Gastrointestinal Microbiota in Gastric Cancer: Potential Mechanisms and Clinical Applications—A Literature Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Gastrointestinal Flora Associated with GC
2.1. H. pylori and GC
2.2. Bacteria Other than H. pylori and GC
2.3. Epstein–Barr Virus and GC
2.4. Fungi and GC
3. The Development of Microbial-Sequencing Technologies
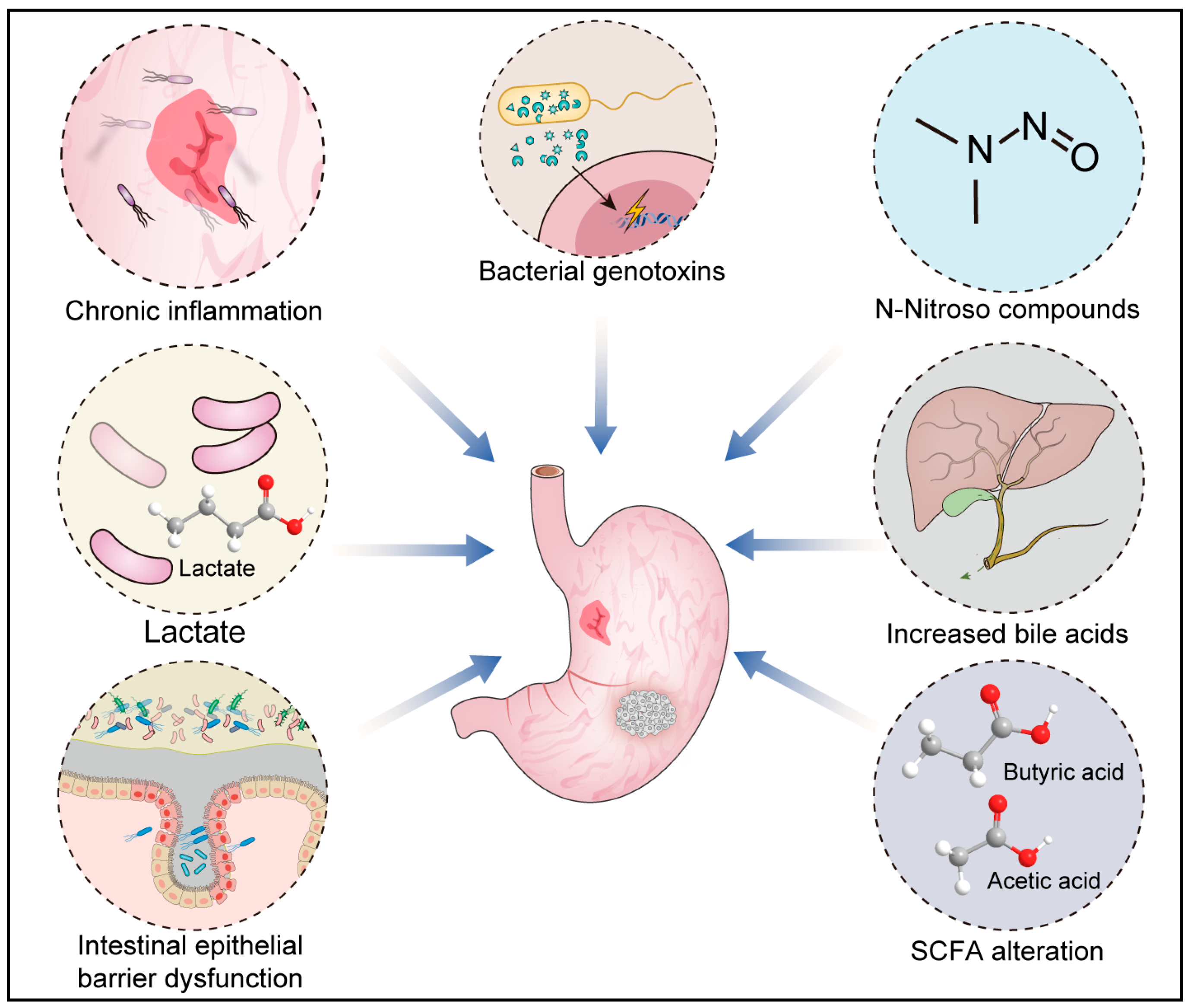
4. The Pathogenic Mechanisms of Microbes in GC
4.1. Chronic Inflammation
4.2. Bacterial Genotoxins
4.3. Short-Chain Fatty Acid (SCFA) Alteration
4.4. Increased Bile Acids
4.5. Intestinal Epithelial Barrier Dysfunction
4.6. N-Nitroso Compounds
4.7. Lactate
5. Influence of Gastrointestinal Microbes on GC Treatment
5.1. Chemotherapy
5.2. Immunotherapy
5.3. Radiotherapy
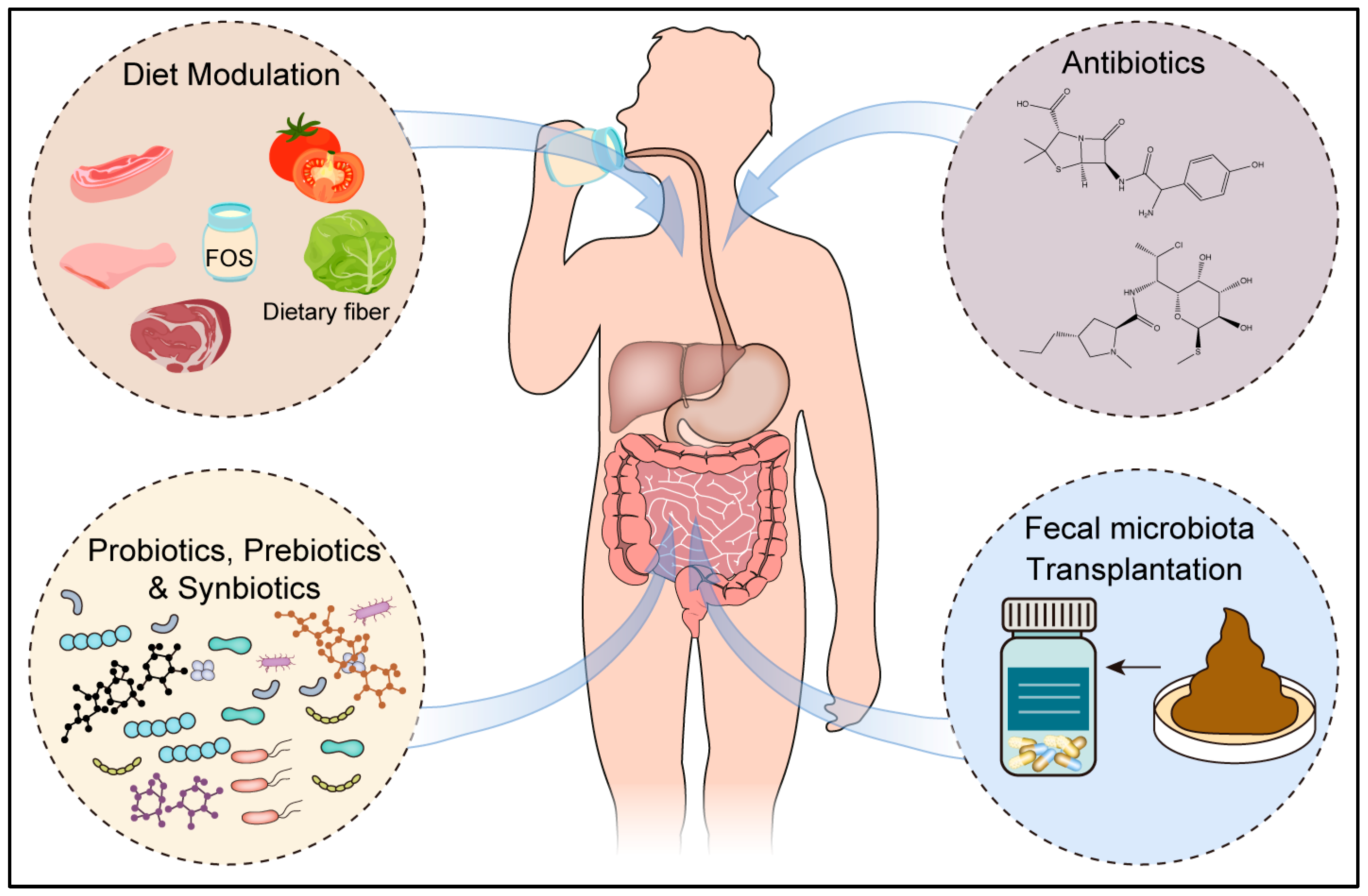
6. Future Therapeutic Approaches Targeting the Microbiome
6.1. Diet
6.2. Antibiotics Act as Modulators of Microbiota
6.3. Probiotics, Prebiotics, and Synbiotics
6.4. Fecal Microbiota Transplantation (FMT)
7. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Cancer Genome Atlas Research Network. Comprehensive Molecular Characterization of Gastric Adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Lauren, P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Haenszel, W.; Cuello, C.; Tannenbaum, S.; Archer, M. A Model for Gastric Cancer Epidemiology. Lancet 1975, 2, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori Infection and the Development of Gastric Cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, A.M.; Shanahan, F. The Gut Flora as a Forgotten Organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef]
- Alarcon, T.; Llorca, L.; Perez-Perez, G. Impact of the Microbiota and Gastric Disease Development by Helicobacter pylori. Curr. Top. Microbiol. Immunol. 2017, 400, 253–275. [Google Scholar]
- Cao, C.; Yue, S.; Lu, A.; Liang, C. Host-Gut Microbiota Metabolic Interactions and Their Role in Precision Diagnosis and Treatment of Gastrointestinal Cancers. Pharmacol. Res. 2024, 207, 107321. [Google Scholar] [CrossRef]
- Warren, J.R.; Marshall, B. Unidentified Curved Bacilli on Gastric Epithelium in Active Chronic Gastritis. Lancet 1983, 1, 1273–1275. [Google Scholar]
- Bik, E.M.; Eckburg, P.B.; Gill, S.R.; Nelson, K.E.; Purdom, E.A.; Francois, F.; Perez-Perez, G.; Blaser, M.J.; Relman, D.A. Molecular Analysis of the Bacterial Microbiota in the Human Stomach. Proc. Natl. Acad. Sci. USA 2006, 103, 732–737. [Google Scholar] [CrossRef]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Leja, M.; Axon, A.; Brenner, H. Epidemiology of Helicobacter pylori Infection. Helicobacter 2016, 21 (Suppl. S1), 3–7. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhu, Y.; Lu, N.H. The Management of Helicobacter pylori Infection and Prevention and Control of Gastric Cancer in China. Front. Cell. Infect. Microbiol. 2022, 12, 1049279. [Google Scholar] [CrossRef] [PubMed]
- Yakirevich, E.; Resnick, M.B. Pathology of Gastric Cancer and Its Precursor Lesions. Gastroenterol. Clin. N. Am. 2013, 42, 261–284. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Yuan, Y.; Moayyedi, P. Helicobacter pylori Eradication Therapy to Prevent Gastric Cancer: Systematic Review and Meta-Analysis. Gut 2020, 69, 2113–2121. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori Infection: The Maastricht VI/Florence Consensus Report. Gut 2022, 7, 1724–1762. [Google Scholar] [CrossRef]
- Liou, J.M.; Malfertheiner, P.; Lee, Y.C.; Sheu, B.S.; Sugano, K.; Cheng, H.C.; Yeoh, K.G.; Hsu, P.I.; Goh, K.L.; Mahachai, V.; et al. Screening and Eradication of Helicobacter pylori for Gastric Cancer Prevention: The Taipei Global Consensus. Gut 2020, 69, 2093–2112. [Google Scholar] [CrossRef]
- Hara, D.; Okamura, T.; Iwaya, Y.; Nagaya, T.; Ota, H.; Umemura, T. Histopathologically Defined Intestinal Metaplasia in Lesser Curvature of Corpus Prior to Helicobacter pylori Eradication Is a Risk Factor for Gastric Cancer Development. Helicobacter 2022, 27, e12934. [Google Scholar] [CrossRef]
- Yu, G.; Torres, J.; Hu, N.; Medrano-Guzman, R.; Herrera-Goepfert, R.; Humphrys, M.S.; Wang, L.; Wang, C.; Ding, T.; Ravel, J.; et al. Molecular Characterization of the Human Stomach Microbiota in Gastric Cancer Patients. Front. Cell. Infect. Microbiol. 2017, 7, 302. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Tung, S.Y.; Pan, H.Y.; Yen, C.W.; Xu, H.W.; Lin, Y.J.; Deng, Y.F.; Hsu, W.T.; Wu, C.S.; Li, C. Increased Abundance of Clostridium and Fusobacterium in Gastric Microbiota of Patients with Gastric Cancer in Taiwan. Sci. Rep. 2018, 8, 158. [Google Scholar] [CrossRef]
- Yang, J.; Xu, J.; Ling, Z.; Zhou, X.; Si, Y.; Liu, X.; Ji, F. Prognostic effects of the Gastric Mucosal Microbiota in Gastric Cancer. Cancer Sci. 2023, 114, 1075–1085. [Google Scholar] [CrossRef]
- de Assumpcao, P.P.; Araujo, T.M.T.; de Assumpcao, P.B.; Barra, W.F.; Khayat, A.S.; Assumpcao, C.B.; Ishak, G.; Nunes, D.N.; Dias-Neto, E.; Coelho, L.G.V. Suicide Journey of H. pylori through Gastric Carcinogenesis: The Role of Non-H. pylori Microbiome and Potential Consequences for Clinical Practice. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- El-Omar, E.M.; Oien, K.; El-Nujumi, A.; Gillen, D.; Wirz, A.; Dahill, S.; Williams, C.; Ardill, J.E.; McColl, K.E. Helicobacter pylori Infection and Chronic Gastric Acid Hyposecretion. Gastroenterology 1997, 113, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Hu, N.; Wang, L.; Wang, C.; Han, X.Y.; Humphry, M.; Ravel, J.; Abnet, C.C.; Taylor, P.R.; Goldstein, A.M. Gastric Microbiota Features Associated with Cancer Risk Factors and Clinical Outcomes: A Pilot Study in Gastric Cardia Cancer Patients from Shanxi, China. Int. J. Cancer 2017, 141, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Stewart, O.A.; Wu, F.; Chen, Y. The Role of Gastric Microbiota in Gastric Cancer. Gut Microbes 2020, 11, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.J.Y.; Coker, O.O.; Chu, E.; Szeto, C.H.; Luk, S.T.Y.; Lau, H.C.H.; Yu, J. Gastric Microbes Associated with Gastric Inflammation, Atrophy and Intestinal Metaplasia 1 Year after Helicobacter pylori Eradication. Gut 2020, 69, 1572–1580. [Google Scholar] [CrossRef]
