Prediction of Subclinical and Clinical Multiple Organ Failure Dysfunction in Breast Cancer Patients—A Review Using AI Tools
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
| Algorithm 1. Hierarchical clustering based on Ward linkage |
| Input: —the database of articles, where each is a tuple containing the title and abstract of the -th article in the database; —the BERT pre-trained large language model. |
| Output: —the dendrogram represented as a set of triplets generated by the hierarchical clustering algorithm. |
| Computation: |
| 1. % Initialize the set of feature vectors with the empty set. |
| 2. for do |
| 3. % Concatenate the title and abstract of the -th article |
| 4. if then % If the text has more than 512 text tokens (words). |
| 5. % Trim to the maximum number of tokens accepted by BERT. |
| 6. endif |
| 7. % Apply BERT to and store the resulting [CLS] token to . |
| 8. % Add the computed feature vector to the set . |
| 9. endfor |
| 10. % Initialize the leaf clusters with one sample per cluster. |
| 11. ; % Initialize the set of clusters. |
| 12. ; % Initialize the dendrogram with the empty set. |
| 13. for do |
| 14. for do |
| 15. for do |
| 16. % Compute Ward criterion. |
| 17. endfor |
| 18. endfor |
| 19. % Find the clusters with the smallest increase in variance. |
| 20. % Merge clusters and into a new cluster denoted as . |
| 21. ; % Remove clusters and from the set of clusters. |
| 22. % Add new cluster to the set of clusters. |
| 23. % Store distance at which clusters and are merged. |
| 24. endfor |
3. Results
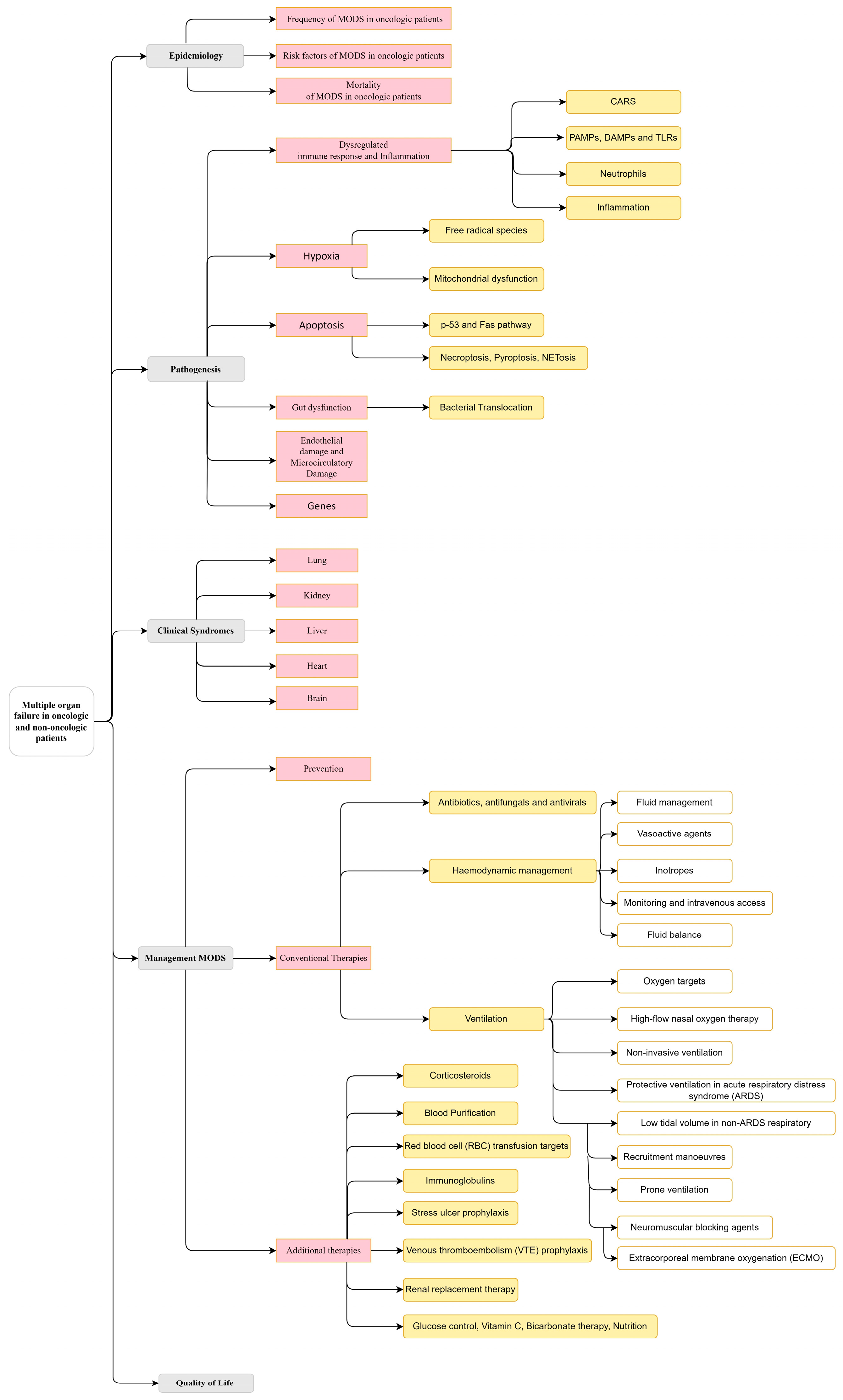
3.1. Multiple Organ Failure in Oncologic and Non-Oncologic Patients
3.1.1. Epidemiology
- Frequency of MODS in oncologic patients
- Risk factors of MODS in oncologic patients
- Mortality of MODS in oncologic patients
3.1.2. Pathogenesis
- Dysregulated immune response and inflammation
- Hypoxia
- Apoptosis
- Gut dysfunction.
