Impact of Nutritional Status of Patients with Head and Neck Squamous Cell Carcinoma on the Expression Profile of Ghrelin, Irisin, and Titin
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Subjects
Nutritional Status of Patients Qualified for the Study Group
2.3. Control Group
2.4. Tissue Collection
2.5. Blood Samples
2.6. RNA Isolation
2.7. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
2.8. Methylation Profile of Irisin, Ghrelin, and Titin
2.9. Enzyme-Linked Immunosorbent Assay Reaction
2.10. Human Papillomavirus Genotyping
2.11. Statistical Analysis
3. Results
3.1. Nutritional Status of Patients with HNSCC before Surgery, on the Day of the Start, and after 6 Months of Chemotherapy
3.2. The Expression Profile of Genes Coding Irisin, Human Acylated Ghrelin, and Titin in Tumor Extracts and on the Margin of Healthy Tissues and Blood Samples Determined Using qRT-PCR
3.3. Irisin, Ghrelin, and Titin Methylation Profile
3.4. The Concentration of Irisin, Ghrelin, and Titin Was Determined Using the ELISA Test in the Tissues and Serum of Patients with HNSCC in Comparison with the Control
3.5. Assessing the Risk of HNSCC According to Blood Type
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chow, L.Q. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Alsahafi, E.; Begg, K.; Amelio, I.; Raulf, N.; Lucarelli, P.; Sauter, T.; Tavassoli, M. Clinical Update on Head and Neck Cancer: Molecular Biology and Ongoing Challenges. Cell Death Dis. 2019, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The Changing Therapeutic Landscape of Head and Neck Cancer. Nat. Rev. Clin. Oncol. 2019, 16, 669–683. [Google Scholar] [CrossRef]
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and Neck Cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; Forastiere, A.A. Head and Neck Cancer: Changing Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2008, 83, 489–501. [Google Scholar]
- Didkowska, J.; Wojciechowska, U.; Zatoński, W. Nowotwory Złośliwe w Polsce w 2011 Roku; Studio Mediana: Gdańsk, Poland, 2013; pp. 1–110. [Google Scholar]
- Graboyes, E.M.; Kompelli, A.R.; Neskey, D.M.; Brennan, E.; Nguyen, S.; Sterba, K.R.; Warren, G.W.; Hughes-Halbert, C.; Nussenbaum, B.; Day, T.A. Association of Treatment Delays with Survival for Patients with Head and Neck Cancer: A Systematic Review. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Vosselman, N.; Alberga, J.; Witjes, M.H.; Raghoebar, G.M.; Reintsema, H.; Vissink, A.; Korfage, A. Prosthodontic Rehabilitation of Head and Neck Cancer Patients—Challenges and New Developments. Oral Dis. 2021, 27, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Daimary, U.D.; Girisa, S.; Kumar, A.; Kunnumakkara, A.B. Tumor Cell Anabolism and Host Tissue Catabolism-Energetic Inefficiency during Cancer Cachexia. Exp. Biol. Med. 2022, 247, 15353702221087962. [Google Scholar] [CrossRef]
- Alexandra, G.; Alexandru, M.; Stefan, C.F.; Petruta-Maria, D.; Gabriel, B.M.; Dragos-Eugen, G.; Teodor, G.M. Blood Group Type Association with Head and Neck Cancer. Hematol. Rep. 2022, 14, 24–30. [Google Scholar] [CrossRef]
- Jing, S.-W.; Xu, Q.; Zhang, X.-Y.; Jing, Z.-H.; Zhao, Z.-J.; Zhang, R.-H.; Wu, F.-P.; Wang, J. Are People With Blood Group O More Susceptible to Nasopharyngeal Carcinoma and Have Worse Survival Rates? A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 698113. [Google Scholar] [CrossRef]
- Kakava, K.; Karelas, I.; Koutrafouris, I.; Damianidis, S.; Stampouloglou, P.; Papadakis, G.; Xenos, A.; Krania, F.; Sarof, P.; Tasopoulos, G.; et al. Relationship between ABO Blood Groups and Head and Neck Cancer among Greek Patients. J. BUON 2016, 21, 594–596. [Google Scholar]
- Talbert, E.E.; Guttridge, D.C. Emerging Signaling Mediators in the Anorexia—Cachexia Syndrome of Cancer. Trends Cancer 2022, 8, 397–403. [Google Scholar] [CrossRef]
- Yin, L.; Cui, J.; Lin, X.; Li, N.; Fan, Y.; Zhang, L.; Liu, J.; Chong, F.; Wang, C.; Liang, T. Identifying Cancer Cachexia in Patients without Weight Loss Information: Machine Learning Approaches to Address a Real-World Challenge. Am. J. Clin. Nutr. 2022, 116, 1229–1239. [Google Scholar] [CrossRef]
- Jones, J.M. The Methodology of Nutritional Screening and Assessment Tools. J. Hum. Nutr. Diet. 2002, 15, 59–71. [Google Scholar] [CrossRef]
- Boutata, F.Z.; Bencharif, M.; Abdessemed, D. Validity of Four Nutritional Screening Tools against Subjective Global Assessment for Inpatients with Non Communicable Diseases (Algeria, 2020). Nutr. Clin. Métabolisme 2022, 36, 54–58. [Google Scholar] [CrossRef]
- Iryzyna—Nowy Mediator Homeostazy Energetycznej|Postępy Higieny i Medycy. Available online: https://phmd.pl/resources/html/article/details?id=141719&language=pl (accessed on 29 December 2023).
