Prognostic Factors for Resolution Delay of Lower Urinary Tract Symptoms in Patients with Prostate Cancer after Low-Dose-Rate Brachytherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Treatment
2.3. Post-Dosimetric Evaluation
2.4. Follow-Up Schedule
2.5. Endpoints and Statistical Analysis
3. Results
3.1. Patient Characteristics
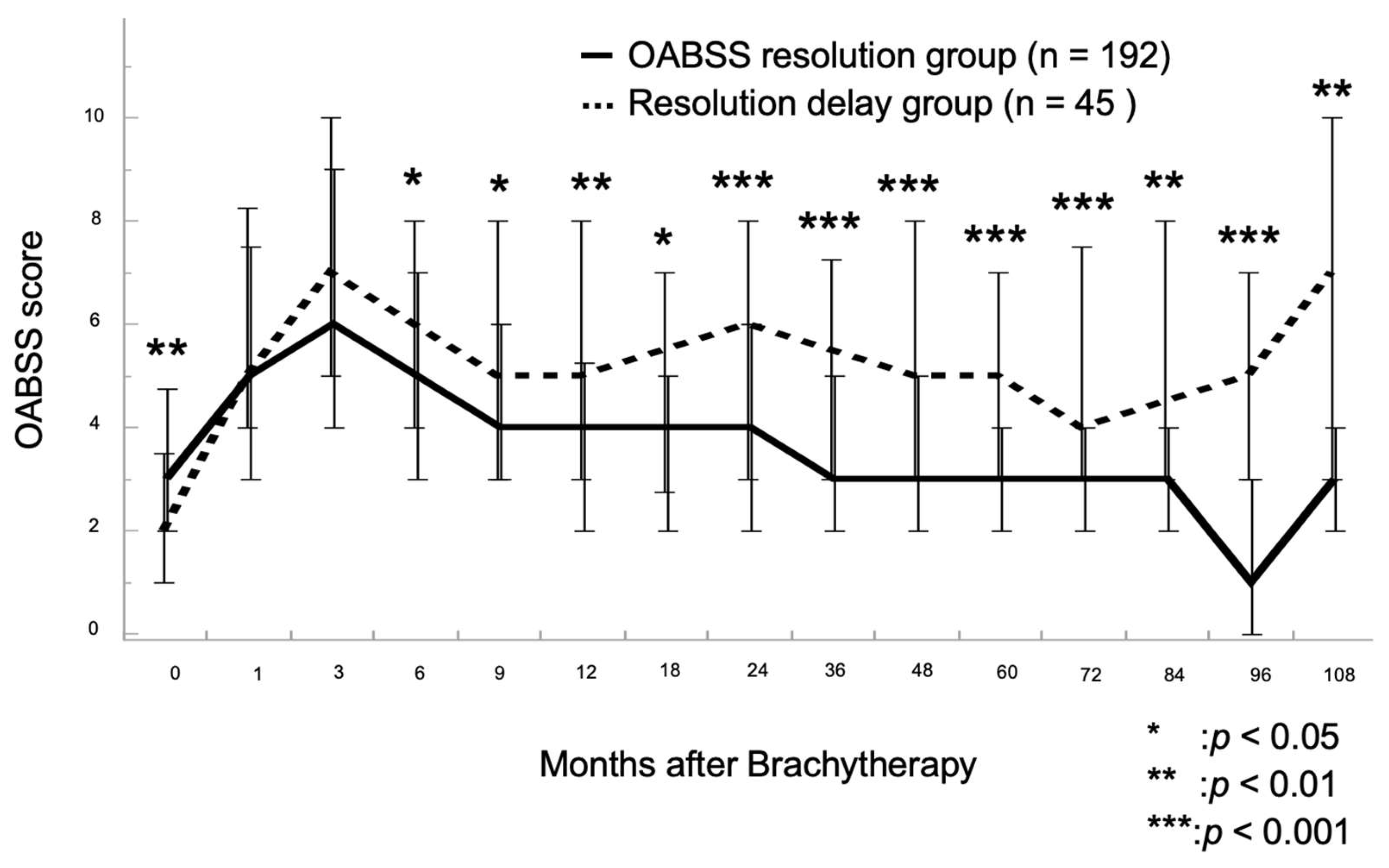
3.2. Chronological Changes in OABSS
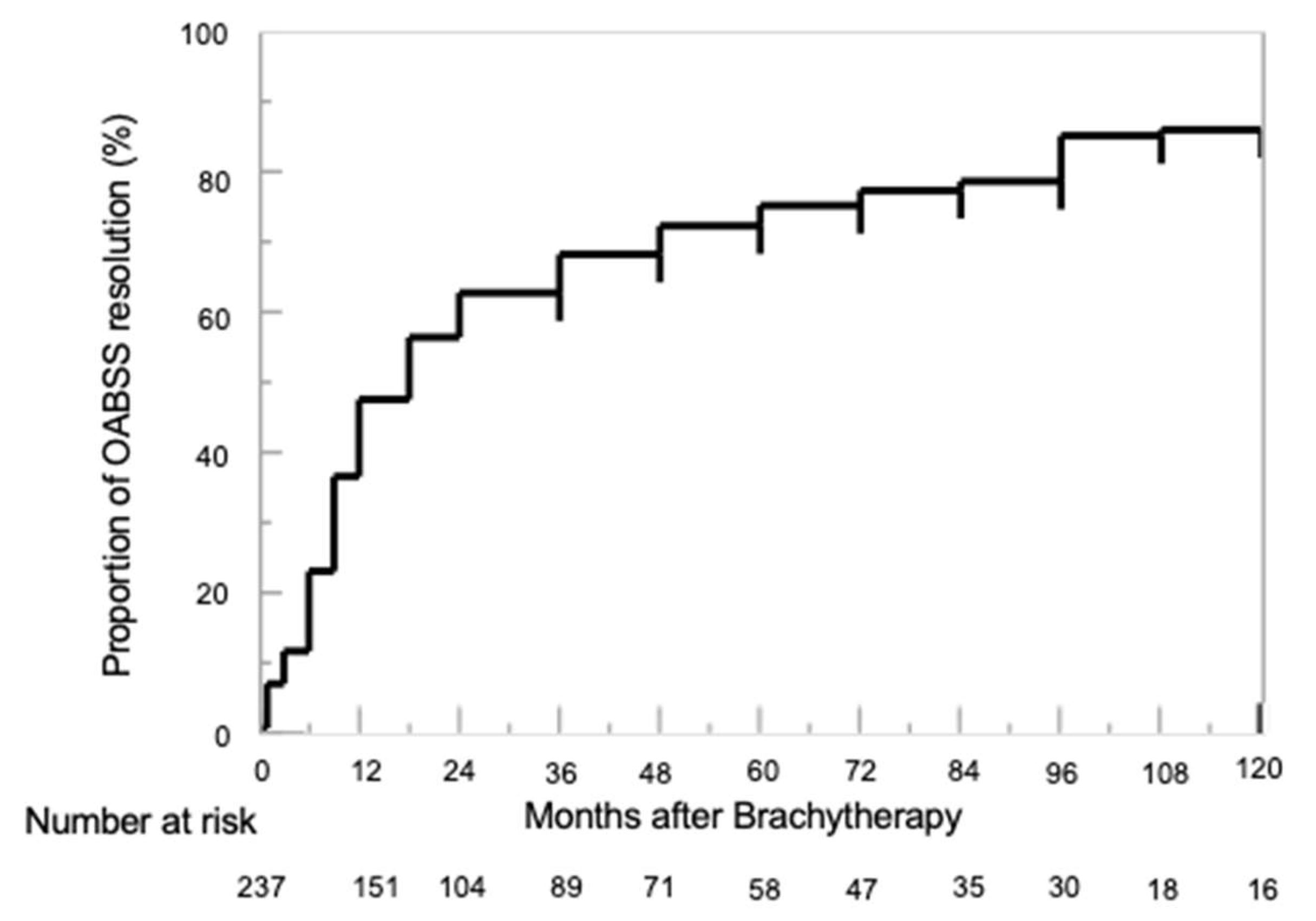
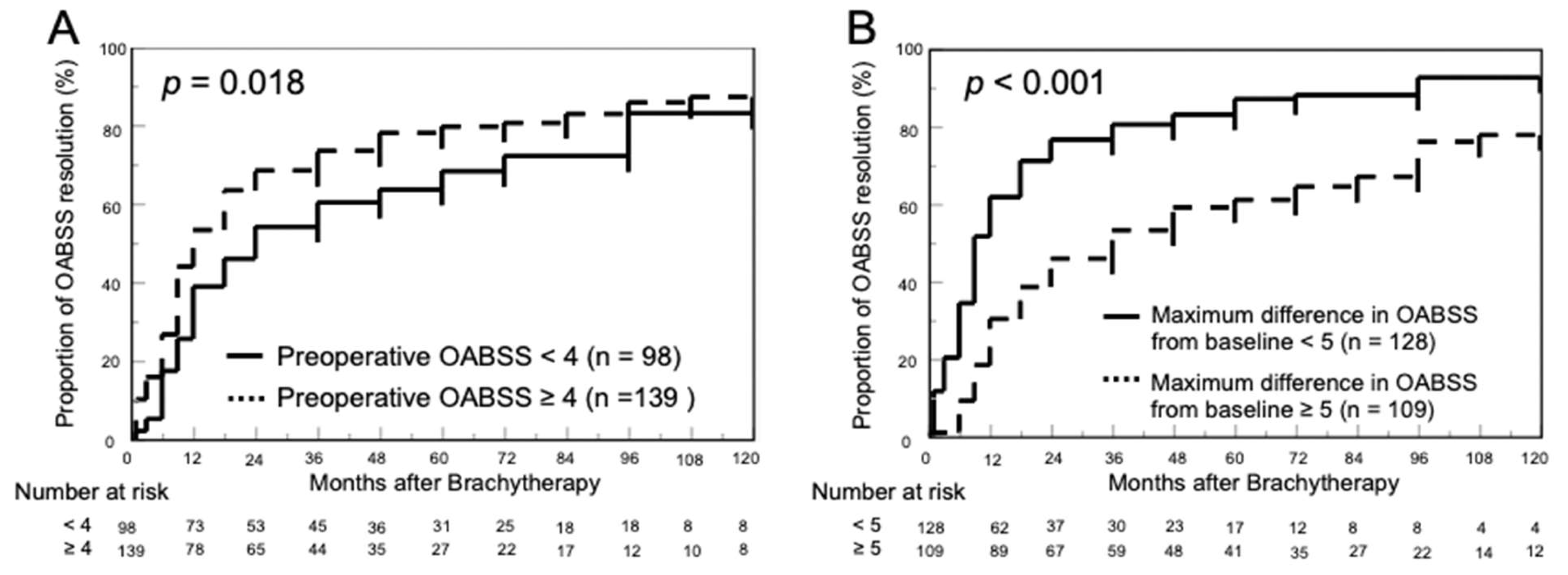
3.3. Proportion of OABSS Resolution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Cancer Registry (Ministry of Health, Labour and Welfare), Tabulated by Cancer Information Service, National Cancer Center, Japan. Available online: https://ganjoho.jp/public/qa_links/report/statistics/2021_en.html (accessed on 6 June 2023).
- Vital Statistics in Japan, Tabulated by Cancer Information Service, National Cancer Center, Japan. Available online: https://ganjoho.jp/reg_stat/statistics/data/dl/en.html (accessed on 6 June 2023).
