Is Unplanned Excision of Soft Tissue Sarcomas Associated with Worse Oncological Outcomes?—A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
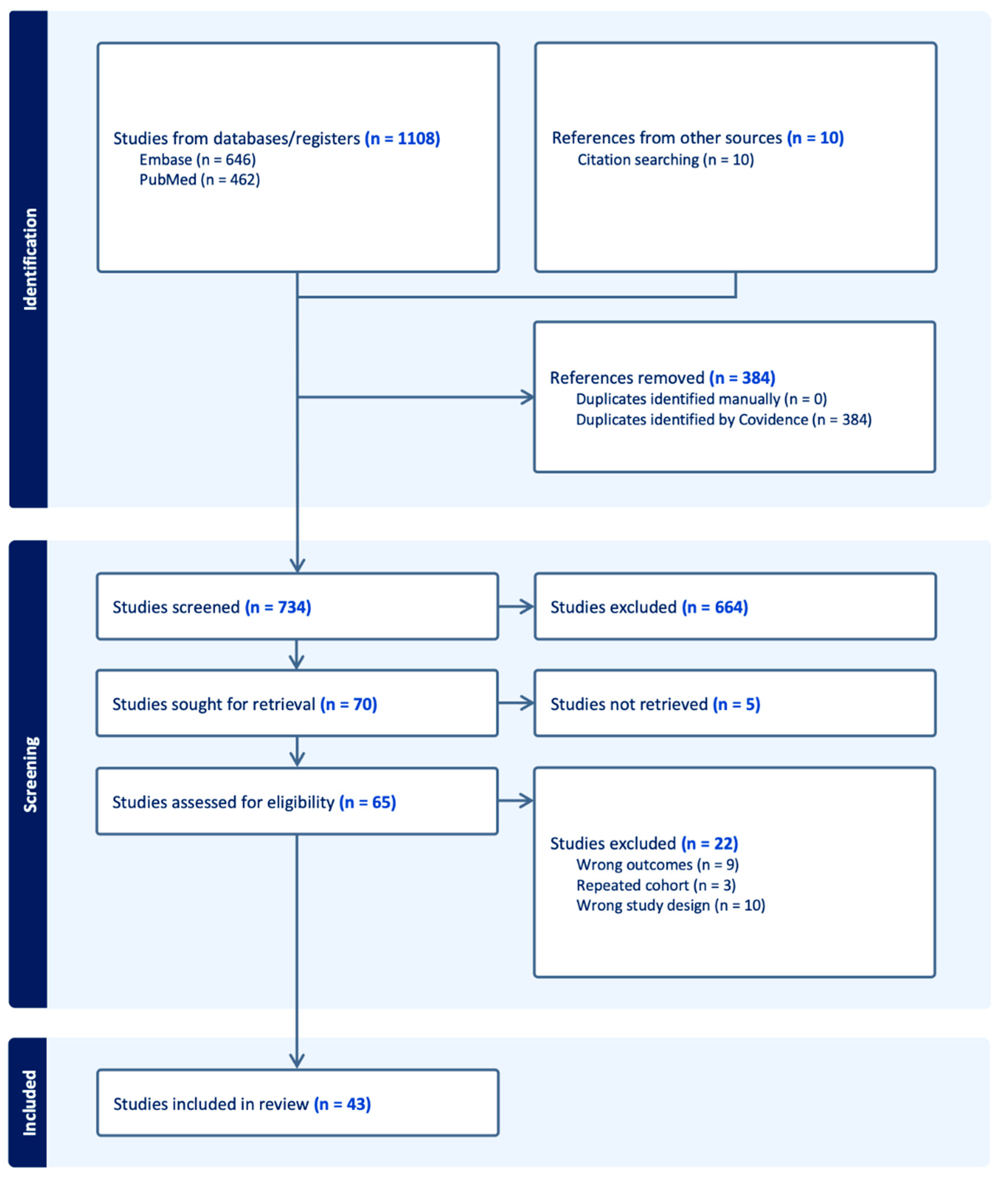
2.3. Selection, Data Collection and Extraction Process
2.4. Study Selection and Characteristics
2.5. Assessment of Study Quality Assessment and Risk of Bias
2.6. Statistical Analysis