- Nuszkiewicz, J.; Czuczejko, J.; Dróżdż, W.; Woźniak, A.; Małkowski, B.; Szewczyk-Golec, K. Concentration of Selected Adipokines and Factors Regulating Carbohydrate Metabolism in Patients with Head and Neck Cancer in Respect to Their Body Mass Index. Int. J. Mol. Sci. 2023, 24, 3283. [Google Scholar] [CrossRef]
- Hicks, D.F.; Bakst, R.; Doucette, J.; Kann, B.H.; Miles, B.; Genden, E.; Misiukiewicz, K.; Posner, M.; Gupta, V. Impact of Obesity on Outcomes for Patients with Head and Neck Cancer. Oral Oncol. 2018, 83, 11–17. [Google Scholar] [CrossRef]
- Sahu, B.; Bal, N.C. Adipokines from White Adipose Tissue in Regulation of Whole Body Energy Homeostasis. Biochimie 2023, 204, 92–107. [Google Scholar] [CrossRef]
- Chapelot, D.; Charlot, K. Physiology of Energy Homeostasis: Models, Actors, Challenges and the Glucoadipostatic Loop. Metabolism 2019, 92, 11–25. [Google Scholar] [CrossRef]
- Mehdar, K.M. The Distribution of Ghrelin Cells in the Human and Animal Gastrointestinal Tract: A Review of the Evidence. Folia Morphol. 2021, 80, 225–236. [Google Scholar] [CrossRef]
- Mihalache, L.; Gherasim, A.; Niţă, O.; Ungureanu, M.C.; Pădureanu, S.S.; Gavril, R.S.; Arhire, L.I. Effects of Ghrelin in Energy Balance and Body Weight Homeostasis. Hormones 2016, 15, 186–196. [Google Scholar] [CrossRef]
- Poher, A.-L.; Tschöp, M.H.; Müller, T.D. Ghrelin Regulation of Glucose Metabolism. Peptides 2018, 100, 236–242. [Google Scholar] [CrossRef]
- Mani, B.K.; Zigman, J.M. Ghrelin as a Survival Hormone. Trends Endocrinol. Metab. 2017, 28, 843–854. [Google Scholar] [CrossRef]
- Arhire, L.I.; Mihalache, L.; Covasa, M. Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front. Endocrinol. 2019, 10, 524. [Google Scholar] [CrossRef]
- Ulupinar, S.; Ozbay, S.; Gencoglu, C.; Altinkaynak, K.; Sebin, E.; Oymak, B. Exercise in the Cold Causes Greater Irisin Release but May Not Be Enough for Adropin. Chin. J. Physiol. 2021, 64, 129. [Google Scholar] [CrossRef]
- Rocha-Rodrigues, S.; Rodríguez, A.; Gouveia, A.M.; Gonçalves, I.O.; Becerril, S.; Ramírez, B.; Beleza, J.; Frühbeck, G.; Ascensão, A.; Magalhães, J. Effects of Physical Exercise on Myokines Expression and Brown Adipose-like Phenotype Modulation in Rats Fed a High-Fat Diet. Life Sci. 2016, 165, 100–108. [Google Scholar] [CrossRef]
- Hofmann, T.; Elbelt, U.; Stengel, A. Irisin as a Muscle-Derived Hormone Stimulating Thermogenesis—A Critical Update. Peptides 2014, 54, 89–100. [Google Scholar] [CrossRef]
- Lagzdina, R.; Rumaka, M.; Gersone, G.; Tretjakovs, P. Circulating Irisin in Healthy Adults: Changes after Acute Exercise, Correlation with Body Composition, and Energy Expenditure Parameters in Cross-Sectional Study. Medicina 2020, 56, 274. [Google Scholar] [CrossRef]
- Niranjan, S.B.; Belwalkar, S.V.; Tambe, S.; Venkataraman, K.; Mookhtiar, K.A. Recombinant Irisin Induces Weight Loss in High Fat DIO Mice through Increase in Energy Consumption and Thermogenesis. Biochem. Biophys. Res. Commun. 2019, 519, 422–429. [Google Scholar] [CrossRef]
- Dong, J.; Dong, Y.; Chen, F.; Mitch, W.E.; Zhang, L. Inhibition of Myostatin in Mice Improves Insulin Sensitivity via Irisin-Mediated Cross Talk between Muscle and Adipose Tissues. Int. J. Obes. 2016, 40, 434–442. [Google Scholar] [CrossRef]
- Van Der Lely, A.J.; Tschop, M.; Heiman, M.L.; Ghigo, E. Biological, Physiological, Pathophysiological, and Pharmacological Aspects of Ghrelin. Endocr. Rev. 2004, 25, 426–457. [Google Scholar] [CrossRef]
- Park, K.H.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.A.; Geladari, E.V.; Huh, J.Y.; Dincer, F.; et al. Circulating Irisin in Relation to Insulin Resistance and the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907. [Google Scholar] [CrossRef]
- Tskhovrebova, L.; Trinick, J. Properties of Titin Immunoglobulin and Fibronectin-3 Domains. J. Biol. Chem. 2004, 279, 46351–46354. [Google Scholar] [CrossRef]
- Herzog, W. The Multiple Roles of Titin in Muscle Contraction and Force Production. Biophys. Rev. 2018, 10, 1187–1199. [Google Scholar] [CrossRef]
- Awano, H.; Matsumoto, M.; Nagai, M.; Shirakawa, T.; Maruyama, N.; Iijima, K.; Nabeshima, Y.; Matsuo, M. Diagnostic and Clinical Significance of the Titin Fragment in Urine of Duchenne Muscular Dystrophy Patients. Clin. Chim. Acta 2018, 476, 111–116. [Google Scholar] [CrossRef]
- Pecoits-Filho, R.; Abensur, H.; Betônico, C.C.; Machado, A.D.; Parente, E.B.; Queiroz, M.; Salles, J.E.N.; Titan, S.; Vencio, S. Interactions between Kidney Disease and Diabetes: Dangerous Liaisons. Diabetol. Metab. Syndr. 2016, 8, 50. [Google Scholar] [CrossRef]
- Toffali, L.; D’Ulivo, B.; Giagulli, C.; Montresor, A.; Zenaro, E.; Delledonne, M.; Rossato, M.; Iadarola, B.; Sbarbati, A.; Bernardi, P.; et al. An Isoform of the Giant Protein Titin Is a Master Regulator of Human T Lymphocyte Trafficking. Cell Rep. 2023, 42, 112516. [Google Scholar] [CrossRef]
- Gaździcka, J.; Gołąbek, K.; Strzelczyk, J.K.; Ostrowska, Z. Epigenetic Modifications in Head and Neck Cancer. Biochem. Genet. 2020, 58, 213–244. [Google Scholar] [CrossRef]
- Hier, J.; Vachon, O.; Bernstein, A.; Ibrahim, I.; Mlynarek, A.; Hier, M.; Alaoui-Jamali, M.A.; Maschietto, M.; da Silva, S.D. Portrait of DNA Methylated Genes Predictive of Poor Prognosis in Head and Neck Cancer and the Implication for Targeted Therapy. Sci. Rep. 2021, 11, 10012. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN) ESPEN Guidelines for Nutrition Screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Lindh, M.; Görander, S.; Andersson, E.; Horal, P.; Mattsby-Balzer, I.; Ryd, W. Real-Time Taqman PCR Targeting 14 Human Papilloma Virus Types. J. Clin. Virol. 2007, 40, 321–324. [Google Scholar] [CrossRef]
- Ingerslev, K.; Hogdall, E.; Skovrider-Ruminski, W.; Schnack, T.H.; Karlsen, M.A.; Nedergaard, L.; Hogdall, C.; Blaakær, J. High-Risk HPV Is Not Associated with Epithelial Ovarian Cancer in a Caucasian Population. Infect. Agents Cancer 2016, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Available online: https://www.who.int (accessed on 10 January 2023).