- Achard, V.; Panje, C.M.; Engeler, D.; Zilli, T.; Putora, P.M. Localized and Locally Advanced Prostate Cancer: Treatment Op-tions. Oncology 2021, 99, 413–421. [Google Scholar] [CrossRef] [PubMed]
- King, M.T.; Keyes, M.; Frank, S.J.; Crook, J.M.; Butler, W.M.; Rossi, P.J.; Cox, B.W.; Showalter, T.N.; Mourtada, F.; Potters, L.; et al. Low Dose Rate Brachytherapy for Primary Treatment of Localized Prostate Cancer: A Systemic Review and Executive Summary of an Evidence-Based Consensus Statement. Brachytherapy 2021, 20, 1114–1129. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Popiolek, M.; Rider, J.R.; Andrén, O.; Andersson, S.-O.; Holmberg, L.; Adami, H.-O.; Johansson, J.-E. Natural History of Early, Localized Prostate Cancer: A Final Report from Three Decades of Follow-Up. Eur. Urol. 2013, 63, 428–435. [Google Scholar] [CrossRef]
- Price, J.G.; Stone, N.N.; Stock, R.G. Predictive Factors and Management of Rectal Bleeding Side Effects Following Prostate Cancer Brachytherapy. Int. J. Radiat. Oncol. 2013, 86, 842–847. [Google Scholar] [CrossRef]
- Onishi, K.; Tanaka, N.; Miyake, M.; Nakai, Y.; Anai, S.; Torimoto, K.; Yamaki, K.; Asakawa, I.; Hasegawa, M.; Fujii, T.; et al. Changes in Lower Urinary Tract Symptoms after Iodine-125 Brachytherapy for Prostate Cancer. Clin. Transl. Radiat. Oncol. 2019, 14, 51–58. [Google Scholar] [CrossRef]
- Iinuma, K.; Nakano, M.; Kato, T.; Kato, D.; Takai, M.; Maekawa, Y.M.; Nakane, K.; Mizutani, K.; Tsuchiya, T.; Ishihara, T.; et al. Assessment of Long-Term Changes in Lower Urinary Tract Symptoms in Patients with Prostate Cancer Who Underwent Low-Dose-Rate Prostate Brachytherapy. Urology 2020, 142, 213–220. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Carroll, P.H.; Mohler, J.L. NCCN Guidelines Updates: Prostate Cancer and Prostate Cancer Early Detection. J. Natl. Compr. Cancer Netw. 2018, 16, 620–623. [Google Scholar] [CrossRef]
- Stock, R.G.; Stone, N.N.; Wesson, M.F.; DeWyngaert, J.K. A Modified Technique Allowing Interactive Ultrasound-Guided Three-Dimensional Transperineal Prostate Implantation. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 219–225. [Google Scholar] [CrossRef]
- Ohashi, T.; Yorozu, A.; Toya, K.; Saito, S.; Momma, T.; Nagata, H.; Kosugi, M.; Shigematsu, N.; Kubo, A. Comparison of Intraoperative Ultrasound with Postimplant Computed Tomography–Dosimetric Values at Day 1 and Day 30 after Prostate Brachytherapy. Brachytherapy 2007, 6, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Lazarev, S.; Thompson, M.R.; Stone, N.N.; Stock, R.G. Low-Dose-Rate Brachytherapy for Prostate Cancer: Outcomes At> 10 Years of Follow-Up. BJU Int. 2018, 121, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Fellin, G.; Mirri, M.A.; Santoro, L.; Jereczek-Fossa, B.A.; Divan, C.; Mussari, S.; Ziglio, F.; La Face, B.; Barbera, F.; Buglione, M. Low Dose Rate Brachytherapy (LDR-BT) as Monotherapy for Early Stage Prostate Cancer in Italy: Practice and Outcome Analysis in a Series of 2237 Patients from 11 Institutions. Br. J. Radiol. 2016, 89, 20150981. [Google Scholar] [CrossRef] [PubMed]
