Investigation of Roles of SLC38A1 in Proliferation and Differentiation of Mouse Tongue Epithelium and Expression in Human Oral Tongue Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Animal Handling
2.3. Measurement of Thickness of Tongue Epithelium in Mice
2.4. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.5. Immunohistochemistry (IHC)
2.6. IHC Assessments of Mice Tongue Specimens
2.7. External Transcriptomic and Genomic Data
2.8. DNA Methylation Profiling
2.9. Western Blotting
2.10. Statistics
3. Results
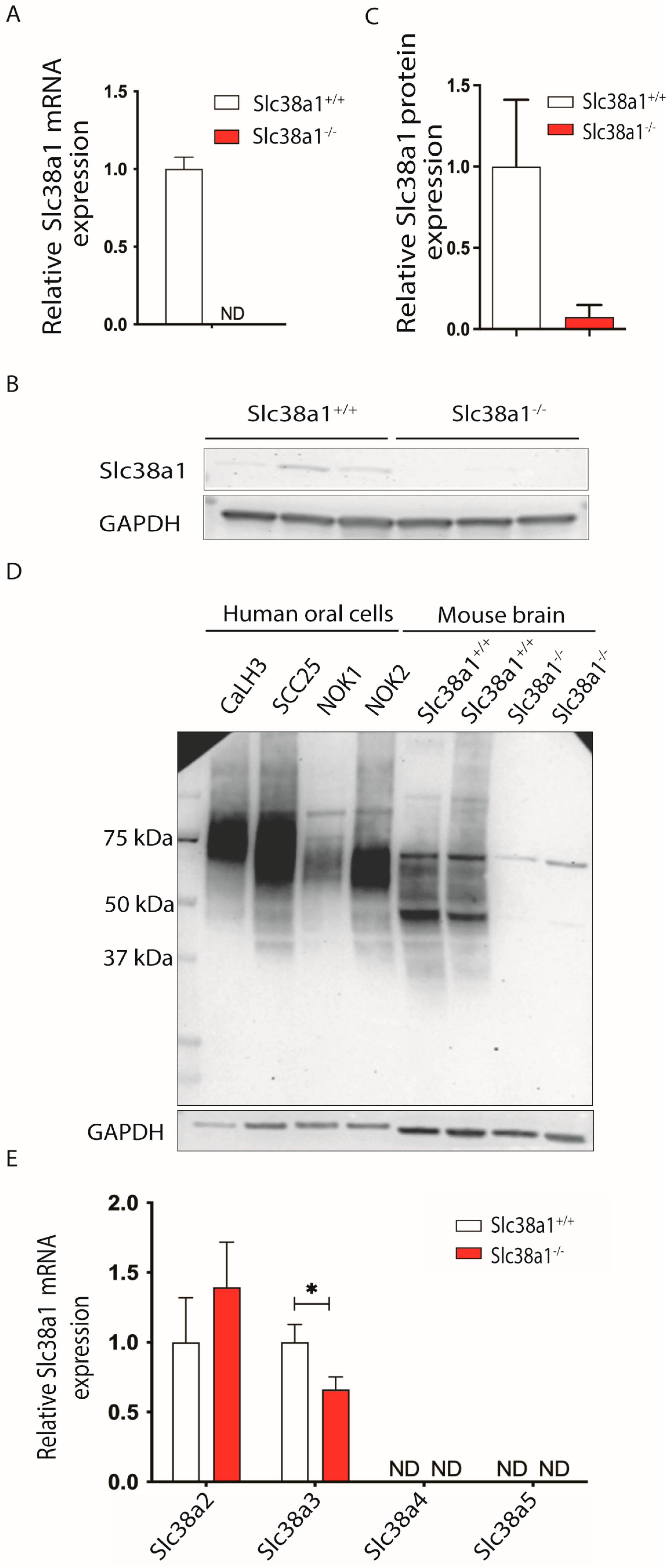
3.1. Slc38a1 mRNA and Protein Are Expressed in Normal and Malignant Oral Cells
3.2. Inactivation of Slc38a1 Gene Was Associated with Significant Down-Regulation of Slc38a3 in Mouse Tongue
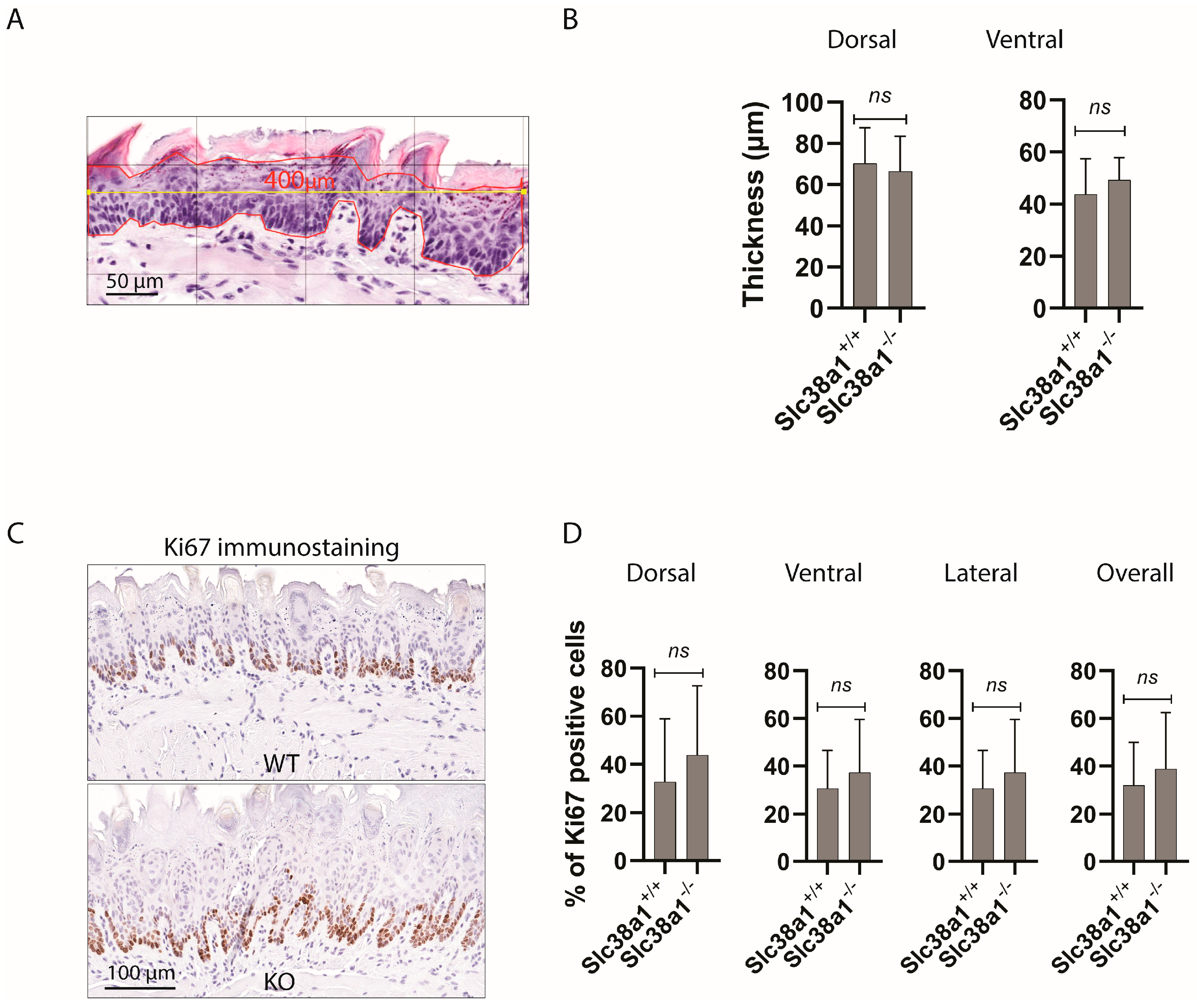
3.3. Slc38a1+/+ and Slc38a1−/− Mice Showed No Significant Difference in Epithelial Thickness and Proliferation and Differentiation Pattern of Tongue Epithelium
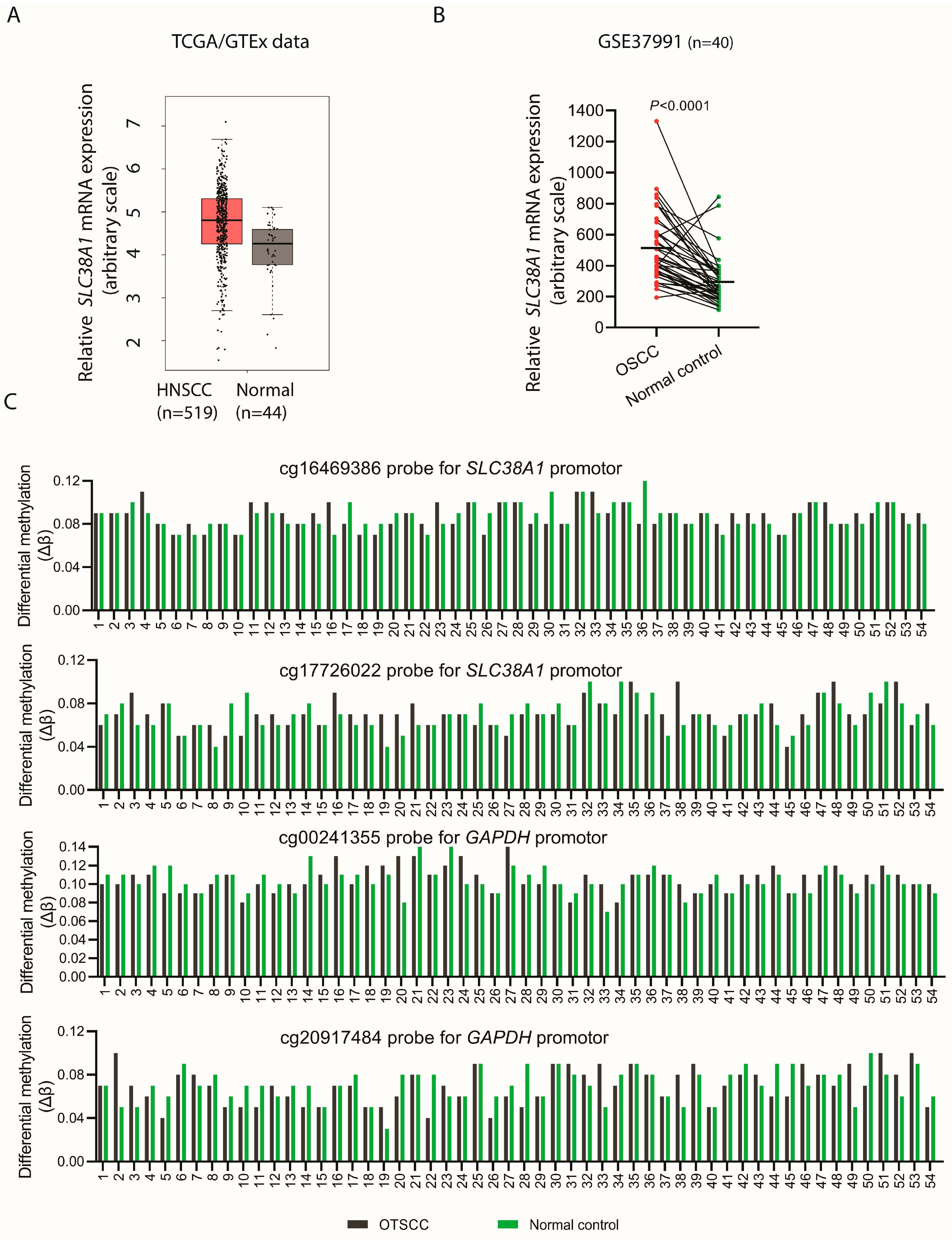
3.4. SLC38A1 mRNA Levels Are Upregulated in OSCC/HNSCC
3.5. SLC38A1 Gene Was Not Amplified in OTSCC Specimens
3.6. Methylation Status of SLC38A1 Gene Promoter in OTSCC Was Similar as Compared to Normal Control Oral Epithelium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaudhry, F.A.; Reimer, R.J.; Edwards, R.H. The glutamine commute: Take the N line and transfer to the A. J. Cell Biol. 2002, 157, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Newman, H.; Shen, L.; Sharma, D.; Hu, G.; Mirando, A.J.; Zhang, H.; Knudsen, E.; Zhang, G.-F.; Hilton, M.J.; et al. Glutamine metabolism regulates proliferation and lineage allocation in skeletal stem cells. Cell Metab. 2019, 29, 966–978.e964. [Google Scholar] [CrossRef] [PubMed]
- Marsboom, G.; Zhang, G.-F.; Pohl-Avila, N.; Zhang, Y.; Yuan, Y.; Kang, H.; Hao, B.; Brunengraber, H.; Malik, A.B.; Rehman, J. Glutamine Metabolism Regulates the Pluripotency Transcription Factor OCT4. Cell Rep. 2016, 16, 323–332. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef]
- Wise, D.R.; Thompson, C.B. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef]