- McGovern, J.; Dolan, R.D.; Skipworth, R.J.; Laird, B.J.; McMillan, D.C. Cancer Cachexia: A Nutritional or a Systemic Inflammatory Syndrome? Br. J. Cancer 2022, 127, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N. ESPEN Expert Group Recommendations for Action against Cancer-Related Malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S. ESPEN Guidelines on Nutrition in Cancer Patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Gangadharan, A.; Choi, S.E.; Hassan, A.; Ayoub, N.M.; Durante, G.; Balwani, S.; Kim, Y.H.; Pecora, A.; Goy, A.; Suh, K.S. Protein Calorie Malnutrition, Nutritional Intervention and Personalized Cancer Care. Oncotarget 2017, 8, 24009. [Google Scholar] [CrossRef]
- Virizuela, J.A.; Camblor-Álvarez, M.; Luengo-Pérez, L.M.; Grande, E.; Álvarez-Hernández, J.; Sendrós-Madroño, M.J.; Jiménez-Fonseca, P.; Cervera-Peris, M.; Ocón-Bretón, M.J. Nutritional Support and Parenteral Nutrition in Cancer Patients: An Expert Consensus Report. Clin. Transl. Oncol. 2018, 20, 619–629. [Google Scholar] [CrossRef]
- Van Baak, M.A.; Mariman, E. Mechanisms of Weight Regain after Weight Loss—The Role of Adipose Tissue. Nat. Rev. Endocrinol. 2019, 15, 274–287. [Google Scholar] [CrossRef]
- Provatopoulou, X.; Georgiou, G.P.; Kalogera, E.; Kalles, V.; Matiatou, M.A.; Papapanagiotou, I.; Sagkriotis, A.; Zografos, G.C.; Gounaris, A. Serum Irisin Levels Are Lower in Patients with Breast Cancer: Association with Disease Diagnosis and Tumor Characteristics. BMC Cancer 2015, 15, 898. [Google Scholar] [CrossRef]
- Gannon, N.P.; Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Trujillo, K.A. Effects of the Exercise-Inducible Myokine Irisin on Malignant and Non-Malignant Breast Epithelial Cell Behavior in Vitro. Int. J. Cancer 2015, 136, E197–E202. [Google Scholar] [CrossRef]
- Shao, L.; Li, H.; Chen, J.; Song, H.; Zhang, Y.; Wu, F.; Wang, W.; Zhang, W.; Wang, F.; Li, H.; et al. Irisin Suppresses the Migration, Proliferation, and Invasion of Lung Cancer Cells via Inhibition of Epithelial-to-Mesenchymal Transition. Biochem. Biophys Res. Commun. 2017, 485, 598–605. [Google Scholar] [CrossRef]
- Moon, H.-S.; Mantzoros, C.S. Regulation of Cell Proliferation and Malignant Potential by Irisin in Endometrial, Colon, Thyroid and Esophageal Cancer Cell Lines. Metab. Clin. Exp. 2014, 63, 188–193. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Moreno-Navarrete, J.M.; Serrano, M.; Ortega, F.; Delgado, E.; Sanchez-Ragnarsson, C.; Valdés, S.; Botas, P.; Ricart, W.; Fernández-Real, J.M. Circulating Irisin Levels Are Positively Associated with Metabolic Risk Factors in Sedentary Subjects. PLoS ONE 2015, 10, e0124100. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.-J.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef] [PubMed]
- Homa-Mlak, I.; Mlak, R.; Brzozowska, A.; Mazurek, M.; Powrózek, T.; Prendecka-Wróbel, M.; Szudy-Szczyrek, A.; Dreher, P.; Kamińska, K.; Małecka-Massalska, T.; et al. High Level of Irisin as a Marker of Malnutrition in Head and Neck Cancer Patients Subjected to Radiotherapy. Med. Sci. Monit. 2022, 28, e936857-1. [Google Scholar] [CrossRef]
- Panagiotou, G.; Triantafyllidou, S.; Tarlatzis, B.C.; Papakonstantinou, E. Serum Levels of Irisin and Omentin-1 in Breast Neoplasms and Their Association with Tumor Histology. Int. J. Endocrinol. 2021, 2021, e6656671. [Google Scholar] [CrossRef]