2.7. Publication Bias
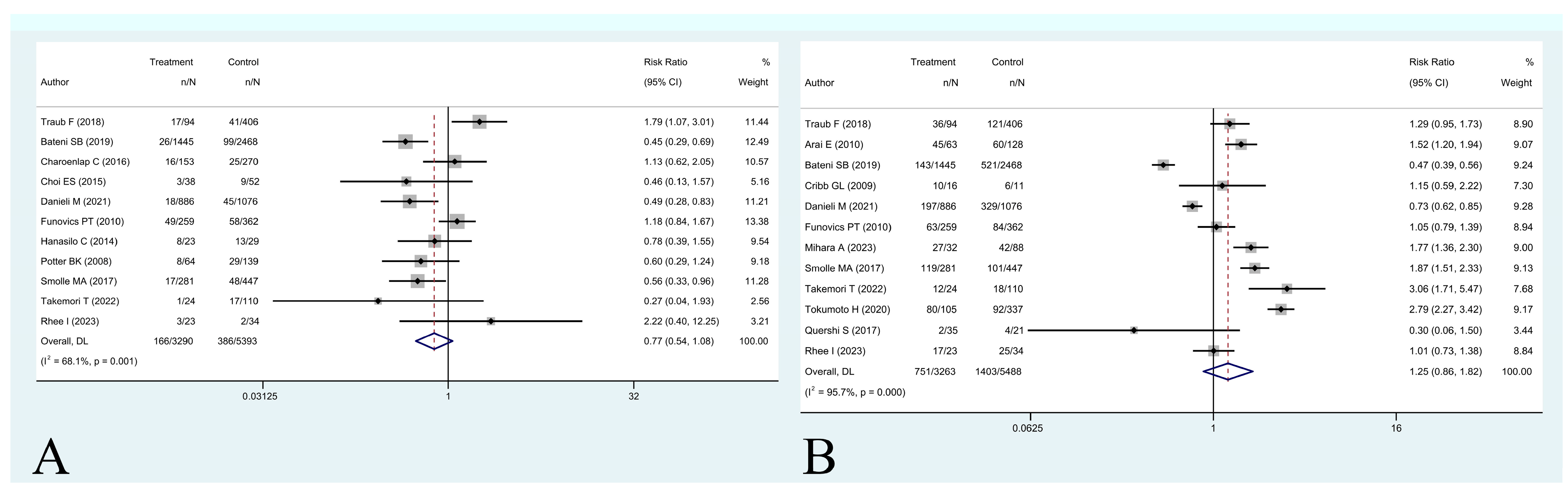
3. Results
- (1)
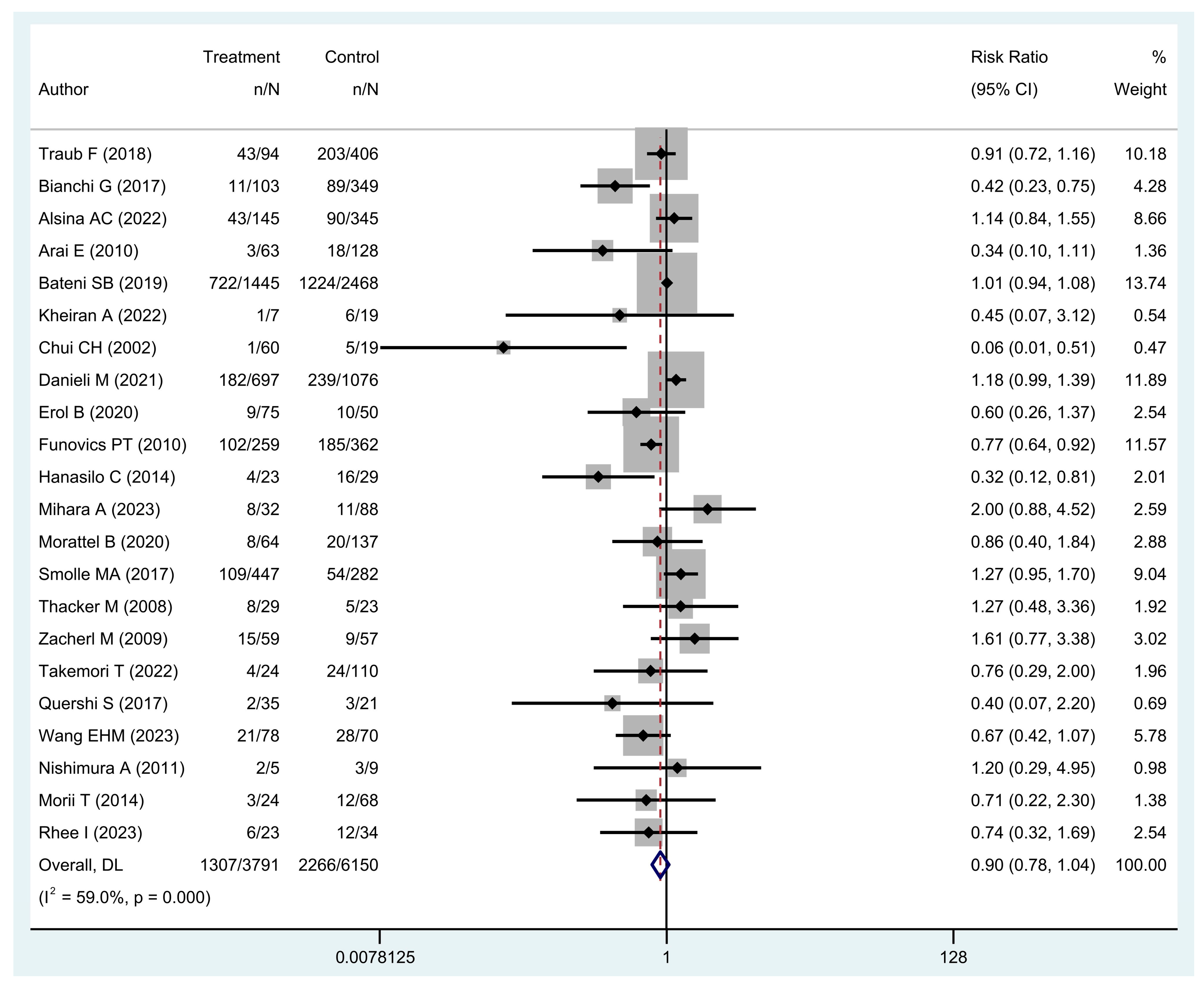
- Is unplanned excision associated with worse five-year overall survival and/or local recurrence-free survival?
- (2)
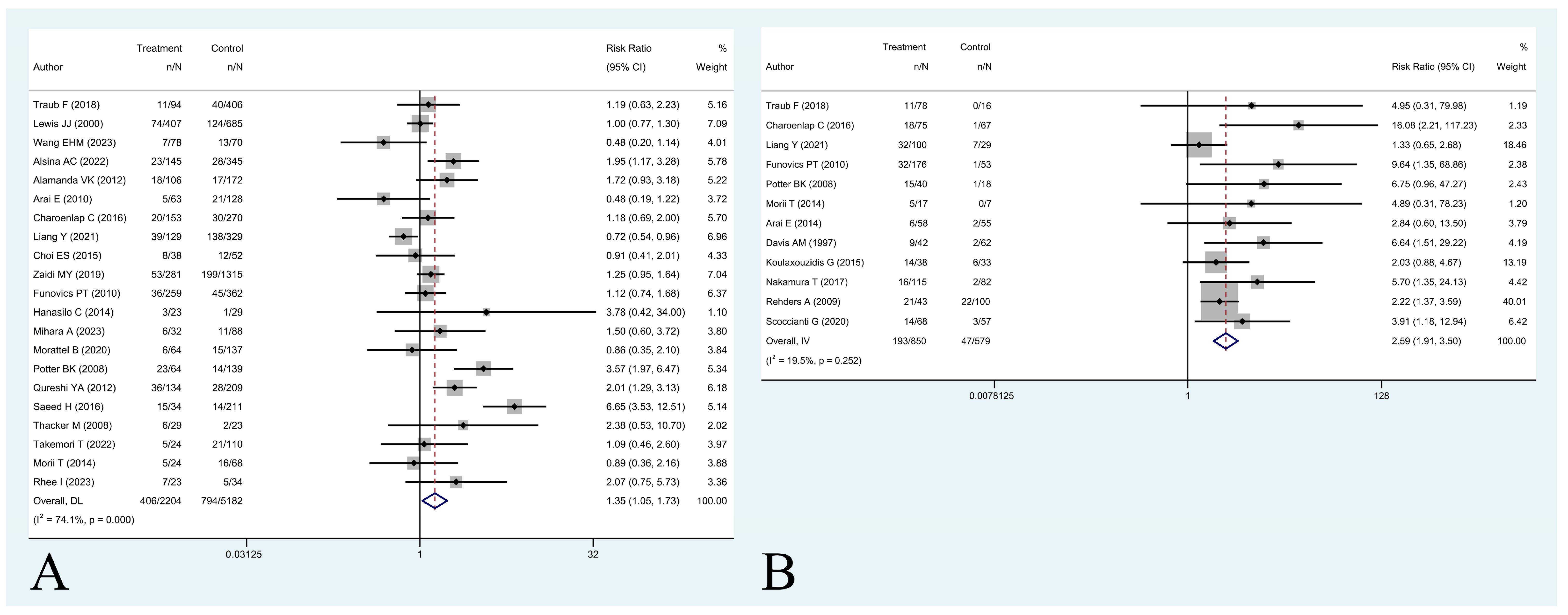
- Is residual disease on the tumor bed associated with worse five-year local recurrence-free survival?