- Wang, K.; Cao, F.; Fang, W.; Hu, Y.; Chen, Y.; Ding, H.; Yu, G. Activation of SNAT1/SLC38A1 in human breast cancer: Correlation with p-Akt overexpression. BMC Cancer 2013, 13, 343. [Google Scholar] [CrossRef]
- Xie, J.; Li, P.; Gao, H.-F.; Qian, J.-X.; Yuan, L.-Y.; Wang, J.-J. Overexpression of SLC38A1 is associated with poorer prognosis in Chinese patients with gastric cancer. BMC Gastroenterol. 2014, 14, 70. [Google Scholar] [CrossRef]
- Wise, D.R.; DeBerardinis, R.J.; Mancuso, A.; Sayed, N.; Zhang, X.-Y.; Pfeiffer, H.K.; Nissim, I.; Daikhin, E.; Yudkoff, M.; McMahon, S.B.; et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA 2008, 105, 18782–18787. [Google Scholar] [CrossRef]
- Gaglio, D.; Metallo, C.M.; Gameiro, P.A.; Hiller, K.; Danna, L.S.; Balestrieri, C.; Alberghina, L.; Stephanopoulos, G.; Chiaradonna, F. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol. Syst. Biol. 2011, 7, 523. [Google Scholar] [CrossRef]
- Barker, G.; Ellory, J. The identification of neutral amino acid transport systems. Exp. Physiol. 1990, 75, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, Y.D.; Ganapathy, V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2016, 1863, 2531–2539. [Google Scholar] [CrossRef]
- Pochini, L.; Scalise, M.; Galluccio, M.; Indiveri, C. Membrane transporters for the special amino acid glutamine: Structure/function relationships and relevance to human health. Front. Chem. 2014, 2, 61. [Google Scholar] [CrossRef] [PubMed]
- Schiöth, H.B.; Roshanbin, S.; Hägglund, M.G.A.; Fredriksson, R. Evolutionary origin of amino acid transporter families SLC32, SLC36 and SLC38 and physiological, pathological and therapeutic aspects. Mol. Asp. Med. 2013, 34, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, F.A.; Reimer, R.J.; Krizaj, D.; Barber, D.; Storm-Mathisen, J.; Copenhagen, D.R.; Edwards, R.H. Molecular analysis of system N suggests novel physiological roles in nitrogen metabolism and synaptic transmission. Cell 1999, 99, 769–780. [Google Scholar] [CrossRef]
