The Effect of Sex on the Therapeutic Efficiency of Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis Based on Randomized Controlled Trials
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Criteria
2.2. Search Strategy
2.3. Data Extraction
2.4. Literature Quality Assessment
2.5. Data Synthesis and Statistical Analysis
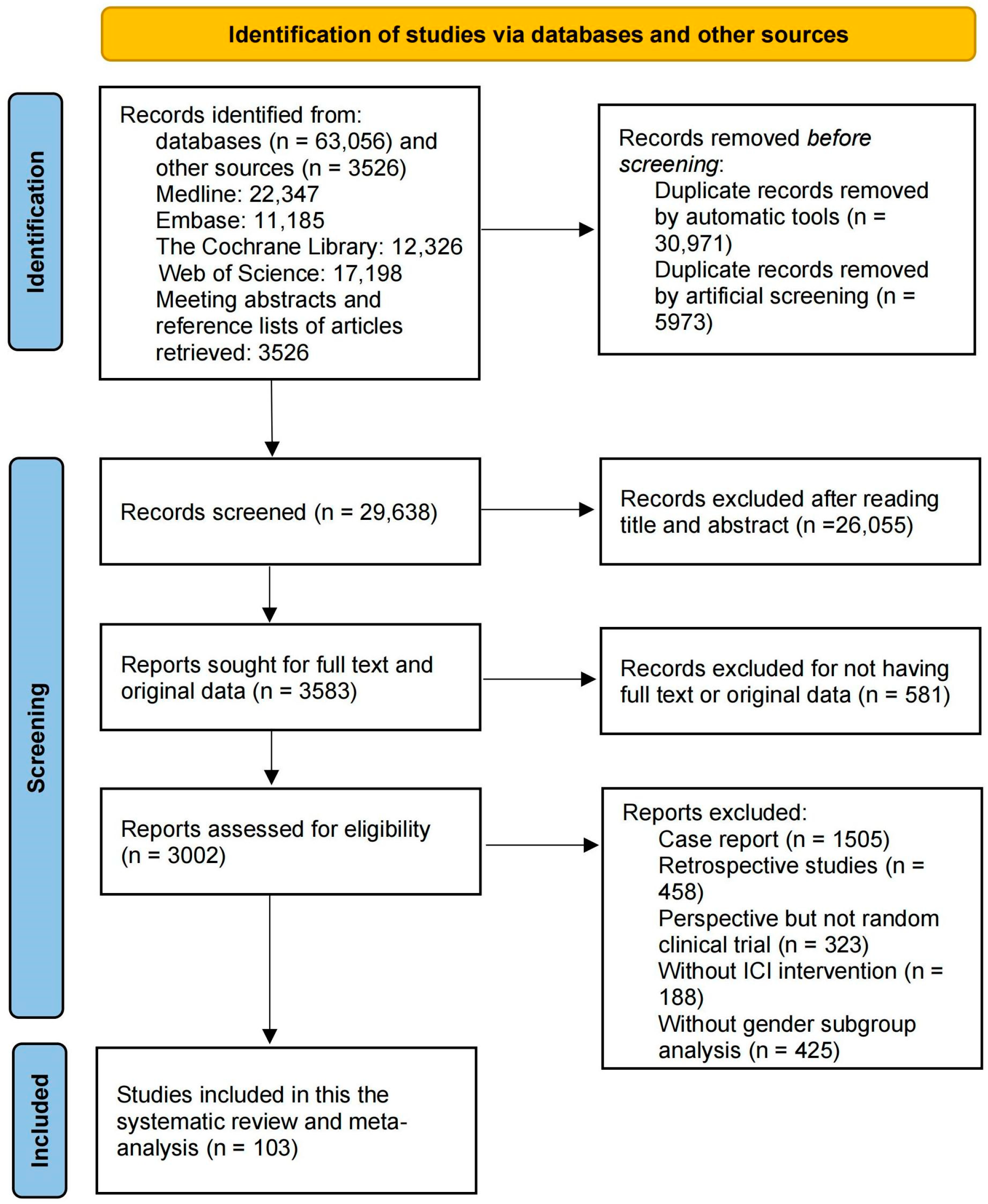
3. Results
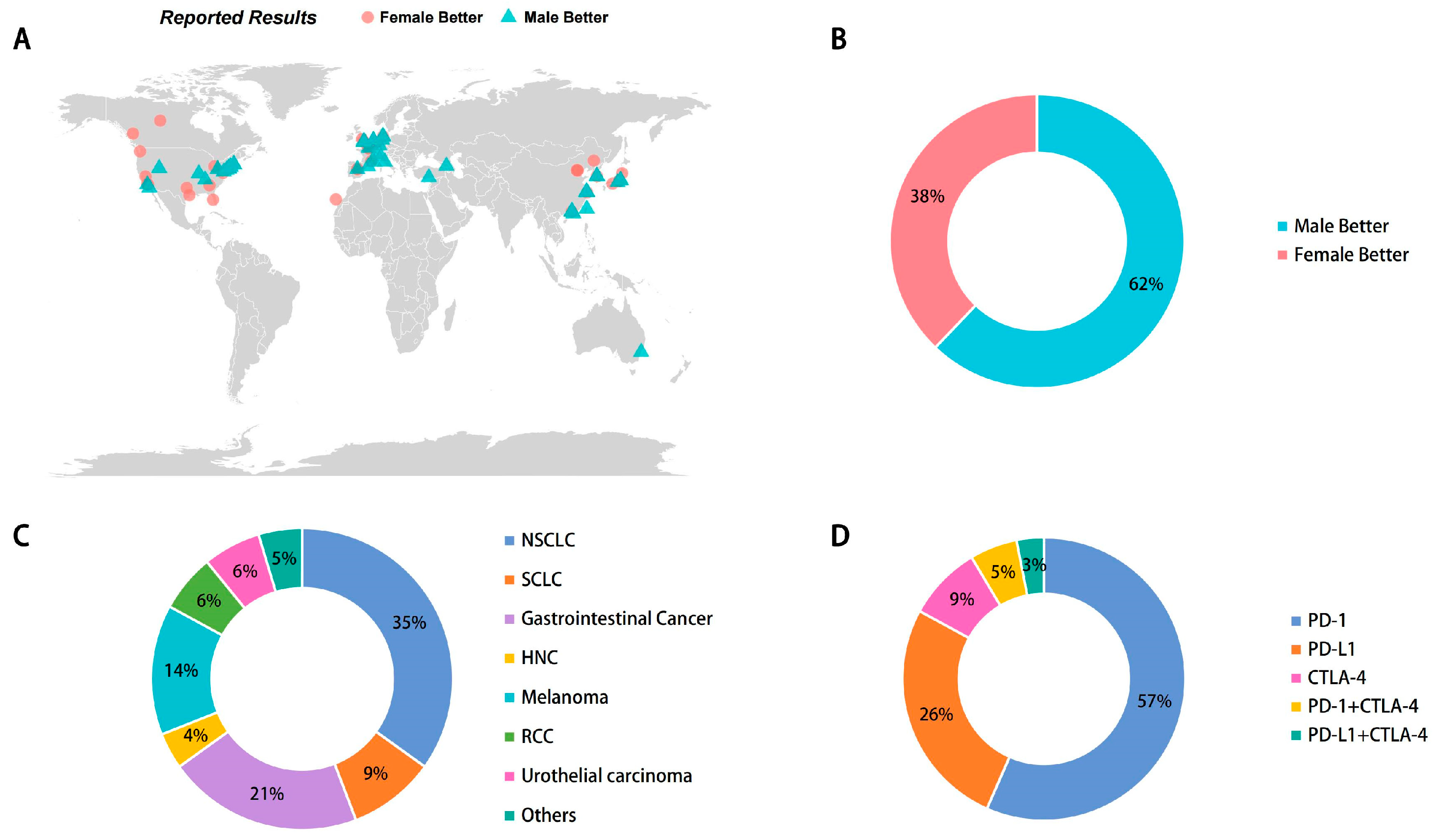
3.1. Characteristics of Included Studies and Patients
3.2. Quality Assessment of the Included Studies
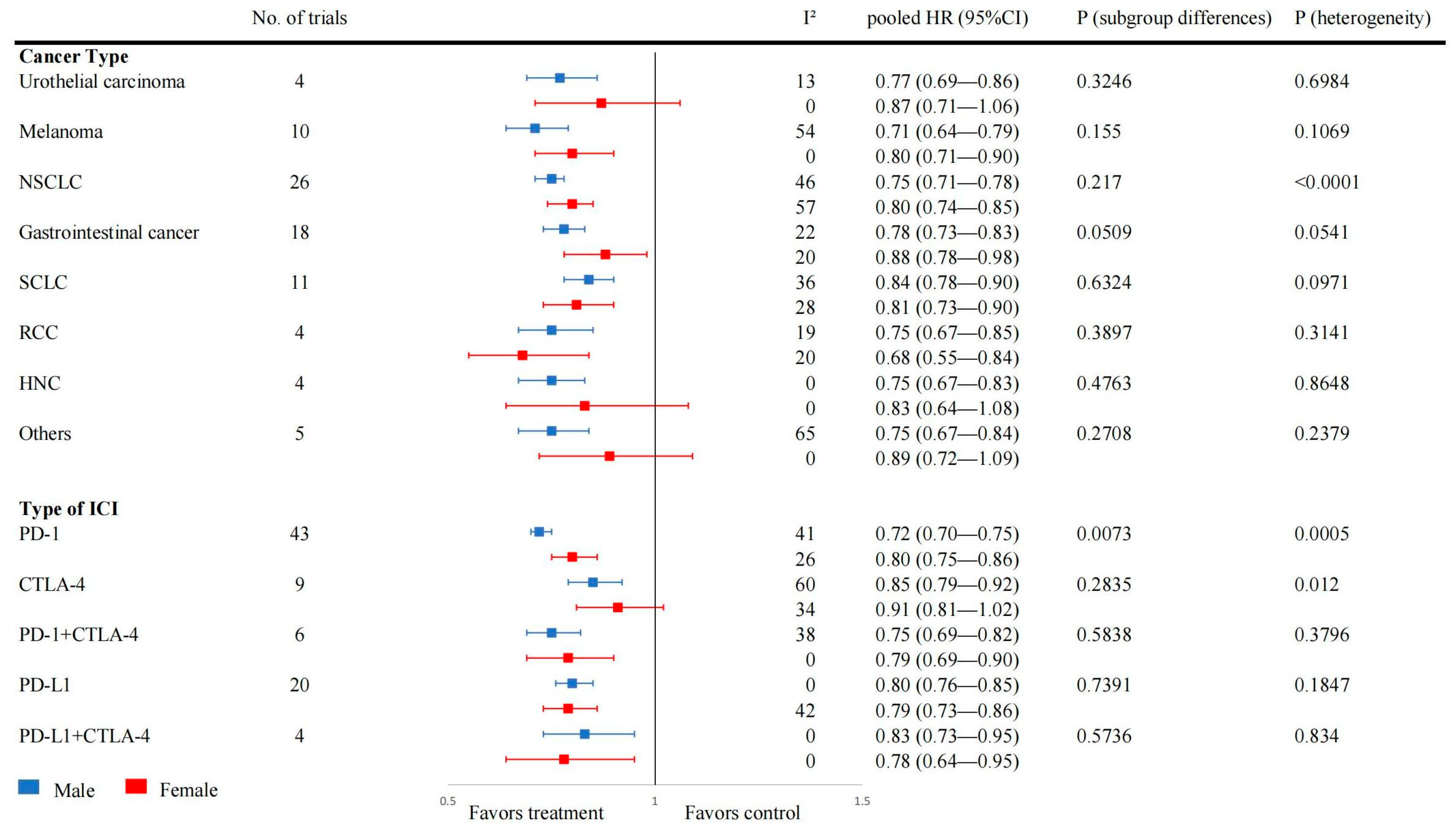
3.3. Effect of Sex on OS after ICI Treatment
3.4. Effect of Sex on PFS and RFS after ICI Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ortona, E.; Pierdominici, M.; Rider, V. Editorial: Sex Hormones and Gender Differences in Immune Responses. Front. Immunol. 2019, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, A.; Anticoli, S.; D’Ambrosio, A.; Giordani, L.; Viora, M. The influence of sex and gender on immunity, infection and vaccination. Ann. Dell’istituto Super. Sanita 2016, 52, 198–204. [Google Scholar] [CrossRef]
- Jacobson, D.L.; Gange, S.J.; Rose, N.R.; Graham, N.M. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin. Immunol. Immunopathol. 1997, 84, 223–243. [Google Scholar] [CrossRef] [PubMed]
- vom Steeg, L.G.; Klein, S.L. SeXX Matters in Infectious Disease Pathogenesis. PLoS Pathog. 2016, 12, e1005374. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.B.; Dawsey, S.M.; Freedman, N.D.; Inskip, P.D.; Wichner, S.M.; Quraishi, S.M.; Devesa, S.S.; McGlynn, K.A. Sex disparities in cancer incidence by period and age. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1174–1182. [Google Scholar] [CrossRef]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef]
- Tan, S.; Li, D.; Zhu, X. Cancer immunotherapy: Pros, cons and beyond. Biomed. Pharmacother. 2020, 124, 109821. [Google Scholar] [CrossRef]
- Marin-Acevedo, J.A.; Kimbrough, E.O.; Lou, Y. Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol. 2021, 14, 45. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Chen, L.T.; Satoh, T.; Ryu, M.H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.S.; Muro, K.; Kang, W.K.; Yeh, K.H.; et al. A phase 3 study of nivolumab in previously treated advanced gastric or gastroesophageal junction cancer (ATTRACTION-2): 2-year update data. Gastric Cancer 2020, 23, 510–519. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Chiarion-Sileni, V.; Grob, J.J.; Dummer, R.; Wolchok, J.D.; Schmidt, H.; Hamid, O.; Robert, C.; Ascierto, P.A.; Richards, J.M.; et al. Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: Long-term follow-up results of the European Organisation for Research and Treatment of Cancer 18071 double-blind phase 3 randomised trial. Eur. J. Cancer 2019, 119, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Ciuleanu, T.E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open 2021, 6, 100273. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Kato, K.; Okada, M.; Chin, K.; Kadowaki, S.; Hamamoto, Y.; Doki, Y.; Kubota, Y.; Kawakami, H.; Ogata, T.; et al. Nivolumab versus chemotherapy in Japanese patients with advanced esophageal squamous cell carcinoma: A subgroup analysis of a multicenter, randomized, open-label, phase 3 trial (ATTRACTION-3). Esophagus 2021, 18, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Sugawara, S.; Fukuda, Y.; Fujimoto, D.; Miura, S.; Ota, K.; Ozawa, Y.; Hara, S.; Tanizaki, J.; Azuma, K.; et al. A Randomized Phase II Study Comparing Nivolumab with Carboplatin-Pemetrexed for EGFR-Mutated NSCLC with Resistance to EGFR Tyrosine Kinase Inhibitors (WJOG8515L). Clin. Cancer Res. 2022, 28, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Shah, M.A.; Muro, K.; Francois, E.; Adenis, A.; Hsu, C.H.; Doi, T.; Moriwaki, T.; Kim, S.B.; Lee, S.H.; et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab versus Chemotherapy in Advanced Esophageal Cancer. J. Clin. Oncol. 2020, 38, 4138–4148. [Google Scholar] [CrossRef] [PubMed]
- Felip, E.; Altorki, N.; Zhou, C.; Csoszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Nishio, M.; Barlesi, F.; West, H.; Ball, S.; Bordoni, R.; Cobo, M.; Longeras, P.D.; Goldschmidt, J., Jr.; Novello, S.; Orlandi, F.; et al. Atezolizumab Plus Chemotherapy for First-Line Treatment of Nonsquamous NSCLC: Results From the Randomized Phase 3 IMpower132 Trial. J. Thorac. Oncol. 2021, 16, 653–664. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ferris, R.L.; Psyrri, A.; Haddad, R.I.; Tahara, M.; Bourhis, J.; Harrington, K.; Chang, P.M.H.; Lin, J.C.; Razaq, M.A.; et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: A randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2021, 22, 450–462. [Google Scholar] [CrossRef]
- Park, K.; Özgüroğlu, M.; Vansteenkiste, J.; Spigel, D.; Yang, J.C.H.; Ishii, H.; Garassino, M.; de Marinis, F.; Szczesna, A.; Polychronis, A.; et al. Avelumab versus Docetaxel in Patients with Platinum-Treated Advanced NSCLC: 2-Year Follow-Up From the JAVELIN Lung 200 Phase 3 Trial. J. Thorac. Oncol. 2021, 16, 1369–1378. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, G.; Huang, Y.; Zhou, J.; Lin, L.; Feng, J.; Wang, Z.; Shu, Y.; Shi, J.; Hu, Y.; et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): A randomised, open-label, multicentre, phase 3 trial. Lancet. Respir. Med. 2021, 9, 305–314. [Google Scholar] [CrossRef]
- Spigel, D.R.; Vicente, D.; Ciuleanu, T.E.; Gettinger, S.; Peters, S.; Horn, L.; Audigier-Valette, C.; Pardo Aranda, N.; Juan-Vidal, O.; Cheng, Y.; et al. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331(☆). Ann. Oncol. 2021, 32, 631–641. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Z.; Sun, Y.; Cao, L.; Ma, Z.; Wu, R.; Yu, Y.; Yao, W.; Chang, J.; Chen, J.; et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): Interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol. 2022, 23, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Kefford, R.; Marshall, M.A.; Punt, C.J.; Haanen, J.B.; Marmol, M.; Garbe, C.; Gogas, H.; Schachter, J.; Linette, G.; et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J. Clin. Oncol. 2013, 31, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.F.; Testori, A.; Grob, J.J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Govindan, R.; Szczesna, A.; Ahn, M.J.; Schneider, C.P.; Gonzalez Mella, P.F.; Barlesi, F.; Han, B.; Ganea, D.E.; Von Pawel, J.; Vladimirov, V.; et al. Phase III Trial of Ipilimumab Combined with Paclitaxel and Carboplatin in Advanced Squamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2017, 35, 3449–3457. [Google Scholar] [CrossRef]