- (3)
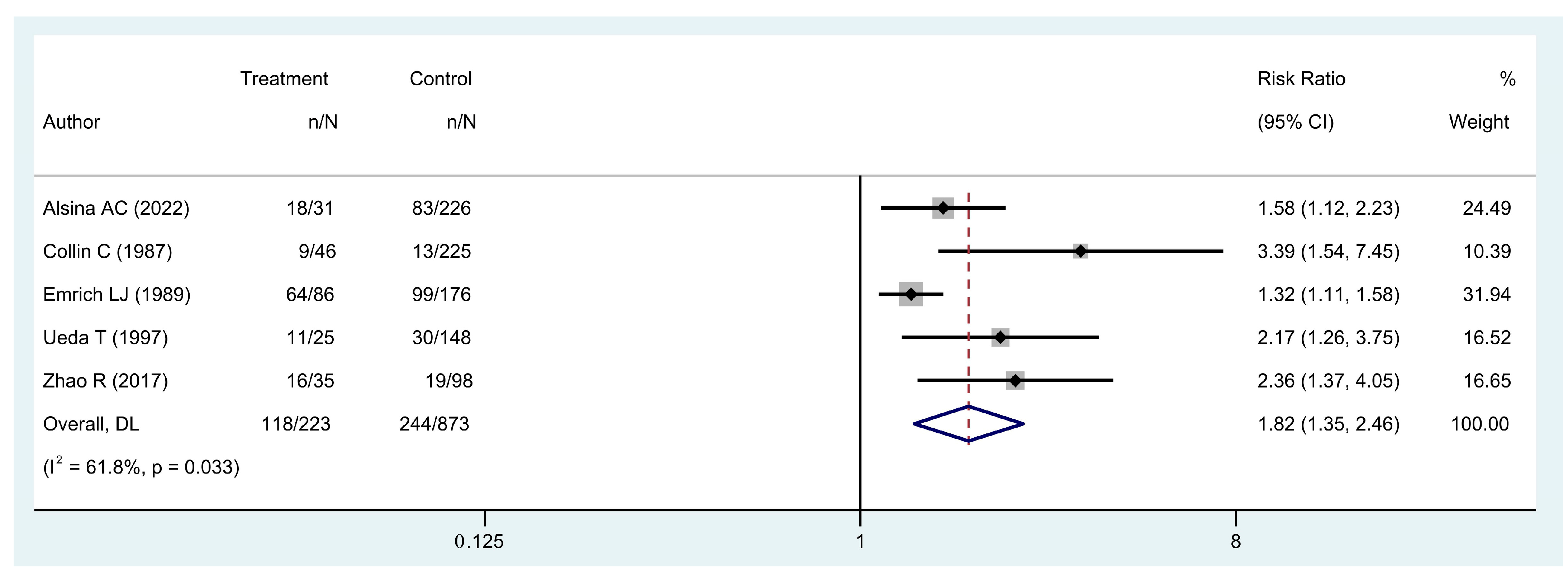
- Is local recurrence associated with worse five-year overall survival?
- (4)
- Is unplanned excision associated with a higher rate of amputation and/or plastic reconstruction surgery?
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Clasby, R.; Tilling, K.; Smith, M.A.; Fletcher, C.D. Variable management of soft tissue sarcoma: Regional audit with implications for specialist care. Br. J. Surg. 1997, 84, 1692–1696. [Google Scholar] [CrossRef]
- Davis, A.M.; Kandel, R.A.; Wunder, J.S.; Unger, R.; Meer, J.; O’Sullivan, B.; Catton, C.N.; Bell, R.S. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J. Surg. Oncol. 1997, 66, 81–87. [Google Scholar] [CrossRef]
- Smolle, M.A.; Tunn, P.-U.; Goldenitsch, E.; Posch, F.; Szkandera, J.; Bergovec, M.; Liegl-Atzwanger, B.; Leithner, A. The Prognostic Impact of Unplanned Excisions in a Cohort of 728 Soft Tissue Sarcoma Patients: A Multicentre Study. Ann. Surg. Oncol. 2017, 24, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Casali, P.G.; Miceli, R.; Mariani, L.; Bertulli, R.; Lozza, L.; Collini, P.; Olmi, P.; Mussi, C.; Gronchi, A. Prognostic effect of re-excision in adult soft tissue sarcoma of the extremity. Ann. Surg. Oncol. 2006, 13, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Pretell-Mazzini, J.; Barton, M.D.; Conway, S.A.; Temple, H.T. Unplanned Excision of Soft-Tissue Sarcomas: Current Concepts for Management and Prognosis. J. Bone Jt. Surg. 2015, 97, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Eilber, F.R. The rationale for planned reoperation after unplanned total excision of soft-tissue sarcomas. J. Clin. Oncol. Off. J. Am. Soc Clin. Oncol. 1985, 3, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Alsina, A.C.; Sacchetti, F.; Kaya, H.; Yaman, B.; Tamsel, İ.; Sabah, D. Impact of the unplanned excision on the oncological outcomes of patients with soft tissue sarcomas: A single-center retrospective review of 490 patients. Acta Orthop. Traumatol. Turc. 2022, 56, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Tokumoto, H.; Akita, S.; Kubota, Y.; Mitsukawa, N. Effect of unplanned excision of soft tissue sarcomas on skin defects and reconstructive procedures. J. Plast. Surg. Hand Surg. 2020, 54, 372–376. [Google Scholar] [CrossRef]
- Wang, E.H.M.; Araneta, K.T.S.; Gaston, C.L.L.; Rubio, D.A.T.; de Dios, A.M.V.; Cañal, J.P.A.; Goleta-Dy, A.N.; Alcasabas, A.P.A.; Odoño, E.G.; Atun, J.M.L.; et al. Unplanned Excision of Soft Tissue Sarcomas of the Extremities in a Low-to-Middle-Income Country. Ann. Surg. Oncol. 2023, 30, 3681–3689. [Google Scholar] [CrossRef]
- Alamanda, V.K.; Crosby, S.N.; Archer, K.R.; Song, Y.; Schwartz, H.S.; Holt, G.E. Primary excision compared with re-excision of extremity soft tissue sarcomas—Is anything new? J. Surg. Oncol. 2012, 105, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Arai, E.; Nishida, Y.; Tsukushi, S.; Wasa, J.; Ishiguro, N. Clinical and treatment outcomes of planned and unplanned excisions of soft tissue sarcomas. Clin. Orthop. 2010, 468, 3028–3034. [Google Scholar] [CrossRef] [PubMed]
- Arai, E.; Sugiura, H.; Tsukushi, S.; Nakashima, H.; Urakawa, H.; Kozawa, E.; Ishiguro, N.; Nishida, Y. Residual tumor after unplanned excision reflects clinical aggressiveness for soft tissue sarcomas. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2014, 35, 8043–8049. [Google Scholar] [CrossRef] [PubMed]
- Bateni, S.B.; Gingrich, A.A.; Jeon, S.Y.; Hoch, J.S.; Thorpe, S.W.; Kirane, A.R.; Bold, R.J.; Canter, R.J. Clinical Outcomes and Costs Following Unplanned Excisions of Soft Tissue Sarcomas in the Elderly. J. Surg. Res. 2019, 239, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Sambri, A.; Cammelli, S.; Galuppi, A.; Cortesi, A.; Righi, A.; Caldari, E.; Ferrari, S.; Donati, D. Impact of residual disease after “unplanned excision” of primary localized adult soft tissue sarcoma of the extremities: Evaluation of 452 cases at a single Institution. Musculoskelet. Surg. 2017, 101, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Charoenlap, C.; Imanishi, J.; Tanaka, T.; Slavin, J.; Ngan, S.Y.; Chander, S.; Dowsey, M.M.; Goyal, C.; Choong, P.F.M. Outcomes of unplanned sarcoma excision: Impact of residual disease. Cancer Med. 2016, 5, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.S.; Han, I.; Cho, H.S.; Kang, H.G.; Kim, J.H.; Kim, H.S. Distinct Clinical Characteristics of Unplanned Excision in Synovial Sarcoma. Clin. Orthop. Surg. 2015, 7, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Chui, C.H.; Spunt, S.L.; Liu, T.; Pappo, A.S.; Davidoff, A.M.; Rao, B.N.; Shochat, S.J. Is reexcision in pediatric nonrhabdomyosarcoma soft tissue sarcoma necessary after an initial unplanned resection? J. Pediatr. Surg. 2002, 37, 1424–1429. [Google Scholar] [CrossRef]