- Endothelial Damage and Microcirculatory Damage
- Genes
3.1.3. Clinical Syndrome
- Respiratory
- Kidney
- Liver
- Cardiocirculatory system
- Brain
3.1.4. Management of MODS
- Prevention
- Conventional Therapies
- Antibiotics, antifungals, and antivirals.
- Hemodynamic management.
- Ventilation.
- Additional Therapies
3.2. Quality of Life
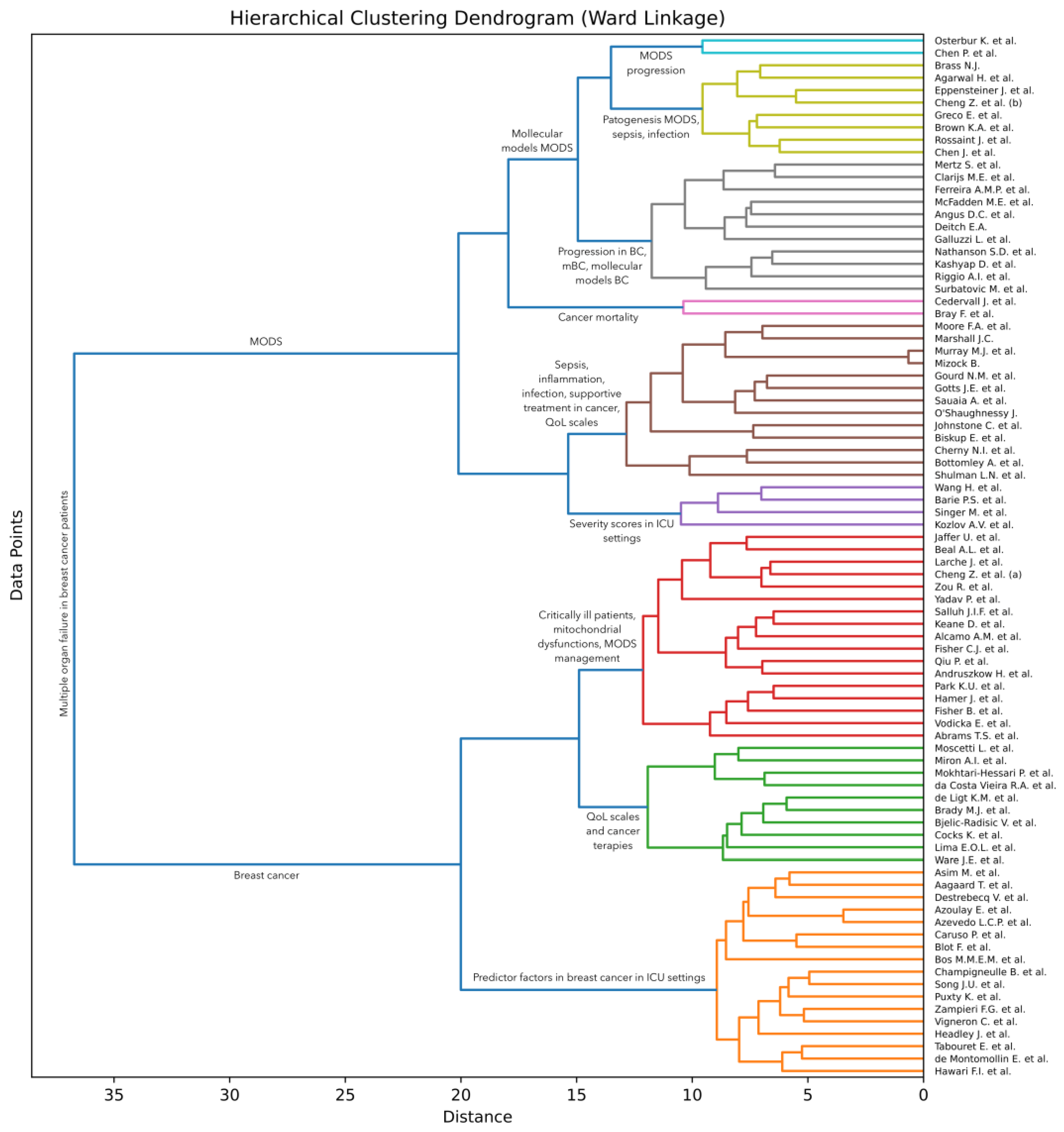
3.3. Analysis of AI-Based Clustering
4. Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McFadden, M.E.; Sartorius, S.E. Multiple Systems Organ Failure in the Patient with Cancer. Part I: Pathophysiologic Perspectives. Oncol. Nurs. Forum 1992, 19, 719–724. [Google Scholar]
- Gourd, N.M.; Nikitas, N. Multiple Organ Dysfunction Syndrome. J. Intensive Care Med. 2020, 35, 1564–1575. [Google Scholar] [CrossRef]
- Fisher, B.; Jeong, J.H.; Dignam, J.; Anderson, S.; Mamounas, E.; Wickerham, D.L.; Wolmark, N. Findings from Recent National Surgical Adjuvant Breast and Bowel Project Adjuvant Studies in Stage I Breast Cancer. J. Natl. Cancer Inst. Monogr. 2001, 2001, 62–66. [Google Scholar] [CrossRef]
- Coniac, S.; Costache Outas, M.C.; Pirvu, E.-E.; Patru, R.-I.; Gainariu, E.; Aldea, C.; Iorga, P.G.; Ambroci, M.; Liscu, H.-D.; Miron, A.-I.; et al. Challenges and Limitations of Endocrine Toxicity Evaluation in Non-Small Cell Lung Cancer Patients Treated with Immunotherapy—Retrospective Study from a Tertiary-Level Hospital in Romania. Diagnostics 2023, 13, 1788. [Google Scholar] [CrossRef]
- Miron, A.-I.; Anghel, A.-V.; Barnonschi, A.-A.; Mitre, R.; Liscu, H.-D.; Găinariu, E.; Pătru, R.; Coniac, S. Real-World Outcomes of CDK4/6 Inhibitors Treatment in Metastatic Breast Cancer in Romania. Diagnostics 2023, 13, 1938. [Google Scholar] [CrossRef]
- Nathanson, S.D.; Detmar, M.; Padera, T.P.; Yates, L.R.; Welch, D.R.; Beadnell, T.C.; Scheid, A.D.; Wrenn, E.D.; Cheung, K. Mechanisms of Breast Cancer Metastasis. Clin. Exp. Metastasis 2022, 39, 117–137. [Google Scholar] [CrossRef]
- Park, K.U.; Chen, Y.; Chitale, D.; Choi, S.; Ali, H.; Nathanson, S.D.; Bensenhaver, J.; Proctor, E.; Petersen, L.; Loutfi, R.; et al. Utilization of the 21-Gene Recurrence Score in a Diverse Breast Cancer Patient Population: Development of a Clinicopathologic Model to Predict High-Risk Scores and Response to Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2018, 25, 1921–1927. [Google Scholar] [CrossRef]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; André, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Vodicka, E.; Kim, K.; Devine, E.B.; Gnanasakthy, A.; Scoggins, J.F.; Patrick, D.L. Inclusion of Patient-Reported Outcome Measures in Registered Clinical Trials: Evidence from ClinicalTrials.Gov (2007–2013). Contemp. Clin. Trials 2015, 43, 1–9. [Google Scholar] [CrossRef]
- Bottomley, A.; Reijneveld, J.C.; Koller, M.; Flechtner, H.; Tomaszewski, K.A.; Greimel, E. 5th EORTC Quality of Life in Cancer Clinical Trials Conference Faculty Current State of Quality of Life and Patient-Reported Outcomes Research. Eur. J. Cancer 2019, 121, 55–63. [Google Scholar] [CrossRef]
