Risk Factors for Bleeding Events in Japanese Patients with Advanced Lung Cancer: Data from the Rising-VTE/NEJ037 Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
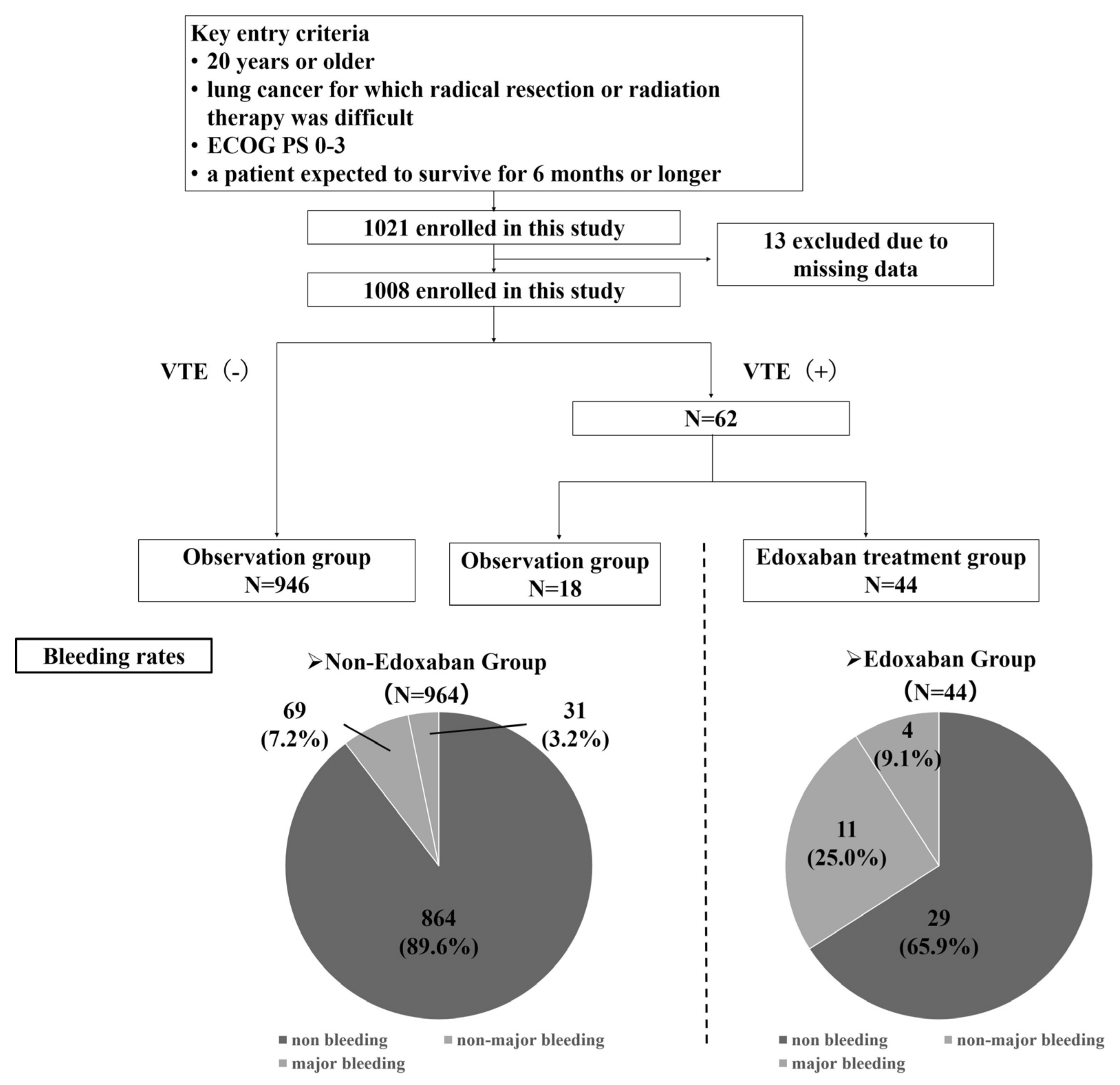
2.1. Patients
2.2. Bleeding Events
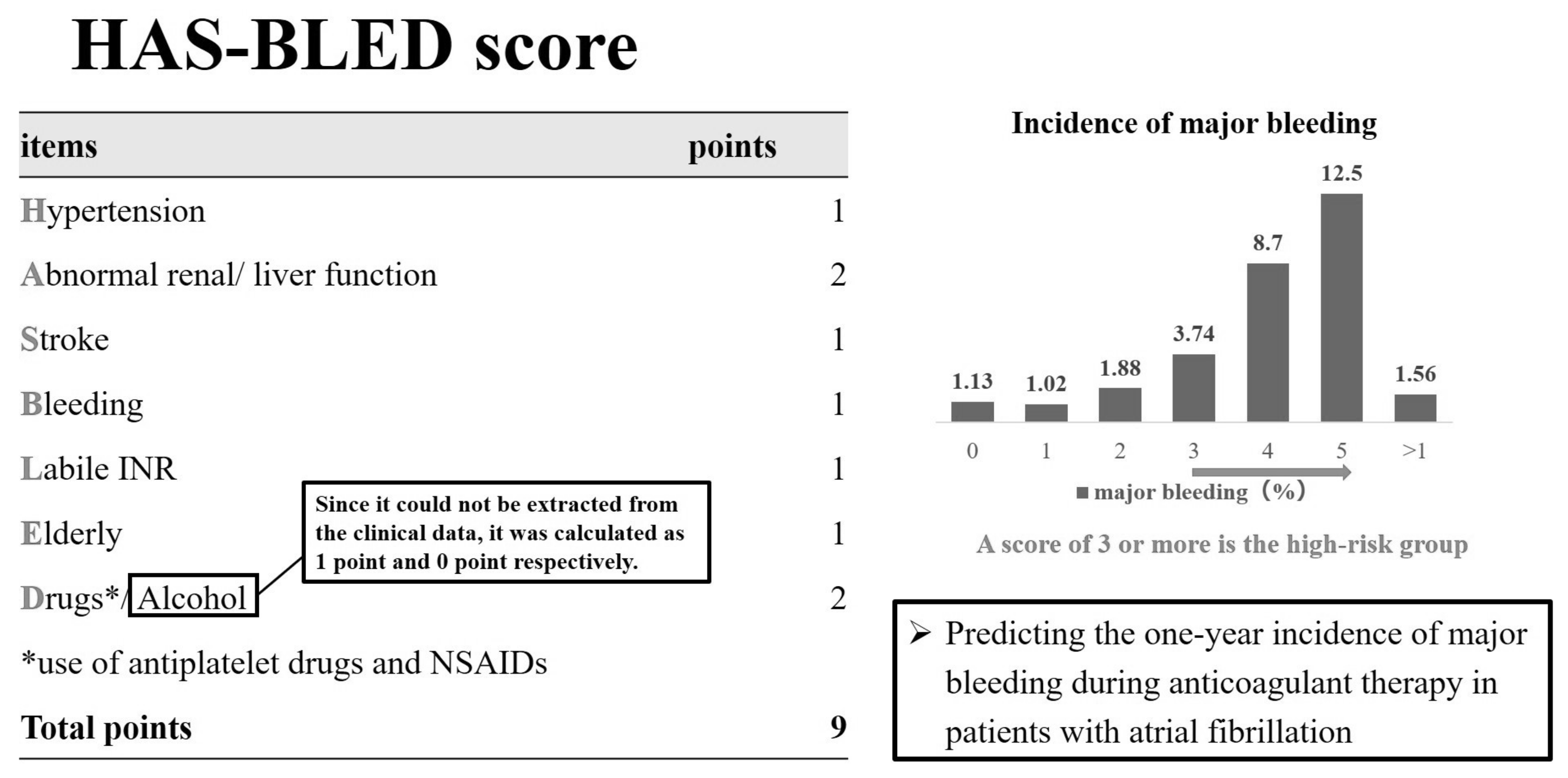
2.3. Hypertension, Abnormal Liver/Renal Function, Stroke History, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly (HAS-BLED) Score
2.4. Venous Thromboembolic Disease and Bleeding (VTE-BLEED) Score
2.5. Statistical Analyses
3. Results
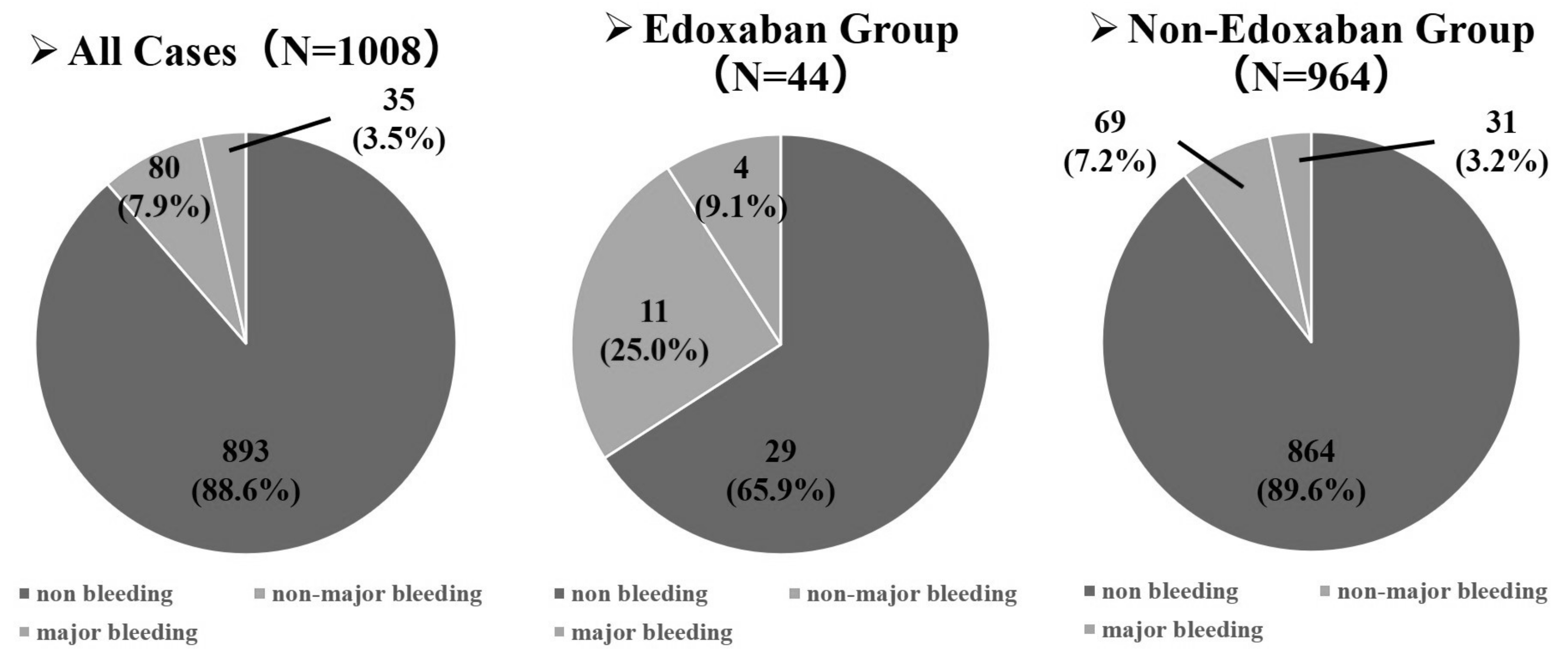
3.1. Patients’ Characteristics and Bleeding Events
3.2. Bleeding Site
3.3. Multivariate Analysis
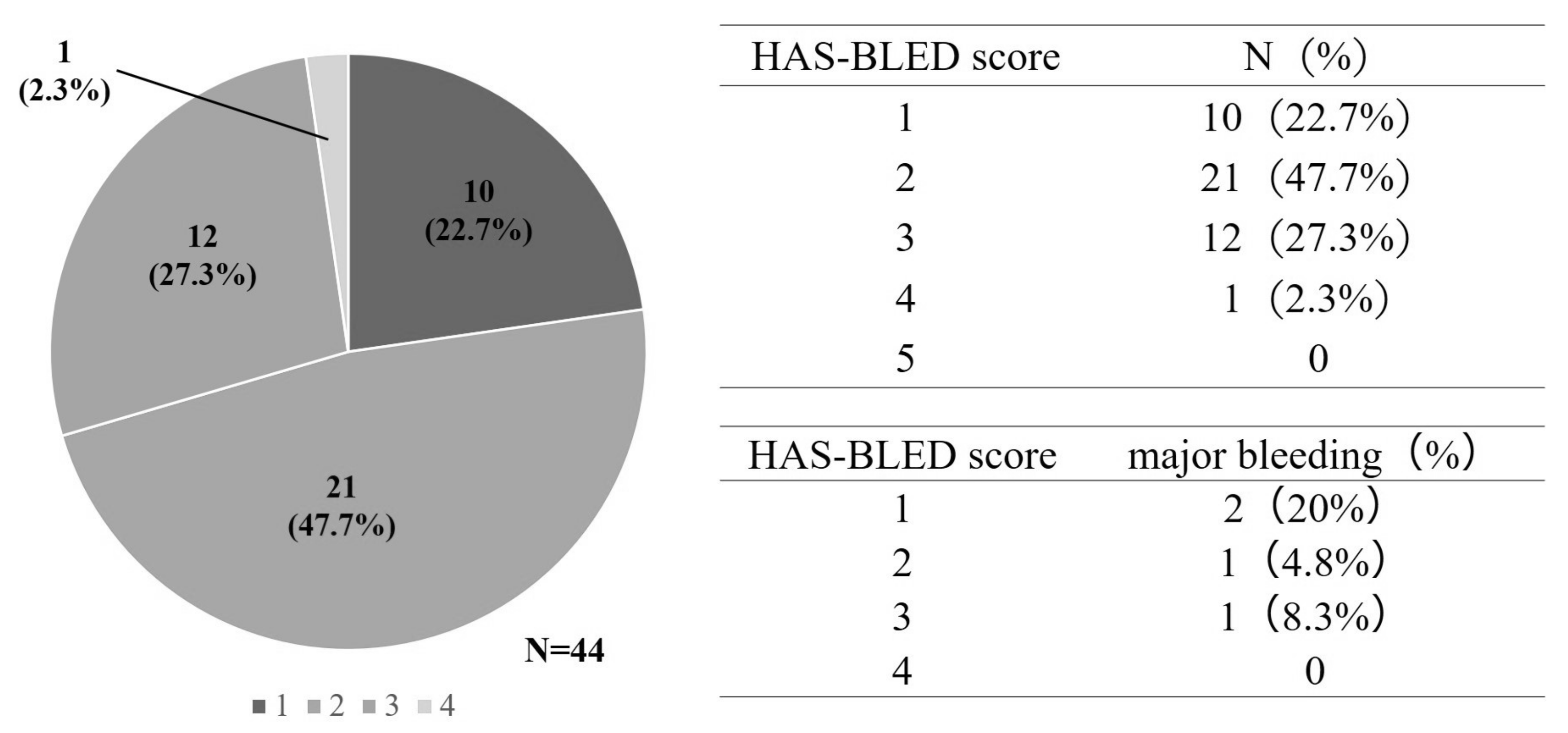
3.4. HAS-BLED Score
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J. Thromb. Haemost. 2007, 5, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Timp, J.F.; Braekkan, S.K.; Versteeg, H.H.; Cannegieter, S.C. Epidemiology of cancer-associated venous thrombosis. Blood 2013, 122, 1712–1723. [Google Scholar] [CrossRef] [PubMed]
- Ay, C.; Pabinger, I.; Cohen, A.T. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb. Haemost. 2017, 117, 219–230. [Google Scholar] [CrossRef]
- Elyamany, G.; Alzahrani, A.M.; Bukhary, E. Cancer-associated thrombosis: An overview. Clin. Med. Insights Oncol. 2014, 8, 129–137. [Google Scholar] [CrossRef]
- Moore, R.A.; Adel, N.; Riedel, E.; Bhutani, M.; Feldman, D.R.; Tabbara, N.E.; Soff, G.; Parameswaran, R.; Hassoun, H. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: A large retrospective analysis. J. Clin. Oncol. 2011, 29, 3466–3473. [Google Scholar] [CrossRef]