- Collin, C.; Godbold, J.; Hajdu, S.; Brennan, M. Localized extremity soft tissue sarcoma: An analysis of factors affecting survival. J. Clin. Oncol. 1987, 5, 601–612. [Google Scholar] [CrossRef]
- Cribb, G.L.; Loo, S.C.S.; Dickinson, I. Limb salvage for soft-tissue sarcomas of the foot and ankle. J. Bone Jt. Surg. Br. 2010, 92, 424–429. [Google Scholar] [CrossRef]
- Danieli, M.; Barretta, F.; Fiore, M.; Radaelli, S.; Sangalli, C.; Barisella, M.; Stacchiotti, S.; Palassini, E.; Miceli, R.; Callegaro, D.; et al. Unplanned Excision of Extremity and Trunk Wall Soft Tissue Sarcoma: To Re-resect or Not to Re-resect? Ann. Surg. Oncol. 2021, 28, 4706–4717. [Google Scholar] [CrossRef] [PubMed]
- Emrich, L.J.; Ruka, W.; Driscoll, D.L.; Karakousis, C.P. The effect of local recurrence on survival time in adult high-grade soft tissue sarcomas. J. Clin. Epidemiol. 1989, 42, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Erol, B.; Baysal, Ö. Does Unplanned Soft Tissue Sarcoma Surgery Have a Negative Effect on Prognosis? J. Investig. Surg. Off. J. Acad. Surg. Res. 2022, 35, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Funovics, P.T.; Vaselic, S.; Panotopoulos, J.; Kotz, R.I.; Dominkus, M. The impact of re-excision of inadequately resected soft tissue sarcomas on surgical therapy, results, and prognosis: A single institution experience with 682 patients. J. Surg. Oncol. 2010, 102, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Hanasilo, C.E.H.; Casadei, M.S.; Auletta, L.; Amstalden, E.M.I.; Matte, S.R.F.; Etchebehere, M. Comparative study of planned and unplanned excisions for the treatment of soft tissue sarcoma of the extremities. Clinics 2014, 69, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Kheiran, A.; Eastley, N.C.; Hanson, J.R.; McCulloch, T.A.; Allen, P.E.; Ashford, R.U. The importance of the early appropriate management of foot and ankle soft tissue sarcomas—Experiences of a regional sarcoma service. Foot 2022, 50, 101866. [Google Scholar] [CrossRef] [PubMed]
- Koulaxouzidis, G.; Schwarzkopf, E.; Bannasch, H.; Stark, G.B. Is revisional surgery mandatory when an unexpected sarcoma diagnosis is made following primary surgery? World J. Surg. Oncol. 2015, 13, 306. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.J.; Leung, D.; Espat, J.; Woodruff, J.M.; Brennan, M.F. Effect of reresection in extremity soft tissue sarcoma. Ann. Surg. 2000, 231, 655–663. [Google Scholar] [CrossRef]
- Liang, Y.; Guo, T.-H.; Xu, B.-S.; Hong, D.-C.; Qiu, H.-B.; Zhou, Z.-W.; Zhang, X. The Impact of Unplanned Excision on the Outcomes of Patients With Soft Tissue Sarcoma of the Trunk and Extremity: A Propensity Score Matching Analysis. Front. Oncol. 2020, 10, 617590. [Google Scholar] [CrossRef]
- Mihara, A.; Iwanaga, R.; Muramatsu, K.; Ihara, K.; Sakai, T. Oncological and functional outcomes of planned and unplanned excision of soft tissue sarcoma: A retrospective study. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2023, 28, 867–873. [Google Scholar] [CrossRef]
- Morattel, B.; Mustaki, L.; Montemurro, M.; Letovanec, I.; Durham, A.D.; Becce, F.; Omoumi, P.; di Summa, P.G.; Matter, M.; Rüdiger, H.A.; et al. Oncological outcome, functional results and costs after unplanned excision of musculoskeletal soft tissue sarcoma. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2020, 46, 898–904. [Google Scholar] [CrossRef]
- Morii, T.; Aoyagi, T.; Tajima, T.; Yoshiyama, A.; Ichimura, S.; Mochizuki, K. Unplanned resection of a soft tissue sarcoma: Clinical characteristics and impact on oncological and functional outcomes. J. Orthop. Sci. Off. J. Jpn Orthop. Assoc. 2015, 20, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Kawai, A.; Sudo, A. Analysis of the patients with soft tissue sarcoma who received additional excision after unplanned excision: Report from the Bone and Soft Tissue Tumor Registry in Japan. Jpn. J. Clin. Oncol. 2017, 47, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Matsumine, A.; Asanuma, K.; Matsubara, T.; Nakamura, T.; Uchida, A.; Kato, K.; Sudo, A. The adverse effect of an unplanned surgical excision of foot soft tissue sarcoma. World J. Surg. Oncol. 