- Nakano, T.; Dohi, O.; Takagi, T.; Naito, Y.; Fukui, H.; Miyazaki, H.; Yasuda, T.; Yoshida, T.; Azuma, Y.; Ishida, T.; et al. Characteristics of Gastric Mucosa-Associated Microbiota in Patients with Early Gastric Cancer After Successful Helicobacter pylori Eradication. Dig. Dis. Sci. 2023, 68, 4398–4406. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, J.; Xin, Y.; Geng, C.; Tian, Z.; Yu, X.; Dong, Q. Bacterial Overgrowth and Diversification of Microbiota in Gastric Cancer. Eur. J. Gastroenterol. Hepatol. 2016, 28, 261–266. [Google Scholar] [CrossRef]
- Liu, X.; Shao, L.; Liu, X.; Ji, F.; Mei, Y.; Cheng, Y.; Liu, F.; Yan, C.; Li, L.; Ling, Z. Alterations of Gastric Mucosal Microbiota across Different Stomach Microhabitats in a Cohort of 276 Patients with Gastric Cancer. EBioMedicine 2019, 40, 336–348. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Zhan, X.; Song, Y.; Xu, M.; Wang, S.; Huang, X.; Xu, Z.Z. Meta-Analysis Reveals Helicobacter pylori Mutual Exclusivity and Reproducible Gastric Microbiome Alterations during Gastric Carcinoma Progression. Gut Microbes 2023, 15, 2197835. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Gerhard, M.; Gao, J.J.; Mejias-Luque, R.; Zhang, L.; Vieth, M.; Ma, J.L.; Bajbouj, M.; Suchanek, S.; et al. Effect of Helicobacter pylori on Gastrointestinal Microbiota: A Population-Based Study in Linqu, a High-Risk Area of Gastric Cancer. Gut 2020, 69, 1598–1607. [Google Scholar] [CrossRef]
- Thorell, K.; Bengtsson-Palme, J.; Liu, O.H.; Palacios Gonzales, R.V.; Nookaew, I.; Rabeneck, L.; Paszat, L.; Graham, D.Y.; Nielsen, J.; Lundin, S.B.; et al. In Vivo Analysis of the Viable Microbiota and Helicobacter pylori Transcriptome in Gastric Infection and Early Stages of Carcinogenesis. Infect. Immun. 2017, 85, e00031-17. [Google Scholar] [CrossRef] [PubMed]
- Ai, B.; Mei, Y.; Liang, D.; Wang, T.; Cai, H.; Yu, D. Uncovering the Special Microbiota Associated with Occurrence and Progression of Gastric Cancer by Using RNA-Sequencing. Sci. Rep. 2023, 13, 5722. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.M.; Yang, Y.S.; Peng, L.H. Microbiota in the Stomach: New Insights. J. Dig. Dis. 2014, 15, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Contreras, A.; Goldfarb, K.C.; Godoy-Vitorino, F.; Karaoz, U.; Contreras, M.; Blaser, M.J.; Brodie, E.L.; Dominguez-Bello, M.G. Structure of the Human Gastric Bacterial Community in Relation to Helicobacter pylori Status. ISME J. 2011, 5, 574–579. [Google Scholar] [CrossRef]
- Park, J.Y.; Seo, H.; Kang, C.S.; Shin, T.S.; Kim, J.W.; Park, J.M.; Kim, J.G.; Kim, Y.K. Dysbiotic Change in Gastric Microbiome and its Functional Implication in Gastric Carcinogenesis. Sci. Rep. 2022, 12, 4285. [Google Scholar] [CrossRef]
- Valentino, M.A.; Brown, J.W.; Cronan-Hillix, W.A. Aesthetic Preference and Lateral Dominance. Percept. Mot. Ski. 1988, 67, 555–561. [Google Scholar] [CrossRef]
- Png, C.W.; Lee, W.J.J.; Chua, S.J.; Zhu, F.; Gastric, C.; Yeoh, K.G.; Zhang, Y. Mucosal Microbiome Associates with Progression to Gastric Cancer. Theranostics 2022, 12, 48–58. [Google Scholar] [CrossRef]
- Dai, D.; Yang, Y.; Yu, J.; Dang, T.; Qin, W.; Teng, L.; Ye, J.; Jiang, H. Interactions between Gastric Microbiota and Metabolites in Gastric Cancer. Cell Death Dis. 2021, 12, 1104. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Barros, A.; Pita, I.; Miranda, I.; Conceicao, G.; Borges-Canha, M.; Leite-Moreira, A.F.; Libanio, D.; Dinis-Ribeiro, M. Gastric Microbiome Profile throughout Gastric Carcinogenesis: Beyond Helicobacter. Scand. J. Gastroenterol. 2021, 56, 708–716. [Google Scholar] [CrossRef]
- Zhou, C.B.; Pan, S.Y.; Jin, P.; Deng, J.W.; Xue, J.H.; Ma, X.Y.; Xie, Y.H.; Cao, H.; Liu, Q.; Xie, W.F.; et al. Fecal Signatures of Streptococcus anginosus and Streptococcus constellatus for Noninvasive Screening and Early Warning of Gastric Cancer. Gastroenterology 2022, 162, 1933–1947.e18. [Google Scholar] [CrossRef]
- Aviles-Jimenez, F.; Vazquez-Jimenez, F.; Medrano-Guzman, R.; Mantilla, A.; Torres, J. Stomach Microbiota Composition Varies between Patients with Non-Atrophic Gastritis and Patients with Intestinal Type of Gastric Cancer. Sci. Rep. 2014, 4, 4202. [Google Scholar] [CrossRef] [PubMed]
- Dicksved, J.; Lindberg, M.; Rosenquist, M.; Enroth, H.; Jansson, J.K.; Engstrand, L. Molecular Characterization of the Stomach Microbiota in Patients with Gastric Cancer and in Controls. J. Med. Microbiol. 2009, 58, 509–516. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wang, F.; Yang, J.; Yang, S. Explore and Analyze the Composition and Characteristics of Intestinal Microbiota between Gastric Cancer Patients and Healthy People. Evid.-Based Complement. Altern. Med. 2022, 2022, 5834293. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, X.; Zeng, R.; Wu, Q.; Sun, H.; Wu, W.; Zhang, X.; Sun, G.; Yan, B.; Wu, L.; et al. Changes of the Gastric Mucosal Microbiome Associated with Histological Stages of Gastric Carcinogenesis. Front. Microbiol. 2020, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Ravegnini, G.; Fosso, B.; Saverio, V.D.; Sammarini, G.; Zanotti, F.; Rossi, G.; Ricci, M.; D’Amico, F.; Valori, G.; Ioli, A.; et al. Gastric Adenocarcinomas and Signet-Ring Cell Carcinoma: Unraveling Gastric Cancer Complexity through Microbiome Analysis-Deepening Heterogeneity for a Personalized Therapy. Int. J. Mol. Sci. 2020, 21, 9735. [Google Scholar] [CrossRef]