- Fernandes, C.J.; Morinaga, L.T.K.; Alves, J.L.; Castro, M.A.; Calderaro, D.; Jardim, C.V.P.; Souza, R. Cancer-associated thrombosis: The when, how and why. Eur. Respir. Rev. 2019, 28, 180119. [Google Scholar] [CrossRef]
- Heit, J.A.; Spencer, F.A.; White, R.H. The epidemiology of venous thromboembolism. J. Thromb. Thrombolysis 2016, 41, 3–14. [Google Scholar] [CrossRef]
- Laporte, S.; Mismetti, P.; Décousus, H.; Uresandi, F.; Otero, R.; Lobo, J.L. Clinical predictors for fatal pulmonary embolism in 15,520 patients with venous thromboembolism: Findings from the Registro Informatizado de la Enfermedad TromboEmbolica venosa (RIETE). Regist. Circ. 2008, 117, 1711–1716. [Google Scholar] [CrossRef]
- Qi, Y.; Hu, X.; Chen, J.; Ying, X.; Shi, Y. The risk factors of VTE and survival prognosis of patients with malignant cancer: Implication for nursing and treatment. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620971053. [Google Scholar] [CrossRef]
- Farge, D.; Debourdeau, P.; Beckers, M.; Baglin, C.; Bauersachs, R.M.; Brenner, B.; Brilhante, D.; Falanga, A.; Gerotzafias, G.T.; Büller, H.R.; et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J. Thromb. Haemost. 2013, 11, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Levine, M.N.; Baker, R.I.; Bowden, C.; Kakkar, A.K.; Prins, M.; Rickles, F.R.; Julian, J.A.; Haley, S.; Kovacs, M.J.; et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N. Engl. J. Med. 2003, 349, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.Y.; Kamphuisen, P.W.; Meyer, G.; Bauersachs, R.; Janas, M.S.; Jarner, M.F.; Khorana, A.A. CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: A randomized clinical trial. JAMA 2015, 314, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Bohlke, K.; Falanga, A.; American Society of Clinical Oncology. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J. Oncol. Pract. 2015, 11, e442–e444. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef] [PubMed]
- Van Es, N.; Coppens, M.; Schulman, S.; Middeldorp, S.; Büller, H.R. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: Evidence from phase 3 trials. Blood 2014, 124, 1968–1975. [Google Scholar] [CrossRef] [PubMed]
- Bleker, S.M.A.; Brekelmans, M.P.A.; Eerenberg, E.S.; Cohen, A.T.; Middeldorp, S.; Raskob, G.; Büller, H.R. Clinical impact of major bleeding in patients with venous thromboembolism treated with factor Xa inhibitors or vitamin K antagonists: An individual patient data meta-analysis. Thromb. Haemost. 2017, 117, 1944–1951. [Google Scholar] [PubMed]
- Stefano, B.; Elisa, R.; Dimitriy, A.; Valeria, C.; Alessandra, M.; Afroditi, A.; Maria, D.G.; Cinzia, C.; Marco, M.; Mariano, C. Risk and Management of Bleeding Complications with Direct Oral Anticoagulants in Patients with Atrial Fibrillation and Venous Thromboembolism: A Narrative Review. Adv. Ther. 2023, 40, 41–66. [Google Scholar]
- Raskob, G.E.; van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F.; et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N. Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef]