- Roach, M.; Hanks, G.; Thames, H.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining Biochemical Failure Following Radiotherapy with or without Hormonal Therapy in Men with Clinically Localized Prostate Cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. 2006, 65, 965–974. [Google Scholar] [CrossRef]
- Tanaka, N.; Asakawa, I.; Hasegawa, M.; Fujimoto, K. Low-dose-rate Brachytherapy for Prostate Cancer: A 15-year Experience in Japan. Int. J. Urol. 2020, 27, 17–23. [Google Scholar] [CrossRef]
- Vuolukka, K.; Auvinen, P.; Palmgren, J.-E.; Voutilainen, T.; Aaltomaa, S.; Kataja, V. Long-Term Efficacy and Urological Toxicity of Low-Dose-Rate Brachytherapy (LDR-BT) as Monotherapy in Localized Prostate Cancer. Brachytherapy 2019, 18, 583–588. [Google Scholar] [CrossRef]
- Tanaka, N.; Yorozu, A.; Kikuchi, T.; Higashide, S.; Kojima, S.; Ohashi, T.; Katayama, N.; Nakamura, K.; Saito, S.; Dokiya, T. Genitourinary Toxicity after Permanent Iodine-125 Seed Implantation: The Nationwide Japanese Prostate Cancer Outcome Study of Permanent Iodine-125 Seed Implantation (J-POPS). Brachytherapy 2019, 18, 484–492. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Pardo, G.S.; Sanchez, A.M.; Lucena, J.B.; Pisano, F.; Cortez, J.C.; Territo, A.; Perez, J.H.; Sopeña, J.G.; Lopez, C.E. Radiation-Induced Haemorrhagic Cystitis after Prostate Cancer Radiotherapy: Factors Associated to Hospitalization and Treatment Strategies. Prostate Int. 2021, 9, 48–53. [Google Scholar] [CrossRef]
- Uematsu, T.; Torimoto, K.; Tanaka, N.; Asakawa, I.; Hori, S.; Yamaki, K.; Nakai, Y.; Miyake, M.; Anai, S.; Hasegawa, M. Factors Affecting Urinary Frequency after Low-Dose-Rate Brachytherapy for Prostate Cancer. LUTS Low. Urin. Tract Symptoms 2022, 14, 4–9. [Google Scholar] [CrossRef]
- Cesaretti, J.A.; Stone, N.N.; Stock, R.G. Urinary Symptom Flare Following I-125 Prostate Brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 1085–1092. [Google Scholar] [CrossRef]
- Sakayori, M.; Ohashi, T.; Momma, T.; Kaneda, T.; Nishimura, S.; Sutani, S.; Yamashita, S.; Shigematsu, N. Quantitative Analysis of Genitourinary Toxicity after Iodine-125 Brachytherapy for Localized Prostate Cancer: Followup of the International Prostate Symptom Score and Overactive Bladder Symptom Score. Brachytherapy 2017, 16, 806–814. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Smith, R.P.; Beriwal, S.; Benoit, R.M. Changes in Lower Urinary Tract Symptoms after Prostate Brachytherapy. J. Contemp. Brachyther. 2011, 3, 115–120. [Google Scholar] [CrossRef]
- Stone, N.N.; Gerber, N.K.; Blacksburg, S.; Stone, J.; Stock, R.G. Factors Influencing Urinary Symptoms 10 Years After Permanent Prostate Seed Implantation. J. Urol. 2012, 187, 117–123. [Google Scholar] [CrossRef]
