Exercise Intervention on Insomnia in Patients with a Cancer: A Systematic Review of the Literature
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Information Sources and Search
2.3. Data Collection Process
3. Results
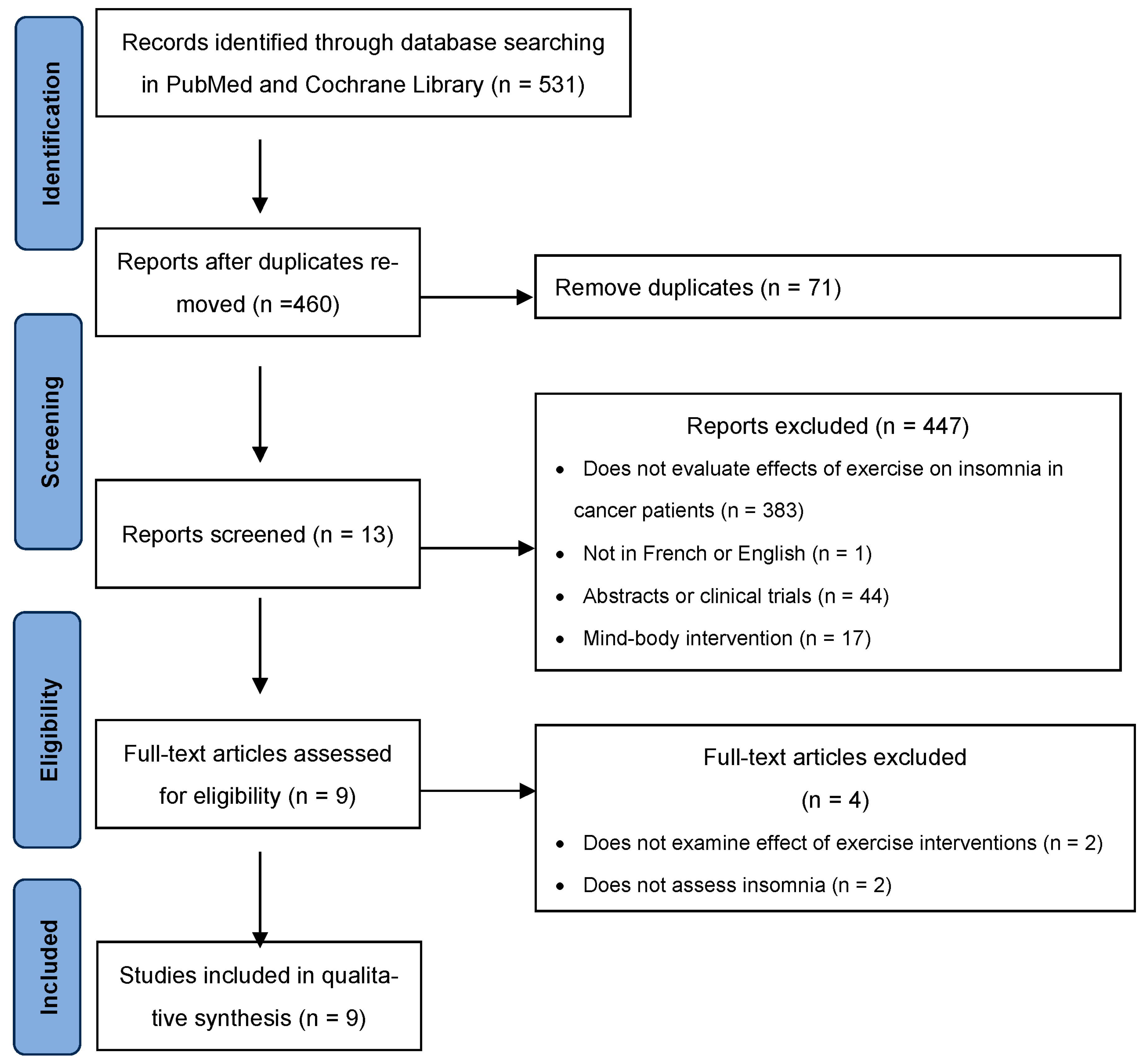
3.1. Study Selection
3.2. Study Characteristics
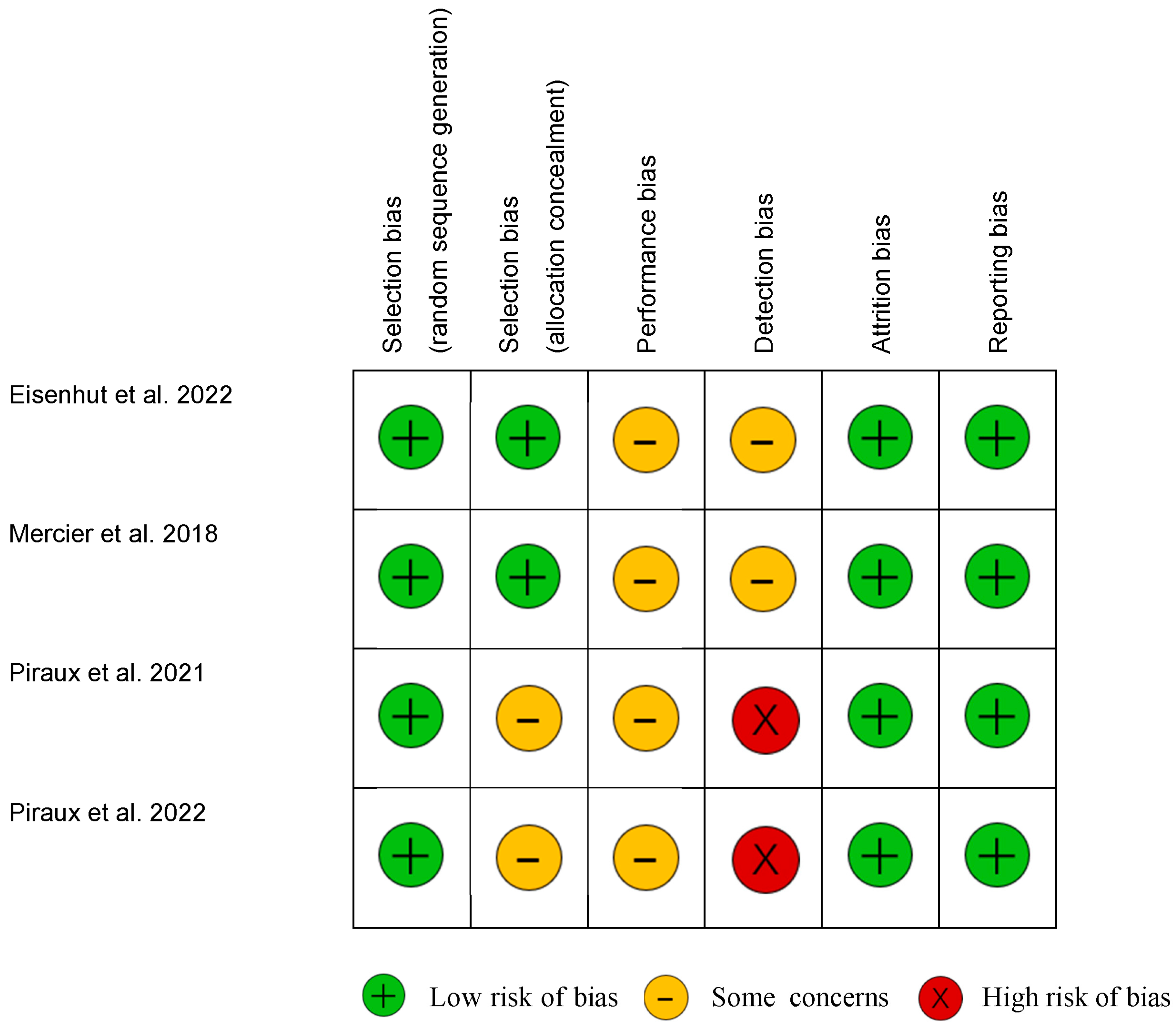
3.3. Quality Evaluation
3.4. Participants
3.5. Exercise Interventions
3.6. Adherence and Compliance
3.7. Sleep Outcomes
3.8. Effect of Exercise Interventions on Insomnia Outcome at Baseline
3.9. Pre- and Post-Intervention Insomnia Outcome
4. Discussion
5. Study Strengths and Limitations
6. Conclusions
7. Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lis, C.G.; Gupta, D.; Grutsch, J.F. The relationship between insomnia and patient satisfaction with quality of life in cancer. Support. Care Cancer 2008, 16, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Kwak, A.; Jacobs, J.; Haggett, D.; Jimenez, R.; Peppercorn, J. Evaluation and management of insomnia in women with breast cancer. Breast Cancer Res. Treat. 2020, 181, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Savard, J.; Simard, S.; Blanchet, J.; Ivers, H.; Morin, C.M. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep 2001, 24, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.R.; MacLean, A.W.; Brundage, M.D.; Schulze, K. Sleep disturbance in cancer patients. Soc. Sci. Med. 2002, 54, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- First, M.B.; Yousif, L.H.; Clarke, D.E.; Wang, P.S.; Gogtay, N.; Appelbaum, P.S. DSM-5-TR: Overview of what’s new and what’s changed. World Psychiatry 2022, 21, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Savard, J.; Savard, M.-H. Insomnia and cancer: Prevalence, nature, and nonpharmacologic treatment. Sleep Med. Clin. 2013, 8, 373–387. [Google Scholar] [CrossRef]