- Tsubata, Y.; Hotta, T.; Hamai, K.; Furuya, N.; Yokoyama, T.; Saito, R.; Nakamura, A.; Masuda, T.; Hamaguchi, M.; Kuyama, S.; et al. Incidence of venous thromboembolism in advanced lung cancer and efficacy and safety of direct oral anticoagulants: A multicenter, prospective, observational study (Rising-VTE/NEJ037 study). Ther. Adv. Med. Oncol. 2022, 14, 17588359221110171. [Google Scholar] [CrossRef]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; de Vos, C.B.; Crijns, H.J.; Lip, G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The euro Heart Survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Hösel, V.; Clemens, A.; Yollo, W.D.; Tilke, C.; Schulman, S.; Lankeit, M.; Konstantinides, S.V. Prediction of bleeding events in patients with venous thromboembolism on stable anticoagulation treatment. Eur. Respir. J. 2016, 48, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; O’Connell, C.; Agnelli, G.; Liebman, H.A.; Lee, A.Y.; Subcommittee on Hemostasis and Malignancy of the SSC of the ISTH. Incidental venous thromboembolism in oncology patients. J. Thromb. Haemost. 2012, 10, 2602–2604. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Crowley, J.; Chansky, K.; Giroux, D.J.; Groome, P.A.; Rami-Porta, R.; Postmus, P.E.; Rusch, V.; Sobin, L.; International Association for the Study of Lung Cancer International Staging Committee; et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J. Thorac. Oncol. 2007, 2, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Huisman, M.V. How I assess and manage the risk of bleeding in patients treated for venous thromboembolism. Blood 2020, 135, 724–734. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, C.; Escalante, C.P.; Goldhaber, S.Z.; McBane, R.; Connors, J.M.; Raskob, G.E. Treatment of Cancer-Associated Venous Thromboembolism with Low-Molecular-Weight Heparin or Direct Oral Anticoagulants: Patient Selection, Controversies, and Caveats. Oncologist 2021, 26, e8–e16. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Carrier, M.; Ay, C.; Di Nisio, M.; Hicks, L.K.; Khorana, A.A.; Leavitt, A.D.; Lee, A.Y.Y.; Macbeth, F.; Morgan, R.L.; et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Adv. 2021, 5, 927–974. [Google Scholar] [CrossRef] [PubMed]
- Chee, C.E.; Ashrani, A.A.; Marks, R.S.; Petterson, T.M.; Bailey, K.R.; Melton, L.J., 3rd; Heit, J.A. Predictors of venous thromboembolism recurrence and bleeding among active cancer patients: A population-based cohort study. Blood 2014, 123, 3972–3978. [Google Scholar] [CrossRef]
- Ohashi, Y.; Ikeda, M.; Kunitoh, H.; Sasako, M.; Okusaka, T.; Mukai, H.; Fujiwara, K.; Nakamura, M.; Oba, M.S.; Kimura, T.; et al. One-year incidence of venous thromboembolism, bleeding, and death in patients with solid tumors newly initiating cancer treatment: Results from the Cancer-VTE Registry. Thromb. Res. 2022, 213, 203–213. [Google Scholar] [CrossRef]
- Hotta, T.; Tsubata, Y.; Hamai, K.; Tanino, A.; Kobayashi, M.; Nakamura, A.; Sugisaka, J.; Hongoh, M.; Ishihara, N.; Ishikawa, N.; et al. Pharmacokinetics of edoxaban in EGFR-mutated non-small cell lung cancer patients with venous thromboembolism. Respir. Investig. 2021, 59, 327–334. [Google Scholar] [CrossRef]