- Shahidi, S.; Hejazi, J.; Moghimi, M.; Borji, S.; Zabihian, S.; Fathi, M. Circulating Irisin Levels and Redox Status Markers in Patients with Gastric Cancer: A Case-Control Study. Asian Pac. J. Cancer Prev. 2020, 21, 2847–2851. [Google Scholar] [CrossRef]
- Altay, D.U.; Keha, E.E.; Karagüzel, E.; Menteşe, A.; Yaman, S.O.; Alver, A. The Diagnostic Value of FNDC5/Irisin in Renal Cell Cancer. Int. Braz. J. Urol. 2018, 44, 734–739. [Google Scholar] [CrossRef]
- Quiñones, M.; Folgueira, C.; Sánchez-Rebordelo, E.; Al-Massadi, O. Circulating Irisin Levels Are Not Regulated by Nutritional Status, Obesity, or Leptin Levels in Rodents. Mediat. Inflamm. 2015, 2015, e620919. [Google Scholar] [CrossRef]
- Kotta, A.S.; Kelling, A.S.; Corleto, K.A.; Sun, Y.; Giles, E.D. Ghrelin and Cancer: Examining the Roles of the Ghrelin Axis in Tumor Growth and Progression. Biomolecules 2022, 12, 483. [Google Scholar] [CrossRef]
- Blatnik, M.; Soderstrom, C.I.; Dysinger, M.; Fraser, S.A. Prandial Ghrelin Attenuation Provides Evidence That Des-Acyl Ghrelin May Be an Artifact of Sample Handling in Human Plasma. Bioanalysis 2012, 4, 2447–2455. [Google Scholar] [CrossRef]
- Gahete, M.D.; Córdoba-Chacón, J.; Salvatori, R.; Castaño, J.P.; Kineman, R.D.; Luque, R.M. Metabolic Regulation of Ghrelin O-Acyl Transferase (GOAT) Expression in the Mouse Hypothalamus, Pituitary, and Stomach. Mol. Cell. Endocrinol. 2010, 317, 154–160. [Google Scholar] [CrossRef]
- Pritchett, N.R.; Maziarz, M.; Shu, X.-O.; Kamangar, F.; Dawsey, S.M.; Fan, J.-H.; Ji, B.-T.; Gao, Y.-T.; Xiang, Y.-B.; Qiao, Y.-L.; et al. Serum Ghrelin and Esophageal and Gastric Cancer in Two Cohorts in China. Int. J. Cancer 2020, 146, 2728–2735. [Google Scholar] [CrossRef]
- Murphy, G.; Cross, A.J.; Dawsey, S.M.; Stanczyk, F.Z.; Kamangar, F.; Weinstein, S.J.; Taylor, P.R.; Männistö, S.; Albanes, D.; Abnet, C.C.; et al. Serum Ghrelin Is Associated with Risk of Colorectal Adenocarcinomas in the ATBC Study. Gut 2018, 67, 1646–1651. [Google Scholar] [CrossRef]
- Murphy, G.; Kamangar, F.; Albanes, D.; Stanczyk, F.Z.; Weinstein, S.J.; Taylor, P.R.; Virtamo, J.; Abnet, C.C.; Dawsey, S.M.; Freedman, N.D. Serum Ghrelin Is Inversely Associated with Risk of Subsequent Oesophageal Squamous Cell Carcinoma. Gut 2012, 61, 1533–1537. [Google Scholar] [CrossRef]
- Venneman, K.; Huybrechts, I.; Gunter, M.J.; Vandendaele, L.; Herrero, R.; Van Herck, K. The Epidemiology of Helicobacter Pylori Infection in Europe and the Impact of Lifestyle on Its Natural Evolution toward Stomach Cancer after Infection: A Systematic Review. Helicobacter 2018, 23, e12483. [Google Scholar] [CrossRef]
- Jarosz, M.; Rychlik, E. Alcohol Consumption and Tobacco Smoking and Selected Gastrointestinal Cancers Morbidity Rates in Poland. Ann. Oncol. 2019, 30, iv37. [Google Scholar] [CrossRef]
- Nunez-Salces, M.; Li, H.; Feinle-Bisset, C.; Young, R.L.; Page, A.J. The Regulation of Gastric Ghrelin Secretion. Acta Physiol. 2021, 231, e13588. [Google Scholar] [CrossRef]
- Zeng: Acylated and Unacylated Ghrelin Relieve Cancer. Available online: https://scholar.google.com/scholar_lookup?title=Acylated+and+unacylated+ghrelin+relieve+cancer+cachexia+in+mice+through+multiple+mechanisms&author=Zeng,+X.&author=Chen,+P.&author=Zhao,+L.&author=Chen,+S.&publication_year=2020&journal=Chin.+J.+Physiol.&volume=63&pages=195%E2%80%93203&doi=10.4103/CJP.CJP_59_20&pmid=33109785 (accessed on 14 January 2023).