- Coker, O.O.; Dai, Z.; Nie, Y.; Zhao, G.; Cao, L.; Nakatsu, G.; Wu, W.K.; Wong, S.H.; Chen, Z.; Sung, J.J.Y.; et al. Mucosal Microbiome Dysbiosis in Gastric Carcinogenesis. Gut 2018, 67, 1024–1032. [Google Scholar] [CrossRef]
- Peng, R.; Liu, S.; You, W.; Huang, Y.; Hu, C.; Gao, Y.; Jia, X.; Li, G.; Xu, Z.; Chen, Y. Gastric Microbiome Alterations Are Associated with Decreased CD8+ Tissue-Resident Memory T Cells in the Tumor Microenvironment of Gastric Cancer. Cancer Immunol. Res. 2022, 10, 1224–1240. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, R.; Chen, S.; Sun, B.; Zhang, K. Analysis of Gastric Microbiome Reveals Three Distinctive Microbial Communities Associated with the Occurrence of Gastric Cancer. BMC Microbiol. 2022, 22, 184. [Google Scholar] [CrossRef]
- Kim, H.N.; Kim, M.J.; Jacobs, J.P.; Yang, H.J. Altered Gastric Microbiota and Inflammatory Cytokine Responses in Patients with Helicobacter pylori-Negative Gastric Cancer. Nutrients 2022, 14, 4981. [Google Scholar] [CrossRef]
- Gantuya, B.; El Serag, H.B.; Matsumoto, T.; Ajami, N.J.; Uchida, T.; Oyuntsetseg, K.; Bolor, D.; Yamaoka, Y. Gastric Mucosal Microbiota in a Mongolian Population with Gastric Cancer and Precursor Conditions. Aliment. Pharmacol. Ther. 2020, 51, 770–780. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Pereira-Marques, J.; Pinto-Ribeiro, I.; Costa, J.L.; Carneiro, F.; Machado, J.C.; Figueiredo, C. Gastric Microbial Community Profiling Reveals a Dysbiotic Cancer-Associated Microbiota. Gut 2018, 67, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Castano-Rodriguez, N.; Goh, K.L.; Fock, K.M.; Mitchell, H.M.; Kaakoush, N.O. Dysbiosis of the Microbiome in Gastric Carcinogenesis. Sci. Rep. 2017, 7, 15957. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, T.; Wang, Y.; Gao, Y.; Kong, Y.; Liu, Z.; Deng, X. A Randomised Trial of Probiotics to Reduce Severity of Physiological and Microbial Disorders Induced by Partial Gastrectomy for Patients with Gastric Cancer. J. Cancer 2019, 10, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zheng, C.; Xu, X.; Jin, R.; Huang, F.; Shi, M.; He, Z.; Luo, Y.; Liu, L.; Liu, Z.; et al. Clostridium butyricum potentially improves inflammation and immunity through alteration of the microbiota and metabolism of gastric cancer patients after gastrectomy. Front. Immunol. 2022, 13, 1076245. [Google Scholar] [CrossRef]
- Chen, C.; Du, Y.; Liu, Y.; Shi, Y.; Niu, Y.; Jin, G.; Shen, J.; Lyu, J.; Lin, L. Characteristics of Gastric Cancer Gut Microbiome according to Tumor Stage and Age Segmentation. Appl. Microbiol. Biotechnol. 2022, 106, 6671–6687. [Google Scholar] [CrossRef]
- Yang, Y.; Dai, D.; Jin, W.; Huang, Y.; Zhang, Y.; Chen, Y.; Wang, W.; Lin, W.; Chen, X.; Zhang, J.; et al. Microbiota and Metabolites Alterations in Proximal and Distal Gastric Cancer Patients. J. Transl. Med. 2022, 20, 439. [Google Scholar] [CrossRef]
- Tavakoli, A.; Monavari, S.H.; Solaymani Mohammadi, F.; Kiani, S.J.; Armat, S.; Farahmand, M. Association between Epstein-Barr Virus Infection and Gastric Cancer: A Systematic Review and Meta-Analysis. BMC Cancer 2020, 20, 493. [Google Scholar] [CrossRef]
- Saito, M.; Kono, K. Landscape of EBV-Positive Gastric Cancer. Gastric Cancer 2021, 24, 983–989. [Google Scholar] [CrossRef]
- Murphy, G.; Pfeiffer, R.; Camargo, M.C.; Rabkin, C.S. Meta-Analysis Shows That Prevalence of Epstein-Barr Virus-Positive Gastric Cancer Differs Based on Sex and Anatomic Location. Gastroenterology 2009, 137, 824–833. [Google Scholar] [CrossRef]
- van Beek, J.; zur Hausen, A.; Klein Kranenbarg, E.; van de Velde, C.J.; Middeldorp, J.M.; van den Brule, A.J.; Meijer, C.J.; Bloemena, E. EBV-Positive Gastric Adenocarcinomas: A Distinct Clinicopathologic Entity with a Low Frequency of Lymph Node Involvement. J. Clin. Oncol. 2004, 22, 664–670. [Google Scholar] [CrossRef]
- Camargo, M.C.; Kim, W.H.; Chiaravalli, A.M.; Kim, K.M.; Corvalan, A.H.; Matsuo, K.; Yu, J.; Sung, J.J.; Herrera-Goepfert, R.; Meneses-Gonzalez, F.; et al. Improved Survival of Gastric Cancer with Tumour Epstein-Barr Virus Positivity: An International Pooled Analysis. Gut 2014, 63, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xie, T.; Wang, Z.; Tong, S.; Zhao, X.; Zhao, F.; Cai, J.; Wei, X.; Peng, Z.; Shen, L. Efficacy and Predictive Biomarkers of Immunotherapy in Epstein-Barr Virus-Associated Gastric Cancer. J. Immunother. Cancer 2022, 10, e004080. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P.; Kramer, J.R.; Hartman, C.M.; Royse, K.; Richardson, P.; Dong, Y.; Raychaudhury, S.; Desiderio, R.; Sanchez, D.; Anandasabapathy, S.; et al. Risk and Predictors of Esophageal and Stomach Cancers in HIV-Infected Veterans: A Matched Cohort Study. J. Acquir. Immune Defic. Syndr. 2019, 81, e65–e72. [Google Scholar] [CrossRef] [PubMed]
- Kayamba, V.; Butt, J.; Waterboer, T.; Besa, E.; Choudhry, N.; Hamasuku, A.; Julius, P.; Heimburger, D.C.; Atadzhanov, M.; Kelly, P. Molecular Profiling of Gastric Cancer in a Population with High HIV Prevalence Reveals a Shift to MLH1 Loss but not the EBV Subtype. Cancer Med. 2020, 9, 3445–3454. [Google Scholar] [CrossRef]
- Wahl, A.; Yao, W.; Liao, B.; Chateau, M.; Richardson, C.; Ling, L.; Franks, A.; Senthil, K.; Doyon, G.; Li, F.; et al. A Germ-Free Humanized Mouse Model Shows the Contribution of Resident Microbiota to Human-Specific Pathogen Infection. Nat. Biotechnol. 2024, 42, 905–915. [Google Scholar] [CrossRef]
- Coker, O.O.; Nakatsu, G.; Dai, R.Z.; Wu, W.K.K.; Wong, S.H.; Ng, S.C.; Chan, F.K.L.; Sung, J.J.Y.; Yu, J. Enteric Fungal Microbiota Dysbiosis and Ecological Alterations in Colorectal Cancer. Gut 2019, 68, 654–662. [Google Scholar] [CrossRef]
- Dohlman, A.B.; Klug, J.; Mesko, M.; Gao, I.H.; Lipkin, S.M.; Shen, X.; Iliev, I.D. A Pan-Cancer Mycobiome Analysis Reveals Fungal Involvement in Gastrointestinal and Lung Tumors. Cell 2022, 185, 3807–3822.e12. [Google Scholar] [CrossRef]
