Validation of a Machine Learning Model to Predict Immunotherapy Response in Head and Neck Squamous Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
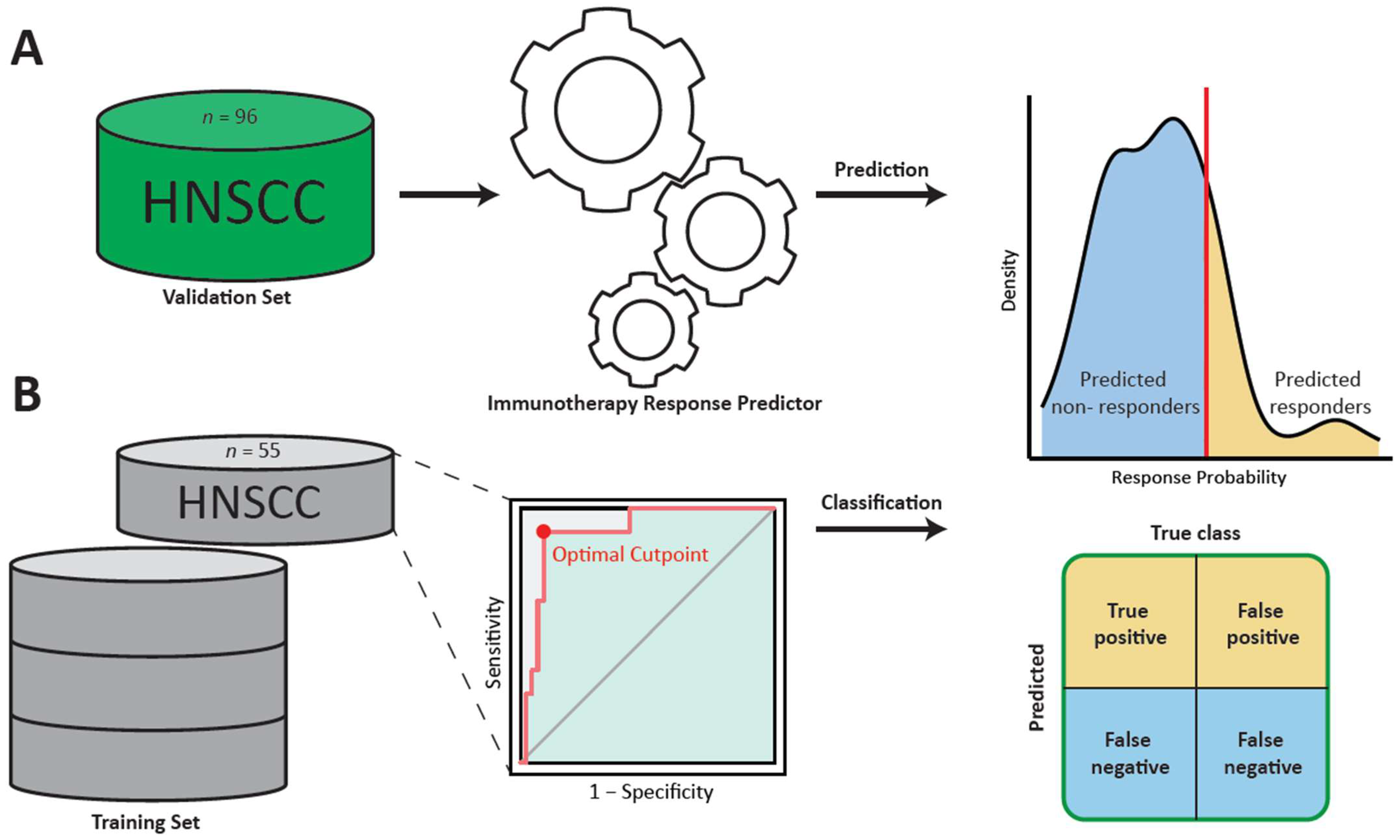
2. Materials and Methods
2.1. Patient Cohort
2.2. Clinical Outcomes
2.3. Genomic, Demographic, Molecular, and Clinical Data
2.4. Application of the Random Forest Classifier
2.5. Performance Metrics and Statistical Analysis
3. Results
3.1. Population Characteristics
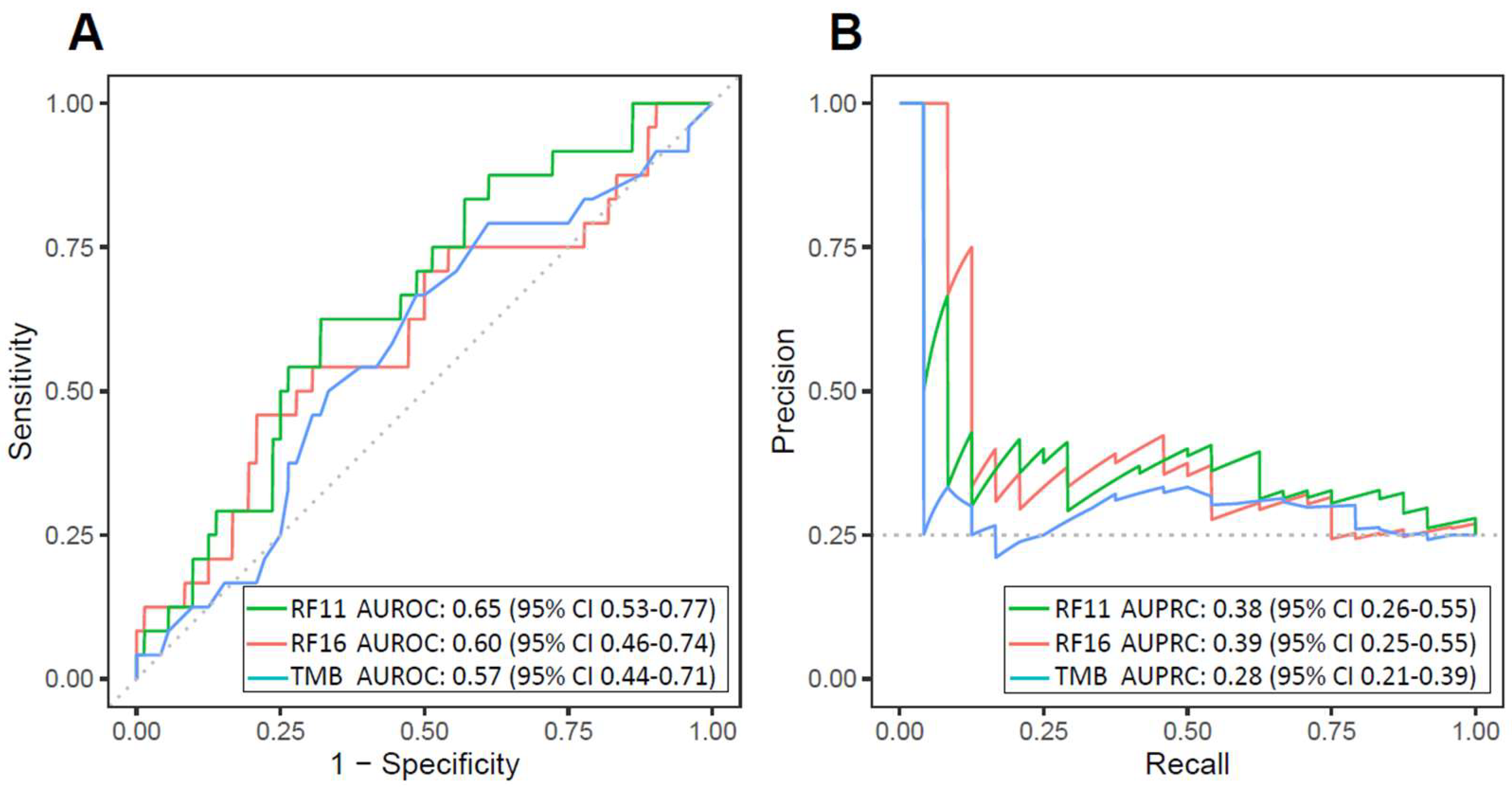
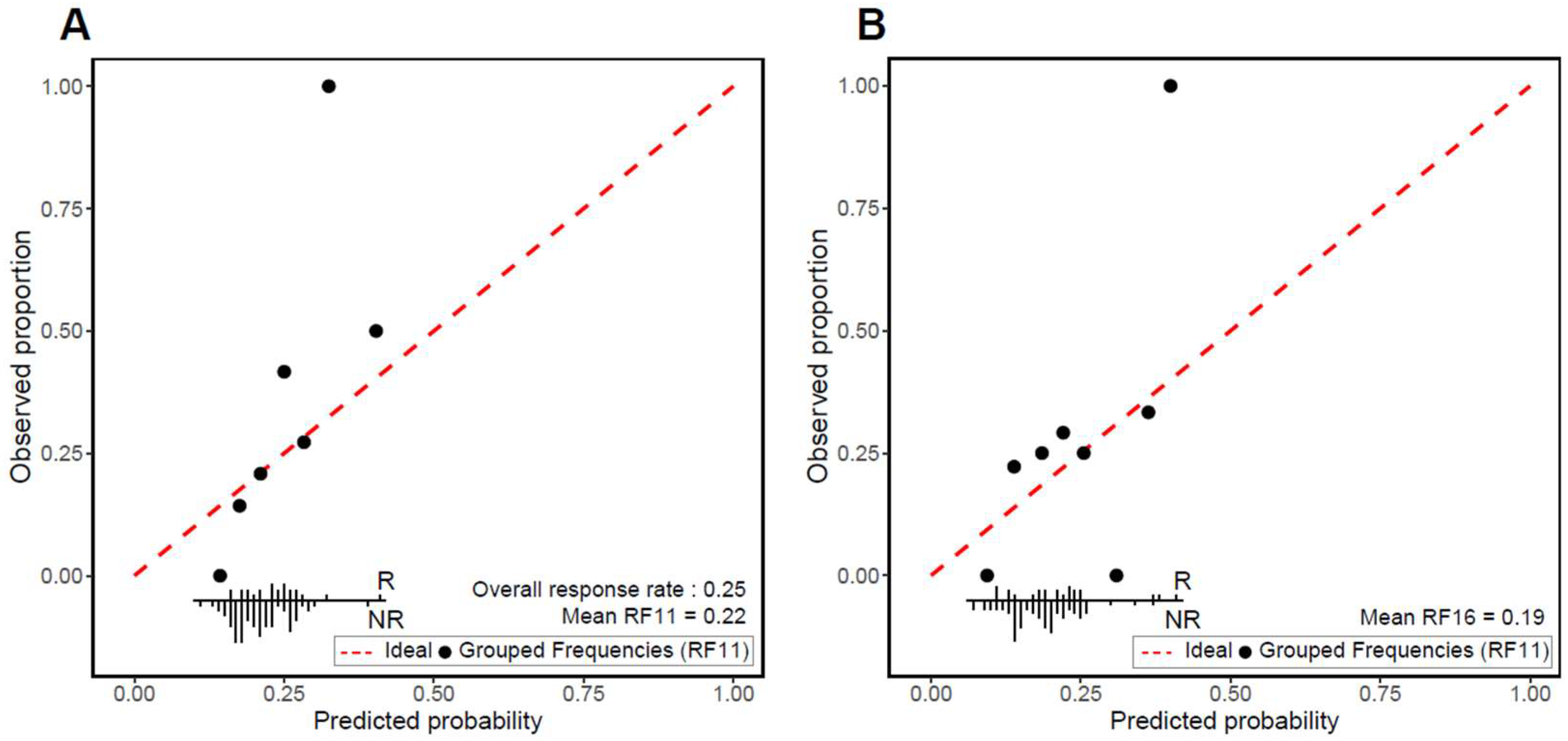
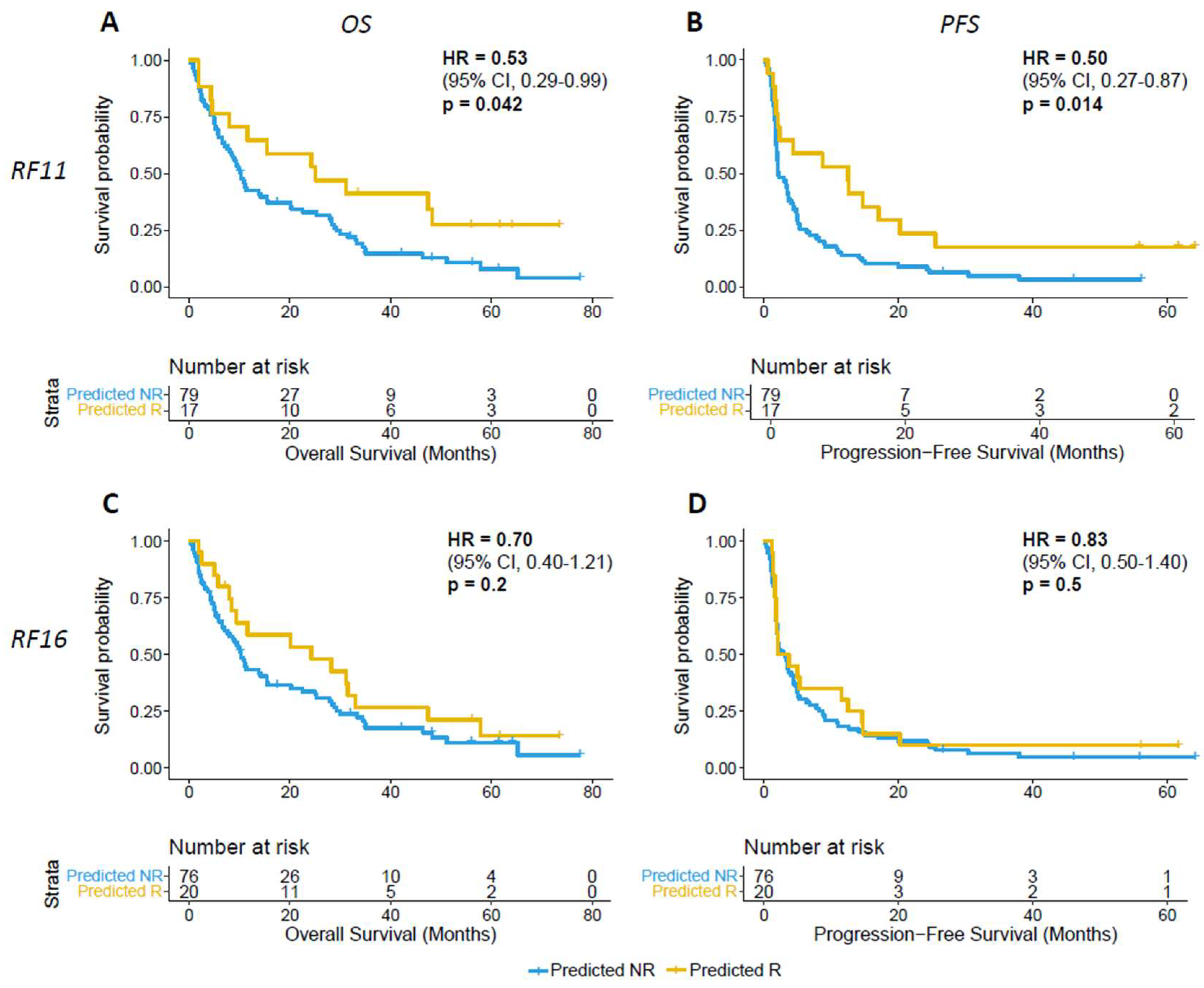
3.2. Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Haring, C.T.; Kana, L.A.; Dermody, S.M.; Brummel, C.; McHugh, J.B.; Casper, K.A.; Chinn, S.B.; Malloy, K.M.; Mierzwa, M.; Prince, M.E.P.; et al. Patterns of Recurrence in Head and Neck Squamous Cell Carcinoma to Inform Personalized Surveillance Protocols. Cancer 2023, 129, 2817–2827. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus Methotrexate, Docetaxel, or Cetuximab for Recurrent or Metastatic Head-and-Neck Squamous Cell Carcinoma (KEYNOTE-040): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Marron, T.U.; Ryan, A.E.; Reddy, S.M.; Kaczanowska, S.; Younis, R.H.; Thakkar, D.; Zhang, J.; Bartkowiak, T.; Howard, R.; Anderson, K.G.; et al. Considerations for Treatment Duration in Responders to Immune Checkpoint Inhibitors. J. Immunother. Cancer 2021, 9, e001901. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Stein, J.E.; Rimm, D.L.; Wang, D.W.; Bell, J.M.; Johnson, D.B.; Sosman, J.A.; Schalper, K.A.; Anders, R.A.; Wang, H.; et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade. JAMA Oncol. 2019, 5, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Valero, C.; Lee, M.; Hoen, D.; Zehir, A.; Berger, M.F.; Seshan, V.E.; Chan, T.A.; Morris, L.G.T. Response Rates to Anti–PD-1 Immunotherapy in Microsatellite-Stable Solid Tumors With 10 or More Mutations per Megabase. JAMA Oncol. 2021, 7, 739–743. [Google Scholar] [CrossRef]
- Haddad, R.I.; Seiwert, T.Y.; Chow, L.Q.M.; Gupta, S.; Weiss, J.; Gluck, I.; Eder, J.P.; Burtness, B.; Tahara, M.; Keam, B.; et al. Influence of Tumor Mutational Burden, Inflammatory Gene Expression Profile, and PD-L1 Expression on Response to Pembrolizumab in Head and Neck Squamous Cell Carcinoma. J. Immunother. Cancer 2022, 10, e003026. [Google Scholar] [CrossRef]
- Corino, V.D.A.; Bologna, M.; Calareso, G.; Licitra, L.; Ghi, M.; Rinaldi, G.; Caponigro, F.; Morelli, F.; Airoldi, M.; Allegrini, G.; et al. A CT-Based Radiomic Signature Can Be Prognostic for 10-Months Overall Survival in Metastatic Tumors Treated with Nivolumab: An Exploratory Study. Diagnostics 2021, 11, 979. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Bertolus, C.; Giraud, P.; Burgun, A.; Saintigny, P.; Bibault, J.-E.; Foy, J.