- Batury, V.-L.; Walton, E.; Tam, F.; Wronski, M.-L.; Buchholz, V.; Frieling, H.; Ehrlich, S. DNA Methylation of Ghrelin and Leptin Receptors in Underweight and Recovered Patients with Anorexia Nervosa. J. Psychiatr. Res. 2020, 131, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Wolf, L.S.; Nicoletti, C.F.; Noronha, N.Y.; de Souza Pinhel, M.A.; Welendorf, C.R.; Delfino, H.B.P.; Salgado Junior, W.; Nonino, C.B. Roux-En-Y Gastric Bypass Can Modulate Methylation Level of Ghrelin Gene: A 6-Month Longitudinal Study. SN Compr. Clin. Med. 2022, 4, 210. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, J.; He, N.; He, J.; Zhu, B. Titin Mutation Associated with Responsiveness to Checkpoint Blockades in Solid Tumors. JCI Insight 2019, 4, e127901. [Google Scholar] [CrossRef]
- Su, C.; Wang, X.; Zhou, J.; Zhao, J.; Zhou, F.; Zhao, G.; Xu, X.; Zou, X.; Zhu, B.; Jia, Q. Titin Mutation in Circulatory Tumor DNA Is Associated with Efficacy to Immune Checkpoint Blockade in Advanced Non-Small Cell Lung Cancer. Transl. Lung Cancer Res. 2021, 10, 1256–1265. [Google Scholar] [CrossRef]
- Han, X.; Chen, J.; Wang, J.; Xu, J.; Liu, Y. TTN Mutations Predict a Poor Prognosis in Patients with Thyroid Cancer. Biosci. Rep. 2022, 42, BSR20221168. [Google Scholar] [CrossRef]
| Factor | Study Group (n = 56) |
|---|---|
| Gender | |
| Male | 24 (43%) |
| Female | 32 (57%) |
| T stage | |
| T3 | 36 (64%) |
| T4 | 20 (36%) |
| N stage | |
| Nx | 2 (3%) |
| N0 | 5 (9%) |
| N1 | 7 (12%) |
| N2 | 38 (68%) |
| N3 | 4 (8%) |
| M stage | |
| Mx | 1 (2%) |
| M0 | 49 (88%) |
| M1 | 6 (10%) |
| Performance status (PS) | |
| Disease stage | |
| I | 0 |
| II | 2 (4%) |
| III | 15 (27%) |
| IVA | 31 (55%) |
| IVB | 5 (9%) |
| IVC | 3 (5%) |
| 0 | 0 |
| 1 | 41 (73%) |
| 2 | 14 (25%) |
| 3 | 1 (2%) |
| 4 | 0 |
| Human Papilloma Virus (HPV)-16 status | |
| Positive | 43 (77%) |
| Negative | 13 (13%) |
| Human Papilloma Virus status-18 | |
| Positive | 41 (73%) |
| Negative | 15 (17%) |
| Factor | Study Group (General) | T3 | T4 | p-Value (Chi-Square Test) |
|---|---|---|---|---|
| Alcohol consumption | 25 (69%) | 18 (50%) | 7 (35%) | 0.28 |
| Social | 20 (80%) | 15 (42%) | 5 (25%) | 0.64 |
| Former alcoholic | 3 (12%) | 2 (6%) | 1 (5%) | |
| Current alcoholic | 2 (8%) | 1 (3%) | 1 (5%) | |
| Never | 31 (55%) | 18 (50%) | 13 (65%) | |
| Smoking status | ||||
| Non-smoker | 14 (25%) | 12 (33%) | 2 (10%) | 0.16 |
| Current smoker | 35 (63%) | 20 (56%) | 15 (75%) | |
| Former smoker | 7 (12%) | 4 (11%) | 3 (15%) | |
| Nutritional status (parenteral nutrition) | ||||
| Yes | 15 (27%) | 11 (31%) | 4 (20%) | 0.39 |
| No | 41 (73%) | 25 (69%) | 16 (80%) | |
| Weight (kg; mean ± SD) | 54.65 ± 8.98 | 54.11 ± 9.18 | 55.19 ± 10.19 | |
| Body Mass Index (BMI) mean ± SD | 20.93 ± 3.12 | 20.76 ± 3.98 | 21.09 ± 4.56 | |
| <18.5 | 38 (68%) | 25 (69%) | 13 (65%) | 0.73 |
| 18.5–24.99 | 18 (32%) | 11 (31%) | 7 (35%) | |
| >25 | 0 (0%) | 0 (0%) | 0 (0%) | |
| SGA | ||||
| A | 23 (41%) | 16 (44%) | 7 (35%) | 0.79 |
| B | 20 (36%) | 12 (33%) | 8 (40%) | |
| C | 13 (23%) | 8 (23%) | 5 (25%) | |
| NRS | ||||