- Zhong, M.; Xiong, Y.; Zhao, J.; Gao, Z.; Ma, J.; Wu, Z.; Song, Y.; Hong, X. Candida Albicans Disorder Is Associated with Gastric Carcinogenesis. Theranostics 2021, 11, 4945–4956. [Google Scholar] [CrossRef]
- Li, X.X.; Wong, G.L.; To, K.F.; Wong, V.W.; Lai, L.H.; Chow, D.K.; Lau, J.Y.; Sung, J.J.; Ding, C. Bacterial Microbiota Profiling in Gastritis without Helicobacter pylori Infection or Non-Steroidal Anti-Inflammatory Drug Use. PLoS ONE 2009, 4, e7985. [Google Scholar] [CrossRef]
- Tong, M.; Jacobs, J.P.; McHardy, I.H.; Braun, J. Sampling of Intestinal Microbiota and Targeted Amplification of Bacterial 16S rRNA Genes for Microbial Ecologic Analysis. Curr. Protoc. Immunol. 2014, 107, 7.41.1–7.41.11. [Google Scholar] [CrossRef]
- Campos-Madueno, E.I.; Aldeia, C.; Endimiani, A. Nanopore R10.4 Metagenomic Detection of bla(CTX-M)/bla(DHA) Antimicrobial Resistance Genes and Their Genetic Environments in Stool. Nat. Commun. 2024, 15, 7450. [Google Scholar] [CrossRef] [PubMed]
- Bars-Cortina, D.; Ramon, E.; Rius-Sansalvador, B.; Guino, E.; Garcia-Serrano, A.; Mach, N.; Khannous-Lleiffe, O.; Saus, E.; Gabaldon, T.; Ibanez-Sanz, G.; et al. Comparison between 16S rRNA and Shotgun Sequencing in Colorectal Cancer, Advanced Colorectal Lesions, and Healthy Human Gut Microbiota. BMC Genom. 2024, 25, 730. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Xu, S.Y.; Ren, Z.G.; Tao, L.; Jiang, J.W.; Zheng, S.S. Application of Metagenomics in the Human Gut Microbiome. World J. Gastroenterol. 2015, 21, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Madhu, B.; Miller, B.M.; Levy, M. Single-Cell Analysis and Spatial Resolution of the Gut Microbiome. Front. Cell. Infect. Microbiol. 2023, 13, 1271092. [Google Scholar] [CrossRef] [PubMed]
- Lamb, A.; Chen, L.F. Role of the Helicobacter pylori-Induced Inflammatory Response in the Development of Gastric Cancer. J. Cell. Biochem. 2013, 114, 491–497. [Google Scholar] [CrossRef]
- Yamaoka, Y. Mechanisms of Disease: Helicobacter pylori Virulence Factors. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 629–641. [Google Scholar] [CrossRef]
- McGee, D.J.; Mobley, H.L. Pathogenesis of Helicobacter pylori Infection. Curr. Opin. Gastroenterol. 2000, 16, 24–31. [Google Scholar] [CrossRef]
- Lamb, A.; Chen, L.F. The Many Roads Traveled by Helicobacter pylori to NFkappaB Activation. Gut Microbes 2010, 1, 109–113. [Google Scholar] [CrossRef]
- Engevik, M.A.; Danhof, H.A.; Ruan, W.; Engevik, A.C.; Chang-Graham, A.L.; Engevik, K.A.; Shi, Z.; Zhao, Y.; Brand, C.K.; Krystofiak, E.S.; et al. Fusobacterium nucleatum Secretes Outer Membrane Vesicles and Promotes Intestinal Inflammation. mBio 2021, 12, e02706-20. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Mei, J.X.; Yu, G.; Lei, L.; Zhang, W.H.; Liu, K.; Chen, X.L.; Kolat, D.; Yang, K.; Hu, J.K. Role of the Gut Microbiota in Anticancer Therapy: From Molecular Mechanisms to Clinical Applications. Signal Transduct. Target. Ther. 2023, 8, 201. [Google Scholar] [CrossRef]
- Druzhinin, V.G.; Matskova, L.V.; Fucic, A. Induction and Modulation of Genotoxicity by the Bacteriome in Mammals. Mutat. Res. Rev. Mutat. Res. 2018, 776, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, K. Genotoxins: The Mechanistic Links between Escherichia coli and Colorectal Cancer. Cancers 2023, 15, 1152. [Google Scholar] [CrossRef] [PubMed]
- Baral, B.; Kandpal, M.; Ray, A.; Jana, A.; Yadav, D.S.; Sachin, K.; Mishra, A.; Baig, M.S.; Jha, H.C. Helicobacter pylori and Epstein-Barr Virus Infection in Cell Polarity Alterations. Folia Microbiol. 2024, 69, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef]
- Mirzaei, R.; Afaghi, A.; Babakhani, S.; Sohrabi, M.R.; Hosseini-Fard, S.R.; Babolhavaeji, K.; Khani Ali Akbari, S.; Yousefimashouf, R.; Karampoor, S. Role of Microbiota-Derived Short-Chain Fatty Acids in Cancer Development and Prevention. Biomed. Pharmacother. 2021, 139, 111619. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between Diet, Gut Microbiota Composition and Gut Metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef]
- Matthews, G.M.; Howarth, G.S.; Butler, R.N. Short-Chain Fatty Acid Modulation of Apoptosis in the Kato III Human Gastric Carcinoma Cell Line. Cancer Biol. Ther. 2007, 6, 1051–1057. [Google Scholar] [CrossRef]
- Kim, Y.L.; Lee, W.; Chung, S.H.; Yu, B.M.; Lee, Y.C.; Hong, J. Metabolic Alterations of Short-Chain Fatty Acids and TCA Cycle Intermediates in Human Plasma from Patients with Gastric Cancer. Life Sci. 2022, 309, 121010. [Google Scholar] [CrossRef]
- Wang, S.; Kuang, J.; Zhang, H.; Chen, W.; Zheng, X.; Wang, J.; Huang, F.; Ge, K.; Li, M.; Zhao, M.; et al. Bile Acid-Microbiome Interaction Promotes Gastric Carcinogenesis. Adv. Sci. 2022, 9, e2200263. [Google Scholar] [CrossRef]
- Winston, J.A.; Theriot, C.M. Diversification of Host Bile Acids by Members of the Gut Microbiota. Gut Microbes 2020, 11, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Wang, D.Q.; Molina-Molina, E.; Lunardi Baccetto, R.; Calamita, G.; Palmieri, V.O.; Portincasa, P. Bile Acids and Cancer: Direct and Environmental-Dependent Effects. Ann. Hepatol. 2017, 16, s87–s105. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.B.; Fukiya, S.; Hagio, M.; Fujii, N.; Ishizuka, S.; Ooka, T.; Ogura, Y.; Hayashi, T.; Yokota, A. Bile Acid Is a Host Factor that Regulates the Composition of the Cecal Microbiota in Rats. Gastroenterology 2011, 141, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, L.; Wang, X.; Kang, H.; Ma, X.; Wang, M.; Lin, S.; Liu, M.; Dai, C.; Dai, Z. Acidified Bile Acids Enhance Tumor Progression and Telomerase Activity of Gastric Cancer in Mice Dependent on c-Myc Expression. Cancer Med. 2017, 6, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Tatsugami, M.; Ito, M.; Tanaka, S.; Yoshihara, M.; Matsui, H.; Haruma, K.; Chayama, K. Bile acid Promotes Intestinal Metaplasia and Gastric Carcinogenesis. Cancer Epidemiol. Biomark. Prev. 2012, 21, 2101–2107. [Google Scholar] [CrossRef]