-P. A Radiomics Approach to Identify Immunologically Active Tumor in Patients with Head and Neck Squamous Cell Carcinomas. Cancers 2023, 15, 5369. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, K.C.; Earls, J.; Schillebeeckx, I.; Hiken, J.; Wellinghoff, R.L.; LaFranzo, N.A.; Bradley, Z.S.; Babbitt, J.; Westra, W.H.; Hsu, R.; et al. Multidimensional Biomarker Predicts Disease Control in Response to Immunotherapy in Recurrent or Metastatic Head and Neck Squamous-Cell Carcinoma. J. Cancer Res. Clin. Oncol. 2023, 149, 14125–14136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yuan, H.; Huang, J.; Hu, D.; Qin, X.; Sun, C.; Chen, G.; Wang, B. Prognostic Value of Immune-Related Genes and Immune Cell Infiltration Analysis in the Tumor Microenvironment of Head and Neck Squamous Cell Carcinoma. Head Neck 2021, 43, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, C.F.; Frederick, M.J.; Thompson, L.D.R.; Corredor, G.; Khalighi, S.; Zhang, Z.; Song, B.; Lu, C.; Nag, R.; Sankar Viswanathan, V.; et al. Machine Learning Driven Index of Tumor Multinucleation Correlates with Survival and Suppressed Anti-Tumor Immunity in Head and Neck Squamous Cell Carcinoma Patients. Oral Oncol. 2023, 143, 106459. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-G.; Wong, A.H.-H.; Wang, H.; Tan, F.; Chen, X.; Jin, S.-H.; He, S.-S.; Shen, G.; Wang, Y.-J.; Frey, B.; et al. Elucidation of the Application of Blood Test Biomarkers to Predict Immune-Related Adverse Events in Atezolizumab-Treated NSCLC Patients Using Machine Learning Methods. Front. Immunol. 2022, 13, 862752. [Google Scholar] [CrossRef]