- Jenstad, M.; Chaudhry, F. The amino acid transporters of the glutamate/GABA-glutamine cycle and their impact on insulin and glucagon secretion. Front. Endocrinol. 2013, 4, 199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qureshi, T.; Sørensen, C.; Berghuis, P.; Jensen, V.; Dobszay, M.B.; Farkas, T.; Dalen, K.T.; Guo, C.; Hassel, B.; Utheim, T.P.; et al. The glutamine transporter Slc38a1 regulates GABAergic neurotransmission and synaptic plasticity. Cereb. Cortex 2019, 29, 5166–5179. [Google Scholar] [CrossRef]
- Chaudhry, F.A.; Schmitz, D.; Reimer, R.J.; Larsson, P.; Gray, A.T.; Nicoll, R.; Kavanaugh, M.; Edwards, R.H. Glutamine uptake by neurons: Interaction of protons with system A transporters. J. Neurosci. 2002, 22, 62–72. [Google Scholar] [CrossRef]
- Menchini, R.J.; Chaudhry, F.A. Multifaceted regulation of the system A transporter Slc38a2 suggests nanoscale regulation of amino acid metabolism and cellular signaling. Neuropharmacology 2019, 161, 107789. [Google Scholar] [CrossRef]
- Yamada, D.; Kawabe, K.; Tosa, I.; Tsukamoto, S.; Nakazato, R.; Kou, M.; Fujikawa, K.; Nakamura, S.; Ono, M.; Oohashi, T.; et al. Inhibition of the glutamine transporter SNAT1 confers neuroprotection in mice by modulating the mTOR-autophagy system. Commun. Biol. 2019, 2, 346. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Jiang, L.; Xu, H.; Wei, J. High expression levels of SLC38A1 are correlated with poor prognosis and defective immune infiltration in hepatocellular carcinoma. J. Oncol. 2021, 2021, 5680968. [Google Scholar] [CrossRef]
- Böhme-Schäfer, I.; Lörentz, S.; Bosserhoff, A.K. Role of amino acid transporter SNAT1/SLC38A1 in human melanoma. Cancers 2022, 14, 2151. [Google Scholar] [PubMed]
- Schreurs, O.; Karatsaidis, A.; Balta, M.G.; Grung, B.; Hals, E.K.B.; Schenck, K. Expression of keratins 8, 18, and 19 in epithelia of atrophic oral lichen planus. Eur. J. Oral Sci. 2020, 128, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Sengüven Toközlü, B.; Sapkota, D.; Vallenari, E.M.; Schreurs, O.; Søland, T.M. Cortactin expression in a Norwegian cohort of human papilloma virus negative oral squamous cell carcinomas of the mobile tongue. Eur. J. Oral Sci. 2023, 131, e12925. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.J.; Piper, K.; Common, J.; Fortune, F.; Mackenzie, I.C. Stem cell patterns in cell lines derived from head and neck squamous cell carcinoma. J. Oral Pathol. Med. 2007, 36, 594–603. [Google Scholar] [CrossRef]