- Reck, M.; Luft, A.; Szczesna, A.; Havel, L.; Kim, S.W.; Akerley, W.; Pietanza, M.C.; Wu, Y.L.; Zielinski, C.; Thomas, M.; et al. Phase III Randomized Trial of Ipilimumab Plus Etoposide and Platinum versus Placebo Plus Etoposide and Platinum in Extensive-Stage Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 3740–3748. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Larkin, J.; Minor, D.; D’Angelo, S.; Neyns, B.; Smylie, M.; Miller, W.H., Jr.; Gutzmer, R.; Linette, G.; Chmielowski, B.; Lao, C.D.; et al. Overall Survival in Patients with Advanced Melanoma Who Received Nivolumab versus Investigator’s Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial. J. Clin. Oncol. 2018, 36, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Maio, M.; Scherpereel, A.; Calabrò, L.; Aerts, J.; Perez, S.C.; Bearz, A.; Nackaerts, K.; Fennell, D.A.; Kowalski, D.; Tsao, A.S.; et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): A multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol. 2017, 18, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Herbst, R.S.; Garon, E.B.; Kim, D.W.; Cho, B.C.; Gervais, R.; Perez-Gracia, J.L.; Han, J.Y.; Majem, M.; Forster, M.D.; Monnet, I.; et al. Five Year Survival Update from KEYNOTE-010: Pembrolizumab versus Docetaxel for Previously Treated, Programmed Death-Ligand 1-Positive Advanced NSCLC. J. Thorac. Oncol. 2021, 16, 1718–1732. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, L.; von Pawel, J.; Park, K.; Rittmeyer, A.; Gandara, D.R.; Ponce Aix, S.; Han, J.Y.; Gadgeel, S.M.; Hida, T.; Cortinovis, D.L.; et al. Updated Efficacy Analysis Including Secondary Population Results for OAK: A Randomized Phase III Study of Atezolizumab versus Docetaxel in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 1156–1170. [Google Scholar] [CrossRef]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Ferrucci, P.F.; Di Giacomo, A.M.; Del Vecchio, M.; Atkinson, V.; Schmidt, H.; Schachter, J.; Queirolo, P.; Long, G.V.; Stephens, R.; Svane, I.M.; et al. KEYNOTE-022 part 3: A randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J. Immunother. Cancer 2020, 8, e001806. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Choueiri, T.K.; McDermott, D.F.; Powles, T.; Vano, Y.A.; Gupta, S.; Yao, J.; Han, C.; Ammar, R.; Papillon-Cavanagh, S.; et al. Biomarker analysis from CheckMate 214: Nivolumab plus ipilimumab versus sunitinib in renal cell carcinoma. J. Immunother. Cancer 2022, 10, e004316. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.J.; Soria, A.; Machiels, J.P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Mok, T.S.; Nishio, M.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodriguez-Abreu, D.; Moro-Sibilot, D.; et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir. Med. 2019, 7, 387–401. [Google Scholar] [CrossRef]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef]
- Jotte, R.; Cappuzzo, F.; Vynnychenko, I.; Stroyakovskiy, D.; Rodríguez-Abreu, D.; Hussein, M.; Soo, R.; Conter, H.J.; Kozuki, T.; Huang, K.C.; et al. Atezolizumab in Combination with Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results from a Randomized Phase III Trial. J. Thorac. Oncol. 2020, 15, 1351–1360. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Özgüroğlu, M.; Bang, Y.J.; Di Bartolomeo, M.; Mandala, M.; Ryu, M.H.; Fornaro, L.; Olesinski, T.; Caglevic, C.; Chung, H.C.; et al. Pembrolizumab versus paclitaxel for previously treated PD-L1-positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial. Gastric Cancer 2022, 25, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Barlesi, F.; Vansteenkiste, J.; Spigel, D.; Ishii, H.; Garassino, M.; de Marinis, F.; Özgüroğlu, M.; Szczesna, A.; Polychronis, A.; Uslu, R.; et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): An open-label, randomised, phase 3 study. Lancet Oncol. 2018, 19, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Bellmunt, J.; Hussain, M.; Gschwend, J.E.; Albers, P.; Oudard, S.; Castellano, D.; Daneshmand, S.; Nishiyama, H.; Majchrowicz, M.; Degaonkar, V.; et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Cho, B.C.; Reinmuth, N.; Lee, K.H.; Luft, A.; Ahn, M.J.; van den Heuvel, M.M.; Cobo, M.; Vicente, D.; Smolin, A.; et al. Durvalumab with or without Tremelimumab vs Standard Chemotherapy in First-line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.G.; Ramalingam, S.S.; Ciuleanu, T.E.; Lee, J.S.; Urban, L.; Caro, R.B.; Park, K.; Sakai, H.; Ohe, Y.; Nishio, M.; et al. First-Line Nivolumab Plus Ipilimumab in Advanced NSCLC: 4-Year Outcomes from the Randomized, Open-Label, Phase 3 CheckMate 227 Part 1 Trial. J. Thorac. Oncol. 2022, 17, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Kenmotsu, H.; Sugawara, S.; Watanabe, Y.; Saito, H.; Okada, M.; Chen-Yoshikawa, T.F.; Ohe, Y.; Nishio, W.; Nakagawa, S.; Nagao, H. Adjuvant atezolizumab in Japanese patients with resected stage IB-IIIA non-small cell lung cancer (IMpower010). Cancer Sci. 2022, 113, 4327–4338. [Google Scholar] [CrossRef]
- Shitara, K.; Van Cutsem, E.; Bang, Y.J.; Fuchs, C.; Wyrwicz, L.; Lee, K.W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Lee, J.; et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients with First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1571–1580. [Google Scholar] [CrossRef]
- O’Brien, M.; Paz-Ares, L.; Marreaud, S.; Dafni, U.; Oselin, K.; Havel, L.; Esteban, E.; Isla, D.; Martinez-Marti, A.; Faehling, M.; et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): An interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022, 23, 1274–1286. [Google Scholar] [CrossRef]
- Grossmann, K.F.; Othus, M.; Patel, S.P.; Tarhini, A.A.; Sondak, V.K.; Knopp, M.V.; Petrella, T.M.; Truong, T.G.; Khushalani, N.I.; Cohen, J.V.; et al. Adjuvant Pembrolizumab versus IFNα2b or Ipilimumab in Resected High-Risk Melanoma. Cancer Discov. 2022, 12, 644–653. [Google Scholar] [CrossRef]
- Powles, T.; van der Heijden, M.S.; Castellano, D.; Galsky, M.D.; Loriot, Y.; Petrylak, D.P.; Ogawa, O.; Park, S.H.; Lee, J.L.; De Giorgi, U.; et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1574–1588. [Google Scholar] [CrossRef]
- Livingstone, E.; Zimmer, L.; Hassel, J.C.; Fluck, M.; Eigentler, T.K.; Loquai, C.; Haferkamp, S.; Gutzmer, R.; Meier, F.; Mohr, P.; et al. Adjuvant nivolumab plus ipilimumab or nivolumab alone versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): Final results of a randomised, double-blind, phase 2 trial. Lancet 2022, 400, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Owonikoko, T.K.; Park, K.; Govindan, R.; Ready, N.; Reck, M.; Peters, S.; Dakhil, S.R.; Navarro, A.; Rodríguez-Cid, J.; Schenker, M.; et al. Nivolumab and Ipilimumab as Maintenance Therapy in Extensive-Disease Small-Cell Lung Cancer: CheckMate 451. J. Clin. Oncol. 2021, 39, 1349–1359. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Cho, B.C.; Takahashi, M.; Okada, M.; Lin, C.Y.; Chin, K.; Kadowaki, S.; Ahn, M.J.; Hamamoto, Y.; Doki, Y.; et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1506–1517. [Google Scholar] [CrossRef]
- Rodríguez-Abreu, D.; Powell, S.F.; Hochmair, M.J.; Gadgeel, S.; Esteban, E.; Felip, E.; Speranza, G.; De Angelis, F.; Dómine, M.; Cheng, S.Y.; et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: Protocol-specified final analysis from KEYNOTE-189. Ann. Oncol. 2021, 32, 881–895. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- Moehler, M.; Dvorkin, M.; Boku, N.; Özgüroğlu, M.; Ryu, M.H.; Muntean, A.S.; Lonardi, S.; Nechaeva, M.; Bragagnoli, A.C.; Coşkun, H.S.; et al. Phase III Trial of Avelumab Maintenance after First-Line Induction Chemotherapy versus Continuation of Chemotherapy in Patients with Gastric Cancers: Results from JAVELIN Gastric 100. J. Clin. Oncol. 2021, 39, 966–977. [Google Scholar] [CrossRef]