| 1 | 8 (14%) | 4 (11%) | 4 (20%) | 0.38 |
| 2 | 9 (16%) | 7 (18%) | 2 (10%) | |
| 3 | 32 (57%) | 22 (59%) | 10 (50%) | |
| >3 | 7 (13%) | 3 (12%) | 4 (20%) | |
| Comorbidities/disease | 27 (48%) | 17 (47%) | 10 (50%) | 0.84 |
| Diabetic mellitus (yes) | 5 (9%) | 3 (8%) | 2 (10%) | 0.83 |
| Hypertension (yes) | 3 (5%) | 2 (6%) | 1 (5%) | 0.93 |
| Hypercholesterolemia (yes) | 1 (2%) | 0 (0%) | 1 (5%) | - |
| Diabetic mellitus plus hypertension | 10 (18%) | 7 (19%) | 3 (15%) | 0.68 |
| Diabetic mellitus plus hypercholesterolemia | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Hypertension plus hypercholesterolemia | 3 (5%) | 2 (6%) | 1 (5%) | 0.93 |
| Diabetic mellitus plus hypercholesterolemia plus hypertension | 5 (9%) | 3 (8%) | 2 (10%) | 0.84 |
| Physical activity | ||||
| Never | 41 (73%) | 25 (69%) | 16 (80%) | 0.65 |
| Occasional | 10 (18%) | 7 (19%) | 3 (15%) | |
| Regular | 5 (9%) | 4 (12%) | 1 (5%) | |
| Dietary | ||||
| Omnivorous | 44 | 29 | 15 | 0.86 |
| Vegetarians | 10 | 6 | 4 | |
| Vegans | 2 | 1 | 1 |
| mRNA | qRT-PCR Amplification Primers (5′-3′) |
|---|---|
| FNDC5 | Forward: GGCTGCACTACAAACCCAAA |
| Reverse: TTGTCATCTCCCAGGGCTTT | |
| GHRL | Forward: AGTTCCCAAGGCATCCATCA |
| Reverse: GGCAGGGATGTTAGCAAAGG | |
| TNT | Forward: CGAAATGCATCAGTCAGCGA |
| Reverse: TCCTTGCAAGCTTGTGTCAC | |
| Β-actin | Forward: TCACCCACACTGTGCCCATCTACGA |
| Reverse: CAGCGGAACCGCTCATTGCCAATGG |
| mRNA | qRT-PCR Amplification Primers (5′-3′) | GC% | ‘C’s | |
|---|---|---|---|---|
| FNDC5 (irisin) | M | Forward: 5′-ATTTTTTAGTAGAAGAAGGATGTGC-3′ Reverse: 5′-AAATCTTAAAAAACACAAACTCGCT-3′ | 52.00 60.00 | 5 8 |
| U | Forward: 5′-TTTTAGTAGAAGAAGGATGTGTGG-3′ Reverse: 5′-AAATCTTAAAAAACACAAACTCACT-3′ | 58.33 60.00 | 4 8 | |
| Ghrelin | M | Forward: 5′-AGGGTTAAGGAGGAGTGTTTGTC-3′ Reverse: 5′-AAATAATTATTTCCCATATTCACGTC-3′ | 65.22 42.31 | 4 4 |
| U | Forward: 5′-AGGGTTAAGGAGGAGTGTTTGTT-3′ Reverse: 5′-AAATAATTATTTCCCATATTCACATC-3′ | 65.22 42.31 | 4 4 | |
| Titin | M | Forward: 5′-GAAAAGTTTATTTTTTTCGAATTGC-3′ Reverse: 5′-CTTTAACTTCCAAATCTTCAAAACG-3′ | 40.00 52.00 | 4 5 |
| U | Forward: 5′-GGAAAAGTTTATTTTTTTTGAATTGT-3′ Reverse: 5′-TTAACTTCCAAATCTTCAAAACACT-3′ | 40.74 52.00 | 4 5 |
| Nutritional Scale | T0 | T3 | T5 | p-Value (Chi-Square Test) |
|---|---|---|---|---|
| SGA | ||||
| A | 8 (14%) | 14 (25%) | 15 (27%) | 0.52 |
| B | 37 (66%) | 32 (57%) | 33 (59%) | |
| C | 11 (20%) | 10 (18%) | 8 (14%) | |
| NRS | ||||
| 1 | 5 (9%) | 6 (11%) | 10 (18%) | 0.91 |
| 2 | 3 (5%) | 10 (18%) | 11 (20%) | |
| 3 | 34 (61%) | 33 (59%) | 28 (50%) | |
| >3 | 14 (25%) | 7 (12%) | 7 (12%) |
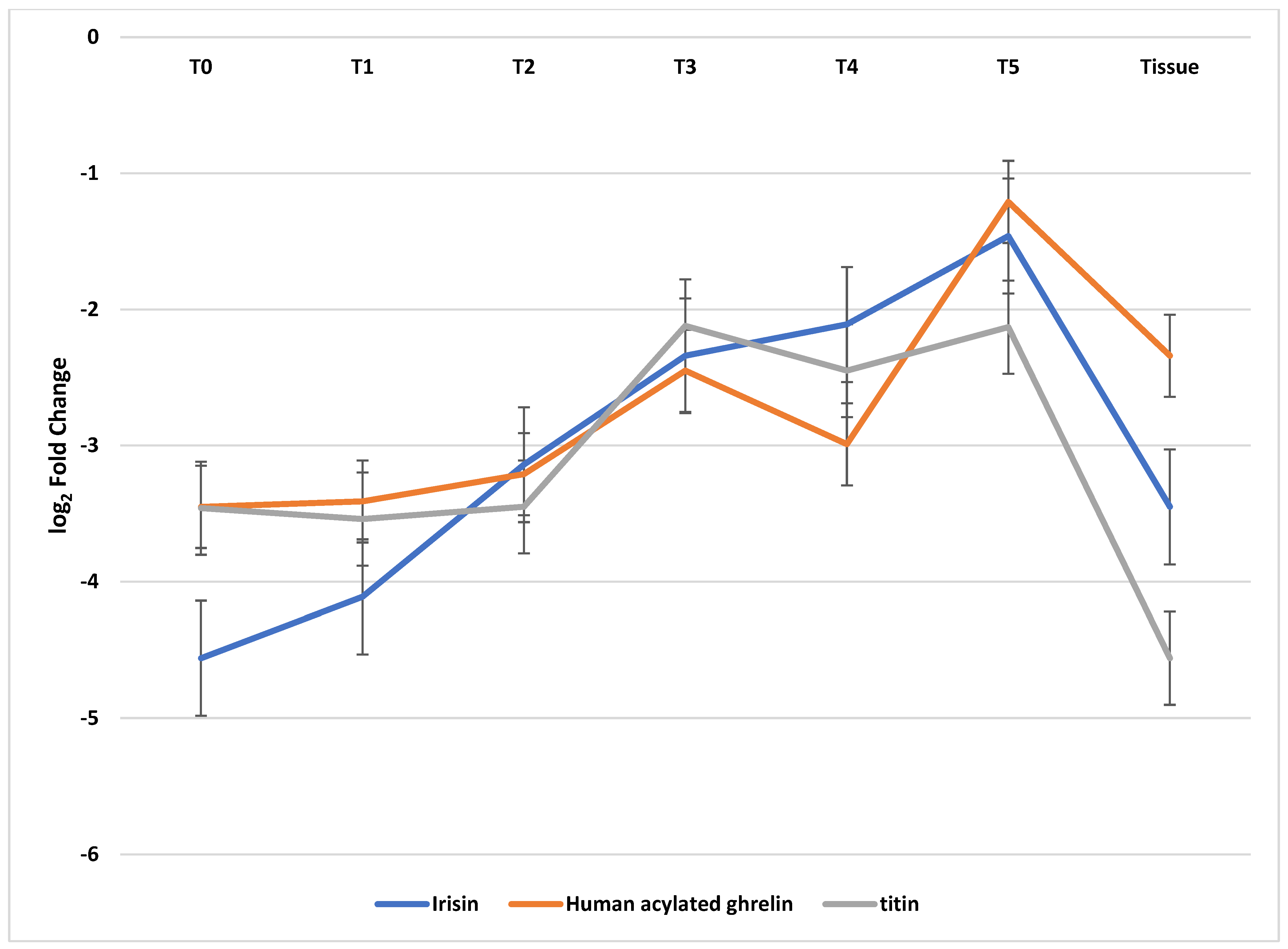
| Type of Tissue | Time | Group According to the Nutrition Status | Log2 Fold Change | ||
|---|---|---|---|---|---|
| Irisin | Human Acylated Ghrelin | Titin | |||
| Tissue | - | General | (−)3.45 ± 0.87 1,2,3 | (−)2.34 ± 0.98 1,2,3 | (−)4.56 ± 0.45 1,2,3 |
| A | (−)1.76± 0.98 | (−)1.87 ± 0.93 | (−)2.34 ± 0.16 | ||
| B | (−)3.21 ± 0.86 | (−)2.13 ± 0.96 | (−)3.98 ± 0.24 | ||
| C | (−)5.38 ± 0.77 | (−)3.02 ± 1.05 | (−)7.36 ± 0.21 | ||