- Cherny, N.I.; Sullivan, R.; Dafni, U.; Kerst, J.M.; Sobrero, A.; Zielinski, C.; de Vries, E.G.E.; Piccart, M.J. A Standardised, Generic, Validated Approach to Stratify the Magnitude of Clinical Benefit That Can Be Anticipated from Anti-Cancer Therapies: The European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann. Oncol. 2015, 26, 1547–1573. [Google Scholar] [CrossRef]
- Mokhtari-Hessari, P.; Montazeri, A. Health-Related Quality of Life in Breast Cancer Patients: Review of Reviews from 2008 to 2018. Health Qual Life Outcomes 2020, 18, 338. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Reddy, C. Data Clustering: Algorithms and Applications, 1st ed.; Chapman and Hall/CRC: New York, NY, USA, 2014; p. 652. [Google Scholar] [CrossRef]
- Ristea, N.-C.; Miron, A.-I.; Savencu, O.; Georgescu, M.-I.; Verga, N.; Khan, F.S.; Ionescu, R.T. CyTran: A Cycle-Consistent Transformer with Multi-Level Consistency for Non-Contrast to Contrast CT Translation. Neurocomputing 2023, 538, 126211. [Google Scholar] [CrossRef]
- Georgescu, M.-I.; Ionescu, R.T.; Miron, A.-I.; Savencu, O.; Ristea, N.-C.; Verga, N.; Khan, F.S. Multimodal Multi-Head Convolutional Attention with Various Kernel Sizes for Medical Image Super-Resolution. In Proceedings of the 2023 IEEE/CVF Winter Conference on Applications of Computer Vision (WACV), Waikoloa, HI, USA, 2–7 January 2023; pp. 2195–2205. [Google Scholar]
- Georgescu, M.-I.; Ionescu, R.T.; Miron, A.I. Diversity-Promoting Ensemble for Medical Image Segmentation. In Proceedings of the Proceedings of the 38th ACM/SIGAPP Symposium on Applied Computing, Tallinn, Estonia, 27–31 March 2023; Association for Computing Machinery: New York, NY, USA, 2023; pp. 599–606. [Google Scholar]
- Ionescu, R.T.; Popescu, M. Learning Based on Similarity. In Knowledge Transfer between Computer Vision and Text Mining: Similarity-based Learning Approaches; Ionescu, R.T., Popescu, M., Eds.; Advances in Computer Vision and Pattern Recognition; Springer International Publishing: Cham, Switzerland, 2016; pp. 15–37. [Google Scholar]
- Soviany, P.; Ionescu, R.T.; Rota, P.; Sebe, N. Curriculum Learning: A Survey. Int. J. Comput. Vis. 2022, 130, 1526–1565. [Google Scholar] [CrossRef]
- Ward, J.H., Jr. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Devlin, J.; Chang, M.-W.; Lee, K.; Toutanova, K. BERT: Pre-Training of Deep Bidirectional Transformers for Language Understanding. In Proceedings of the 2019 Conference of the North American Chapter of the Association for Computational Linguistics: Human Language Technologies, Volume 1 (Long and Short Papers), Minneapolis, MN, USA, 2–7 June 2019; Burstein, J., Doran, C., Solorio, T., Eds.; Association for Computational Linguistics: Minneapolis, MN, USA, 2019; pp. 4171–4186. [Google Scholar]
- BERT. Available online: https://huggingface.co/docs/transformers/model_doc/bert (accessed on 16 December 2023).