- Bang, Y.J.; Ruiz, E.Y.; Van Cutsem, E.; Lee, K.W.; Wyrwicz, L.; Schenker, M.; Alsina, M.; Ryu, M.H.; Chung, H.C.; Evesque, L.; et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: Primary analysis of JAVELIN Gastric 300. Ann. Oncol. 2018, 29, 2052–2060. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Motzer, R.J.; Rini, B.I.; Haanen, J.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Gravis-Mescam, G.; Uemura, M.; Lee, J.L.; et al. Updated efficacy results from the JAVELIN Renal 101 trial: First-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann. Oncol. 2020, 31, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cao, Y.; Liu, W.; Ju, X.; Zhao, X.; Jiang, L.; Ye, Y.; Jin, G.; Zhang, H. Stereotactic body radiotherapy plus pembrolizumab and trametinib versus stereotactic body radiotherapy plus gemcitabine for locally recurrent pancreatic cancer after surgical resection: An open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2022, 23, e105–e115. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lièvre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Chen, L.T.; Ryu, M.H.; Oh, D.Y.; Oh, S.C.; Chung, H.C.; Lee, K.W.; Omori, T.; Shitara, K.; Sakuramoto, S.; et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.V.; Reck, M.; Mansfield, A.S.; Mok, T.; Scherpereel, A.; Reinmuth, N.; Garassino, M.C.; De Castro Carpeno, J.; Califano, R.; Nishio, M.; et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients with Extensive-Stage Small-Cell Lung Cancer Treated with Atezolizumab, Carboplatin, and Etoposide (IMpower133). J. Clin. Oncol. 2021, 39, 619–630. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Arija, J.Á.A.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-Del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izumi, K.; et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1547–1557. [Google Scholar] [CrossRef]
- Motzer, R.; Alekseev, B.; Rha, S.Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef]
- Chen, E.X.; Jonker, D.J.; Loree, J.M.; Kennecke, H.F.; Berry, S.R.; Couture, F.; Ahmad, C.E.; Goffin, J.R.; Kavan, P.; Harb, M.; et al. Effect of Combined Immune Checkpoint Inhibition vs Best Supportive Care Alone in Patients with Advanced Colorectal Cancer: The Canadian Cancer Trials Group CO.26 Study. JAMA Oncol. 2020, 6, 831–838. [Google Scholar] [CrossRef]
- Liu, T.; Bai, Y.; Lin, X.; Li, W.; Wang, J.; Zhang, X.; Pan, H.; Bai, C.; Bai, L.; Cheng, Y.; et al. First-line nivolumab plus chemotherapy vs chemotherapy in patients with advanced gastric, gastroesophageal junction, and esophageal adenocarcinoma: CheckMate 649 Chinese subgroup analysis. Int. J. Cancer 2023, 152, 749–760. [Google Scholar] [CrossRef]
- Renouf, D.J.; Loree, J.M.; Knox, J.J.; Topham, J.T.; Kavan, P.; Jonker, D.; Welch, S.; Couture, F.; Lemay, F.; Tehfe, M.; et al. The CCTG PA.7 phase II trial of gemcitabine and nab-paclitaxel with or without durvalumab and tremelimumab as initial therapy in metastatic pancreatic ductal adenocarcinoma. Nat. Commun. 2022, 13, 5020. [Google Scholar] [CrossRef]
- Peters, S.; Scherpereel, A.; Cornelissen, R.; Oulkhouir, Y.; Greillier, L.; Kaplan, M.A.; Talbot, T.; Monnet, I.; Hiret, S.; Baas, P.; et al. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann. Oncol. 2022, 33, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Gutzmer, R.; Stroyakovskiy, D.; Gogas, H.; Robert, C.; Lewis, K.; Protsenko, S.; Pereira, R.P.; Eigentler, T.; Rutkowski, P.; Demidov, L.; et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): Primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 395, 1835–1844. [Google Scholar] [CrossRef]
- Popat, S.; Curioni-Fontecedro, A.; Dafni, U.; Shah, R.; O’Brien, M.; Pope, A.; Fisher, P.; Spicer, J.; Roy, A.; Gilligan, D.; et al. A multicentre randomised phase III trial comparing pembrolizumab versus single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: The European Thoracic Oncology Platform (ETOP 9-15) PROMISE-meso trial. Ann. Oncol. 2020, 31, 1734–1745. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Uzzo, R.; Karam, J.A.; Master, V.A.; Donskov, F.; Suarez, C.; Albiges, L.; Rini, B.; Tomita, Y.; Kann, A.G.; et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2022, 400, 1103–1116. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; Garassino, M.C.; et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open 2022, 7, 100408. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J.L.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Uwer, L.; Hureaux, J.; Guisier, F.; Carmier, D.; Madelaine, J.; Otto, J.; et al. A Randomized Non-Comparative Phase II Study of Anti-Programmed Cell Death-Ligand 1 Atezolizumab or Chemotherapy as Second-Line Therapy in Patients with Small Cell Lung Cancer: Results From the IFCT-1603 Trial. J. Thorac. Oncol. 2019, 14, 903–913. [Google Scholar] [CrossRef]
- Fennell, D.A.; Ewings, S.; Ottensmeier, C.; Califano, R.; Hanna, G.G.; Hill, K.; Danson, S.; Steele, N.; Nye, M.; Johnson, L.; et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): A multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1530–1540. [Google Scholar] [CrossRef]
- Rudin, C.M.; Awad, M.M.; Navarro, A.; Gottfried, M.; Peters, S.; Csőszi, T.; Cheema, P.K.; Rodriguez-Abreu, D.; Wollner, M.; Yang, J.C.; et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J. Clin. Oncol. 2020, 38, 2369–2379. [Google Scholar] [CrossRef]
- Sezer, A.; Kilickap, S.; Gümüş, M.; Bondarenko, I.; Özgüroğlu, M.; Gogishvili, M.; Turk, H.M.; Cicin, I.; Bentsion, D.; Gladkov, O.; et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 2021, 397, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xu, J.; Chen, Y.; Zhuang, W.; Zhang, Y.; Chen, Z.; Chen, J.; Zhang, H.; Niu, Z.; Fan, Q.; et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): A multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020, 21, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Lee, J.S.; Kang, J.H.; Kim, H.R.; Inui, N.; Hida, T.; Lee, K.H.; Yoshida, T.; Tanaka, H.; Yang, C.T.; et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann. Oncol. 2021, 32, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Hajek, J.; Gurney, H.; Chang, Y.H.; Lee, J.L.; Sarwar, N.; et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Boyer, M.; Sendur, M.A.N.; Rodriguez-Abreu, D.; Park, K.; Lee, D.H.; Cicin, I.; Yumuk, P.F.; Orlandi, F.J.; Leal, T.A.; Molinier, O.; et al. Pembrolizumab Plus Ipilimumab or Placebo for Metastatic Non-Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score >= 50%: Randomized, Double-Blind Phase III KEYNOTE-598 Study. J. Clin. Oncol. 2021, 39, 2327–2338. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, D.; Fan, Y.; Yu, X.; Liu, Y.; Shu, Y.; Ma, Z.; Wang, Z.; Cheng, Y.; Wang, J.; et al. Tislelizumab versus Docetaxel in Patients with Previously Treated Advanced NSCLC (RATIONALE-303): A Phase 3, Open-Label, Randomized Controlled Trial. J. Thorac. Oncol. 2023, 18, 93–105. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.Q.; Chen, Q.Y.; Chen, D.; Hu, C.; Yang, K.; Wen, J.; Li, J.; Shi, Y.R.; Jin, F.; Xu, R.; et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: A multicenter randomized phase 3 trial. Nat. Med. 2021, 27, 1536–1543. [Google Scholar] [CrossRef]