- Lewinson, R.T.; Meyers, D.E.; Vallerand, I.A.; Suo, A.; Dean, M.L.; Cheng, T.; Bebb, D.G.; Morris, D.G. Machine Learning for Prediction of Cutaneous Adverse Events in Patients Receiving Anti–PD-1 Immunotherapy. J. Am. Acad. Dermatol. 2021, 84, 183–185. [Google Scholar] [CrossRef]
- Moons, K.G.M.; Kengne, A.P.; Grobbee, D.E.; Royston, P.; Vergouwe, Y.; Altman, D.G.; Woodward, M. Risk Prediction Models: II. External Validation, Model Updating, and Impact Assessment. Heart 2012, 98, 691–698. [Google Scholar] [CrossRef]
- Chowell, D.; Yoo, S.-K.; Valero, C.; Pastore, A.; Krishna, C.; Lee, M.; Hoen, D.; Shi, H.; Kelly, D.W.; Patel, N.; et al. Improved Prediction of Immune Checkpoint Blockade Efficacy across Multiple Cancer Types. Nat. Biotechnol. 2022, 40, 499–506. [Google Scholar] [CrossRef]
- Cheng, D.T.; Mitchell, T.N.; Zehir, A.; Shah, R.H.; Benayed, R.; Syed, A.; Chandramohan, R.; Liu, Z.Y.; Won, H.H.; Scott, S.N.; et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J. Mol. Diagn. 2015, 17, 251–264. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-Based to a More “Personalized” Approach to Cancer Staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Seshan, V.E. FACETS: Allele-Specific Copy Number and Clonal Heterogeneity Analysis Tool for High-Throughput DNA Sequencing. Nucleic Acids Res. 2016, 44, e131. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Ye, K.; Zhang, Q.; Lu, C.; Xie, M.; McLellan, M.D.; Wendl, M.C.; Ding, L. MSIsensor: Microsatellite Instability Detection Using Paired Tumor-Normal Sequence Data. Bioinformatics 2014, 30, 1015–1016. [Google Scholar] [CrossRef] [PubMed]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating; Statistics for Biology and Health; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-03016-398-3. [Google Scholar]
- Steyerberg, E.W.; Vickers, A.J.; Cook, N.R.; Gerds, T.; Gonen, M.; Obuchowski, N.; Pencina, M.J.; Kattan, M.W. Assessing the Performance of Prediction Models: A Framework for Some Traditional and Novel Measures. Epidemiology 2010, 21, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Van Calster, B.; Nieboer, D.; Vergouwe, Y.; De Cock, B.; Pencina, M.J.; Steyerberg, E.W. A Calibration Hierarchy for Risk Models Was Defined: From Utopia to Empirical Data. J. Clin. Epidemiol. 2016, 74, 167–176. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. BMC Med. 2015, 13, 1. [Google Scholar] [CrossRef]
- Van Calster, B.; McLernon, D.J.; van Smeden, M.; Wynants, L.; Steyerberg, E.W.; Bossuyt, P.; Collins, G.S.; Macaskill, P.; McLernon, D.J.; Moons, K.G.M.; et al. Calibration: The Achilles Heel of Predictive Analytics. BMC Med. 2019, 17, 230. [Google Scholar] [CrossRef]