- Noto, J.M.; Piazuelo, M.B.; Shah, S.C.; Romero-Gallo, J.; Hart, J.L.; Di, C.; Carmichael, J.D.; Delgado, A.G.; Halvorson, A.E.; Greevy, R.A.; et al. Iron Deficiency Linked to Altered Bile Acid Metabolism Promotes Helicobacter pylori-Induced Inflammation-Driven Gastric Carcinogenesis. J. Clin. Investig. 2022, 132, e147822. [Google Scholar] [CrossRef]
- Jin, D.; Huang, K.; Xu, M.; Hua, H.; Ye, F.; Yan, J.; Zhang, G.; Wang, Y. Deoxycholic Acid Induces Gastric Intestinal Metaplasia by Activating STAT3 Signaling and Disturbing Gastric Bile Acids Metabolism and Microbiota. Gut Microbes 2022, 14, 2120744. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of Inflammatory Responses by Gut Microbiota and Chemoattractant Receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional Characterization of Human Receptors for Short Chain Fatty Acids and Their Role in Polymorphonuclear Cell Activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef]
- Grander, C.; Adolph, T.E.; Wieser, V.; Lowe, P.; Wrzosek, L.; Gyongyosi, B.; Ward, D.V.; Grabherr, F.; Gerner, R.R.; Pfister, A.; et al. Recovery of Ethanol-Induced Akkermansia Muciniphila Depletion Ameliorates Alcoholic Liver Disease. Gut 2018, 67, 891–901. [Google Scholar] [CrossRef]
- Ewaschuk, J.B.; Diaz, H.; Meddings, L.; Diederichs, B.; Dmytrash, A.; Backer, J.; Looijer-van Langen, M.; Madsen, K.L. Secreted Bioactive Factors from Bifidobacterium Infantis Enhance Epithelial Cell Barrier Function. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G1025–G1034. [Google Scholar] [CrossRef] [PubMed]
- Beil, W.; Enss, M.L.; Muller, S.; Obst, B.; Sewing, K.F.; Wagner, S. Role of vacA and cagA in Helicobacter pylori Inhibition of Mucin Synthesis in Gastric Mucous Cells. J. Clin. Microbiol. 2000, 38, 2215–2218. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Dagnault, A.; Desjardins, J. A Randomized Double-Blind Controlled Trial: Impact of Probiotics on Diarrhea in Patients Treated with Pelvic Radiation. Clin. Nutr. 2014, 33, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Reed, P.I.; Smith, P.L.; Haines, K.; House, F.R.; Walters, C.L. Gastric Juice N-Nitrosamines in Health and Gastroduodenal Disease. Lancet 1981, 2, 550–552. [Google Scholar] [CrossRef]
- Correa, P. Human Gastric Carcinogenesis: A Multistep and Multifactorial Process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992, 52, 6735–6740. [Google Scholar]
- Vinasco, K.; Mitchell, H.M.; Kaakoush, N.O.; Castano-Rodriguez, N. Microbial Carcinogenesis: Lactic Acid Bacteria in Gastric Cancer. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 188309. [Google Scholar] [CrossRef]
- Ambs, S.; Ogunfusika, M.O.; Merriam, W.G.; Bennett, W.P.; Billiar, T.R.; Harris, C.C. Up-Regulation of Inducible Nitric Oxide Synthase Expression in Cancer-Prone p53 Knockout Mice. Proc. Natl. Acad. Sci. USA 1998, 95, 8823–8828. [Google Scholar] [CrossRef]
- Winter, S.E.; Winter, M.G.; Xavier, M.N.; Thiennimitr, P.; Poon, V.; Keestra, A.M.; Laughlin, R.C.; Gomez, G.; Wu, J.; Lawhon, S.D.; et al. Host-Derived Nitrate Boosts Growth of E. coli in the Inflamed Gut. Science 2013, 339, 708–711. [Google Scholar] [CrossRef]
- Forsythe, S.J.; Cole, J.A. Nitrite Accumulation during Anaerobic Nitrate Reduction by Binary Suspensions of Bacteria Isolated from the Achlorhydric Stomach. J. Gen. Microbiol. 1987, 133, 1845–1849. [Google Scholar]
- Calmels, S.; Ohshima, H.; Crespi, M.; Leclerc, H.; Cattoen, C.; Bartsch, H. N-Nitrosamine Formation by Microorganisms Isolated from Human Gastric Juice and Urine: Biochemical Studies on Bacteria-Catalysed Nitrosation; IARC Scientific Publications: Lyon, France, 1987; pp. 391–395. [Google Scholar]
- Wagner, W.; Ciszewski, W.M.; Kania, K.D. L- and D-Lactate Enhance DNA Repair and Modulate the Resistance of Cervical Carcinoma Cells to Anticancer Drugs via Histone Deacetylase Inhibition and Hydroxycarboxylic Acid Receptor 1 Activation. Cell Commun. Signal. 2015, 13, 36. [Google Scholar] [CrossRef]
- San-Millan, I.; Brooks, G.A. Reexamining Cancer Metabolism: Lactate Production for Carcinogenesis Could be the Purpose and Explanation of the Warburg Effect. Carcinogenesis 2017, 38, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.L.; Coleman, D.T.; Shi, R.; Cardelli, J.A. Monocarboxylate Transporter 1 contributes to Growth Factor-Induced Tumor Cell Migration Independent of Transporter Activity. Oncotarget 2016, 7, 32695–32706. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, L.; Ma, Y.; Wang, B.; Ci, C.; Zhang, J.; Zhou, Y.; Dou, C.; Gu, Q.; An, Y.; et al. Study on the Characteristics of Intestinal Flora Composition in Gastric Cancer Patients and Healthy People in the Qinghai-Tibet Plateau. Appl. Biochem. Biotechnol. 2022, 194, 1510–1526. [Google Scholar] [CrossRef] [PubMed]
- Bourriaud, C.; Robins, R.J.; Martin, L.; Kozlowski, F.; Tenailleau, E.; Cherbut, C.; Michel, C. Lactate Is Mainly Fermented to Butyrate by Human Intestinal Microfloras but Inter-Individual Variation Is Evident. J. Appl. Microbiol. 2005, 99, 201–212. [Google Scholar] [CrossRef]
- Lertpiriyapong, K.; Whary, M.T.; Muthupalani, S.; Lofgren, J.L.; Gamazon, E.R.; Feng, Y.; Ge, Z.; Wang, T.C.; Fox, J.G. Gastric Colonisation with a Restricted Commensal Microbiota Replicates the Promotion of Neoplastic Lesions by Diverse Intestinal Microbiota in the Helicobacter pylori INS-GAS Mouse Model of Gastric Carcinogenesis. Gut 2014, 63, 54–63. [Google Scholar] [CrossRef]