| Blood samples | T0 | General | (−)4.56 ± 0.54 1,2,3 | (−)3.45 ± 0.23 2 | (−)3.46 ± 0.84 1,2,3 |
| A | (−)2.87 ± 0.45 | (−)3.12 ± 0.13 | (−)1.98 ± 0.79 | ||
| B | (−)4.01 ± 0.65 | (−)3.35 ± 0.24 | (−)3.67 ± 0.98 | ||
| C | (−)6.80 ± 0.51 | (−)3.98 ± 0.31 | (−)4.73 ± 0.76 | ||
| T1 | General | (−)4.11 ± 0.921,2,3 | (−)3.41 ± 0.12 1,2,3 | (−)3.54 ± 0.98 1,2,3 | |
| A | (−)2.98 ± 0.87 | (−)1.59 ± 0.11 | (−)2.00 ± 0.99 | ||
| B | (−)4.12 ± 0.99 | (−)2.98 ± 0.13 | (−)3.87 ± 0.91 | ||
| C | (−)5.23 ± 0.91 | (−)5.66 ± 0.12 | (−)4.75 ± 1.04 | ||
| T2 | General | (−)3.14 ± 0.23 1,2,3 | (−)3.21 ± 0.18 1,2,3 | (−)3.45 ± 0.19 1,2,3 | |
| A | (−)2.78 ± 0.24 | (−)1.84 ± 0.19 | (−)2.01 ± 0.21 | ||
| B | (−)2.97 ± 0.21 | (−)3.44 ± 0.19 | (−)3.51 ± 0.20 | ||
| C | (−)3.76 ± 0.24 | (−)4.35 ± 0.16 | (−)4.83 ± 0.16 | ||
| T3 | General | (−)2.34 ± 0.19 1,2,3 | (−)2.45 ± 0.12 1,2,3 | (−)2.12 ± 0.18 1,2,3 | |
| A | (−)1.06 ± 0.16 | (−)1.87 ± 0.12 | (−)1.56 ± 0.25 | ||
| B | (−)2.18 ± 0.21 | (−)2.50 ± 0.13 | (−)1.98 ± 0.16 | ||
| C | (−)3.78 ± 0.20 | (−)2.98 ± 0.11 | (−)2.82 ± 0.13 | ||
| T4 | General | (−)2.11 ± 0.65 1,2,3 | (−)2.99 ± 0.87 1,2,3 | (−)2.45 ± 0.77 1,2,3 | |
| A | (−)1.43 ± 0.45 | (−)1.21 ± 0.98 | (−)1.77 ± 0.80 | ||
| B | (−)2.12 ± 0.87 | (−)3.80 ± 0.71 | (−)2.13 ± 0.67 | ||
| C | (−)2.78 ± 0.56 | (−)3.96 ± 0.92 | (−)3.45 ± 0.84 | ||
| T5 | General | (−)1.56 ± 0.29 1,2,3 | (−)1.21 ± 0.98 | (−)2.13 ± 0.99 1 | |
| A | (−)1.12 ± 0.23 | (−)1.11 ± 1.01 | (−)2.01 ± 1.04 | ||
| B | (−)1.58 ± 0.31 | (−)1.23 ± 0.89 | (−)2.07 ± 1.01 | ||
| C | (−)1.87 ± 0.33 | (−)1.19 ± 1.04 | (−)2.31 ± 0.92 | ||
| Type of Tissue | Time | Irisin | Human Acylated Ghrelin | Titin | |||
|---|---|---|---|---|---|---|---|
| Methylated | Non-Methylated | Methylated | Non-Methylated | Methylated | Non-Methylated | ||
| Cancer tissue | - | 55 (98%) | 1 (2%) | 55 (98%) | 1 (2%) | 54 (96%) | 2 (4%) |
| Margin tissue | - | 1 (2%) | 55 (98%) | 1 (2%) | 55 (98%) | 2 (4%) | 54 (96%) |
| Blood samples | C | 1 (2%) | 69 (99%) | 0 (0%) | 70 (100%) | 2 (4%) | 68 (96%) |
| T0 | 56 (100%) | 0 (0%) | 56 (100%) | 0 (0%) | 56 (100%) | 0 (0%) | |
| T1 | 56 (100%) | 0 (0%) | 56 (100%) | 0 (0%) | 56 (100%) | 0 (0%) | |
| T2 | 56 (100%) | 0 (0%) | 56 (100%) | 0 (0%) | 56 (100%) | 0 (0%) | |
| T3 | 56 (100%) | 0 (0%) | 56 (100%) | 0 (0%) | 56 (100%) | 0 (0%) | |
| T4 | 56 (100%) | 0 (0%) | 56 (100%) | 0 (0%) | 56 (100%) | 0 (0%) | |
| T5 | 56 (100%) | 0 (0%) | 56 (100%) | 0 (0%) | 56 (100%) | 0 (0%) | |
| Type of Tissue | Time | Group | Irisin [ng/mL] | Human Acylated Ghrelin [pg/mL] | Titin [pg/mL] |
|---|---|---|---|---|---|
| Margin tissue (control) | - | - | 103.24 ± 0.76 | 141.12 ± 2.23 | 51.11 ± 1.23 |
| Cancer tissue | - | General | 21.42 ± 0.19 1,2,3 | 37.12 ± 1.23 1,2,3 | 24.56 ± 0.12 1,2,3 |
| A | 24.56 ± 0.23 | 43.21 ± 1.56 | 27.98 ± 0.13 | ||
| B | 21.09 ± 0.21 | 39.24 ± 1.21 | 27.69 ± 0.11 | ||
| C | 15.9 ± 0.13 | 28.91 ± 0.92 | 18.01 ± 0.12 | ||
| Blood samples | - | Control | 78.17 ± 0.98 | 189.09 ± 1.34 | 102.98 ± 1.45 |
| T0 | General | 21.14 ± 0.44 1,2,3 | 21.98 ± 0.13 1,2,3 | 28.98 ± 0.12 1,2,3 | |