- Spielman, A.J.; Caruso, L.S.; Glovinsky, P.B. A behavioral perspective on insomnia treatment. Psychiatr. Clin. N. Am. 1987, 10, 541–553. [Google Scholar] [CrossRef]
- Garland, S.N.; Johnson, J.A.; Savard, J.; Gehrman, P.; Perlis, M.; Carlson, L.; Campbell, T. Sleeping well with cancer: A systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr. Dis. Treat. 2014, 10, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- O‘Donnell, J.F. Insomnia in cancer patients. Clin. Cornerstone 2004, 6, S6–S14. [Google Scholar] [CrossRef]
- Howell, D.; Oliver, T.K.; Keller-Olaman, S.; Davidson, J.R.; Garland, S.; Samuels, C.; Savard, J.; Harris, C.; Aubin, M.; Olson, K.; et al. Sleep disturbance in adults with cancer: A systematic review of evidence for best practices in assessment and management for clinical practice. Ann. Oncol. 2014, 25, 791–800. [Google Scholar] [CrossRef]
- Ma, Y.; Hall, D.L.; Ngo, L.H.; Liu, Q.; Bain, P.A.; Yeh, G.Y. Efficacy of cognitive behavioral therapy for insomnia in breast cancer: A meta-analysis. Sleep Med. Rev. 2021, 55, 101376. [Google Scholar] [CrossRef] [PubMed]
- Quesnel, C.; Savard, J.; Simard, S.; Ivers, H.; Morin, C.M. Efficacy of cognitive-behavioral therapy for insomnia in women treated for nonmetastatic breast cancer. J. Consult. Clin. Psychol. 