- Wang, J.; Lu, S.; Yu, X.; Hu, Y.; Sun, Y.; Wang, Z.; Zhao, J.; Yu, Y.; Hu, C.; Yang, K.; et al. Tislelizumab Plus Chemotherapy vs Chemotherapy Alone as First-line Treatment for Advanced Squamous Non-Small-Cell Lung Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 709–717. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Fang, J.; Yu, Q.; Han, B.; Cang, S.; Chen, G.; Mei, X.; Yang, Z.; Stefaniak, V.; et al. Final overall survival data of sintilimab plus pemetrexed and platinum as First-Line treatment for locally advanced or metastatic nonsquamous NSCLC in the Phase 3 ORIENT-11 study. Lung Cancer 2022, 171, 56–60. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Fang, J.; Yu, Q.; Han, B.; Cang, S.; Chen, G.; Mei, X.; Yang, Z.; Ma, R.; et al. Efficacy and Safety of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC: A Randomized, Double-Blind, Phase 3 Study (Oncology pRogram by InnovENT anti-PD-1-11). J. Thorac. Oncol. 2020, 15, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Lu, J.; Bai, Y.; Mao, T.; Wang, J.; Fan, Q.; Zhang, Y.; Zhao, K.; Chen, Z.; Gao, S.; et al. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients with Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA 2021, 326, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, C.; Yao, W.; Wang, Q.; Min, X.; Chen, G.; Xu, X.; Li, X.; Xu, F.; Fang, Y.; et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022, 23, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, M.; Jiang, O.; Pan, Y.; Hu, D.; Lin, Q.; Wu, G.; Cui, J.; Chang, J.; Cheng, Y.; et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): Interim results of a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2022, 23, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wu, L.; Jian, H.; Chen, Y.; Wang, Q.; Fang, J.; Wang, Z.; Hu, Y.; Sun, M.; Han, L.; et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): First interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2022, 23, 1167–1179. [Google Scholar] [CrossRef]
- Wang, Z.X.; Cui, C.; Yao, J.; Zhang, Y.; Li, M.; Feng, J.; Yang, S.; Fan, Y.; Shi, J.; Zhang, X.; et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell 2022, 40, 277–288.e273. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Han, L.; Wu, L.; Chen, J.; Sun, H.; Wen, G.; Ji, Y.; Dvorkin, M.; Shi, J.; Pan, Z.; et al. Effect of First-Line Serplulimab vs Placebo Added to Chemotherapy on Survival in Patients with Extensive-Stage Small Cell Lung Cancer: The ASTRUM-005 Randomized Clinical Trial. JAMA 2022, 328, 1223–1232. [Google Scholar] [CrossRef]
- Gogishvili, M.; Melkadze, T.; Makharadze, T.; Giorgadze, D.; Dvorkin, M.; Penkov, K.; Laktionov, K.; Nemsadze, G.; Nechaeva, M.; Rozhkova, I.; et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: A randomized, controlled, double-blind phase 3 trial. Nat. Med. 2022, 28, 2374–2380. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Khattak, A.; Carlino, M.S.; et al. Association between Immune-Related Adverse Events and Recurrence-Free Survival among Patients with Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2020, 6, 519–527. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Oremus, M.; Wolfson, C.; Perrault, A.; Demers, L.; Momoli, F.; Moride, Y. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer’s disease drug trials. Dement. Geriatr. Cogn. Disord. 2001, 12, 232–236. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ (Clin. Res. Ed.) 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin. Res. Ed.) 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Schwarzer, G. meta: An R package for meta-analysis. R News 2007, 7, 40–45. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011; pp. 12–21. [Google Scholar]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Markovic, S.N.; Molina, J.R.; Halfdanarson, T.R.; Pagliaro, L.C.; Chintakuntlawar, A.V.; Li, R.; Wei, J.; Wang, L.; Liu, B.; et al. Association of Sex, Age, and Eastern Cooperative Oncology Group Performance Status with Survival Benefit of Cancer Immunotherapy in Randomized Clinical Trials: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2012534. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.B.; McGlynn, K.A.; Devesa, S.S.; Freedman, N.D.; Anderson, W.F. Sex disparities in cancer mortality and survival. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Straface, E.; Gambardella, L.; Brandani, M.; Malorni, W. Sex differences at cellular level: “cells have a sex”. Handb. Exp. Pharmacol. 2012, 214, 49–65. [Google Scholar] [CrossRef]
- Zhu, Y.; Shao, X.; Wang, X.; Liu, L.; Liang, H. Sex disparities in cancer. Cancer Lett. 2019, 466, 35–38. [Google Scholar] [CrossRef]
- Pala, L.; De Pas, T.; Catania, C.; Giaccone, G.; Mantovani, A.; Minucci, S.; Viale, G.; Gelber, R.D.; Conforti, F. Sex and cancer immunotherapy: Current understanding and challenges. Cancer Cell 2022, 40, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.L.; Nofchissey, R.A.; Khan, M.A.; Reidy, M.A.; Lerner, M.R.; Wu, X.; Guo, S.; Hill, S.L.; Weygant, N.; Adams, S.F.; et al. The role of sex in the innate and adaptive immune environment of metastatic colorectal cancer. Br. J. Cancer 2020, 123, 624–632. [Google Scholar] [CrossRef]
- Lee, J.; Nicosia, M.; Hong, E.S.; Silver, D.J.; Li, C.; Bayik, D.; Watson, D.C.; Lauko, A.; Kay, K.E.; Wang, S.Z.; et al. Sex-Biased T-cell Exhaustion Drives Differential Immune Responses in Glioblastoma. Cancer Discov. 2023, 13, 2090–2105. [Google Scholar] [CrossRef]
- Manson, Q.F.; Ter Hoeve, N.D.; Buerger, H.; Moelans, C.B.; van Diest, P.J. PD-1 and PD-L1 Expression in Male Breast Cancer in Comparison with Female Breast Cancer. Target. Oncol. 2018, 13, 769–777. [Google Scholar] [CrossRef]
- Gu, Y.; Tang, Y.Y.; Wan, J.X.; Zou, J.Y.; Lu, C.G.; Zhu, H.S.; Sheng, S.Y.; Wang, Y.F.; Liu, H.C.; Yang, J.; et al. Sex difference in the expression of PD-1 of non-small cell lung cancer. Front. Immunol. 2022, 13, 1026214. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Pagan, E.; Corti, C.; Bagnardi, V.; Queirolo, P.; Catania, C.; De Pas, T.; Giaccone, G. Sex-based differences in response to anti-PD-1 or PD-L1 treatment in patients with non-small-cell lung cancer expressing high PD-L1 levels. A systematic review and meta-analysis of randomized clinical trials. ESMO Open 2021, 6, 100251. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Polesso, F.; Wang, C.; Sehrawat, A.; Hawkins, R.M.; Murray, S.E.; Thomas, G.V.; Caruso, B.; Thompson, R.F.; Wood, M.A.; et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature 2022, 606, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Dehm, S.M.; Sharifi, N. Targeting the Androgen Signaling Axis in Prostate Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 4267–4278. [Google Scholar] [CrossRef]