- Rheinwald, J.G.; Beckett, M.A. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981, 41, 1657–1663. [Google Scholar]
- Qureshi, T.; Bjørkmo, M.; Nordengen, K.; Gundersen, V.; Utheim, T.P.; Watne, L.O.; Storm-Mathisen, J.; Hassel, B.; Chaudhry, F.A. Slc38a1 conveys astroglia-derived glutamine into GABAergic interneurons for neurotransmitter GABA synthesis. Cells 2020, 9, 1686. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Solbu, T.T.; Bjørkmo, M.; Berghuis, P.; Harkany, T.; Chaudhry, F. SAT1, a glutamine transporter, is preferentially expressed in GABAergic neurons. Front. Neuroanat. 2010, 4, 2010. [Google Scholar] [CrossRef]
- GTEx. The genotype-tissue expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Wong, T.-S.; Chan, J.Y.-W.; Lu, S.-C.; Lin, P.; Cheng, A.-J.; Chen, Y.-J.; Chang, J.S.-M.; Hsiao, S.-H.; Leu, Y.-W.; et al. Epigenetic regulation of the X-linked tumour suppressors BEX1 and LDOC1 in oral squamous cell carcinoma. J. Pathol. 2013, 230, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.M.; Dhas, K.; Nair, J.; Palve, V.; Bagwan, J.; Siddappa, G.; Suresh, A.; Kekatpure, V.D.; Kuriakose, M.A.; Panda, B. A minimal DNA methylation signature in oral tongue squamous cell carcinoma links altered methylation with tumor attributes. Mol. Cancer Res. 2016, 14, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Solbu, T.T.; Boulland, J.-L.; Zahid, W.; Lyamouri Bredahl, M.K.; Amiry-Moghaddam, M.; Storm-Mathisen, J.; Roberg, B.A.; Chaudhry, F.A. Induction and targeting of the glutamine transporter SN1 to the basolateral membranes of cortical kidney tubule cells during chronic metabolic acidosis suggest a role in pH regulation. J. Am. Soc. Nephrol. 2005, 16, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Gammelsaeter, R.; Jenstad, M.; Bredahl, M.K.L.; Gundersen, V.; Chaudhry, F.A. Complementary expression of SN1 and SAT2 in the islets of Langerhans suggests concerted action of glutamine transport in the regulation of insulin secretion. Biochem. Biophys. Res. Commun. 2009, 381, 378–382. [Google Scholar] [CrossRef]