- Scarlat, F.; Scarisoreanu, A.; Verga, N. Absorbed dose distributions using the isodensitometric method for exposures with filter employed for mammographies. Rom. Rep. Phys. 2013, 65, 168–177. [Google Scholar]
- Alcamo, A.M.; Pang, D.; Bashir, D.A.; Carcillo, J.A.; Nguyen, T.C.; Aneja, R.K. Role of Damage-Associated Molecular Patterns and Uncontrolled Inflammation in Pediatric Sepsis-Induced Multiple Organ Dysfunction Syndrome. J. Pediatr. Intensive Care 2019, 8, 25–31. [Google Scholar] [CrossRef]
- Ferreira, A.M.P.; Sakr, Y. Organ Dysfunction: General Approach, Epidemiology, and Organ Failure Scores. Semin. Respir. Crit. Care Med. 2011, 32, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of Severe Sepsis in the United States: Analysis of Incidence, Outcome, and Associated Costs of Care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef]
- Hawari, F.I.; Nazer, L.H.; Addassi, A.; Rimawi, D.; Jamal, K. Predictors of ICU Admission in Patients with Cancer and the Related Characteristics and Outcomes: A 5-Year Registry-Based Study. Crit. Care Med. 2016, 44, 548–553. [Google Scholar] [CrossRef]
- de Montmollin, E.; Tandjaoui-Lambiotte, Y.; Legrand, M.; Lambert, J.; Mokart, D.; Kouatchet, A.; Lemiale, V.; Pène, F.; Bruneel, F.; Vincent, F.; et al. Outcomes in critically ill cancer patients with septic shock of pulmonary origin. Shock 2013, 39, 250–254. [Google Scholar] [CrossRef]
- Cheng, Z.; Abrams, S.T.; Toh, J.; Wang, S.S.; Wang, Z.; Yu, Q.; Yu, W.; Toh, C.-H.; Wang, G. The Critical Roles and Mechanisms of Immune Cell Death in Sepsis. Front. Immunol. 2020, 11, 1918. [Google Scholar] [CrossRef] [PubMed]
- Puxty, K.; McLoone, P.; Quasim, T.; Sloan, B.; Kinsella, J.; Morrison, D.S. Risk of Critical Illness Among Patients With Solid Cancers: A Population-Based Observational Study. JAMA Oncol. 2015, 1, 1078–1085. [Google Scholar] [CrossRef]
- Bos, M.M.E.M.; Verburg, I.W.M.; Dumaij, I.; Stouthard, J.; Nortier, J.W.R.; Richel, D.; van der Zwan, E.P.A.; de Keizer, N.F.; de Jonge, E. Intensive Care Admission of Cancer Patients: A Comparative Analysis. Cancer Med. 2015, 4, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, F.G.; Romano, T.G.; Salluh, J.I.F.; Taniguchi, L.U.; Mendes, P.V.; Nassar, A.P.; Costa, R.; Viana, W.N.; Maia, M.O.; Lima, M.F.A.; et al. Trends in Clinical Profiles, Organ Support Use and Outcomes of Patients with Cancer Requiring Unplanned ICU Admission: A Multicenter Cohort Study. Intensive Care Med. 2021, 47, 170–179. [Google Scholar] [CrossRef]
- Champigneulle, B.; Merceron, S.; Lemiale, V.; Geri, G.; Mokart, D.; Bruneel, F.; Vincent, F.; Perez, P.; Mayaux, J.; Cariou, A.; et al. What is the outcome of cancer patients admitted to the ICU after cardiac arrest? Results from a multicenter study. Resuscitation 2015, 92, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-U.; Suh, G.Y.; Chung, M.P.; Kim, H.; Kwon, O.J.; Jung, C.W.; Kang, W.K.; Park, K.; Jeon, K. Risk Factors to Predict Outcome in Critically Ill Cancer Patients Receiving Chemotherapy in the Intensive Care Unit. Support. Care Cancer 2011, 19, 491–495. [Google Scholar] [CrossRef]
- Vigneron, C.; Charpentier, J.; Coussy, F.; Alexandre, J.; Pène, F.; Jamme, M. When Breast Cancer Comes to the ICU: Outcomes and Prognostic Factors. Acta Oncol. 2023, 62, 358–363. [Google Scholar] [CrossRef]
- Mizock, B. The Multiple Organ Dysfunction Syndrome. Dis.-a-Mon. DM 2009, 55, 476–526. [Google Scholar] [CrossRef]
- Barie, P.S.; Hydo, L.J. Epidemiology of Multiple Organ Dysfunction Syndrome in Critical Surgical Illness. Surg. Infect. 2000, 1, 173–186. [Google Scholar] [CrossRef]
- Aagaard, T.; Reekie, J.; Jørgensen, M.; Roen, A.; Daugaard, G.; Specht, L.; Sengeløv, H.; Mocroft, A.; Lundgren, J.; Helleberg, M. Mortality and Admission to Intensive Care Units after Febrile Neutropenia in Patients with Cancer. Cancer Med. 2020, 9, 3033–3042. [Google Scholar] [CrossRef] [PubMed]