- Yang, C.; Jin, J.; Yang, Y.; Sun, H.; Wu, L.; Shen, M.; Hong, X.; Li, W.; Lu, L.; Cao, D.; et al. Androgen receptor-mediated CD8(+) T cell stemness programs drive sex differences in antitumor immunity. Immunity 2022, 55, 1268–1283.e9. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Schafer, J.M.; Song, N.J.; Kaneko, S.; Li, A.; Xiao, T.; Ma, A.; Allen, C.; Das, K.; Zhou, L.; et al. Androgen conspires with the CD8(+) T cell exhaustion program and contributes to sex bias in cancer. Sci. Immunol. 2022, 7, eabq2630. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Artomov, M.; Goggins, W.; Daly, M.; Tsao, H. Gender Disparity and Mutation Burden in Metastatic Melanoma. J. Natl. Cancer Inst. 2015, 107, djv221. [Google Scholar] [CrossRef] [PubMed]
- Van Allen, E.M.; Miao, D.; Schilling, B.; Shukla, S.A.; Blank, C.; Zimmer, L.; Sucker, A.; Hillen, U.; Foppen, M.H.G.; Goldinger, S.M.; et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 2015, 350, 207–211. [Google Scholar] [CrossRef]
- Paolino, M.; Koglgruber, R.; Cronin, S.J.F.; Uribesalgo, I.; Rauscher, E.; Harreiter, J.; Schuster, M.; Bancher-Todesca, D.; Pranjic, B.; Novatchkova, M.; et al. RANK links thymic regulatory T cells to fetal loss and gestational diabetes in pregnancy. Nature 2021, 589, 442–447. [Google Scholar] [CrossRef]
- Kieffer, T.E.; Faas, M.M.; Scherjon, S.A.; Prins, J.R. Pregnancy persistently affects memory T cell populations. J. Reprod. Immunol. 2017, 119, 1–8. [Google Scholar] [CrossRef]
- Venanzi, F.M.; Bini, M.; Nuccio, A.; De Toma, A.; Lambertini, M.; Ogliari, F.R.; Oresti, S.; Viganò, M.G.; Brioschi, E.; Polignano, M.; et al. Sex dimorphism and cancer immunotherapy: May pregnancy solve the puzzle? Cancer Treat. Rev. 2023, 121, 102648. [Google Scholar] [CrossRef]
- Yacouba, A.; Tidjani Alou, M.; Lagier, J.C.; Dubourg, G.; Raoult, D. Urinary microbiota and bladder cancer: A systematic review and a focus on uropathogens. Semin. Cancer Biol. 2022, 86, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Thackray, V.G. Sex, Microbes, and Polycystic Ovary Syndrome. Trends Endocrinol. Metab. TEM 2019, 30, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ju, Q.; Jia, K.; Yu, J.; Shi, H.; Wu, H.; Jiang, M. Correlation between sex and efficacy of immune checkpoint inhibitors (PD-1 and CTLA-4 inhibitors). Int. J. Cancer 2018, 143, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Grassadonia, A.; Sperduti, I.; Vici, P.; Iezzi, L.; Brocco, D.; Gamucci, T.; Pizzuti, L.; Maugeri-Saccà, M.; Marchetti, P.; Cognetti, G.; et al. Effect of Gender on the Outcome of Patients Receiving Immune Checkpoint Inhibitors for Advanced Cancer: A Systematic Review and Meta-Analysis of Phase III Randomized Clinical Trials. J. Clin. Med. 2018, 7, 542. [Google Scholar] [CrossRef]
- Lahiri, A.; Maji, A.; Potdar, P.D.; Singh, N.; Parikh, P.; Bisht, B.; Mukherjee, A.; Paul, M.K. Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol. Cancer 2023, 22, 40. [Google Scholar] [CrossRef]
- Piombino, C.; Tonni, E.; Oltrecolli, M.; Pirola, M.; Pipitone, S.; Baldessari, C.; Dominici, M.; Sabbatini, R.; Vitale, M.G. Immunotherapy in urothelial cancer: Current status and future directions. Expert Rev. Anticancer. Ther. 2023, 23, 1141–1155. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]

| Phase | Tumor Type | Line | Treatment Groups | Number of Patients | Number of Men (%) | Number of Women (%) | Median Age, Years | Median Follow-Up, Months | Outcome | Overall HR (95% CI) | HR (95% CI) for Men | HR (95% CI) for Women | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li-Tzong Chen et al. (2020) [10] | 3 | Gastrointestinal cancer | >1 | nivolumab vs. placebo | 493 | 348 (71%) | 145 (29%) | 61.7 | 27.3 | OS | 0.66 (0.54–0.80) | 0.60 (0.48–0.76) | 0.79 (0.55–1.15) |
| Alexander M M Eggermont et al. (2019) [11] | 3 | Melanoma | >1 | ipilimumab vs. placebo | 951 | 589 (62%) | 362 (38%) | NA | 82.8 | OS | 0.73 (0.60–0.90) | 0.76 (0.55–1.05) | 0.69 (0.45–1.07) |
| NA | NA | RFS | 0.76 (0.64–0.89) | 0.76 (0.58–0.99) | 0.76 (0.54–1.07) | ||||||||
| M. Reck et al. (2021) [12] | 3 | NSCLC | 1 | nivolumab + ipilimumab + chemo vs. chemo | 719 | 504 (70%) | 215 (30%) | NA | 30.7 | OS | 0.73 (0.61–0.87) | 0.72 (0.59–0.88) | 0.75 (0.54–1.04) |
| Masanobu Takahashi et al. (2021) [13] | 3 | Gastrointestinal cancer | >1 | nivolumab vs. paclitaxel or docetaxel | 274 | 230 (84%) | 44 (16%) | NA | NA | OS | 0.77 (0.59–1.01) | 0.79 (0.59–1.06) | 0.75 (0.37–1.55) |
| Hidetoshi Hayashi et al. (2022) [14] | 2 | NSCLC | >1 | nivolumab vs. pemetrexed + carboplatin | 102 | 43 (42%) | 59 (58%) | NA | NA | PFS | 1.92 (1.61–2.29) | 1.92 (1.01–3.67) | 1.79 (1.04–3.07) |
| Takashi Kojima et al. (2020) [15] | 3 | Gastrointestinal cancer | >1 | pembrolizumab vs. chemo | 628 | 544 (87%) | 84 (13%) | NA | NA | PFS | 0.89 (0.75–1.05) | 0.89 (0.75–1.07) | 0.90 (0.57–1.43) |
| Enriqueta Felip et al. (2021) [16] | 3 | NSCLC | >1 | atezolizumab vs. best supportive care after adjuvant cisplatin-based chemo | 1764 | 1178 (67%) | 586 (33%) | NA | 35.3 | RFS | 0.79 (0.64–0.96) | 0.76 (0.59–0.99) | 0.80 (0.57–1.13) |
| Makoto Nishio et al. (2021) [17] | 3 | NSCLC | 1 | atezolizumab + pemetrexed vs. pemetrexed | 578 | 384 (66%) | 194 (34%) | 63.5 (31–85) | NA | PFS | 0.60 (0.49–0.72) | 0.64 (0.51–0.79) | 0.51 (0.36–0.71) |
| OS | 0.86 (0.71–1.06) | 0.93 (0.73–1.18) | 0.76 (0.54–1.09) | ||||||||||
| Nancy Y Lee et al. (2021) [18] | 3 | HNC | >1 | avelumab + chemo vs. placebo + chemo | 697 | 575 (82%) | 122 (18%) | NA | 14.6 (8.5–19.6) | PFS | 1.21 (0.93–1.57) | 1.21 (0.91–1.61) | 1.16 (0.58–2.33) |
| Keunchil Park et al. (2021) [19] | 3 | NSCLC | 2 | avelumab vs. docetaxel | 529 | 367 (69%) | 162 (31%) | NA | NA | OS | 0.87 (0.71–1.05) | 0.82 (0.65–1.03) | 1.02 (0.72–1.45) |
| Caicun Zhou et al. (2021) [20] | 3 | NSCLC | >1 | camrelizumab + carboplatin + pemetrexed vs. chemo | 412 | 295 (72%) | 117 (28%) | NA | 11.9 | PFS | 0.60 (0.45–0.79) | 0.55 (0.40–0.75) | 0.78 (0.45–1.37) |
| D. R. Spigel et al. (2021) [21] | 3 | SCLC | >1 | nivolumab vs. chemo | 569 | 351 (62%) | 218 (38%) | NA | 15.8 | OS | 0.87 (0.73–1.05) | 0.80 (0.63–1.01) | 1.03 (0.77–1.38) |
| Caicun Zhou et al. (2022) [22] | 3 | NSCLC | 1 | sugemalimab vs. placebo | 479 | 383 (80%) | 96 (20%) | NA | 8.6 | PFS | 0.48 (0.39–0.60) | 0.48 (0.38–0.62) | 0.6090.37–0.99) |
| F Stephen Hodi et al. (2010) [23] | 3 | Melanoma | >1 | A: ipilimumab + gp100 vs. gp100 | 676 | 401 (59%) | 275 (41%) | 56.2 (NA) | 21.0 vs. 27.8 vs. 17.2 | OS | 0.68 (NA) | 0.66 (0.50–0.87) | 0.72 (0.52–0.99) |
| B: ipilimumab vs. gp100 | 0.66 (NA) | 0.54 (0.37–0.77) | 0.81 (0.55–1.20) | ||||||||||
| Antoni Ribas et al. (2013) [24] | 3 | Melanoma | 1 | tremelimumab vs. chemo | 655 | 372 (57%) | 283 (43%) | 56.5 (22–90) | NA | OS | 0.88 (NA) | 0.93 (0.74–1.17) | 0.81 (0.62–1.06) |
| Caroline Robert et al. (2011) [25] | 3 | Melanoma | 1 | lpilimumab + dacarbazine vs. dacarbazine + placebo | 502 | 301 (60%) | 201 (40%) | 56.9 (NA) | 54 | OS | 0.72 (0.59–0.87) | 0.70 (0.55–0.90) | 0.86 (0.63–1.17) |
| Ramaswamy Govindan et al. (2017) [26] | 3 | NSCLC | 1 | Ipilimumab + paclitaxel + carboplatin vs. paclitaxel + carboplatin + placebo | 749 | 635 (85%) | 114 (15%) | 64 (28–85) | 12.5 vs. 11.8 | OS | 0.91 (0.77–1.07) | 0.85 (0.71–1.02) | 1.33 (0.84–2.11) |