- Kim, M.-H.; Kim, H. The roles of glutamine in the intestine and its implication in intestinal diseases. Int. J. Mol. Sci. 2017, 18, 1051. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ling, Z.; Li, W.; Su, Z.; Lu, J.; Zeng, Q.; Cheng, B.; Tao, X. Glutamine promotes the proliferation of epithelial cells via mTOR/S6 pathway in oral lichen planus. J. Oral Pathol. Med. 2023, 52, 150–160. [Google Scholar] [CrossRef]
- Ogura, M.; Kakuda, T.; Takarada, T.; Nakamichi, N.; Fukumori, R.; Kim, Y.-H.; Hinoi, E.; Yoneda, Y. Promotion of both proliferation and neuronal differentiation in pluripotent P19 cells with stable overexpression of the glutamine transporter slc38a1. PLoS ONE 2012, 7, e48270. [Google Scholar] [CrossRef]
- Palacin, M.; Estevez, R.; Bertran, J.; Zorzano, A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol. Rev. 1998, 78, 969–1054. [Google Scholar] [CrossRef] [PubMed]
- Bröer, A.; Rahimi, F.; Bröer, S. Deletion of amino acid transporter ASCT2 (SLC1A5) reveals an essential role for transporters SNAT1 (SLC38A1) and SNAT2 (SLC38A2) to sustain glutaminolysis in cancer cells. J. Biol. Chem. 2016, 291, 13194–13205. [Google Scholar] [CrossRef]
- Morotti, M.; Bridges, E.; Valli, A.; Choudhry, H.; Sheldon, H.; Wigfield, S.; Gray, N.; Zois, C.E.; Grimm, F.; Jones, D.; et al. Hypoxia-induced switch in SNAT2/SLC38A2 regulation generates endocrine resistance in breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 12452–12461. [Google Scholar] [CrossRef] [PubMed]
- Nissen-Meyer, L.S.; Chaudhry, F. Protein kinase C phosphorylates the system N glutamine transporter SN1 (Slc38a3) and regulates Its membrane trafficking and degradation. Front. Endocrinol. 2013, 4, 138. [Google Scholar] [CrossRef]
- Sutinen, E.; Jyrkkiö, S.; Alanen, K.; Någren, K.; Minn, H. Uptake of [N-methyl-11C]α-methylaminoisobutyric acid in untreated head and neck cancer studied by PET. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Wang, H. Long non-coding RNA 01559 mediates the malignant phenotypes of hepatocellular carcinoma cells through targeting miR-511. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101648. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Lin, J.; Li, J.; Wang, X.-D.; Xu, L.-M.; Chen, D.-Z.; Chen, Y.-P. miRNA-432 and SLC38A1 as predictors of hepatocellular carcinoma complicated with alcoholic steatohepatitis. Oxidative Med. Cell. Longev. 2022, 2022, 4832611. [Google Scholar] [CrossRef] [PubMed]