- Brass, N.J. Predisposition to Multiple Organ Dysfunction. Crit. Care Nurs. Q. 1994, 16, 1–7. [Google Scholar] [CrossRef]
- Salluh, J.I.F.; Soares, M.; De Meis, E. Antiphospholipid Antibodies and Multiple Organ Failure in Critically Ill Cancer Patients. Clinics 2009, 64, 79–82. [Google Scholar] [CrossRef][Green Version]
- Azoulay, E.; Lemiale, V.; Mokart, D.; Pène, F.; Kouatchet, A.; Perez, P.; Vincent, F.; Mayaux, J.; Benoit, D.; Bruneel, F.; et al. Acute Respiratory Distress Syndrome in Patients with Malignancies. Intensive Care Med. 2014, 40, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Beal, A.L. Multiple Organ Failure Syndrome in the 1990s: Systemic Inflammatory Response and Organ Dysfunction. JAMA 1994, 271, 226. [Google Scholar] [CrossRef]
- Caruso, P.; Ferreira, A.C.; Laurienzo, C.E.; Titton, L.N.; Terabe, D.S.M.; Carnieli, D.S.; Deheinzelin, D. Short- and Long-Term Survival of Patients with Metastatic Solid Cancer Admitted to the Intensive Care Unit: Prognostic Factors. Eur. J. Cancer Care 2010, 19, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Tabouret, E.; Boucard, C.; Devillier, R.; Barrie, M.; Boussen, S.; Autran, D.; Chinot, O.; Bruder, N. Neuro-Oncological Patients Admitted in Intensive-Care Unit: Predictive Factors and Functional Outcome. J. Neurooncol 2016, 127, 111–117. [Google Scholar] [CrossRef]
- Blot, F.; Guiguet, M.; Nitenberg, G.; Leclercq, B.; Gachot, B.; Escudier, B. Prognostic Factors for Neutropenic Patients in an Intensive Care Unit: Respective Roles of Underlying Malignancies and Acute Organ Failures. Eur. J. Cancer 1997, 33, 1031–1037. [Google Scholar] [CrossRef]
- Headley, J.; Theriault, R.; Smith, T.L. Independent Validation of APACHE II Severity of Illness Score for Predicting Mortality in Patients with Breast Cancer Admitted to the Intensive Care Unit. Cancer 1992, 70, 497–503. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Asim, M.; Amin, F.; El-Menyar, A. Multiple Organ Dysfunction Syndrome: Contemporary Insights on the Clinicopathological Spectrum. Qatar Med. J. 2020, 2020, 22. [Google Scholar] [CrossRef]
- Marshall, J.C. Inflammation, Coagulopathy, and the Pathogenesis of Multiple Organ Dysfunction Syndrome. Crit. Care Med. 2001, 29, S99–S106. [Google Scholar] [CrossRef] [PubMed]
- Ader, R.; Cohen, N.; Felten, D. Psychoneuroimmunology: Interactions between the Nervous System and the Immune System. Lancet 1995, 345, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, S. The Cytokine Storm and Factors Determining the Sequence and Severity of Organ Dysfunction in Multiple Organ Dysfunction Syndrome. Am. J. Emerg. Med. 2008, 26, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Osterbur, K.; Mann, F.A.; Kuroki, K.; DeClue, A. Multiple Organ Dysfunction Syndrome in Humans and Animals. J. Vet. Intern. Med. 2014, 28, 1141–1151. [Google Scholar] [CrossRef]
- Moore, F.A.; Moore, E.E. Evolving Concepts in the Pathogenesis of Postinjury Multiple Organ Failure. Surg. Clin. N. Am. 1995, 75, 257–277. [Google Scholar] [CrossRef]
- Groza, A.; Iconaru, S.L.; Jiga, G.; Chapon, P.; Gaiaschi, S.; Verga, N.; Beuran, M.; Prodan, A.M.; Matei, M.; Marinescu, S.A.; et al. The Effect of the Ionizing Radiation on Hydroxyapatite–Polydimethylsiloxane Layers. Polym. Eng. Sci. 2019, 59, 2406–2412. [Google Scholar] [CrossRef]
- Eppensteiner, J.; Kwun, J.; Scheuermann, U.; Barbas, A.; Limkakeng, A.T.; Kuchibhatla, M.; Elster, E.A.; Kirk, A.D.; Lee, J. Damage- and Pathogen-Associated Molecular Patterns Play Differential Roles in Late Mortality after Critical Illness. JCI Insight 2019, 4, e127925. [Google Scholar] [CrossRef]
- Brown, K.A.; Brain, S.D.; Pearson, J.D.; Edgeworth, J.D.; Lewis, S.M.; Treacher, D.F. Neutrophils in Development of Multiple Organ Failure in Sepsis. Lancet 2006, 368, 157–169. [Google Scholar] [CrossRef]