- Chrysostomou, D.; Roberts, L.A.; Marchesi, J.R.; Kinross, J.M. Gut Microbiota Modulation of Efficacy and Toxicity of Cancer Chemotherapy and Immunotherapy. Gastroenterology 2023, 164, 198–213. [Google Scholar]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut Microbiota Modulation of Chemotherapy Efficacy and Toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef]
- Lehouritis, P.; Cummins, J.; Stanton, M.; Murphy, C.T.; McCarthy, F.O.; Reid, G.; Urbaniak, C.; Byrne, W.L.; Tangney, M. Local Bacteria Affect the Efficacy of Chemotherapeutic Drugs. Sci. Rep. 2015, 5, 14554. [Google Scholar] [CrossRef]
- Buysse, A.; Van Oost, P. ‘Appropriate’ Male and Female Safer Sexual Behaviour in Heterosexual Relationships. AIDS Care 1997, 9, 549–561. [Google Scholar] [CrossRef]
- Encarnacao, J.C.; Pires, A.S.; Amaral, R.A.; Goncalves, T.J.; Laranjo, M.; Casalta-Lopes, J.E.; Goncalves, A.C.; Sarmento-Ribeiro, A.B.; Abrantes, A.M.; Botelho, M.F. Butyrate, a Dietary Fiber Derivative that Improves Irinotecan Effect in Colon Cancer Cells. J. Nutr. Biochem. 2018, 56, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Yue, B.; Gao, R.; Wang, Z.; Dou, W. Microbiota-Host-Irinotecan Axis: A New Insight Toward Irinotecan Chemotherapy. Front. Cell. Infect. Microbiol. 2021, 11, 710945. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal Bacteria Control Cancer Response to Therapy by Modulating the Tumor Microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef]
- Sougiannis, A.T.; VanderVeen, B.N.; Davis, J.M.; Fan, D.; Murphy, E.A. Understanding Chemotherapy-Induced Intestinal Mucositis and Strategies to Improve Gut Resilience. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G712–G719. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The Commensal Microbiome Is Associated with Anti-PD-1 Efficacy in Metastatic Melanoma Patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti-PD-L1 Efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Vetizou, M.; Pitt, J.M.; Daillere, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer Immunotherapy by CTLA-4 Blockade Relies on the Gut Microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillere, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1-Based Immunotherapy against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Nasr, R.; Shamseddine, A.; Mukherji, D.; Nassar, F.; Temraz, S. The Crosstalk between Microbiome and Immune Response in Gastric Cancer. Int. J. Mol. Sci. 2020, 21, 6586. [Google Scholar] [CrossRef]
- Liu, X.; Choi, M.G.; Kim, K.; Kim, K.M.; Kim, S.T.; Park, S.H.; Cristescu, R.; Peter, S.; Lee, J. High PD-L1 Expression in Gastric Cancer (GC) Patients and Correlation with Molecular Features. Pathol. Res. Pract. 2020, 216, 152881. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Suarez, G.; Beswick, E.J.; Sierra, J.C.; Graham, D.Y.; Reyes, V.E. Expression of B7-H1 on Gastric Epithelial Cells: Its Potential Role in Regulating T Cells during Helicobacter pylori Infection. J. Immunol. 2006, 176, 3000–3009. [Google Scholar] [CrossRef] [PubMed]
- Clavel, T.; Gomes-Neto, J.C.; Lagkouvardos, I.; Ramer-Tait, A.E. Deciphering Interactions between the Gut Microbiota and the Immune System via Microbial Cultivation and Minimal Microbiomes. Immunol. Rev. 2017, 279, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Castle, L.; Mayo, A.; Gilbert, J. Migration of Plasticizers from Printing Inks into Foods. Food Addit. Contam. 1989, 6, 437–443. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Herrera, F.G.; Bourhis, J.; Coukos, G. Radiotherapy Combination Opportunities Leveraging Immunity for the Next Oncology Practice. CA Cancer J. Clin. 2017, 67, 65–85. [Google Scholar] [CrossRef]
- Uribe-Herranz, M.; Rafail, S.; Beghi, S.; Gil-de-Gomez, L.; Verginadis, I.; Bittinger, K.; Pustylnikov, S.; Pierini, S.; Perales-Linares, R.; Blair, I.A.; et al. Gut Microbiota Modulate Dendritic Cell Antigen Presentation and Radiotherapy-Induced Antitumor Immune Response. J. Clin. Investig. 2020, 130, 466–479. [Google Scholar] [CrossRef]
- Shiao, S.L.; Kershaw, K.M.; Limon, J.J.; You, S.; Yoon, J.; Ko, E.Y.; Guarnerio, J.; Potdar, A.A.; McGovern, D.P.B.; Bose, S.; et al. Commensal Bacteria and Fungi Differentially Regulate Tumor Responses to Radiation Therapy. Cancer Cell 2021, 39, 1202–1213.e6. [Google Scholar] [CrossRef]
- Guo, H.; Chou, W.C.; Lai, Y.; Liang, K.; Tam, J.W.; Brickey, W.J.; Chen, L.; Montgomery, N.D.; Li, X.; Bohannon, L.M.; et al. Multi-Omics Analyses of Radiation Survivors Identify Radioprotective Microbes and Metabolites. Science 2020, 370, eaay9097. [Google Scholar] [CrossRef]
- Ren, H.; Wu, Q.; Sun, Z.; Fang, M.; Liu, J.; Luo, J. Research Progress and Treatment of Radiation Enteritis and Gut Microbiota. Radiat. Oncol. J. 2023, 41, 61–68. [Google Scholar] [CrossRef]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia Muciniphila and Its Role in Regulating Host Functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Kanarek, N.; Petrova, B.; Sabatini, D.M. Dietary Modifications for Enhanced Cancer Therapy. Nature 2020, 579, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Mana, M.D.; Hussey, A.M.; Tzouanas, C.N.; Imada, S.; Barrera Millan, Y.; Bahceci, D.; Saiz, D.R.; Webb, A.T.; Lewis, C.A.; Carmeliet, P.; et al. High-Fat Diet-Activated Fatty Acid Oxidation Mediates Intestinal Stemness and Tumorigenicity. Cell Rep. 2021, 35, 109212. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.; van Limpt, K.; Kegler, D.; Luiking, Y.; Garssen, J.; van Helvoort, A.; Vos, A.P.; Knol, J. Bacterial Translocation Is Reduced by a Specific Nutritional Combination in Mice with Chemotherapy-Induced Neutropenia. J. Nutr. 2011, 141, 1292–1298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, X.B.; Farhangfar, A.; Valcheva, R.; Sawyer, M.B.; Dieleman, L.; Schieber, A.; Ganzle, M.G.; Baracos, V. The Role of Intestinal Microbiota in Development of Irinotecan Toxicity and in Toxicity Reduction through Dietary Fibres in Rats. PLoS ONE 2014, 9, e83644. [Google Scholar] [CrossRef]