- Shroff, E.H.; Eberlin, L.S.; Dang, V.M.; Gouw, A.M.; Gabay, M.; Adam, S.J.; Bellovin, D.I.; Tran, P.T.; Philbrick, W.M.; Garcia-Ocana, A.; et al. MYC oncogene overexpression drives renal cell carcinoma in a mouse model through glutamine metabolism. Proc. Natl. Acad. Sci. USA 2015, 112, 6539–6544. [Google Scholar] [CrossRef]
- Tameire, F.; Verginadis, I.I.; Leli, N.M.; Polte, C.; Conn, C.S.; Ojha, R.; Salas Salinas, C.; Chinga, F.; Monroy, A.M.; Fu, W.; et al. ATF4 couples MYC-dependent translational activity to bioenergetic demands during tumour progression. Nat. Cell Biol. 2019, 21, 889–899. [Google Scholar] [CrossRef]
- Morotti, M.; Zois, C.E.; El-Ansari, R.; Craze, M.L.; Rakha, E.A.; Fan, S.-J.; Valli, A.; Haider, S.; Goberdhan, D.C.I.; Green, A.R.; et al. Increased expression of glutamine transporter SNAT2/SLC38A2 promotes glutamine dependence and oxidative stress resistance, and is associated with worse prognosis in triple-negative breast cancer. Br. J. Cancer 2021, 124, 494–505. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, L.; Cui, M.; Wang, Y.; Xu, Y.; Li, M.; Mi, J. Amino acid transporter SLC38A3 promotes metastasis of non-small cell lung cancer cells by activating PDK1. Cancer Lett. 2017, 393, 8–15. [Google Scholar] [CrossRef]
- Huang, L.; Li, L.; Cheng, B.; Xing, T. SLC38A6, regulated by EP300-mediated modifications of H3K27ac, promotes cell proliferation, glutamine metabolism and mitochondrial respiration in hepatocellular carcinoma. Carcinogenesis 2022, 43, 885–894. [Google Scholar] [CrossRef]
- Verdon, Q.; Boonen, M.; Ribes, C.; Jadot, M.; Gasnier, B.; Sagné, C. SNAT7 is the primary lysosomal glutamine exporter required for extracellular protein-dependent growth of cancer cells. Proc. Natl. Acad. Sci. USA 2017, 114, E3602–E3611. [Google Scholar] [CrossRef]
| CK4 | CK10 | ||||
|---|---|---|---|---|---|
| Sample | Genotype | Dorsal | Ventral | Dorsal | Ventral |