| Martin Reck et al. (2016) [27] | 3 | SCLC | 1 | Ipilimumab + etoposide + platinum vs. placebo + etoposide + platinum | 954 | 643 (67%) | 311 (33%) | 62.5 (36–85) | 10.5 vs. 10.2 | OS | 0.94 (0.81–1.09) | 1.07 (0.89–1.28) | 1.06 (0.81–1.37) |
| Julie Brahmer et al. (2015) [28] | 3 | NSCLC | >1 | nivolumab vs. docetaxel | 272 | 208 (76%) | 64 (24%) | 63 (39–85) | 11 | OS | 0.59 (0.44–0.79) | 0.57 (0.41–0.78) | 0.67 (0.36–1.25) |
| Robert J Motzer et al. (2015) [29] | 3 | RCC | >1 | nivolumab vs. everolimus | 821 | 619 (75%) | 202 (25%) | 62 (18–88) | 14 | OS | 0.73 (0.57–0.93) | 0.73 (0.58–0.92) | 0.84 (0.57–1.24) |
| Hossein Borghaei et al. (2015) [30] | 3 | NSCLC | >1 | nivolumab vs. docetaxel | 582 | 319 (55%) | 263 (45%) | 62 (21–85) | 13.2 | OS | 0.73 (0.59–0.89) | 0.73 (0.56–0.96) | 0.78 (0.58–1.04) |
| James Larkin et al. (2018) [31] | 3 | Melanoma | >1 | nivolumab vs. chemo | 405 | 261 (64%) | 144 (36%) | 60 (23–88) | NA | OS | 0.92 (0.71–1.18) | 0.85 (0.62–1.17) | 1.07 (0.69–1.65) |
| Caroline Robert et al. (2015) [32] | 3 | Melanoma | 1 | nivolumab vs. dacarbazine | 418 | 246 (59%) | 172 (41%) | 65 (18–87) | 8.9 vs. 6.8 | OS | 0.42 (0.25–0.73) | 0.34 (0.22–0.54) | 0.56 (0.33–0.95) |
| Michele Maio et al. (2017) [33] | 2b | Others | >1 | tremelimumab vs. placebo | 571 | 434 (76%) | 137 (24%) | 66.5 (60–73) | NA | OS | 0.92 (0.76–1.12) | 0.91 (0.73–1.13) | 1.12 (0.72–1.75) |
| Roy S Herbst et al. (2016) [34] | 2/3 | NSCLC | >1 | pembrolizumab vs. docetaxel | 1033 | 634 (61%) | 399 (39%) | 62.5 (54–70) | 13.1 | PFS | 0.85 (0.73–0.98) | 0.78 (0.64–0.94) | 1.02 (0.78–1.32) |
| Roy S. Herbst et al. (2021) [35] | 2/3 | NSCLC | >1 | pembrolizumab vs. docetaxel | 1033 | 634 (61%) | 399 (39%) | NA | 67.4 | OS | 0.70 (0.61–0.80) | 0.71 (0.60–0.86) | 0.66 (0.53–0.84) |
| Louis Fehrenbacher et al. (2018) [36] | 3 | NSCLC | >1 | atezolizumab vs. docetaxel | 1225 | 467 (38%) | 758 (62%) | 63.5 (25–85) | 28 | OS | 0.80 (0.70–0.92) | 0.81 (0.65–1.01) | 0.79 (0.66–0.93) |
| David P Carbone et al. (2017) [37] | 3 | NSCLC | 1 | nivolumab vs. chemo | 541 | 332 (61%) | 209 (39%) | 64 (29–89) | 13.5 | OS | 1.02 (0.80–1.30) | 0.97 (0.74–1.26) | 1.15 (0.79–1.66) |
| Robert L Ferris et al. (2016) [38] | 3 | HNC | >1 | nivolumab vs. chemo | 361 | 300 (83%) | 61 (17%) | 60 (28–83) | 5.1 | OS | 0.70 (0.51–0.96) | 0.65 (0.48–0.88) | 0.93 (0.47–1.85) |
| Scott J Antonia et al. (2018) [39] | 3 | NSCLC | >1 | durvalumab vs. placebo | 713 | 500 (70%) | 213 (30%) | NA | 25.2 | OS | 0.68 (0.54–0.86) | 0.78 (0.59–1.03) | 0.46 (0.30–0.73) |
| Pier Francesco Ferrucci et al. (2020) [40] | 2 | Melanoma | >1 | pembrolizumab + dabrafenib + trametinib vs. placebo + dabrafenib + trametinib | 120 | 69 (58%) | 51 (43%) | 56 (18–83) | 36.6 | PFS | 0.53 (0.34–0.83) | 0.45 (0.25–0.82) | 0.79 (0.41–1.53) |
| OS | 0.64 (0.38–1.06) | 0.62 (0.31–1.25) | 0.64 (0.30–1.38) | ||||||||||
| Martin Reck et al. (2019) [41] | 3 | NSCLC | 1 | pembrolizumab vs. platinum-based chemo | 305 | 187 (61%) | 118 (39%) | 65.2 (33–90) | 25.2 | OS | 0.63 (0.47–0.86) | 0.54 (0.36–0.79) | 0.95 (0.56–1.62) |
| Tony S K Mok et al. (2019) [42] | 3 | NSCLC | 1 | pembrolizumab vs. chemo | 1274 | 902 (71%) | 372 (29%) | 63 (57–69) | 12.8 | OS | 0.81 (0.71–0.93) | 0.80 (0.68–0.94) | 0.89 (0.68–1.17) |
| Robert J Motzer et al. (2022) [43] | 3 | RCC | 1 | nivolumab + ipilimumab vs. sunitinib | 636 | 477 (75%) | 159 (25%) | 62 (21–85) | 67.7 | OS | 0.94 (0.65–1.37) | 0.81 (0.68–0.97) | 0.62 (0.45–0.84) |
| Ezra E W Cohen et al. (2019) [44] | 3 | HNC | >1 | pembrolizumab vs. standard of care | 495 | 412 (83%) | 83 (17%) | 60.0 (54–66) | 7.5 | OS | 0.80 (0.65–0.98) | 0.77 (0.62–0.96) | 0.94 (0.54–1.63) |
| Joaquim Bellmunt et al. (2017) [45] | 3 | Urothelial carcinoma | >1 | pembrolizumab vs. chemo | 542 | 402 (74%) | 140 (26%) | 66 (26–88) | 14.1 | OS | 0.73 (0.59–0.91) | 0.73 (0.56–0.94) | 0.78 (0.49–1.24) |
| Barbara Burtness et al. (2019) [46] | 3 | HNC | 1 | A: pembrolizumab vs. cetuximab + chemo | 601 | 511 (85%) | 90 (15%) | 61.3 (54.5–68) | 11.5 vs. 13.0 vs. 10.7 | OS | 0.81 (0.68–0.97) | 0.80 (0.66–0.97) | 0.89 (0.56–1.41) |
| B: pembrolizumab + chemo vs. cetuximab + chemo | 559 | 466 (83%) | 93 (17%) | 0.72 (0.60–0.86) | 0.72 (0.59–0.89) | 0.67 (0.42–1.06) | |||||||
| Alexander M M Eggermont et al. (2021) [47] | 3 | Melanoma | >1 | pembrolizumab vs. placebo | 1019 | 628 (62%) | 391 (38%) | NA | 42.3 | PFS | 0.60 (0.49–0.73) | 0.56 (0.41–0.77) | 0.68 (0.44–1.05) |
| Martin Reck et al. (2019) [48] | 3 | NSCLC | 1 | atezolizumab + bevacizumab + carboplatin + paclitaxel vs. bevacizumab + carboplatin + paclitaxel | 800 | 479 (60%) | 321 (40%) | 63 (31–90) | 19.6 vs. 19.7 | OS | 0.76 (0.63–0.93) | 0.73 (0.57–0.93) | 0.82 (0.61–1.12) |
| Howard West et al. (2019) [49] | 3 | NSCLC | 1 | atezolizumab + carboplatin + nabpaclitaxel vs. chemo | 679 | 400 (59%) | 279 (41%) | 64.3 (18–86) | 18.5 vs. 19.2 | OS | 0.80 (0.65–0.99) | 0.87 (0.66–1.15) | 0.66 (0.46–0.93) |
| Robert Jotte et al. (2020) [50] | 3 | NSCLC | 1 | atezolizumab + carboplatin + nab-paclitaxel vs. carboplatin + nab-paclitaxel | 683 | 557 (82%) | 126 (18%) | 65 (23–83) vs. 65 (38–86) | 26.8 vs. 24.8 | OS | 0.88 (0.73–1.05) | 0.91 (0.75–1.12) | 0.68 (0.44–1.04) |
| PFS | 0.71 (0.60–0.85) | 0.71 (0.59–0.85) | 0.66 (0.45–0.97) | ||||||||||
| Charles S. Fuchs et al. (2022) [51] | 3 | Gastrointestinal cancer | >1 | pembrolizumab vs. paclitaxel | 395 | 286 (72%) | 109 (28%) | NA | NA | OS | 0.81 (0.66–1.00) | 0.87 (0.68–1.11) | 0.77 (0.50–1.18) |
| Fabrice Barlesi et al. (2018) [52] | 3 | NSCLC | >1 | avelumab vs. docetaxel | 529 | 367 (69%) | 162 (31%) | 63.5 (56–70) | 18.3 | OS | 0.90 (0.73–1.12) | 0.83 (0.64–1.08) | 1.08 (0.74–1.59) |
| Roy S Herbst et al. (2020) [53] | 3 | NSCLC | 1 | atezolizumab vs. chemo | 205 | 143 (70%) | 62 (30%) | NA | NA | OS | 0.59 (0.40–0.89) | 0.57 (0.35–0.93) | 0.69 (0.34–1.39) |
| Joaquim Bellmunt et al. (2021) [54] | 3 | Urothelial carcinoma | >1 | atezolizumab vs. observation | 809 | 638 (79%) | 171 (21%) | NA | 21.9 | RFS | 0.89 (0.74–1.08) | 0.91 (0.73–1.13) | 1.00 (0.79–1.29) |
| Naiyer A Rizvi et al. (2020) [55] | 3 | NSCLC | 1 | durvalumab vs. chemo | 325 | 219 (67%) | 106 (33%) | 64.2 (32–85) | 30.2 | OS | 0.76 (0.56–1.02) | 0.81 (0.59–1.11) | 0.66 (0.41–1.04) |
| Luis G Paz-Ares et al. (2022) [56] | 3 | NSCLC | 1 | nivolumab + ipilimumab vs. chemo | 1166 | 778 (67%) | 388 (33%) | NA | 54.8 | OS | 0.74 (0.65–0.84) | 0.67 (0.57–0.78) | 0.88 (0.70–1.11) |
| Hirotsugu Kenmotsu et al. (2022) [57] | 3 | NSCLC | >1 | atezolizumab vs. best supportive care | 74 | 58 (78%) | 16 (22%) | 64.0 (40–75) vs. 68.0 (37–74) | 38.3 | RFS | 0.52 (0.25–1.08) | 0.50 (0.21–1.17) | 0.66 (0.16–2.73) |
| Kohei Shitara et al. (2020) [58] | 3 | Gastrointestinal cancer | 1 | A: pembrolizumab vs. chemo | 506 | 359 (71%) | 147 (29%) | 61.7 (20–87) | 29.4 | OS | 0.91 (0.74–1.1) | 0.88 (0.70–1.11) | 0.90 (0.63–1.27) |
| B: pembrolizumab + chemo vs. chemo | 507 | 374 (74%) | 133 (26%) | 62.2 (22–87) | OS | 0.85 (0.7–1.03) | 0.84 (0.67–1.05) | 0.89 (0.62–1.29) | |||||
