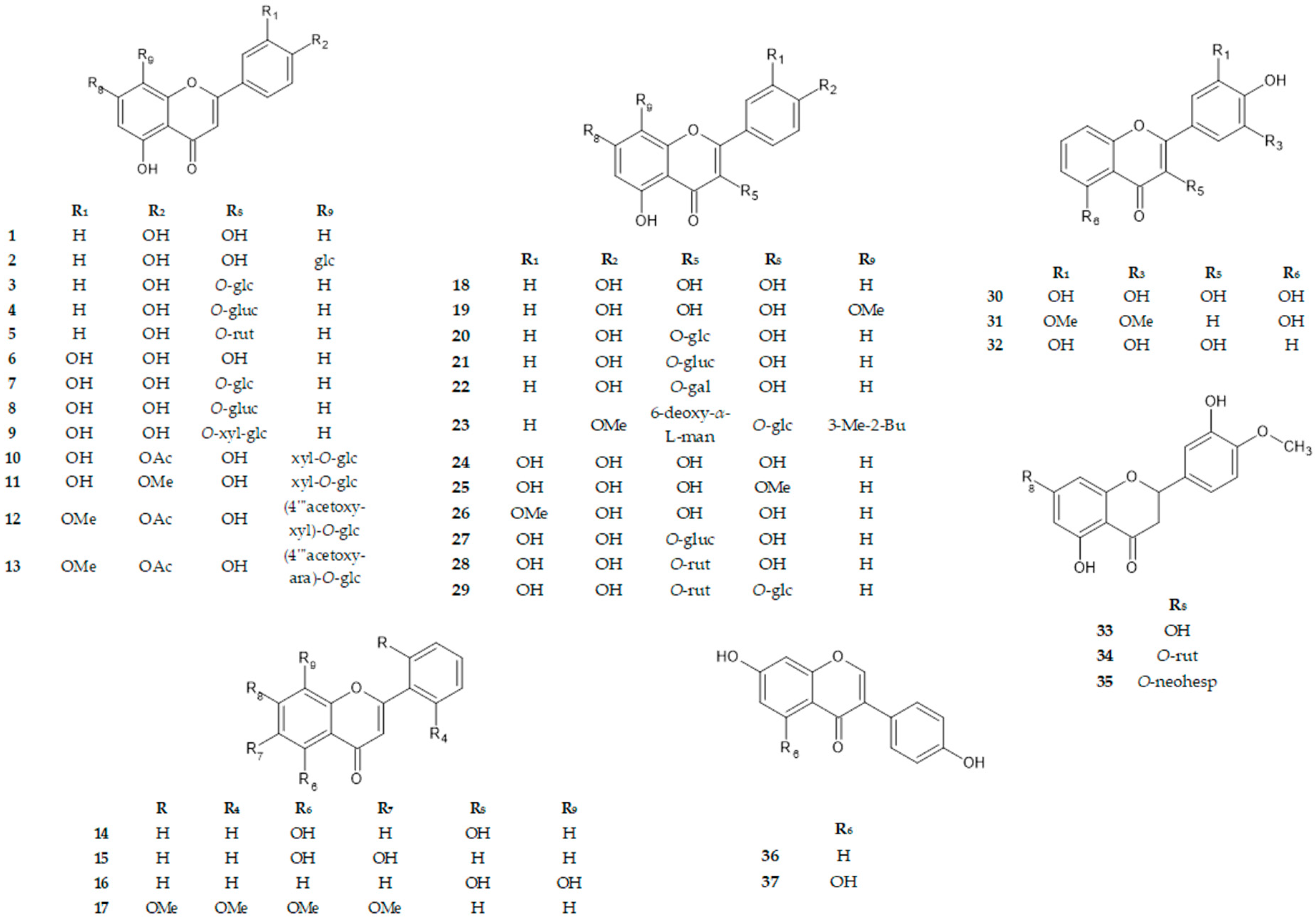

| No. | π C2-C3 | B-Ring | Benzo-γ-Pyrane Ring | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C2′ (R) r (R) | C3′ (R1) | C4′ (R2) | C5′ (R3) | C6′ (R4) | C3 (R5) | C5 (R6) | C6 (R7) | C7 (R8) | C8 (R9) | ||
| 1 | + | H | H | OH | H | H | H | OH | H | OH | H |
| 2 | + | H | H | OH | H | H | H | OH | H | OH | glc |
| 3 | + | H | H | OH | H | H | H | OH | H | O-glc | H |
| 4 | + | H | H | OH | H | H | H | OH | H | O-gluc | H |
| 5 | + | H | H | OH | H | H | H | OH | H | O-rut | H |
| 6 | + | H | OH | OH | H | H | H | OH | H | OH | H |
| 7 | + | H | OH | OH | H | H | H | OH | H | O-glc | H |
| 8 | + | H | OH | OH | H | H | H | OH | H | O-gluc | H |
| 9 | + | H | OH | OH | H | H | H | OH | H | O-xyl-glc | H |
| 10 | + | h | OH | OAc | H | H | H | OH | H | OH | xyl-O-glc |
| 11 | + | h | OH | OMe | H | H | H | OH | H | OH | xyl-O-glc |
| 12 | + | H | OMe | OAc | H | H | H | OH | H | OH | (4‴acetoxy-xyl)-O-glc |
| 13 | + | H | OMe | OAc | H | H | H | OH | H | OH | (4‴acetoxy-ara)-O-glc |
| 14 | + | H | H | H | H | H | H | OH | H | OH | H |
| 15 | + | H | H | H | H | H | H | OH | OH | H | H |
| 16 | + | H | H | H | H | H | H | H | H | OH | OH |
| 17 | + | OMe | H | H | H | OMe | H | OMe | OMe | H | H |
| 18 | + | H | H | OH | H | H | OH | OH | H | OH | H |
| 19 | + | H | H | OH | H | H | OH | OH | h | OH | OMe |
| 20 | + | H | H | OH | H | H | O-glc | OH | H | OH | H |
| 21 | + | H | H | OH | H | H | O-gluc | OH | H | OH | H |
| 22 | + | H | H | OH | H | H | O-gal | OH | H | OH | H |
| 23 | + | H | H | OMe | H | H | 6-deoxy-α-L-man | OH | H | O-glc | 3-Me-2-Bu |
| 24 | + | H | OH | OH | H | H | OH | OH | H | OH | H |
| 25 | + | H | OH | OH | H | H | OH | oH | H | OMe | H |
| 26 | + | H | OMe | OH | H | H | OH | OH | H | OH | H |
| 27 | + | H | OH | OH | H | H | O-gluc | OH | H | OH | H |
| 28 | + | H | OH | OH | H | H | O-rut | OH | H | OH | H |
| 29 | + | H | OH | OH | H | H | O-rut | OH | H | O-glc | H |
| 30 | + | H | OH | OH | OH | H | OH | OH | H | OH | H |
| 31 | + | H | OMe | OH | OMe | H | H | OH | H | OH | H |
| 32 | + | H | OH | OH | OH | H | OH | H | H | OH | H |
| 33 | − | h | OH | OMe | H | H | H | OH | H | OH | H |
| 34 | − | h | OH | OMe | H | H | H | OH | H | O-rut | H |
| 35 | − | h | OH | OMe | H | H | H | OH | H | O-neohesp | H |
| 36 | + | H | H | OH | H | H | 4-Hp | H | H | OH | H |
| 37 | + | H | H | OH | H | H | 4-Hp | OH | H | OH | H |
| No. | A375 | C32 | Normal Cells | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| 1 | >200 | 31.9 ± 3.0 | 20.7 ± 1.5 | >200 | 63.1 ± 4.7 | 31.8 ± 2.5 | >200 | >200 | 100–200 |