2011, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Potter, B.K.; Adams, S.C.; Pitcher, J.D.; Temple, H.T. Local recurrence of disease after unplanned excisions of high-grade soft tissue sarcomas. Clin. Orthop. 2008, 466, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.S.; Prabhu, A.; Bhagat, M.; Kembhavi, S.; Vora, T.; Chinnaswamy, G.; Ramadwar, M.; Laskar, S.; Talole, S. Re-excision after unplanned resection of nonmetastatic nonrhabdomyosarcoma soft tissue sarcoma in children: Comparison with planned excision. J. Pediatr. Surg. 2017, 52, 1340–1343. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, Y.A.; Huddy, J.R.; Miller, J.D.; Strauss, D.C.; Thomas, J.M.; Hayes, A.J. Unplanned excision of soft tissue sarcoma results in increased rates of local recurrence despite full further oncological treatment. Ann. Surg. Oncol. 2012, 19, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Rehders, A.; Stoecklein, N.H.; Poremba, C.; Alexander, A.; Knoefel, W.T.; Peiper, M. Reexcision of soft tissue sarcoma: Sufficient local control but increased rate of metastasis. World J. Surg. 2009, 33, 2599–2605. [Google Scholar] [CrossRef] [PubMed]
- Rhee, I.; Spazzoli, B.; Stevens, J.; Hansa, A.; Spelman, T.; Pang, G.; Guiney, M.; Powell, G.; Choong, P.; Di Bella, C. Oncologic outcomes in myxofibrosarcomas: The role of a multidisciplinary approach and surgical resection margins. ANZ J. Surg. 2023, 93, 577–584. [Google Scholar] [CrossRef]
- Saeed, H.; King, D.M.; Johnstone, C.A.; Charlson, J.A.; Hackbarth, D.A.; Neilson, J.C.; Bedi, M. Preoperative Radiation Therapy Followed by Reexcision May Improve Local Control and Progression-Free Survival in Unplanned Excisions of Soft Tissue Sarcomas of the Extremity and Chest-Wall. Int. J. Surg. Oncol. 2016, 2016, 5963167. [Google Scholar] [CrossRef]
- Scoccianti, G.; Innocenti, M.; Frenos, F.; Muratori, F.; Sacchetti, F.; Beltrami, G.; Capanna, R.; Campanacci, D.A. Re-excision after unplanned excision of soft tissue sarcomas: Long-term results. Surg. Oncol. 2020, 34, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Takemori, T.; Kawamoto, T.; Hara, H.; Fukase, N.; Fujiwara, S.; Kitayama, K.; Yahiro, S.; Miyamoto, T.; Mifune, Y.; Hoshino, Y.; et al. Clinical Outcomes and Prognostic Factors in Soft Tissue Sarcoma Patients After Unplanned Excision. Cancer Manag. Res. 2022, 14, 1815–1824. [Google Scholar] [CrossRef] [PubMed]
- Thacker, M.M.; Potter, B.K.; Pitcher, J.D.; Temple, H.T. Soft tissue sarcomas of the foot and ankle: Impact of unplanned excision, limb salvage, and multimodality therapy. Foot Ankle Int. 2008, 29, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Traub, F.; Griffin, A.M.; Wunder, J.S.; Ferguson, P.C. Influence of unplanned excisions on the outcomes of patients with stage III extremity soft-tissue sarcoma. Cancer 2018, 124, 3868–3875. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Yoshikawa, H.; Mori, S.; Araki, N.; Myoui, A.; Kuratsu, S.; Uchida, A. Influence of local recurrence on the prognosis of soft-tissue sarcomas. J. Bone Jt. Surg. Br. 1997, 79, 553–557. [Google Scholar] [CrossRef]
- Zacherl, M.; Giessauf, C.; Glehr, M.; Gruber, G.; Maurer-Ertl, W.; Schwantzer, G.; Liegl-Atzwanger, B.; Koch, H.; Leithner, A.; Windhager, R. Revision of inadequately treated soft-tissue sarcoma is associated with increased need for plastic or reconstructive surgery. Eur. Surg. 2009, 41, 155–162. [Google Scholar] [CrossRef]
- Zaidi, M.Y.; Ethun, C.G.; Liu, Y.; Poultsides, G.; Howard, J.H.; Mogal, H.; Tseng, J.; Votanopoulos, K.; Fields, R.C.; Cardona, K. The impact of unplanned excisions of truncal/extremity soft tissue sarcomas: A multi-institutional propensity score analysis from the US Sarcoma Collaborative. J. Surg. Oncol. 2019, 120, 332–339. [Google Scholar] [CrossRef]
- Zhao, R.; Yu, X.; Feng, Y.; Yang, Z.; Chen, X.; Wand, J.; Ma, S.; Zhang, Z.; Guo, X. Local recurrence is correlated with decreased overall survival in patients with intermediate high-grade localized primary soft tissue sarcoma of extremity and abdominothoracic wall. Asia Pac. J. Clin. Oncol. 2018, 14, e109–e115. [Google Scholar] [CrossRef]
- Gonzalez, M.R.; Bedi, A.D.S.; Karczewski, D.; Lozano-Calderon, S.A. Treatment and Outcomes of Fungal Prosthetic Joint Infections: A Systematic Review of 225 Cases. J. Arthroplast. 2023, 38, 2464–2471.e1. [Google Scholar] [CrossRef]