- Agarwal, H.; Reddy, S.; Sharma, K. Aberrant Cellular Signaling in Multiple Organ Failure: Mechanism, Consequences and Therapeutic Applications. J. Drug Deliv. Ther. 2014, 4, 86–92. [Google Scholar] [CrossRef]
- Surbatovic, M.; Veljovic, M.; Jevdjic, J.; Popovic, N.; Djordjevic, D.; Radakovic, S. Immunoinflammatory Response in Critically Ill Patients: Severe Sepsis and/or Trauma. Mediat. Inflamm. 2013, 2013, 362793. [Google Scholar] [CrossRef] [PubMed]
- Andruszkow, H.; Fischer, J.; Sasse, M.; Brunnemer, U.; Andruszkow, J.H.K.; Gänsslen, A.; Hildebrand, F.; Frink, M. Interleukin-6 as Inflammatory Marker Referring to Multiple Organ Dysfunction Syndrome in Severely Injured Children. Scand. J. Trauma Resusc. Emerg. Med. 2014, 22, 16. [Google Scholar] [CrossRef]
- Jaffer, U.; Wade, R.G.; Gourlay, T. Cytokines in the Systemic Inflammatory Response Syndrome: A Review. HSR Proc. Intensive Care Cardiovasc. Anesth. 2010, 2, 161–175. [Google Scholar]
- Qiu, P.; Cui, X.; Sun, J.; Welsh, J.; Natanson, C.; Eichacker, P.Q. Antitumor Necrosis Factor Therapy Is Associated with Improved Survival in Clinical Sepsis Trials: A Meta-Analysis. Crit. Care Med. 2013, 41, 2419–2429. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.V.; Grillari, J. Pathogenesis of Multiple Organ Failure: The Impact of Systemic Damage to Plasma Membranes. Front. Med. 2022, 9, 806462. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, J.; Zarbock, A. Pathogenesis of Multiple Organ Failure in Sepsis. Crit. Rev. Immunol. 2015, 35, 277–291. [Google Scholar] [CrossRef]
- Spapen, H.D.; Jacobs, R.; Honoré, P.M. Sepsis-Induced Multi-Organ Dysfunction Syndrome—A Mechanistic Approach. J. Emerg. Crit. Care Med. 2017, 1, 27. [Google Scholar] [CrossRef]
- Larche, J.; Lancel, S.; Hassoun, S.M.; Favory, R.; Decoster, B.; Marchetti, P.; Chopin, C.; Neviere, R. Inhibition of Mitochondrial Permeability Transition Prevents Sepsis-Induced Myocardial Dysfunction and Mortality. J. Am. Coll. Cardiol. 2006, 48, 377–385. [Google Scholar] [CrossRef]
- Zou, R.; Tao, J.; Qiu, J.; Lu, H.; Wu, J.; Zhu, H.; Li, R.; Mui, D.; Toan, S.; Chang, X.; et al. DNA-PKcs promotes sepsis-induced multiple organ failure by triggering mitochondrial dysfunction. J. Adv. Res. 2022, 41, 39–48. [Google Scholar] [CrossRef]
- Fisher, C.J.; Agosti, J.M.; Opal, S.M.; Lowry, S.F.; Balk, R.A.; Sadoff, J.C.; Abraham, E.; Schein, R.M.; Benjamin, E. Treatment of Septic Shock with the Tumor Necrosis Factor Receptor:Fc Fusion Protein. The Soluble TNF Receptor Sepsis Study Group. N. Engl. J. Med. 1996, 334, 1697–1702. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Abrams, S.T.; Morton, B.; Alhamdi, Y.; Alsabani, M.; Lane, S.; Welters, I.D.; Wang, G.; Toh, C.H. A Novel Assay for Neutrophil Extracellular Trap Formation Independently Predicts Disseminated Intravascular Coagulation and Mortality in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2019, 200, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Abrams, S.T.; Alhamdi, Y.; Toh, J.; Yu, W.; Wang, G.; Toh, C.-H. Circulating Histones Are Major Mediators of Multiple Organ Dysfunction Syndrome in Acute Critical Illnesses. Crit. Care Med. 2019, 47, e677–e684. [Google Scholar] [CrossRef]
- Cedervall, J.; Zhang, Y.; Olsson, A.-K. Tumor-Induced NETosis as a Risk Factor for Metastasis and Organ Failure. Cancer Res. 2016, 76, 4311–4315. [Google Scholar] [CrossRef]
- Chen, P. Gut Microbiota and Pathogenesis of Organ Injury, 1st ed.; Springer: Singapore, 2020; pp. 195–202. [Google Scholar] [CrossRef]
- Petca, A.; Miron, B.C.; Pacu, I.; Dumitrașcu, M.C.; Mehedințu, C.; Șandru, F.; Petca, R.-C.; Rotar, I.C. HELLP Syndrome-Holistic Insight into Pathophysiology. Medicina 2022, 58, 326. [Google Scholar] [CrossRef] [PubMed]
- Sandru, F.; Petca, R.-C.; Costescu, M.; Dumitrașcu, M.C.; Popa, A.; Petca, A.; Miulescu, R.-G. Cutaneous Mastocytosis in Childhood—Update from the Literature. J. Clin. Med. 2021, 10, 1474. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, H. Immune Intervention in Sepsis. Front. Pharmacol. 2021, 12, 718089. [Google Scholar] [CrossRef]