| 2 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 3 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 4 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 5 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 6 | >200 | 38.2 ± 4.1 | 24.6 ± 1.6 | >200 | 42.1 ± 3.5 | 13.1 ± 1.3 | >200 | 69.7 ± 5.2 | 20.1 ± 1.4 |
| 7 | >200 | >200 | 37.1 ± 3.0 | >200 | 60.5 ± 4.6 | 40.7 ± 2.9 | >200 | >200 | >200 |
| 8 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 9 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 10 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 11 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 12 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 13 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 14 | 100–200 | 66.4 ± 4.5 | 34.4 ± 2.8 | 100–200 | 96.4 ± 7.0 | 58.9 ± 4.5 | >200 | >200 | 71.2 ± 4.5 |
| 15 | 86.0 ± 6.3 | 36.4 ± 2.0 | 26.4 ± 1.2 | 100–200 | 47.5 ± 3.5 | 23.5 ± 1.9 | >200 | >200 | >200 |
| 16 | 100–200 | 33.16 ± 3.2 | 20.5 ± 2.2 | 100–200 | 39.2± 2.6 | 26.2 ± 2.8 | >200 | 100–200 | 93.3 ± 8.0 |
| 17 | 97.9 ± 7.3 | 31.1 ± 2.9 | 20.5 ± 1.9 | 100–200 | 26.5 ± 1.3 | 13.9 ± 1.2 | >200 | 78.0 ± 5.2 | 53.9 ± 3.6 |
| 18 | 100–200 | 100–200 | 75.8 ± 5.8 | 100–200 | 100–200 | 58.4 ± 3.5 | 100–200 | 100–200 | 55.8 ± 4.5 |
| 19 | 100–200 | 100–200 | 61.8 ± 3.1 | 100–200 | 100–200 | 100–200 | >200 | >200 | 100–200 |
| 20 | 94.2 ± 6.9 | 46.9 ± 3.5 | 21.5 ± 2.2 | >200 | >200 | >200 | >200 | >200 | >200 |
| 21 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 22 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 23 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 24 | >200 | >200 | 74.4 ± 5.0 | >200 | >200 | 68.1 ± 4.6 | >200 | >200 | 36.1 ± 2.0 |
| 25 | 100–200 | 42.1 ± 3.0 | 25.1 ± 2.3 | >200 | 100–200 | 37.7 ± 2.5 | 100–200 | 45.3 ± 2.3 | 35.7 ± 2.9 |
| 26 | 86.30 ± 5.9 | 68.5 ± 3.9 | 50.8 ± 4.0 | >200 | 100–200 | 49.3 ± 3.6 | 100–200 | 68.5 ± 5.4 | 46.2 ± 3.3 |
| 27 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 28 | >200 | 57.0 ± 3.1 | 57.8 ± 4.8 | >200 | >200 | >200 | >200 | >200 | >200 |
| 29 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 30 | >200 | >200 | 75.3 ± 4.8 | >200 | >200 | >200 | >200 | >200 | >200 |
| 31 | >200 | 100–200 | 100–200 | >200 | 100–200 | 100–200 | 100–200 | 80.3 ± 6.6 | 56.4 ± 4.7 |
| 32 | >200 | >200 | >100–200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 33 | 100–200 | 100–200 | 74.5 ± 6.4 | >200 | 100–200 | 76.5 ± 6.3 | >200 | >200 | >200 |