- Ma, D.C.; Anderson, C.M.; Rodman, S.N.; Buranasudja, V.; McCormick, M.L.; Davis, A.; Loth, E.; Bodeker, K.L.; Ahmann, L.; Parkhurst, J.R.; et al. Ketogenic Diet with Concurrent Chemoradiation in Head and Neck Squamous Cell Carcinoma: Preclinical and Phase 1 Trial Results. Radiat. Res. 2021, 196, 213–224. [Google Scholar] [CrossRef]
- Yuan, W.; Xiao, X.; Yu, X.; Xie, F.; Feng, P.; Malik, K.; Wu, J.; Ye, Z.; Zhang, P.; Li, X. Probiotic Therapy (BIO-THREE) Mitigates Intestinal Microbial Imbalance and Intestinal Damage Caused by Oxaliplatin. Probiotics Antimicrob. Proteins 2022, 14, 60–71. [Google Scholar] [CrossRef]
- Pinato, D.J.; Howlett, S.; Ottaviani, D.; Urus, H.; Patel, A.; Mineo, T.; Brock, C.; Power, D.; Hatcher, O.; Falconer, A.; et al. Association of Prior Antibiotic Treatment with Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients with Cancer. JAMA Oncol. 2019, 5, 1774–1778. [Google Scholar] [CrossRef]
- Wilson, B.E.; Routy, B.; Nagrial, A.; Chin, V.T. The Effect of Antibiotics on Clinical Outcomes in Immune-Checkpoint Blockade: A Systematic Review and Meta-Analysis of Observational Studies. Cancer Immunol. Immunother. 2020, 69, 343–354. [Google Scholar] [CrossRef]
- Raman, M.; Ambalam, P.; Kondepudi, K.K.; Pithva, S.; Kothari, C.; Patel, A.T.; Purama, R.K.; Dave, J.M.; Vyas, B.R. Potential of Probiotics, Prebiotics and Synbiotics for Management of Colorectal Cancer. Gut Microbes 2013, 4, 181–192. [Google Scholar] [CrossRef]
- Geier, M.S.; Butler, R.N.; Howarth, G.S. Probiotics, Prebiotics and Synbiotics: A Role in Chemoprevention for Colorectal Cancer? Cancer Biol. Ther. 2006, 5, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, T.; Lu, J.; Wei, K.; Tian, H.; Liu, W.; Xu, T.; Wang, X.; Wang, S.; Yang, R.; et al. Adjuvant Treatment and Molecular Mechanism of Probiotic Compounds in Patients with Gastric Cancer after Gastrectomy. Food Funct. 2021, 12, 6294–6308. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Yang, H. Using Probiotics as Supplementation for Helicobacter pylori Antibiotic Therapy. Int. J. Mol. Sci. 2020, 21, 1136. [Google Scholar] [CrossRef] [PubMed]
- Taper, H.S.; Roberfroid, M.B. Possible Adjuvant Cancer Therapy by Two Prebiotics—Inulin or Oligofructose. In Vivo 2005, 19, 201–204. [Google Scholar] [PubMed]
- Taper, H.S.; Roberfroid, M.B. Non-Toxic Potentiation of Cancer Radiotherapy by Dietary Oligofructose or Inulin. Anticancer Res. 2002, 22, 3319–3323. [Google Scholar]
- Wang, C.; Lan, T.; Chen, Z.; Wang, X.; Han, Y.; Yang, N.; Xu, Z.; Li, H.; Tao, M.; Song, Y. The Preventive Effects of Inulin, Cellulose, and Their Mixture on Colorectal Cancer Liver Metastasis in Mice by Regulating Gut Microbiota. J. Food Sci. 2023, 88, 4705–4717. [Google Scholar] [CrossRef]
- Sugimoto, T.; Atobe, S.; Kado, Y.; Takahashi, A.; Motoori, M.; Sugimura, K.; Miyata, H.; Yano, M.; Tanaka, K.; Doki, Y.; et al. Gut Microbiota Associated with the Mitigation Effect of Synbiotics on Adverse Events of Neoadjuvant Chemotherapy in Patients with Esophageal Cancer: A Retrospective Exploratory Study. J. Med. Microbiol. 2023, 72, 001723. [Google Scholar] [CrossRef]
- Mahdavi, R.; Faramarzi, E.; Nikniaz, Z.; FarshiRadvar, F. Role of Probiotics and Synbiotics in Preventing Chemoradiotherapy-Associated Toxicity in Colorectal Cancer Patients: A Systematic Review. Iran. J. Med. Sci. 2023, 48, 110–117. [Google Scholar]
- Khazaei, Y.; Basi, A.; Fernandez, M.L.; Foudazi, H.; Bagherzadeh, R.; Shidfar, F. The Effects of Synbiotics Supplementation on Reducing Chemotherapy-Induced Side Effects in Women with Breast Cancer: A Randomized Placebo-Controlled Double-Blind Clinical Trial. BMC Complement. Med. Ther. 2023, 23, 339. [Google Scholar] [CrossRef]
- Boicean, A.; Ichim, C.; Todor, S.B.; Anderco, P.; Popa, M.L. The Importance of Microbiota and Fecal Microbiota Transplantation in Pancreatic Disorders. Diagnostics 2024, 14, 861. [Google Scholar] [CrossRef]
- Borody, T.J.; Khoruts, A. Fecal Microbiota Transplantation and Emerging Applications. Nat. Rev. Gastroenterol. Hepatol. 2011, 9, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Petithory, J.C.; Pariente, P.; Milgram, M.; Rismondo, J.; Sebbah, J.L.; Sloven, P.; Tardy, M.; de Loye, J. Occurrence of L.A.V. Infection in Drug Addicts in the Northern Suburbs of Paris. Bull. Acad. Natl. Med. 1986, 170, 689–696. [Google Scholar] [PubMed]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal Microbiota Transplant Overcomes Resistance to Anti-PD-1 Therapy in Melanoma Patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhang, X.; Xie, T.; Cheng, S.; Han, Z.; Wang, S.; Ban, Z.; Xu, X.; Zhu, Z.; Zhu, J.; et al. Efficacy of Fecal Microbiota Transplantation in Patients with Anti-PD-1–Resistant/Refractory Gastrointestinal Cancers. J. Clin. Oncol. 2023, 41, 389. [Google Scholar] [CrossRef]
- Delgado, S.; Cabrera-Rubio, R.; Mira, A.; Suarez, A.; Mayo, B. Microbiological Survey of the Human Gastric Ecosystem Using Culturing and Pyrosequencing Methods. Microb. Ecol. 2013, 65, 763–772. [Google Scholar] [CrossRef]
| Time | Region | Key Results | Method | Sample Size | Sample Type | References |
|---|---|---|---|---|---|---|
| 2023 | China | Hp increased significantly in peritumoral microhabitat of patients with good prognoses | 16S rRNA | 132 GC | Tissues | [20] |
| 2023 | Japan | In GC patients treated with Hp eradication, the average abundance of Unclassified Oxalobacteraceae, Capnocytophaga, and Haemophilus increased | 16S rRNA | 8 EGC vs. 9 NC | Tissues | [26] |
| 2023 | China | Helicobacter and Lysobacter were notably more abundant in normal tissues, whereas Pseudomonas was more prevalent in tumor tissues | RNA-Seq | 727 GC | Tissues | [32] |