| Mary O’Brien et al. (2022) [59] | 3 | NSCLC | >1 | Pembrolizumab vs. placebo | 1177 | 804 (68%) | 373 (32%) | 65 (59–70) | 35.6 | RFS | 0.76 (0.63–0.91) | 0.81 (0.65–1.01) | 0.73 (0.54–1.00) |
| Kenneth F Grossmann et al. (2022) [60] | 3 | Melanoma | >1 | pembrolizumab vs. standard of care | 1201 | 678 (56%) | 523 (44%) | 53 (20, 82) vs. 54 (18, 86) | 47.5 | OS | 0.82 (0.62–1.07) | 0.7 (0.43–1.15) | 0.87 (0.63–1.21) |
| RFS | 0.76 (0.64–0.91) | 0.75 (0.55–1.03) | 0.76 (0.62–0.93) | ||||||||||
| Thomas Powles et al. (2020) [61] | 3 | Urothelial carcinoma | 1 | durvalumab + tremelimumab vs. chemo | 686 | 530 (77%) | 156 (23%) | 68 (60–73) | 41.2 | OS | 0.85 (0.72–1.02) | 0.84 (0.69–1.02) | 0.90 (0.63–1.30) |
| Elisabeth Livingstone et al. (2022) [62] | 2 | Melanoma | >1 | A: nivolumab + ipilimumab vs. placebo | 108 | 64 (59%) | 44 (41%) | 55.0 (46.0–65.0) | 49.2 | RFS | 0.25 (0.13–0.48) | 0.33 (0.17–0.66) | 0.17 (0.07–0.44) |
| B: nivolumab vs. placebo | 111 | 64 (58%) | 47 (42%) | 55.0 (46.0–65.1) | 49.2 | RFS | 0.60 (0.36–1.00) | 0.80 (0.45–1.41) | 0.43 (0.21–0.87) | ||||
| Taofeek K Owonikoko et al. (2021) [63] | 3 | SCLC | >1 | A: chemo + nivolumab + ipilimumab | 554 | 355 (64%) | 199 (36%) | NA | NA | OS | 0.92 (0.76–1.12) | 0.99 (0.78–1.27) | 0.82 (0.58–1.14) |
| B: chemo +nivolumab vs. chemo + placebo | 555 | 352 (63%) | 203 (37%) | OS | 0.83 (0.63–1.01) | 0.89 (0.70–1.14) | 0.75 (0.54–1.04) | ||||||
| Luis A Diaz Jr. et al. (2022) [64] | 3 | Gastrointestinal cancer | 1 | pembrolizumab vs. mFOLFOX6 | 307 | 153 (50%) | 154 (50%) | 63 (50–73) | NA | OS | 0.74 (0.53–1.03) | 0.61 (0.38–0.99) | 0.88 (0.55–1.41) |
| Thierry André et al. (2020) [65] | 3 | Gastrointestinal cancer | 1 | pembrolizumab vs. chemo | 307 | 153 (50%) | 154 (50%) | 63 (24–93) | 32.4 | PFS | 0.60 (0.45–0.80) | 0.59 (0.38–0.90) | 0.58 (0.39–0.87) |
| Ken Kato et al. (2019) [66] | 3 | Gastrointestinal cancer | 2 | nivolumab vs. chemo | 419 | 364 (87%) | 55 (13%) | 65.5 (57–72) | 10.5 vs. 8.0 | OS | 0.77 (0.62–0.95) | 0.79 (0.63–0.99) | 0.72 (0.38–1.36) |
| D Rodríguez-Abreu et al. (2021) [67] | 3 | NSCLC | 1 | pemetrexed + platinum + pembrolizumab vs. pemetrexed + platinum | 616 | 363 (59%) | 253 (41%) | 64.5 (34–84) | 31 | OS | 0.56 (0.46–0.69) | 0.74 (0.56–0.96) | 0.41 (0.30–0.56) |
| PFS | 0.49 (0.41–0.59) | 0.58 (0.46–0.74) | 0.39 (0.29–0.52) | ||||||||||
| Thomas Powles et al. (2020) [68] | 3 | Urothelial carcinoma | >1 | avelumab + best support care vs. best support care | 700 | 541 (77%) | 159 (23%) | 68.5 (32–90) | NA | OS | 0.69 (0.56–0.86) | 0.64 (0.50–0.83) | 0.89 (0.56–1.41) |
| PFS | 0.62 (0.52–0.75) | 0.60 (0.49–0.74) | 0.69 (0.47–1.01) | ||||||||||
| Markus Moehler et al. (2021) [69] | 3 | Gastrointestinal cancer | 1 | avelumab vs. chemo | 499 | 331 (66%) | 168 (34%) | NA | NA | OS | 0.90 (0.74–1.11) | 0.83 (0.64–1.07) | 1.06 (0.76–1.49) |
| Y-J Bang et al. (2018) [70] | 3 | Gastrointestinal cancer | >1 | avelumab vs. chemo | 371 | 267 (72%) | 104 (28%) | 60 (18–86) | 10.6 | OS | 1.1 (0.90–1.40) | 0.99 (0.75–1.32) | 1.54 (0.99–2.38) |
| D.F. Bajorin et al. (2021) [71] | 3 | Urothelial carcinoma | >1 | nivolumab vs. placebo | 709 | 540 (76%) | 169 (24%) | NA | NA | RFS | 0.70 (0.57–0.86) | 0.68 (0.54–0.87) | 0.76 (0.50–1.16) |
| T. K. Choueiri et al. (2020) [72] | 3 | RCC | 1 | avelumab + axitinib vs. sunitinib | 886 | 660 (74%) | 226 (26%) | NA | NA | PFS | 0.688 (0.574–0.825) | 0.647 (0.524–0.798) | 0.862 (0.604–1.229) |
| OS | 0.796 (0.616- 1.027) | 0.797 (0.594–1.071) | 0.814 (0.486–1.364) | ||||||||||
| Xiaofei Zhu et al. (2022) [73] | 2 | Gastrointestinal cancer | >1 | stereotactic body radiotherapy + pembrolizumab + trametinib vs. SBRT + gemcitabine | 170 | 105 (62%) | 65 (38%) | NA | 13.1 | OS; PFS | OS: 0.69 (0.51–0.95); PFS: 0.60 (0.44–0.81) | OS: 0.62 (0.42–0.92); PFS: 0.52 (0.35–0.77) | OS: 0.86 (0.52–1.40); PFS: 0.76 (0.47–1.24) |
| R.J. Kelly et al. (2021) [74] | 3 | Gastrointestinal cancer | >1 | nivolumab vs. placebo | 532 | 671 (126%) | 123 (23%) | NA | 24.4 | RFS | 0.70 (0.58–0.86) | 0.73 (0.59–0.91) | 0.59 (0.35–1.00) |
| Yoon-Koo Kang et al. (2022) [75] | 2/3 | Gastrointestinal cancer | >1 | nivolumab + chemo vs. placebo + chemo | 724 | 523 (72%) | 201 (28%) | NA | 11.6 | PFS | 0.68 (0.51–0.90) | 0.70 (0.54–0.90) | 0.72 (0.48–1.10) |
| OS | 0.90 (0.75–1.08) | 0.87 (0.70–1.07) | 0.99 (0.70–1.39) | ||||||||||
| Stephen V. Liu et al. (2021) [76] | 1/3 | SCLC | 1 | carboplatin + etoposide (CP/ET) + atezolizumab vs. CP/ET + placebo | 403 | 261 (65%) | 142 (35%) | NA | NA | OS | 0.76 (0.60–0.95) | 0.83 (0.63–1.10) | 0.64 (0.43–0.94) |
| Luis Paz-Ares et al. (2018) [77] | 3 | NSCLC | 1 | pembrolizumab + chemo vs. placebo + chemo | 559 | 455 (81%) | 104 (19%) | 65 (29–88) | 7.8 | OS | 0.64 (0.49–0.85) | 0.69 (0.51–0.94) | 0.42 (0.22–0.81) |
| Matthew D Galsky et al. (2020) [78] | 3 | Urothelial carcinoma | >1 | atezolizumab + chemo vs. chemo | 1213 | 636 (52%) | 215 (18%) | 69 (62–75) vs. 67 (61–73) | 11.8 | OS | 0.84 (0.69–1.00) | 0.83 (0.67–1.02) | 0.88 (0.61–1.26) |
| PFS | 0.81 (0.69–0.94) | 0.83 (0.69–0.99) | 0.75 (0.55–1.02) | ||||||||||
| R. Motzer et al. (2021) [79] | 3 | RCC | >1 | lenvatinib + pembrolizumab vs. sunitinib | 712 | 530 (74%) | 209 (26%) | NA | NA | PFS | 0.39 (0.32–0.49) | 0.38 (0.30–0.49) | 0.42 (0.27–0.66) |
| Eric X. Chen et al. (2020) [80] | 2 | Gastrointestinal cancer | >1 | tremelimumab + durvalumab + BSC vs. BSC | 180 | 121 (67%) | 59 (33%) | 65 (36–87) | 15.2 | OS | 0.72 (0.54–0.97) | 0.79 (0.57–1.10) | 0.55 (0.32–0.95) |
| Tianshu Liu et al. (2022) [81] | 3 | Gastrointestinal cancer | 1 | nivolumab + chemo vs. chemo | 156 | 105 (67%) | 51 (33%) | 60.5 (23–85) | 14.0 vs. 9.9 | OS | 0.54 (0.37–0.79) | 0.52 (0.33–0.83) | 0.68 (0.30–1.11) |
| Daniel J. Renouf et al. (2022) [82] | 2 | Gastrointestinal cancer | >1 | gemcitabine + nab-paclitaxel + durvalumab + tremelimumab vs. gemcitabine + nab-paclitaxe | 180 | 93 (52%) | 87 (48%) | 64.3 (29–84) | 28.5 | OS | 0.94 (0.71–1.25) | 1.00 (0.66–1.64) | 0.84 (0.57–1.26) |
| S. Peters et al. (2022) [83] | 3 | Others | 1 | nivolumab + ipilimumab vs. chemo | 605 | 467 (77%) | 138 (23%) | 69 (62–75) | 43.1 | OS | 0.75 (0.63–0.90) | 0.73 (0.60–0.90) | 0.82 (0.56–1.20) |
| Ralf Gutzmer et al. (2020) [84] | 3 | Melanoma | 1 | atezolizumab + vemurafenib + cobimetinib vs. placebo + vemurafenib + cobimetinib | 514 | 299 (58%) | 215 (42%) | 54.0 (44.8–64.0) vs. 53.5 (43.0–63.8) | 18.9 | PFS | 0.77 (0.62–0.96) | 0.75 (0.56–1.01) | 0.81 (0.58–1.13) |
| S. Popat et al. (2020) [85] | 3 | Others | >1 | pembrolizumab vs. chemo | 144 | 118 (82%) | 26 (18%) | NA | NA | OS | 1.11 (0.73–1.66) | 1.16 (0.74–1.82) | 0.91 (0.34–2.45) |
| Sumanta Kumar Pal et al. (2022) [86] | 3 | RCC | >1 | atezolizumab vs. placebo | 778 | 565 (73%) | 213 (27%) | 60 (52–69) | 45 | RFS | 0.93 (0.75–1.15) | 1.08 (0.84–1.39) | 0.61 (0.40–0.94) |
| L. Paz-Ares et al. (2022) [87] | 3 | SCLC | 1 | A: durvalumab + EP vs. EP | 537 | 374 (70%) | 163 (30%) | NA | 39.4 | OS | 0.71 (0.60–0.86) | 0.76 (0.62–0.95) | 0.60 (0.42–0.84) |
| B: durvalumab + tremelimumab + EP vs. EP | 537 | 386 (72%) | 151 (28%) | NA | 39.4 | OS | 0.81 (0.67–0.97) | 0.81 (0.65–1.00) | 0.74 (0.52–1.05) | ||||