| A | 25.19 ± 0.46 | 27.87 ± 0.12 | 34.87 ± 0.12 | ||
| B | 21.12 ± 0.42 | 20.55 ± 0.15 | 33.09 ± 0.15 | ||
| C | 17.10 ± 0.44 | 17.52 ± 0.12 | 18.98 ± 0.09 | ||
| T1 | General | 18.76 ± 0.14 1,2,3 | 22.13 ± 0.87 1,2,3 | 28.09 ± 0.92 1,2,3 | |
| A | 21.09 ± 0.16 | 25.14 ± 0.98 | 32.98 ± 0.98 | ||
| B | 19.30 ± 0.12 | 23.19 ± 0.54 | 26.87 ± 0.95 | ||
| C | 15.90 ± 0.14 | 18.06 ± 1.09 | 24.42 ± 0.83 | ||
| T2 | General | 19.09 ± 0.76 1,2,3 | 22.23 ± 0.99 1,2,3 | 30.01 ± 0.82 1,2,3 | |
| A | 21.76 ± 0.85 | 25.09 ± 1.02 | 35.98 ± 0.54 | ||
| B | 19.87 ± 0.54 | 22.19 ± 0.97 | 27.13 ± 0.86 | ||
| C | 15.64 ± 0.89 | 19.41 ± 0.95 | 26.92 ± 1.06 | ||
| T3 | General | 24.89 ± 0.12 1,2,3 | 29.98 ± 0.45 1,2,3 | 34.98 ± 1.33 1,2,3 | |
| A | 29.17 ± 0.15 | 36.78 ± 0.23 | 41.11 ± 1.54 | ||
| B | 23.45 ± 0.09 | 31.09 ± 0.65 | 34.56 ± 1.01 | ||
| C | 22.05 ± 0/12 | 22.07 ± 0.47 | 29.27 ± 1.44 | ||
| T4 | General | 25.09 ± 0.13 1,2,3 | 29.01 ± 0.89 1,2,3 | 35.61 ± 0.99 1,2,3 | |
| A | 27.18 ± 0.12 | 35.11 ± 0.76 | 41.23 ± 1.21 | ||
| B | 23.56 ± 0.14 | 30.98 ± 0.99 | 36.78 ± 0.43 | ||
| C | 24.53 ± 0.13 | 20.94 ± 0.92 | 28.82 ± 1.33 | ||
| T5 | General | 25.15 ± 0.37 1,2,3 | 29.54 ± 1.65 1,2,3 | 35.18 ± 1.41 1,2,3 | |
| A | 27.11 ± 0.30 | 34.56 ± 1.76 | 38.98 ± 1.43 | ||
| B | 22.98 ± 0.41 | 29.18 ± 1.00 | 32.11 ± 1.41 | ||
| C | 25.36 ± 0.40 | 24.88 ± 1.11 | 34.45 ± 1.39 |
| Blood Group | A n (%) | B | AB | 0 |
|---|---|---|---|---|
| Study group | 34 (61) | 6 (11) | 3 (5) | 13 (23) |
| Control group | 27 (38) | 6 (9) | 3 (4) | 34 (49) |
| Odds ratio | 2.46 | 1.28 | 1.26 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andruszko, A.; Szydłowski, J.; Grabarek, B.O.; Mazur, K.; Sirek, T.; Ossowski, P.; Kozikowski, M.; Kaminiów, K.; Zybek-Kocik, A.; Banaszewski, J. Impact of Nutritional Status of Patients with Head and Neck Squamous Cell Carcinoma on the Expression Profile of Ghrelin, Irisin, and Titin. Cancers 2024, 16, 437. https://doi.org/10.3390/cancers16020437
Andruszko A, Szydłowski J, Grabarek BO, Mazur K, Sirek T, Ossowski P, Kozikowski M, Kaminiów K, Zybek-Kocik A, Banaszewski J. Impact of Nutritional Status of Patients with Head and Neck Squamous Cell Carcinoma on the Expression Profile of Ghrelin, Irisin, and Titin. Cancers. 2024; 16(2):437. https://doi.org/10.3390/cancers16020437
Chicago/Turabian StyleAndruszko, Agata, Jarosław Szydłowski, Beniamin Oskar Grabarek, Katarzyna Mazur, Tomasz Sirek, Piotr Ossowski, Mieszko Kozikowski, Konrad Kaminiów, Ariadna Zybek-Kocik, and Jacek Banaszewski. 2024. "Impact of Nutritional Status of Patients with Head and Neck Squamous Cell Carcinoma on the Expression Profile of Ghrelin, Irisin, and Titin" Cancers 16, no. 2: 437. https://doi.org/10.3390/cancers16020437
APA StyleAndruszko, A., Szydłowski, J., Grabarek, B. O., Mazur, K., Sirek, T., Ossowski, P., Kozikowski, M., Kaminiów, K., Zybek-Kocik, A., & Banaszewski, J. (2024). Impact of Nutritional Status of Patients with Head and Neck Squamous Cell Carcinoma on the Expression Profile of Ghrelin, Irisin, and Titin. Cancers, 16(2), 437. https://doi.org/10.3390/cancers16020437