| All n = 1008 | All n = 1008 | With Hemorrhagic Events n = 115 | Without Hemorrhagic Events n = 893 |
|---|---|---|---|
| Age [years] | |||
| Median | 70 | 71 | 71 |
| Range | 30–94 | 44–88 | 30–94 |
| Sex [%] | |||
| Male | 714 [70.8] | 96 [83.5] | 618 [69.2] |
| Female | 294 [29.2] | 19 [16.5] | 275 [30.8] |
| ECOG PS [%] | |||
| 0 | 403 [40.0] | 27 [23.5] | 376 [42.1] |
| 1 | 490 [40.6] | 77 [67.0] | 413 [46.2] |
| 2 | 74 [7.3] | 6 [5.2] | 68 [7.7] |
| 3 | 41 [4.1] | 5 [4.3] | 36 [4.0] |
| Histological type [%] | |||
| Adenocarcinoma | 641 [63.6] | 65 [56.5] | 576 [64.5] |
| Squamous | 187 [18.6] | 29 [25.2] | 158 [17.7] |
| Small cell | 137 [13.6] | 14 [12.2] | 123 [13.8] |
| Others | 43 [4.3] | 7 [6.1] | 36 [4.0] |
| Clinical stage [%] | |||
| T factor | |||
| T1 | 160 [16.8] | 15 [13.0] | 145 [16.2] |
| T2 | 255 [26.8] | 29 [25.2] | 226 [25.3] |
| T3 | 213 [22.4] | 26 [22.6] | 187 [20.9] |
| T4 | 287 [30.1] | 32 [27.8] | 255 [28.6] |
| Tx | 37 [3.9] | 2 [1.7] | 35 [3.9] |
| Missing | 56 | 11 [9.6] | 45 [5.0] |
| N factor | |||
| N0 | 195 [20.2] | 20 [17.4] | 175 [19.6] |
| N1 | 98 [10.2] | 8 [7.0] | 90 [10.1] |
| N2 | 268 [27.8] | 32 [27.8] | 236 [26.4] |
| N3 | 402 [41.7] | 47 [40.9] | 355 [39.8] |
| Missing | 45 | 8 [7.0] | 37 [4.1] |
| M factor | |||
| M0 | 192 [20.0] | 29 [25.2] | 163 [18.3] |
| M1a | 228 [23.8] | 19 [16.5] | 209 [23.4] |
| M1b | 540 [56.3] | 59 [51.3] | 481 [53.9] |
| Missing | 48 | 8 [7.0] | 40 [4.5] |