- Neill, M.; Studer, G.; Le, L.; McLean, M.; Yeung, I.; Pond, G.; Crook, J.M. The nature and extent of urinary morbidity in relation to prostate brachytherapy urethral dosimetry. Brachytherapy 2007, 6, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Stone, N.N.; Stock, R.G. Long-term urinary, sexual, and rectal morbidity in patients treated with iodine-125 prostate brachytherapy followed up for a minimum of 5 years. Urology 2007, 69, 338–342. [Google Scholar] [CrossRef]
- Boettcher, M.; Haselhuhn, A.; Jakse, G.; Brehmer, B.; Kirschner-Hermanns, R. Overactive Bladder Syndrome: An Underestimated Long-Term Problem after Treatment of Patients with Localized Prostate Cancer? BJU Int. 2012, 109, 1824–1830. [Google Scholar] [CrossRef]
- Keyes, M.; Miller, S.; Moravan, V.; Pickles, T.; McKenzie, M.; Pai, H.; Liu, M.; Kwan, W.; Agranovich, A.; Spadinger, I.; et al. Predictive factors for acute and late urinary toxicity after permanent prostate brachytherapy: Long-term outcome in 712 consecutive patients. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Hikita, K.S.; Honda, M.; Hirano, S.; Kawamoto, B.; Panagiota, T.; Muraoka, K.; Sejima, T.; Takenaka, A. Comparison of the overactive bladder symptom score and the overactive bladder symptom score derived from the bladder diaries. Neurourol. Urodyn. 2016, 35, 349–353. [Google Scholar] [CrossRef] [PubMed]

| Covariates | OABSS Resolution Group (n = 192) | Delayed Resolution Group (n = 45) | p-Value * |
|---|---|---|---|
| Age (year, median, IQR) | 66.5 (63.0–71.0) | 64.0 (60.0–69.0) | 0.023 |
| BMI (median, IQR) | 23.6 (21.9–25.3) | 23.0 (21.8–25.6) | 0.921 |
| Initial PSA (ng/mL, median, IQR) | 6.3 (5.0–8.7) | 5.4 (4.5–7.6) | 0.029 |
| Prostate volume (mL, median, IQR) | 24.9 (18.6–34.9) | 24.0 (18.5–35.1) | 0.664 |
| Biopsy Gleason grade group (number, %) | 0.355 | ||
| 1 | 88 (42.7) | 25 (25.9) | |
| 2 | 65 (34.8) | 13 (29.6) | |
| 3 | 23 (13.4) | 3 (22.2) | |
| 4 | 11 (6.4) | 1 (7.4) | |
| 5 | 5 (2.8) | 3 (14.8) | |
| Clinical T stage (number, %) | 0.167 | ||
| 1 | 134 (69.8) | 33 (73.3) | |
| 2 | 58 (30.2) | 11 (24.4) | |
| 3 | 0 (0) | 1 (2.2) | |
| NCCN risk classification (number, %) | 0.555 | ||
| Low | 80 (41.7) | 24 (53.3) | |
| Favorable intermediate | 69 (35.9) | 14 (31.1) | |
| Unfavorable intermediate | 26 (13.5) | 3 (6.7) | |
| High | 16 (8.3) | 4 (8.9) | |
| Very High | 1 (0.5) | 0 (0) | |
| Inserted needles (number, median, IQR) | 21 (19–24) | 22 (20–24) | 0.269 |
| Inserted seeds (number, median, IQR) | 65 (54–84) | 70 (60–85) | 0.323 |
| BED (Gy, median, IQR) | 192 (173–205) | 192 (176–212) | 0.590 |
| D90 (Gy, median, IQR) | 160 (130–175) | 168 (137–183) | 0.055 |
| V100 (%, median, IQR) | 96 (94–98) | 97 (95–98) | 0.266 |
| UD30 (Gy, median, IQR) | 200 (169–221) | 207 (181–239) | 0.124 |
| RV100 (cc, median, IQR) | 0.25 (0.08–0.79) | 0.54 (0.15–0.88) | 0.072 |
| Combined ADT (number, %) | 160 (83.3) | 35 (77.8) | 0.389 |
| Supplementary EBRT (number, %) | 62 (32.3) | 11 (24.4) | 0.371 |
| Preoperative maximal flow rate (mL/s, median, IQR) | 18 (14–23) | 18 (15–22) | 0.754 |