| 34 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 35 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 36 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 37 | 93.1 ± 5.8 | 60.9 ± 4.2 | 24.2 ± 2.6 | >200 | >200 | 51.1 ± 3.5 | >200 | 100–200 | 56.8 ± 4.1 |
| cPt A | 15.6 ± 2.3 | 8.1 ± 1.1 | 8.8 ± 1.1 | 18.3 ± 2.3 | 6.6 ± 1.1 | 6.2 ± 1.1 | 100–200 | 40.4 ± 4.5 | 24.5 ± 1.5 |
| No. | A375 | C32 | Normal Cells | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| 1 | 64.5 ± 4.3 | 24.7 ± 2.4 | 21.2 ± 1.4 | 100–200 | 25.7 ± 1.6 | 22.4 ± 1.5 | >200 | 87.8 ± 5.8 | 58.0 ± 3.2 |
| 2 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 3 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 4 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 5 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 6 | 58.1 ± 4.2 | 25.7 ± 1.5 | 23.9 ± 0.23 | 41.3 ± 3.8 | 13.1 ± 0.11 | 12.6 ± 1.2 | >200 | 45.2 ± 2.6 | 21.4 ± 1.7 |
| 7 | >200 | 56.7 ± 3.8 | 41.5 ± 3.3 | >200 | 29.6 ± 2.0 | 23.0 ± 2.1 | >200 | 100–200 | 100–200 |
| 8 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 9 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 10 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 11 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 12 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 13 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 14 | 52.4 ± 3.5 | 34.5 ± 2.3 | 33.1 ± 2.8 | 89.2 ± 7.8 | 44.7 ± 3.9 | 25.6 ± 2.4 | 100–200 | 65.7 ± 4.2 | 76.3 ± 4.7 |
| 15 | 53.3 ± 4.6 | 16.0 ± 1.1 | 13.6 ± 1.1 | 72.6 ± 5.5 | 18.2 ± 2.1 | 15.4 ± 1.1 | >200 | 100–200 | 100–200 |
| 16 | 100–200 | 36.0 ± 3.5 | 27.4 ± 2.4 | >200 | 19.9 ± 1.2 | 16.5 ± 2.1 | >200 | 100–200 | 72.9 ± 5.6 |
| 17 | 42.6 ± 3.2 | 14.7 ± 1.2 | 14.6 ± 1.2 | >200 | 14.7 ± 2.2 | 13.9 ± 2.1 | >200 | 59.4 ± 5.2 | 43.5 ± 2.9 |
| 18 | 100–200 | 57.4 ± 4.6 | 47.7 ± 3.9 | >200 | 62.8 ± 3.5 | 34.4 ± 2.9 | >200 | 69.8 ± 5.8 | 51.1 ± 3.2 |
| 19 | 100–200 | 100–200 | 100–200 | 100–200 | 88.8 ± 7.0 | 71.9 ± 7.0 | >200 | >200 | 64.8 ± 5.0 |
| 20 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 21 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 22 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 23 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 24 | 100–200 | 84.7 ± 6.5 | 79.2 ± 7.3 | >200 | 58.0 ± 3.5 | 47.5 ± 4.3 | >200 | 92.6 ± 4.2 | 36.4 ± 0.23 |
| 25 | >200 | 63.4 ± 4.8 | 43.7 ± 3.5 | >200 | 55.4 ± 4.3 | 28.4 ± 2.5 | >200 | 66.3 ± 5.6 | 38.4 ± 3.4 |
| 26 | >200 | 100–200 | 78.1 ± 6.5 | >200 | 100–200 | 65.1 ± 4.0 | >200 | 78.7 ± 5.5 | 69.2 ± 4.6 |
| 27 | >200 | >200 | >200 | >200 | >200 | 100–200 | >200 | >200 | >200 |