| Complications of atrial fibrillation [%] | 32 [3.2] | 7 [6.1] | 25 [2.8] |
| Complications of vascular disease 1 [%] | 88 [8.7] | 10 [8.7] | 78 [8.7] |
| History of anticoagulant use [%] | 59 [5.9] | 11 [9.6] | 48 [5.4] |
| N | HR | 95% CI | p Value | |
|---|---|---|---|---|
| VTE (+) [vs. VTE (−)] | 100 | 4.003 | 2.470–6.488 | <0.001 |
| Female (vs. male) | 294 | 0.454 | 0.277–0.742 | 0.002 |
| NSCLC (vs. SCLC) | 871 | 0.867 | 0.496–1.516 | 0.616 |
| Adenocarcinoma (vs. SCLC) | 641 | 0.731 | 0.506–1.058 | 0.096 |
| Squamous (vs. SCLC) | 187 | 1.522 | 0.999–2.318 | 0.051 |
| Others (vs. SCLC) | 43 | 1.323 | 0.582–3.010 | 0.504 |
| T type | ||||
| T1a | 28 | 1.000 | Reference | |
| T1b | 132 | 0.574 | 0.183–1.801 | 0.341 |
| T2a | 195 | 0.866 | 0.301–2.497 | 0.790 |
| T2b | 60 | 0.569 | 0.153–2.120 | 0.401 |
| T3 | 213 | 0.848 | 0.296–2.430 | 0.759 |
| T4 | 287 | 0.787 | 0.278–2.224 | 0.651 |
| TX | 37 | 0.372 | 0.068–2.029 | 0.253 |
| N type | ||||
| N0 | 195 | 1.000 | Reference | |
| N1 | 98 | 0.789 | 0.347–1.790 | 0.570 |
| N2 | 268 | 1.170 | 0.669–2.045 | 0.582 |
| N3 | 402 | 1.156 | 0.685–1.951 | 0.587 |
| M type | ||||
| M0 | 192 | 1.000 | Reference | |
| M1a | 228 | 0.542 | 0.304–0.966 | 0.038 |
| M1b | 540 | 0.732 | 0.469–1.142 | 0.169 |
| ECOG PS | ||||
| 0 | 403 | 1.000 | Reference | |
| 1 | 490 | 2.476 | 1.597–3.838 | 0.000 |
| 2 | 74 | 1.249 | 0.516–3.024 | 0.623 |
| 3 | 41 | 1.996 | 0.769–5.183 | 0.156 |
| NSCLC Stage | ||||
| Ⅰa | 0 | - | - | - |
| Ⅰb | 2 | 5.23 | 0.701–39.007 | 0.107 |
| Ⅱa | 4 | 1.795 | 0.241–13.380 | 0.568 |