- Budach, V.; Tinhofer, I. Novel Prognostic Clinical Factors and Biomarkers for Outcome Prediction in Head and Neck Cancer: A Systematic Review. Lancet Oncol. 2019, 20, e313–e326. [Google Scholar] [CrossRef]
| Characteristics and Outcomes | Validation Cohort (n = 96) | HNSCC Patients in Development Cohort, Training Set (n = 55) | Development Cohort (n = 1479) |
|---|---|---|---|
| Age, median, years (IQR) | 62 (54–69) | 62 (52–67) | 64 (55–71) |
| Sex, n (%) | |||
| Female | 20 (21) | 14 (26) | 668 (45.2) |
| Male | 76 (79) | 41 (74) | 811 (54.8) |
| Cancer type, n (%) | |||
| Head and neck | 96 (100) | 55 (100) | 69 (4.67) |
| Stage, n (%) | |||
| I–III | 0 (0) | 1 (2) | 97 (6.6) |
| IV | 96 (100) | 54 (98) | 1382 (93.4) |
| Chemotherapy prior to ICB, n (%) | |||
| Yes | 92 (96) | 51 (93) | 1016 (68.7) |
| No | 4 (4) | 4 (7) | 463 (31.3) |
| Drug class, n (%) | |||
| PD-1/PD-L1 | 67 (93) | 53 (96) | 1221 (82.6) |
| CTLA-4 | 0 (0) | 1 (2) | 5 (0.3) |
| Combo | 7 (7) | 1 (2) | 253 (17.1) |
| ICB response, n (%) | |||
| Yes | 24 (25) | 11 (20) | 409 (27.6) |
| No | 72 (75) | 44 (80) | 1070 (72.4) |
| Metric (95% CI) | Development RF11 | Development RF 16 | Validation RF11 | Validation RF16 |
|---|---|---|---|---|
| Sensitivity | 0.46 (0.18–0.73) | 0.82 (0.55–1.00) | 0.29 (0.13–0.50) | 0.29 (0.13–0.50) |
| Specificity | 0.66 (0.52–0.80) | 0.91 (0.82–0.98) | 0.86 (0.78–0.93) | 0.82 (0.72–0.90) |
| PPV | 0.25 (0.11–0.41) | 0.69 (0.50–0.91) | 0.41 (0.20–0.64) | 0.35 (0.17–0.54) |