| 2022 | China | Gastric surgery can reduce the diversity of the gut microbiota in GC | 16S rRNA | 100 GC | Feces | [54] |
| 2022 | Korea | Gastric mucosal microbiota in GC patients showed reduced diversity and increased abundance of Actinobacteria, Bacteroidetes, and Firmicutes | 16S rRNA | 45 GC vs. 92 HC | Tissues | [49] |
| 2022 | Korea | The microbial diversity continuously decreased continuously from gastritis to GC | 16S rRNA | 88 GC | Gastric juice | [35] |
| 2022 | Singapore | GC development was marked by increased Proteobacteria and decreased Bacteroidetes | 16S rRNA | 89 (43 GC vs. others) | Tissues | [37] |
| 2022 | China | GC patients had significantly lower levels of Faecalibacterium, Bifidobacterium, and Subdoligranulum, and higher levels of Enterococcus, Streptococcus, and Bacteroides, compared to healthy individuals | 16S rRNA | 30 GC vs. 30 Normal | Feces | [43] |
| 2022 | China | Microbial composition and metabolic products differ between proximal and distal GC, though species diversity and abundance remain similar | 16S rRNA | 29 GC vs. NT | Tissues | [56] |
| 2022 | USA | High Candida levels were associated with pro-inflammatory immune pathways | External ITS sequencing | 321 (from TCGA) | Tissues | [67] |
| 2022 | China | Streptococcus anginosus and Streptococcus constellatus were more common in GC tumor tissues | 16S rRNA | 1043 GC | Tissues and feces | [40] |
| 2022 | China | The microbial diversity of GC microbiota was reduced | 16S rRNA | 53 GC vs. 30 CG | Tissues | [47] |
| 2022 | China | The composition of intestinal flora was different in different stages of GC | 16S rRNA | 226 GC | Feces | [55] |
| 2021 | China | The abundance of Lactobacillus, Streptococcus, Bacteroides, and Prevotella was increased in tumor tissues compared to non-tumor tissues | 16S rRNA | 37 GC vs. NT | Tissues | [38] |
| 2021 | Portugal | GC tissues were enriched with Firmicutes, Gemella, and Streptococcus, while Proteobacteria were reduced | 16S rRNA | 77 GC | Tissues | [39] |
| 2021 | China | The species richness, diversity, and evenness of fungal components tended to decrease with gastric carcinogenesis, and the fungal community structure changed significantly. Albicans may be a biomarker for GC | ITS rDNA gene analysis | 45 GC | Tissues | [68] |
| 2020 | USA/Japan | The overall bacterial alpha diversity metrics in the control group was higher than the cancer groups | 16S rRNA | 48 GC and 120 NC | Tissues | [50] |
| 2020 | HK | Eradication of Hp treatment can lead to an increase in bacterial diversity | 16S rRNA | 202 GC | Tissues | [25] |
| 2020 | China | Bacterial diversity and phyla Armatimonadetes, Chloroflexi, Elusimicrobia, Nitrospirae, Planctomycetes, Verrucomicrobia, and WS3 decreased from CG to GC, while Actinobacteria, Bacteroides, Firmicutes, Fusobacteria, SR1, and TM7 increased in IN and GC | 16S rRNA | 132 (29 GC vs. others) | Tissues | [44] |
| 2020 | China | In advanced gastric lesion patients, Helicobacter showed strong avoidance of co-occurrence with Fusobacterium, Neisseria, Prevotella, Veillonella, and Rothia | 16S rRNA | 115 GC | Tissues and feces | [30] |
| 2019 | China | Bacterial richness decreased in peritumoral and tumoral microhabitats, and the correlation network of abundant gastric bacteria was simplified in the tumoral microhabitat | 16S rRNA | 276 GC | Tissues | [28] |
| 2018 | China | Hp abundance was lower in tumor tissues compared to adjacent non-tumor tissues | 16S rRNA | 11 GC vs. 16 NC | Tissues | [19] |
| 2018 | Portugal | Patients with GC exhibit a dysbiotic microbial community with genotoxic potential | 16S rRNA | 54 GC and 81 CG | Tissues | [51] |
| 2017 | USA | Hp dominated the non-malignant gastric tissue microbiota in many GC patients | 16S rRNA | 160 GC | Tissues | [23] |
| 2017 | Australia | Increased richness and phylogenetic diversity in GC | 16S rRNA | 12 GC vs. 20 FD | Tissues | [52] |
| 2017 | Sweden | Hp abundance was positively correlated with Campylobacter, Deinococcus, and Sulfurospirillum | Metatranscriptomic RNA sequencing | 149 GC | Tissues | [31] |
| 2017 | China | P. stomatis, D. pneumosintes, S. exigua, P. micra, and S. anginosus may play important roles in GC progression | 16S rRNA | 200 (GC, AG, IM, SG) | Tissues | [46] |
| 2016 | China | The microbiota structure in GC was more diverse | 16S rRNA | 12 GC | Tissues | [27] |
| 2014 | Mexico | Bacterial diversity decreases from NAG to IM to intestinal-type GC | G3 PhyloChip | 5 GC | Tissues | [41] |
| 2010 | USA | Hp GC showed increased levels of Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes | 16S rRNA | 12 GC | Tissues | [24] |
| 2006 | USA | Hp presence did not affect gastric community composition. The gastric flora comprises 128 diverse phylotypes | 16S rRNA | 23 GC | Tissues | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Tian, C.; Zou, Z.; Jin, M.; Liu, H. Gastrointestinal Microbiota in Gastric Cancer: Potential Mechanisms and Clinical Applications—A Literature Review. Cancers 2024, 16, 3547. https://doi.org/10.3390/cancers16203547
Wu M, Tian C, Zou Z, Jin M, Liu H. Gastrointestinal Microbiota in Gastric Cancer: Potential Mechanisms and Clinical Applications—A Literature Review. Cancers. 2024; 16(20):3547. https://doi.org/10.3390/cancers16203547
Chicago/Turabian StyleWu, Mengjiao, Chenjun Tian, Zhenwei Zou, Min Jin, and Hongli Liu. 2024. "Gastrointestinal Microbiota in Gastric Cancer: Potential Mechanisms and Clinical Applications—A Literature Review" Cancers 16, no. 20: 3547. https://doi.org/10.3390/cancers16203547
APA StyleWu, M., Tian, C., Zou, Z., Jin, M., & Liu, H. (2024). Gastrointestinal Microbiota in Gastric Cancer: Potential Mechanisms and Clinical Applications—A Literature Review. Cancers, 16(20), 3547. https://doi.org/10.3390/cancers16203547