- Gonzalez, M.R.; Castillo-Flores, S.; Portmann-Baracco, A.; Pretell-Mazzini, J. Ganglion Cysts Arising From the Proximal Tibiofibular Joint: Treatment Approach and Associated Outcomes—A Systematic Review. HSS J. Musculoskelet. J. Hosp. Spec. Surg. 2023. [Google Scholar] [CrossRef]
- Inchaustegui, M.L.; Ruiz, K.; Gonzalez, M.R.; Pretell-Mazzini, J. Surgical Management of Metastatic Pathologic Subtrochanteric Fractures: Treatment Modalities and Associated Outcomes. JBJS Rev. 2023, 11, e22.00232. [Google Scholar] [CrossRef] [PubMed]
- Atal, I.; Porcher, R.; Boutron, I.; Ravaud, P. The statistical significance of meta-analyses is frequently fragile: Definition of a fragility index for meta-analyses. J. Clin. Epidemiol. 2019, 111, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, F.; Alsina, A.C.; Morganti, R.; Innocenti, M.; Andreani, L.; Muratori, F.; Scoccianti, G.; Totti, F.; Campanacci, D.A.; Capanna, R. Re-excision after unplanned excision of soft tissue sarcoma: A systematic review and metanalysis. The rationale of systematic re-excision. J. Orthop. 2021, 25, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, C.R.; Wafa, H.; Grimer, R.J.; Carter, S.R.; Tillman, R.M.; Abudu, A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J. Bone Jt. Surg. Br. 2008, 90, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Decanter, G.; Stoeckle, E.; Honore, C.; Meeus, P.; Mattei, J.C.; Dubray-Longeras, P.; Ferron, G.; Carrere, S.; Causeret, S.; Guilloit, J.-M.; et al. Watch and Wait Approach for Re-excision After Unplanned Yet Macroscopically Complete Excision of Extremity and Superficial Truncal Soft Tissue Sarcoma is Safe and Does Not Affect Metastatic Risk or Amputation Rate. Ann. Surg. Oncol. 2019, 26, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Lo Vullo, S.; Colombo, C.; Collini, P.; Stacchiotti, S.; Mariani, L.; Fiore, M.; Casali, P.G. Extremity Soft Tissue Sarcoma in a Series of Patients Treated at a Single Institution: Local Control Directly Impacts Survival. Ann. Surg. 2010, 251, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Zagars, G.K.; Ballo, M.T.; Pisters, P.W.T.; Pollock, R.E.; Patel, S.R.; Benjamin, R.S.; Evans, H.L. Prognostic factors for patients with localized soft-tissue sarcoma treated with conservation surgery and radiation therapy: An analysis of 1225 patients. Cancer 2003, 97, 2530–2543. [Google Scholar] [CrossRef]
- Bonvalot, S.; Levy, A.; Terrier, P.; Tzanis, D.; Bellefqih, S.; Le Cesne, A.; Le Péchoux, C. Primary Extremity Soft Tissue Sarcomas: Does Local Control Impact Survival? Ann. Surg. Oncol. 2017, 24, 194–201. [Google Scholar] [CrossRef]
- Matsumoto, S.; Ahmed, A.R.; Kawaguchi, N.; Manabe, J.; Matsushita, Y. Results of surgery for malignant fibrous histiocytomas of soft tissue. Int. J. Clin. Oncol. 2003, 8, 104–109. [Google Scholar] [CrossRef]
- Lewis, J.J.; Leung, D.; Heslin, M.; Woodruff, J.M.; Brennan, M.F. Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J. Clin. Oncol. 1997, 15, 646–652. [Google Scholar] [CrossRef]
- Kwee, R.M.; Kwee, T.C. Diagnostic performance of MRI in detecting residual soft tissue sarcoma after unplanned excision: Systematic review and meta-analysis. Eur. J. Radiol. 2021, 145, 110049. [Google Scholar] [CrossRef]
| Author | Sample (n) | PE (n) | UE (n) | Age (Years) e | Follow-Up (Months) e | Outcomes |
|---|---|---|---|---|---|---|
| Alamanda et al. [11] | 278 | 172 | 106 | 58.5 +/55.5 + | 37.2 + | 5y LRFS |
| Alsina et al. [8] | 490 | 345 | 145 | 50.1 */47.3 * | 57.1 */55.8 * | 5y OS c, 5y LRFS |
| Arai et al. [12] | 191 | 128 | 63 | 50.5 */51 * | 55 * | 5y OS, 5y LRFS, PRS |
| Arai et al. [13] b | 113 | 0 | 113 | -/49 * | 69 * | 5y LRFS (UE) |
| Bateni et al. [14] | 3913 | 2468 | 1445 | 77.4/77.6 | 42 + | 5y OS, AMP, PRS |
| Bianchi et al. [15] | 452 | 349 | 103 | 59 +/51 + | 98 +/46+ | 5y OS |
| Charoenlap et al. [16] | 451 | 290 | 161 | - | 72.6 + | 5y LRFS a, AMP |
| Choi et al. [17] | 90 | 52 | 38 | 31.3 */34.7 * | 72 * | 5y LRFS, AMP |
| Chui et al. [18] | 79 | 19 | 60 | - | - | 5y OS |
| Collin et al. [19] d | 271 | - | - | - | 98.4 + | 5y OS |
| Cribb et al. [20] | 27 | 11 | 16 | 45.9 */59.1 * | 90 * | PRS |
| Danieli et al. [21] | 1962 | 1076 | 886 | 59 +/- | 85.2 + | 5y OS, AMP, PRS |
| Davis et al. [3] b | 104 | 0 | 104 | - | 36.8 * | 5y LRFS (UE) |
| Emrich et al. [22] d | 262 | - | - | - | 64 + | 5y OS |
| Erol et al. [23] | 125 | 75 | 50 | 47.2 */47.3 * | - | 5y OS |
| Funovics et al. [24] | 621 | 362 | 259 | 49 */53 * | - | 5y OS, 5y LRFS a, AMP, PRS |
| Hanasilo et al. [25] | 52 | 29 | 23 | - | 39.9 * | 5y OS, 5y LRFS, AMP |