| 28 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 29 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 30 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 31 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 32 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 33 | 100–200 | 100–200 | 100–200 | 69.0 ± 5.1 | 58.3 ± 5.6 | 53.7 ± 4.0 | >200 | >200 | 100–200 |
| 34 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 35 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 36 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 |
| 37 | 49.2 ± 3.2 | 32.3 ± 3.5 | 19.3 ± 2.2 | 100–200 | 31.5 ± 2.8 | 14.4 ± 2.1 | >200 | 57.8 ± 4.0 | 38.6 ± 3.7 |
| cPt A | 16.6 ± 2.1 | 14.4 ± 1.1 | 9.9 ± 1.1 | 15.7 ± 1.2 | 15.0 ± 1.2 | 13.8 ± 1.5 | 100–200 | 47.5 ± 4.9 | 24.4 ± 3.5 |
| A375 | C32 | Normal Cells | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| 1 | 87.7 ± 4.2 | 28.6 ± 1.8 | 23.4 ± 1.6 | >200 | 27.0 ± 1.4 | 22.5 ± 1.2 | 187.5 ± 8.1 | 188.6 ± 9.5 | >200 |
| 6 | >200 | 38.5 ± 2.9 | 30.9 ± 1.8 | >200 | 30.9 ± 2.2 | 24.5 ± 1.81 | >200 | >200 | >200 |
| 15 | >200 | 32.4 ± 2.4 | 31.4 ± 1.7 | >200 | 20.0 ± 1.9 | 20.3 ± 1.2 | 174.9 ± 7.6 | >200 | >200 |
| 16 | 62.1 ± 3.9 | 29.7 ± 1.6 | 20.5 ± 2.2 | 38.1 ± 1.5 | 18.4 ± 1.4 | 12.8 ± 0.9 | 81.6 ± 5.8 | 64.6 ± 2.8 | 37.0 ± 2.5 |
| 17 | >200 | 6.0 ± 0.2 | 5.34 ± 0.4 | 58.7 ± 3.8 | 9.1 ± 1.2 | 5.8 ± 3.1 | >200 | >200 | 87.9 ± 4.0 |
| 37 | 153.5 ± 10.8 | 28.0 ± 0.8 | 24.2 ± 1.5 | 25.4 ± 1.3 | 18.5 ± 0.7 | 19.1 ± 0.3 | >200 | >200 | >200 |
| cPt | 3.8 ± 0.2 | 3.9 ± 0.5 | 3.4 ± 0.3 | 6.8 ± 0.4 | 5.7 ± 0.3 | 4.4 ± 0.4 | 7.1 ± 0.9 | 6.1 ± 0.5 | 6.1 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakimiuk, K.; Szoka, Ł.; Surażyński, A.; Tomczyk, M. Using Flavonoid Substitution Status to Predict Anticancer Effects in Human Melanoma Cancers: An In Vitro Study. Cancers 2024, 16, 487. https://doi.org/10.3390/cancers16030487
Jakimiuk K, Szoka Ł, Surażyński A, Tomczyk M. Using Flavonoid Substitution Status to Predict Anticancer Effects in Human Melanoma Cancers: An In Vitro Study. Cancers. 2024; 16(3):487. https://doi.org/10.3390/cancers16030487
Chicago/Turabian StyleJakimiuk, Katarzyna, Łukasz Szoka, Arkadiusz Surażyński, and Michał Tomczyk. 2024. "Using Flavonoid Substitution Status to Predict Anticancer Effects in Human Melanoma Cancers: An In Vitro Study" Cancers 16, no. 3: 487. https://doi.org/10.3390/cancers16030487
APA StyleJakimiuk, K., Szoka, Ł., Surażyński, A., & Tomczyk, M. (2024). Using Flavonoid Substitution Status to Predict Anticancer Effects in Human Melanoma Cancers: An In Vitro Study. Cancers, 16(3), 487. https://doi.org/10.3390/cancers16030487