| 16A | WT | + | − | +++ | +++ |
| 13A | WT | + | + | ++ | +++ |
| 12A | WT | + | − | + | ++ |
| 14A | WT | + | + | +++ | +++ |
| 26A | WT | + | − | ++ | +++ |
| 29A | WT | ++ | + | +++ | +++ |
| 19A | WT | + | + | +++ | +++ |
| 30A | WT | + | − | +++ | +++ |
| 24A | WT | + | + | +++ | +++ |
| 23A | WT | + | − | +++ | +++ |
| 15A | KO | ++ | + | ++ | +++ |
| 18A | KO | ++ | + | +++ | +++ |
| 17A | KO | ++ | + | +++ | +++ |
| 11A | KO | + | − | ++ | +++ |
| 21A | KO | + | − | NA | NA |
| 28A | KO | ++ | + | +++ | +++ |
| 27A | KO | + | + | +++ | +++ |
| 20A | KO | + | + | +++ | +++ |
| 31A | KO | + | − | +++ | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapkota, D.; Wang, D.; Schreurs, O.; Vallenari, E.M.; Pandey Dhakal, S.; Küntziger, T.; Toközlü, B.S.; Utheim, T.P.; Chaudhry, F.A. Investigation of Roles of SLC38A1 in Proliferation and Differentiation of Mouse Tongue Epithelium and Expression in Human Oral Tongue Squamous Cell Carcinoma. Cancers 2024, 16, 405. https://doi.org/10.3390/cancers16020405
Sapkota D, Wang D, Schreurs O, Vallenari EM, Pandey Dhakal S, Küntziger T, Toközlü BS, Utheim TP, Chaudhry FA. Investigation of Roles of SLC38A1 in Proliferation and Differentiation of Mouse Tongue Epithelium and Expression in Human Oral Tongue Squamous Cell Carcinoma. Cancers. 2024; 16(2):405. https://doi.org/10.3390/cancers16020405
Chicago/Turabian StyleSapkota, Dipak, Daxin Wang, Olaf Schreurs, Evan M. Vallenari, Sushma Pandey Dhakal, Thomas Küntziger, Burcu Sengüven Toközlü, Tor Paaske Utheim, and Farrukh Abbas Chaudhry. 2024. "Investigation of Roles of SLC38A1 in Proliferation and Differentiation of Mouse Tongue Epithelium and Expression in Human Oral Tongue Squamous Cell Carcinoma" Cancers 16, no. 2: 405. https://doi.org/10.3390/cancers16020405
APA StyleSapkota, D., Wang, D., Schreurs, O., Vallenari, E. M., Pandey Dhakal, S., Küntziger, T., Toközlü, B. S., Utheim, T. P., & Chaudhry, F. A. (2024). Investigation of Roles of SLC38A1 in Proliferation and Differentiation of Mouse Tongue Epithelium and Expression in Human Oral Tongue Squamous Cell Carcinoma. Cancers, 16(2), 405. https://doi.org/10.3390/cancers16020405