| Preoperative PVR (mL, median, IQR) | 15 (7–25) | 13 (4–20) | 0.224 |
| Preoperative IPSS (median, IQR) | 5 (3–9) | 6 (3–11) | 0.693 |
| Preoperative QOL (median, IQR) | 2 (1–3) | 2 (1–3) | 0.826 |
| Preoperative OABSS (median, IQR) | 3 (2–5) | 2 (1–4) | 0.004 |
| Change from baseline to maximum in OABSS (median, IQR) | 4 (2–6) | 6 (4–8) | <0.001 |
| Time to maximal OABSS (month, median, IQR) | 3 (1–3) | 3 (1–8) | 0.081 |
| Time to the resolution of OABSS (month, median, IQR) | 12 (6–24) | - | - |
| Biochemical recurrence (number, %) | 3 (1.6) | 1 (2.2) | 0.572 |
| Follow-up period (month, median, IQR) | 87 (68–120) | 87 (72–108) | 0.461 |
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age (continuous) | 1.023 (0.997–1.050) | 0.080 | 1.014 (0.989–1.041) | 0.276 |
| BMI (continuous) | 0.994 (0.936–1.054) | 0.840 | ||
| Initial PSA (continuous) | 0.987 (0.954–1.016) | 0.393 | ||
| Prostate volume (continuous) | 1.000 (0.999–1.001) | 0.366 | 1.001 (1.000–1.002) | 0.077 |
| BED (continuous) | 0.995 (0.989–1.001) | 0.109 | ||
| UD30 (continuous) | 0.998 (0.994–1.001) | 0.232 | 1.000 (0.997–1.004) | 0.835 |
| Combined ADT (vs. none) | 1.304 (0.904–1.941) | 0.160 | ||
| Supplementary EBRT (vs. none) | 0.971 (0.712–1.309) | 0.847 | ||
| Preoperative IPSS (continuous) | 1.001 (0.972–1.030) | 0.941 | ||
| Preoperative OABSS (continuous) | 1.192 (1.115–1.272) | <0.001 | 1.155 (1.079–1.234) | <0.001 |
| Change from baseline to maximum in OABSS (continuous) | 0.772 (0.721–0.825) | <0.001 | 0.785 (0.733–0.839) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taniguchi, T.; Kawase, M.; Nakane, K.; Nakano, M.; Iinuma, K.; Kato, D.; Takai, M.; Tobisawa, Y.; Mori, T.; Takano, H.; et al. Prognostic Factors for Resolution Delay of Lower Urinary Tract Symptoms in Patients with Prostate Cancer after Low-Dose-Rate Brachytherapy. Cancers 2023, 15, 4048. https://doi.org/10.3390/cancers15164048
Taniguchi T, Kawase M, Nakane K, Nakano M, Iinuma K, Kato D, Takai M, Tobisawa Y, Mori T, Takano H, et al. Prognostic Factors for Resolution Delay of Lower Urinary Tract Symptoms in Patients with Prostate Cancer after Low-Dose-Rate Brachytherapy. Cancers. 2023; 15(16):4048. https://doi.org/10.3390/cancers15164048
Chicago/Turabian StyleTaniguchi, Tomoki, Makoto Kawase, Keita Nakane, Masahiro Nakano, Koji Iinuma, Daiki Kato, Manabu Takai, Yuki Tobisawa, Takayuki Mori, Hirota Takano, and et al. 2023. "Prognostic Factors for Resolution Delay of Lower Urinary Tract Symptoms in Patients with Prostate Cancer after Low-Dose-Rate Brachytherapy" Cancers 15, no. 16: 4048. https://doi.org/10.3390/cancers15164048
APA StyleTaniguchi, T., Kawase, M., Nakane, K., Nakano, M., Iinuma, K., Kato, D., Takai, M., Tobisawa, Y., Mori, T., Takano, H., Kumano, T., Matsuo, M., Ito, T., & Koie, T. (2023). Prognostic Factors for Resolution Delay of Lower Urinary Tract Symptoms in Patients with Prostate Cancer after Low-Dose-Rate Brachytherapy. Cancers, 15(16), 4048. https://doi.org/10.3390/cancers15164048