| Kheiran et al. [26] | 26 | 19 | 7 | 54.4 */65.9 * | 75.6 * | 5y OS |
| Koulaxouzidis et al. [27] b | 71 | 0 | 71 | -/55.9 + | - | 5y LRFS (UE) |
| Lewis et al. [28] | 1092 | 685 | 407 | - | 57.6 + | 5y LRFS |
| Liang et al. [29] | 458 | 329 | 129 | - | 112.2 + | 5y LRFS a, 5y MFS |
| Mihara et al. [30] | 120 | 88 | 32 | 58.9 */60.9 * | 48.3 */50.8 * | 5y OS, 5y LRFS, PRS |
| Morattel et al. [31] | 201 | 137 | 64 | 55.9 +/52.5 + | 89 */99 * | 5y OS, 5y LRFS |
| Morii et al. [32] | 92 | 68 | 24 | 58.1 */61.8 * | 60 * | 5y OS, 5y LRFS a |
| Nakamura et al. [33] b | 197 | 0 | 197 | -/54 * | 48 * | 5y LRFS (UE) |
| Nishimura et al. [34] | 14 | 9 | 5 | 31 */34 * | 56.4 * | 5y OS |
| Potter et al. [35] | 203 | 139 | 64 | 57 +/53 + | 63 */48 * | 5y LRFS a, AMP |
| Quershi et al. [36] | 56 | 21 | 35 | - | - | 5y OS, PRS |
| Qureshi et al. [37] | 343 | 209 | 134 | 60 +/56 + | 51.6 + | 5y LRFS |
| Rehders et al. [38] b | 143 | 0 | 143 | -/50 + | 109 + | 5y LRFS (UE) |
| Rhee et al. [39] | 57 | 34 | 23 | 65.7 +/66.2 + | 64.7 + | 5y OS, 5y LRFS, AMP, PRS |
| Saeed et al. [40] | 245 | 211 | 34 | 57 +/64 + | 33.6 + | 5y LRFS, AMP |
| Scoccianti et al. [41] b | 125 | 0 | 125 | -/50 * | 130.8 * | 5y LRFS (UE) |
| Smolle et al. [4] | 728 | 447 | 281 | - | - | 5y OS, AMP, PRS |
| Takemori et al. [42] | 134 | 110 | 24 | 58 +/61 + | 89.5 +/95.5 + | 5y OS, 5y LRFS, AMP, PRS |
| Thacker et al. [43] | 52 | 23 | 29 | - | 99 * | 5y OS, 5y LRFS |
| Tokumoto et al. [9] | 442 | 337 | 105 | 56.7 */62.1 * | - | PRS |
| Traub et al. [44] | 500 | 406 | 94 | 58.8 */59.4 * | 54 */61.6 * | 5y OS, 5y LRFS a, AMP, PRS |
| Ueda et al. [45] d | 173 | - | - | - | 48 + | 5y OS |
| Wang et al. [10] | 148 | 70 | 78 | - | 52.8 + | 5y OS, 5y LRFS |
| Zacherl et al. [46] | 116 | 57 | 59 | 50.4/57.8 | 64.7 * | 5y OS |
| Zaidi et al. [47] | 1596 | 1315 | 281 | 57.6 */54.9 * | - | 5y LRFS |
| Zhao et al. [48] d | 133 | - | - | - | 68 + | 5y OS |
| Author | M:F Ratio | Size (cm) b | Deep Tumor (%) c | High Grade (%) c | Final Positive Margin (%) c |
|---|---|---|---|---|---|
| Alamanda et al. [11] | 1.29/1.1 | 12 +/5 + | 91.9/77.4 | 75.6/74.5 | 9.3/5.7 |
| Alsina et al. [8] | - | 10.2 */6.2 * | - | 86.9/88.3 | - |
| Arai et al. [12] | 1.21/1 | 9 */4.6 * | 70/13 | 62/52 | - |
| Arai et al. [13] | -/0.9 | -/4.5 * | -/15.9 | -/77 | -/3.4 |
| Bateni et al. [14] | 1/1.2 | - | 37.4/23.9 | 54.6/46.9 | - |
| Bianchi et al. [15] | 1.27/1.1 | 78%/43% | 94/72 | 100/100 | 3/17 |
| Charoenlap et al. [16] | 1.18/1.4 | 74.5%/42.9% | 85.9/53.3 | 91/73.4 | 8/5 |
| Choi et al. [17] | 1.74/1.5 | 9.1 */5.2 * | 96/71 | 100/95 | 13/21 |
| Chui et al. [18] | - | 52.6%/21.7% | - | 57.9/40 | 0/0 |
| Collin et al. [19] a | - | - | - | - | - |
| Cribb et al. [20] | - | - | - | - | - |
| Danieli et al. [21] | 1.19/1.3 | 9 +/6 + | 86.1/47.9 | 80.8/75.6 | - |
| Davis et al. [3] | - | - | - | - | - |
| Emrich et al. [22] a | - | - | - | 100 | - |
| Erol et al. [23] | 0.74/1.3 | 9.6 +/5.6 + | 100/84 | 82.7/76 | - |
| Funovics et al. [24] | 1.23/1.1 | - | - | 86.5/88.4 | 10.5/10.8 |
| Hanasilo et al. [25] | 1.23/1.6 | 89.7%/87% | 100/78.3 | 100/82.6 | 3.4/17.4 |
| Kheiran et al. [26] | 0.58/0.8 | ≥4 cm: 42.1%/≥4 cm: 14.3% | 63.2/85.7 | 84.2/85.7 | - |
| Koulaxouzidis et al. [27] | -/1.2 | -/48.2% | -/65.9 | -/36.5 | - |
| Lewis et al. [28] | 1.24/1.1 | 74.4%/40.3% | 88.2/60.7 | 67.6/62.7 | 25.5/9.1 |
| Liang et al. [29] | 1.49/1.6 | 52%/29.5% | 62/44.2 | 67.4/76 | - |
| Mihara et al. [30] | 0.91/1.9 | 8.9 */3.89 * | 39/46 | 78/74 | - |
| Morattel et al. [31] | 1.36/1.2 | 75.9%/39.1% | 81/43.8 | 36.5/37.5 | - |
| Morii et al. [32] | 1.06/0.8 | - | 73/20.8 | 80/70 | 13/16 |
| Nakamura et al. [33] | -/1.3 | -/4.7 * | -/33 | -/68.5 | -/3.6 |
| Nishimura et al. [34] | 3.5/0.7 | - | - | 22.2/40 | - |
| Potter et al. [35] | 1.14/1.6 | 11.5 */8.9 * | 75/33 | 100/100 | 1/6 |
| Quershi et al. [36] | 4.25/1.7 | 54%/34.2% | 100/82.8 | 41.6/77 | - |
| Qureshi et al. [37] | 1.3/1.4 | 87.7%/40.3% | 74.2/25.4 | 64.6/55.3 | - |
| Rehders et al. [38] | -/1.3 | -/66% | - | -/72 | - |
| Rhee et al. [39] | 1/0.9 | 70.6%/69.6% | 70.6/52.2 | 100/100 | - |
| Saeed et al. [40] | - | 8.5 +/4 + | - | 75.5/82.3 | - |
| Scoccianti et al. [41] | -/0.8 | - | - | -/72.5 | -/6.1 |
| Smolle et al. [4] | 0.95/1.3 | 28%/20% | 78.7/49.8 | 77.4/74.3 | - |
| Takemori et al. [42] | 0.9/2.4 | 7.50 +/3.55 + | 78.2/29.2 | - | 11.8/12.5 |
| Thacker et al. [43] | - | - | - | - | - |