| NPV | 0.83 (0.75–0.92) | 0.95 (0.89–1.00) | 0.78 (0.74–0.84) | 0.78 (0.73–0.83) |
| Accuracy | 0.62 (0.49–0.75) | 0.89 (0.80–0.96) | 0.72 (0.65–0.79) | 0.69 (0.60–0.76) |
| F1 score | 0.32 (0.14–0.50) | 0.75 (0.56–0.91) | 0.34 (0.16–0.53) | 0.31 (0.15–0.48) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.S.; Valero, C.; Yoo, S.-k.; Vos, J.L.; Chowell, D.; Morris, L.G.T. Validation of a Machine Learning Model to Predict Immunotherapy Response in Head and Neck Squamous Cell Carcinoma. Cancers 2024, 16, 175. https://doi.org/10.3390/cancers16010175
Lee AS, Valero C, Yoo S-k, Vos JL, Chowell D, Morris LGT. Validation of a Machine Learning Model to Predict Immunotherapy Response in Head and Neck Squamous Cell Carcinoma. Cancers. 2024; 16(1):175. https://doi.org/10.3390/cancers16010175
Chicago/Turabian StyleLee, Andrew Sangho, Cristina Valero, Seong-keun Yoo, Joris L. Vos, Diego Chowell, and Luc G. T. Morris. 2024. "Validation of a Machine Learning Model to Predict Immunotherapy Response in Head and Neck Squamous Cell Carcinoma" Cancers 16, no. 1: 175. https://doi.org/10.3390/cancers16010175
APA StyleLee, A. S., Valero, C., Yoo, S.-k., Vos, J. L., Chowell, D., & Morris, L. G. T. (2024). Validation of a Machine Learning Model to Predict Immunotherapy Response in Head and Neck Squamous Cell Carcinoma. Cancers, 16(1), 175. https://doi.org/10.3390/cancers16010175