- Teodorescu, C.O.D.; Șandru, F.; Charkaoui, A.; Teodorescu, A.; Popa, A.R.; Miron, A.-I. The Dynamic Changes in the Pattern of Liver Function Tests in Pregnant Obese Women. Exp. Ther. Med. 2021, 22, 986. [Google Scholar] [CrossRef]
- Sauaia, A.; Moore, F.A.; Moore, E.E. Postinjury Inflammation and Organ Dysfunction. Crit. Care Clin. 2017, 33, 167–191. [Google Scholar] [CrossRef]
- Azevedo, L.C.P.; Caruso, P.; Silva, U.V.A.; Torelly, A.P.; Silva, E.; Rezende, E.; Netto, J.J.; Piras, C.; Lobo, S.M.A.; Knibel, M.F.; et al. Outcomes for Patients with Cancer Admitted to the ICU Requiring Ventilatory Support: Results from a Prospective Multicenter Study. Chest 2014, 146, 257–266. [Google Scholar] [CrossRef]
- Sandru, F.; Petca, A.; Dumitrascu, M.C.; Petca, R.C.; Carsote, M. Peutz-Jeghers syndrome: Skin manifestations and endocrine anomalies (Review). Exp. Ther. Med. 2021, 22, 1387. [Google Scholar] [CrossRef] [PubMed]
- Greco, E.; Lupia, E.; Bosco, O.; Vizio, B.; Montrucchio, G. Platelets and Multi-Organ Failure in Sepsis. Int. J. Mol. Sci. 2017, 18, 2200. [Google Scholar] [CrossRef] [PubMed]
- Deitch, E.A. Multiple Organ Failure. Pathophysiology and Potential Future Therapy. Ann. Surg. 1992, 216, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Clarijs, M.E.; Thurell, J.; Kühn, F.; Uyl-de Groot, C.A.; Hedayati, E.; Karsten, M.M.; Jager, A.; Koppert, L.B. Measuring Quality of Life Using Patient-Reported Outcomes in Real-World Metastatic Breast Cancer Patients: The Need for a Standardized Approach. Cancers 2021, 13, 2308. [Google Scholar] [CrossRef]
- de Ligt, K.M.; de Rooij, B.H.; Hedayati, E.; Karsten, M.M.; Smaardijk, V.R.; Velting, M.; Saunders, C.; Travado, L.; Cardoso, F.; Lopez, E.; et al. International Development of a Patient-Centered Core Outcome Set for Assessing Health-Related Quality of Life in Metastatic Breast Cancer Patients. Breast Cancer Res. Treat. 2023, 198, 265–281. [Google Scholar] [CrossRef]
- Liscu, H.-D.; Liscu, B.-R.; Mitre, R.; Anghel, I.-V.; Antone-Iordache, I.-L.; Balan, A.; Coniac, S.; Miron, A.-I.; Halcu, G. The Conditioning of Adjuvant Chemotherapy for Stage II and III Rectal Cancer Determined by Postoperative Pathological Characteristics in Romania. Medicina 2023, 59, 1224. [Google Scholar] [CrossRef]
- Keane, D.; Phillips, G.; Mitchell, N.; Connolly, R.M.; Hegarty, J. Improving Quality of Life and Symptom Experience in Patients with Metastatic Breast Cancer: A Systematic Review of Supportive Care Interventions. Psychooncology 2023, 32, 1192–1207. [Google Scholar] [CrossRef]
- Mertz, S.; Benjamin, C.; Girvalaki, C.; Cardone, A.; Gono, P.; May, S.G.; Comerford, E.; Than, K.-S.; Birch, K.; Roach, M.; et al. Progression-Free Survival and Quality of Life in Metastatic Breast Cancer: The Patient Perspective. Breast 2022, 65, 84–90. [Google Scholar] [CrossRef]
- Lima, E.d.O.L.; Silva, M.M. da Quality of Life of Women with Locally Advanced or Metastatic Breast Cancer. Rev. Gauch. Enferm. 2020, 41, e20190292. [Google Scholar] [CrossRef]
- Cocks, K.; Wells, J.R.; Johnson, C.; Schmidt, H.; Koller, M.; Oerlemans, S.; Velikova, G.; Pinto, M.; Tomaszewski, K.A.; Aaronson, N.K.; et al. Content Validity of the EORTC Quality of Life Questionnaire QLQ-C30 for Use in Cancer. Eur. J. Cancer 2023, 178, 128–138. [Google Scholar] [CrossRef]
- Bjelic-Radisic, V.; Cardoso, F.; Cameron, D.; Brain, E.; Kuljanic, K.; da Costa, R.A.; Conroy, T.; Inwald, E.C.; Serpentini, S.; Pinto, M.; et al. An International Update of the EORTC Questionnaire for Assessing Quality of Life in Breast Cancer Patients: EORTC QLQ-BR45. Ann. Oncol. 2020, 31, 283–288. [Google Scholar] [CrossRef]