| Tokumoto et al. [9] | 1.13/1.6 | - | - | 70.6/48.6 | - |
| Traub et al. [44] | 1.33/1.3 | 12.5 */10.3 * | - | 100/100 | 16.3/12.8 |
| Ueda et al. [45] a | 1.4 | 59.5% | 65.9 | 72.3 | - |
| Wang et al. [10] | 1.26/1 | 11 +/8.8 + | 87.1/76.9 | 50/69.2 | 14.3/21.8 |
| Zacherl et al. [46] | 1.59/1 | 83.6%/72.6% | 90.9/51.9 | 77/76 | 3.5/1.7 |
| Zaidi et al. [47] | - | 10.7 +/4.6 + | 91/74 | 71/74 | 17/14 |
| Zhao et al. [48] a | 1.6 | 56.4% | - | 100 | 6.8 |
| Author | 5-Year OS a (%) | 5-Year LRFS a (%) | PRS Rate a (%) | AMP Rate a (%) | 5-Year LRFS (UE Group [RD+ vs. RD-]) b (%) | 5-Year OS (LR+ vs. LR-) c (%) |
|---|---|---|---|---|---|---|
| Alamanda et al. [11] | - | 90.2/83 | - | - | - | - |
| Alsina et al. [8] | 73.9/70.3 | 91.9/84.1 | - | - | - | 42.7/63.2 |
| Arai et al. [12] | 85.7/95.7 | 83.4/92.2 | 47/71 | - | - | - |
| Arai et al. [13] | - | - | - | - | 89/97 | - |
| Bateni et al. [14] | 50.4/50 | - | 30.1/24.2 | 6.8/4.9 | - | - |
| Bianchi et al. [15] | 74.4/89.4 | - | - | - | - | - |
| Charoenlap et al. [16] | - | 88.9/86.9 | - | 9.4/10.7 | 75.7/98.6 | - |
| Choi et al. [17] | - | 77/79 | - | 17/8 | - | - |
| Chui et al. [18] | 73.7/98.2 | - | - | - | - | - |
| Collin et al. [19] | - | - | - | - | - | 80/94 |
| Cribb et al. [20] | - | - | 54.5/62.5 | - | - | - |
| Danieli et al. [21] | 77.8/86.9 | - | 30.6/22.2 | 4.2/2 | - | - |
| Davis et al. [3] | - | - | - | - | 79.5/97 | - |
| Emrich et al. [22] | - | - | - | - | - | 26/44 |
| Erol et al. [23] | 88/80 | - | - | - | - | - |
| Funovics et al. [24] | 48.8/60.5 | 87.6/86.1 | 23.2/24.3 | 16/18.9 | 82/98.2 | - |
| Hanasilo et al. [25] | 96.5/86.2 | 46.1/82.7 | - | 44.8/34.8 | - | - |
| Kheiran et al. [26] | 68.4/85.6 | - | - | - | - | - |
| Koulaxouzidis et al. [27] | - | - | - | - | 62.5/83.3 | - |
| Lewis et al. [28] | - | 81.9/81.9 | - | - | - | - |
| Liang et al. [29] | - | 58/70 | - | - | 68.1/75.9 | - |
| Mihara et al. [30] | 87.1/76.5 | 87.2/79.9 | 48/84 | - | - | - |
| Morattel et al. [31] | 85.4/87.8 | 89.3/90.6 | - | - | - | - |
| Morii et al. [32] | 82/87.8 | 76.3/79.8 | - | - | 72.1/100 | - |
| Nakamura et al. [33] | - | - | - | - | 86.2/97.3 | - |
| Nishimura et al. [34] | 65.6/60 | - | - | - | - | - |
| Potter et al. [35] | - | 89.7/63.7 | - | 21/13 | 61.3/94.4 | - |
| Quershi et al. [36] | 84.9/93.5 | - | 20.8/6 | - | - | - |
| Qureshi et al. [37] | - | 86.8/73.1 | - | - | - | - |
| Rehders et al. [38] | - | - | - | - | 50.4/77.7 | - |
| Rhee et al. [39] | 64.5/75.9 | 84.4/70.1 | 73.5/73.9 | 5.9/13 | - | - |
| Saeed et al. [40] | - | 93.2/56.1 | - | 0/0 | - | - |
| Scoccianti et al. [41] | - | - | - | - | 79.7/94.7 | - |
| Smolle et al. [4] | 75.6/81.9 | - | 22.6/42.3 | 10.7/6.0 | - | - |
| Takemori et al. [42] | 77.9/83.3 | 81.2/78.1 | 16.3/0.5 | 15.4/4.1 | - | - |
| Thacker et al. [43] | 80.2/73.4 | 90.8/79 | - | - | - | - |
| Tokumoto et al. [9] | - | - | 27.3/76.2 | - | - | - |
| Traub et al. [44] | 50.1/54 | 90.1/88.3 | 39.9/56.4 | 10.1/18.1 | 85.5/100 | - |
| Ueda et al. [45] | - | - | - | - | - | 54.1/79.5 |
| Wang et al. [10] | 60/73 | 82/91 | - | - | - | - |
| Zacherl et al. [46] | 84.2/74.5 | - | - | - | - | - |
| Zaidi et al. [47] | - | 84.9/81.2 | - | - | - | - |
| Zhao et al. [48] | - | - | - | - | - | 53.6/80.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larios, F.; Gonzalez, M.R.; Ruiz-Arellanos, K.; Aquilino E Silva, G.; Pretell-Mazzini, J. Is Unplanned Excision of Soft Tissue Sarcomas Associated with Worse Oncological Outcomes?—A Systematic Review and Meta-Analysis. Cancers 2024, 16, 443. https://doi.org/10.3390/cancers16020443
Larios F, Gonzalez MR, Ruiz-Arellanos K, Aquilino E Silva G, Pretell-Mazzini J. Is Unplanned Excision of Soft Tissue Sarcomas Associated with Worse Oncological Outcomes?—A Systematic Review and Meta-Analysis. Cancers. 2024; 16(2):443. https://doi.org/10.3390/cancers16020443
Chicago/Turabian StyleLarios, Felipe, Marcos R. Gonzalez, Kim Ruiz-Arellanos, George Aquilino E Silva, and Juan Pretell-Mazzini. 2024. "Is Unplanned Excision of Soft Tissue Sarcomas Associated with Worse Oncological Outcomes?—A Systematic Review and Meta-Analysis" Cancers 16, no. 2: 443. https://doi.org/10.3390/cancers16020443
APA StyleLarios, F., Gonzalez, M. R., Ruiz-Arellanos, K., Aquilino E Silva, G., & Pretell-Mazzini, J. (2024). Is Unplanned Excision of Soft Tissue Sarcomas Associated with Worse Oncological Outcomes?—A Systematic Review and Meta-Analysis. Cancers, 16(2), 443. https://doi.org/10.3390/cancers16020443