| Ⅱb | 4 | 1.671 | 0.224–12.451 | 0.616 |
| Ⅲa | 28 | 1.198 | 0.450–3.192 | 0.718 |
| Ⅲb | 73 | 1.248 | 0.621–2.508 | 0.535 |
| Ⅳ | 623 | 0.657 | 0.396–1.090 | 0.104 |
| Postoperative recurrence | 137 | 1.000 | Reference |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawakado, K.; Tsubata, Y.; Hotta, T.; Yamasaki, M.; Ishikawa, N.; Fujitaka, K.; Kubota, T.; Kobayashi, K.; Isobe, T. Risk Factors for Bleeding Events in Japanese Patients with Advanced Lung Cancer: Data from the Rising-VTE/NEJ037 Study. Cancers 2024, 16, 301. https://doi.org/10.3390/cancers16020301
Kawakado K, Tsubata Y, Hotta T, Yamasaki M, Ishikawa N, Fujitaka K, Kubota T, Kobayashi K, Isobe T. Risk Factors for Bleeding Events in Japanese Patients with Advanced Lung Cancer: Data from the Rising-VTE/NEJ037 Study. Cancers. 2024; 16(2):301. https://doi.org/10.3390/cancers16020301
Chicago/Turabian StyleKawakado, Keita, Yukari Tsubata, Takamasa Hotta, Masahiro Yamasaki, Nobuhisa Ishikawa, Kazunori Fujitaka, Tetsuya Kubota, Kunihiko Kobayashi, and Takeshi Isobe. 2024. "Risk Factors for Bleeding Events in Japanese Patients with Advanced Lung Cancer: Data from the Rising-VTE/NEJ037 Study" Cancers 16, no. 2: 301. https://doi.org/10.3390/cancers16020301
APA StyleKawakado, K., Tsubata, Y., Hotta, T., Yamasaki, M., Ishikawa, N., Fujitaka, K., Kubota, T., Kobayashi, K., & Isobe, T. (2024). Risk Factors for Bleeding Events in Japanese Patients with Advanced Lung Cancer: Data from the Rising-VTE/NEJ037 Study. Cancers, 16(2), 301. https://doi.org/10.3390/cancers16020301