- Brady, M.J.; Cella, D.F.; Mo, F.; Bonomi, A.E.; Tulsky, D.S.; Lloyd, S.R.; Deasy, S.; Cobleigh, M.; Shiomoto, G. Reliability and Validity of the Functional Assessment of Cancer Therapy-Breast Quality-of-Life Instrument. J. Clin. Oncol. 1997, 15, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-Item Short-Form Health Survey (SF-36). I. Conceptual Framework and Item Selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Jianu, D.M.; Marin, A. Invited Discussion on: Evaluation of Chlorhexidine Concentration on the Skin After Preoperative Surgical Site Preparation in Breast Surgery-A Randomized Controlled Trial. Aesthetic Plast. Surg. 2022, 46, 1523–1524. [Google Scholar] [CrossRef] [PubMed]
- Destrebecq, V.; Lieveke, A.; Berghmans, T.; Paesmans, M.; Sculier, J.-P.; Meert, A.-P. Are Intensive Cares Worthwhile for Breast Cancer Patients: The Experience of an Oncological ICU. Front. Med. 2016, 3, 50. [Google Scholar] [CrossRef][Green Version]
- Moscetti, L.; Sperduti, I.; Frassoldati, A.; Musolino, A.; Nasso, C.; Toss, A.; Omarini, C.; Dominici, M.; Piacentini, F. Quality of Life of Therapies for Hormone Receptor Positive Advanced/Metastatic Breast Cancer: Regulatory Aspects and Clinical Impact in Europe. Breast 2021, 59, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Hamer, J.; McDonald, R.; Zhang, L.; Verma, S.; Leahey, A.; Ecclestone, C.; Bedard, G.; Pulenzas, N.; Bhatia, A.; Chow, R.; et al. Quality of Life (QOL) and Symptom Burden (SB) in Patients with Breast Cancer. Support. Care Cancer 2017, 25, 409–419. [Google Scholar] [CrossRef]
- Biskup, E.; Cai, F.; Vetter, M.; Marsch, S. Oncological Patients in the Intensive Care Unit: Prognosis, Decision-Making, Therapies and End-of-Life Care. Swiss Med. Wkly. 2017, 147, w14481. [Google Scholar] [CrossRef]
- O’Shaughnessy, J. Extending Survival with Chemotherapy in Metastatic Breast Cancer. Oncologist 2005, 10 (Suppl. S3), 20–29. [Google Scholar] [CrossRef]
- Reshma, I.A.; Franchet, C.; Gaspard, M.; Ionescu, R.T.; Mothe, J.; Cussat-Blanc, S.; Luga, H.; Brousset, P. Finding a Suitable Class Distribution for Building Histological Images Datasets Used in Deep Model Training—The Case of Cancer Detection. J. Digit. Imaging 2022, 35, 1326–1349. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J.; Coursin, D.B. Multiple Organ Dysfunction Syndrome. Yale J. Biol. Med. 1993, 66, 501–510. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, A.-I.; Atasiei, D.-I.; Ionescu, R.-T.; Ultimescu, F.; Barnonschi, A.-A.; Anghel, A.-V.; Anghel, C.-A.; Antone-Iordache, I.-L.; Mitre, R.; Bobolocu, A.M.; et al. Prediction of Subclinical and Clinical Multiple Organ Failure Dysfunction in Breast Cancer Patients—A Review Using AI Tools. Cancers 2024, 16, 381. https://doi.org/10.3390/cancers16020381
Ionescu A-I, Atasiei D-I, Ionescu R-T, Ultimescu F, Barnonschi A-A, Anghel A-V, Anghel C-A, Antone-Iordache I-L, Mitre R, Bobolocu AM, et al. Prediction of Subclinical and Clinical Multiple Organ Failure Dysfunction in Breast Cancer Patients—A Review Using AI Tools. Cancers. 2024; 16(2):381. https://doi.org/10.3390/cancers16020381
Chicago/Turabian StyleIonescu (Miron), Andreea-Iuliana, Dimitrie-Ionut Atasiei, Radu-Tudor Ionescu, Flavia Ultimescu, Andrei-Alexandru Barnonschi, Alexandra-Valentina Anghel, Cătălin-Alexandru Anghel, Ionuț-Lucian Antone-Iordache, Ruxandra Mitre, Alexandra Maria Bobolocu, and et al. 2024. "Prediction of Subclinical and Clinical Multiple Organ Failure Dysfunction in Breast Cancer Patients—A Review Using AI Tools" Cancers 16, no. 2: 381. https://doi.org/10.3390/cancers16020381
APA StyleIonescu, A.-I., Atasiei, D.-I., Ionescu, R.-T., Ultimescu, F., Barnonschi, A.-A., Anghel, A.-V., Anghel, C.-A., Antone-Iordache, I.-L., Mitre, R., Bobolocu, A. M., Zamfir, A., Lișcu, H.-D., Coniac, S., & Șandru, F. (2024). Prediction of Subclinical and Clinical Multiple Organ Failure Dysfunction in Breast Cancer Patients—A Review Using AI Tools. Cancers, 16(2), 381. https://doi.org/10.3390/cancers16020381