| Jean-Louis Pujol et al. (2019) [88] | 2 | SCLC | 2 | atezolizumab vs. chemo | 73 | 43 (59%) | 30 (41%) | 64.7 (51.1–85.5) | 13.7 | OS | 0.84 (0.45–1.58) | 0.82 (0.36–1.91) | 0.74 (0.26–2.07) |
| Dean A. Fennell et al. (2021) [89] | 3 | Others | >1 | nivolumab vs. placebo | 332 | 253 (76%) | 79 (24%) | NA | 11.6 | PFS | 0.67 (0.53–0.85) | 0.65 (0.49–0.86) | 0.69 (0.42–1.14) |
| OS | 0.69 (0.52–0.91) | 0.63 (0.46–0.87) | 0.96 (0.51–1.79) | ||||||||||
| Charles M Rudin et al. (2020) [90] | 3 | SCLC | 1 | pembrolizumab + EP vs. placebo + EP | 453 | 294 (65%) | 159 (35%) | 64.5 (24–83) | NA | PFS | 0.73 (0.60–0.88) | 0.66 (0.52–0.84) | 0.76 (0.54–1.06) |
| OS | 0.80 (0.64–0.98) | 0.76 (0.59–0.98) | 0.88 (0.61–1.26) | ||||||||||
| Ahmet Sezer et al. (2021) [91] | 3 | NSCLC | 1 | cemiplimab vs. platinum-doublet chemo | 463 | 479 (103%) | 84 (18%) | NA | NA | OS | 0.57 (0.42–0.77) | 0.50 (0.36–0.69) | 1.11 (0.49–2.52) |
| PFS | 0.54 (0.43–0.68) | 0.50 (0.40–0.64) | 0.79 (0.43–1.46) | ||||||||||
| Jing Huang et al. (2020) [92] | 3 | Gastrointestinal cancer | 2 | camrelizumab vs. chemo | 448 | 400 (89%) | 48 (11%) | 60 (54–65) | 8.3 vs. 6.2 | OS | 0.71 (0.57–0.87) | 0.75 (0.60–0.93) | 0.45 (0.21–0.93) |
| S. Sugawara et al. (2021) [93] | 3 | NSCLC | 1 | nivolumab + carboplatin, paclitaxel and bevacizumab vs. chemo + carboplatin, paclitaxel and bevacizumab | 550 | 411 (75%) | 139 (25%) | NA | NA | PFS | 0.57 (0.46–0.72) | 0.53 (0.41–0.69) | 0.72 (0.45–1.15) |
| Thomas Powles et al. (2022) [94] | 3 | RCC | >1 | pembrolizumab vs. placebo | 994 | 706 (71%) | 288 (29%) | NA | 30.1 | RFS | 0.63 (0.50–0.80) | 0.60 (0.45–0.80) | 0.73 (0.48–1.13) |
| Michael Boyer et al. (2021) [95] | 3 | NSCLC | >1 | ipilimumab vs. saline placebo | 568 | 393 (69%) | 174 (31%) | NA | NA | OS | 1.08 (0.85–1.37) | 0.97 (0.73–1.29) | 1.29 (0.84–1.99) |
| PFS | 1.06 (0.86–1.30) | 1.02 (0.80–1.29) | 1.15 (0.78–1.69) | ||||||||||
| Caicun Zhou et al. (2022) [96] | 3 | NSCLC | >1 | tislelizumab vs. docetaxel | 805 | 622 (77%) | 183 (23%) | NA | 16 | OS | 0.66 (0.56–0.79) | 0.61 (0.50–0.74) | 0.95 (0.65–1.38) |
| Richard S. Finn et al. (2020) [97] | 3 | Gastrointestinalcancer | >1 | atezolizumab + bevacizumab vs. sorafenib | 501 | 414 (83%) | 87 (17%) | NA | NA | OS | 0.60 (0.44–0.82) | 0.66 (0.47–0.92) | 0.35 (0.15–0.81) |
| PFS | 0.59 (0.47–0.75) | 0.59 (0.45–0.77) | 0.60 (0.34–1.06) | ||||||||||
| Hai-Qiang Mai et al. (2021) [98] | 3 | Others | 1 | toripalimab + GP vs. placebo + GP | 289 | 240 (83%) | 49 (17%) | 48 (19–72) | NA | PFS | 0.51 (0.356–0.728) | 0.54 (0.360–0.806) | 0.41 (0.187–0.889) |
| Jie Wang et al. (2021) [99] | 3 | NSCLC | >1 | A: tislelizumab + PC vs. PC | 241 | 218 (90%) | 23 (10%) | 62 (34–74) | NA | PFS | 0.52 (0.37–0.74) | 0.53 (0.37–0.76) | 0.53 (0.17–1.61) |
| B: tislelizumab + nab-PC vs. PC | 240 | 223 (93%) | 17 (7%) | 0.48 (0.34–0.68) | 0.50 (0.35–0.71) | 0.36 (0.09–1.47) | |||||||
| Li Zhang et al. (2022) [100] | 3 | NSCLC | 1 | sintilimab + chemo vs. placebo + chemo | 397 | 303 (76%) | 94 (24%) | NA | 30.8 | OS | 0.65 (0.50–0.85) | 0.57 (0.43–0.77) | 0.99 (0.56–1.77) |
| Yunpeng Yang et al. (2020) [101] | 3 | NSCLC | 1 | sintilimab + chemo vs. placebo + chemo | 397 | 303 (76%) | 94 (24%) | 61 (30, 75) | 8.9 | PFS | 0.482 (0.362–0.643) | 0.443 (0.320–0.612) | 0.603 (0.332–1.098) |
| Huiyan Luo et al. (2021) [102] | 3 | Gastrointestinal cancer | >1 | camrelizumab + chemo vs. placebo + chemo | 596 | 523 (88%) | 73 (12%) | NA | 10.8 | OS | 0.70 (0.56–0.88) | 0.69 (0.55–0.88) | 0.87 (0.42–1.81) |
| Jie Wang et al. (2022) [103] | 3 | SCLC | 1 | adebrelimab + chemo vs. placebo + chemo | 462 | 372 (81%) | 90 (19%) | 62 (56–66) | 13.5 | OS | 0.72 (0.58–0.90) | 0.72 (0.57–0.92) | 0.62 (0.37–1.05) |
| Qing Zhou et al. (2022) [104] | 3 | NSCLC | >1 | sugemalimab vs. placebo | 381 | 351 (92%) | 30 (8%) | NA | NA | PFS | 0.64 (0.48–0.85) | 0.61 (0.45–0.82) | 1.40 (0.55–3.57) |
| Shun Lu et al. (2022) [105] | 3 | NSCLC | >1 | sintilimab + bevacizumab biosimilar IBI305 + pemetrexed + cisplatin vs. pemetrexed + cisplatin | 299 | 123 (41%) | 176 (59%) | NA | NA | PFS | 0.46 (0.34–0.64) | 0.67 (0.41–1.09) | 0.44 (0.30–0.66) |
| Zi-Xian Wang et al. (2022) [106] | 3 | Gastrointestinal cancer | >1 | toripalimab + TP vs. placebo + TP | 514 | 437 (85%) | 77 (15%) | NA | NA | PFS | 0.57 (0.45–0.72) | 0.51 (0.40–0.66) | 0.96 (0.53–1.75) |
| OS | 0.59 (0.43–0.80) | 0.50 (0.36–0.70) | 1.40 (0.60–3.28) | ||||||||||
| Ying Cheng et al. (2022) [107] | 3 | SCLC | 1 | serplulimab + chemo vs. placebo + chemo | 585 | 481 (82%) | 104 (18%) | 61.1 | 12.3 | OS | 0.63 (0.49–0.82) | 0.64 (0.48–0.84) | 0.57 (0.30–1.06) |
| Miranda Gogishvili et al. (2022) [108] | 3 | NSCLC | 1 | cemiplimab + chemo vs. placebo + chemo | 312 | 268 (86%) | 44 (14%) | 63.0 (57–68) | 16.3 vs. 16.7 | OS | 0.71 (0.53–0.93) | 0.55 (0.41–0.74) | 2.11 (0.89–5.03) |
| PFS | 0.56 (0.44–0.70) | 0.48 (0.37–0.61) | 0.90 (0.50–1.62) | ||||||||||
| Alexander M. M. Eggermont et al. (2020) [109] | 3 | Melanoma | >1 | pembrolizumab vs. placebo | 1019 | 628 (62%) | 391 (38%) | 54 (19–88) | 36.6 | RFS | 0.56 (0.47–0.68) | 0.55 (0.40–0.74) | 0.60 (0.40–0.90) |
| Richard S. Finn et al. (2020) [110] | 3 | Gastrointestinal cancer | >1 | pembrolizumab + BSC vs. placebo + BSC | 413 | 338 (82%) | 75 (18%) | 13.8 | NA | OS | 0.78 (0.61–1.00) | 0.76 (0.58–1.00) | 0.80 (0.44–1.47) |
| PFS | 0.72 (0.57–0.90) | 0.74 (0.57–0.95) | 0.59 (0.33–1.06) | ||||||||||
| F. Stephen Hodi et al. (2016) [111] | 2 | Melanoma | >1 | nivolumab and ipilimumab vs. ipilimumab | 142 | 95 (67%) | 47 (33%) | 65 | 24.5 | OS | 0.74 (0.43–1.26) | 0.65 (0.33–1.26) | 0.89 (0.36–2.19) |
| Brian I. Rini et al. (2019) [112] | 3 | RCC | >1 | pembrolizumab + axitinib vs. sunitinib | 861 | 628 (73%) | 233 (27%) | 61.5 | 12.8 | OS | 0.53 (0.38–0.74) | 0.54 (0.37–0.80) | 0.45 (0.25–0.83) |
| PFS | 0.69 (0.57–0.84) | 0.77 (0.61–0.97) | 0.54 (0.37–0.81) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, X.; Sun, J.; Zeng, N.; Xiong, Y.; An, Y.; Wang, S.; Xia, Q. The Effect of Sex on the Therapeutic Efficiency of Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis Based on Randomized Controlled Trials. Cancers 2024, 16, 382. https://doi.org/10.3390/cancers16020382
Zhong X, Sun J, Zeng N, Xiong Y, An Y, Wang S, Xia Q. The Effect of Sex on the Therapeutic Efficiency of Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis Based on Randomized Controlled Trials. Cancers. 2024; 16(2):382. https://doi.org/10.3390/cancers16020382
Chicago/Turabian StyleZhong, Xingyu, Jianxuan Sun, Na Zeng, Yifan Xiong, Ye An, Shaogang Wang, and Qidong Xia. 2024. "The Effect of Sex on the Therapeutic Efficiency of Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis Based on Randomized Controlled Trials" Cancers 16, no. 2: 382. https://doi.org/10.3390/cancers16020382
APA StyleZhong, X., Sun, J., Zeng, N., Xiong, Y., An, Y., Wang, S., & Xia, Q. (2024). The Effect of Sex on the Therapeutic Efficiency of Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis Based on Randomized Controlled Trials. Cancers, 16(2), 382. https://doi.org/10.3390/cancers16020382