2003, 71, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Palesh, O.; Scheiber, C.; Kesler, S.; Janelsins, M.C.; Guido, J.J.; Heckler, C.; Cases, M.G.; Miller, J.; Chrysson, N.G.; Mustian, K.M. Feasibility and acceptability of brief behavioral therapy for cancer-related insomnia: Effects on insomnia and circadian rhythm during chemotherapy: A phase II randomised multicentre controlled trial. Br. J. Cancer 2018, 119, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Savard, J.; Simard, S.; Ivers, H.; Morin, C.M. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: Sleep and psychological effects. J. Clin. Oncol. 2005, 23, 6083–6096. [Google Scholar] [CrossRef] [PubMed]
- Ballesio, A.; Aquino, M.R.J.V.; Feige, B.; Johann, A.F.; Kyle, S.D.; Spiegelhalder, K.; Lombardo, C.; Rücker, G.; Riemann, D.; Baglioni, C. The effectiveness of behavioural and cognitive behavioural therapies for insomnia on depressive and fatigue symptoms: A systematic review and network meta-analysis. Sleep Med. Rev. 2018, 37, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Espie, C.A.; Fleming, L.; Cassidy, J.; Samuel, L.; Taylor, L.M.; White, C.A.; Douglas, N.J.; Engleman, H.M.; Kelly, H.-L.; Paul, J. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J. Clin. Oncol. 2008, 26, 4651–4658. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.S.; Suh, S.; Youn, S.; Chung, S. Adapting cognitive-behavior therapy for insomnia in cancer patients. Sleep Med. Res. 2017, 8, 51–61. [Google Scholar] [CrossRef]
- Koffel, E.; Bramoweth, A.D.; Ulmer, C.S. Increasing access to and utilization of cognitive behavioral therapy for insomnia (CBT-I): A narrative review. J. Gen. Intern. Med. 2018, 33, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Speck, R.M.; Rye, S.A.; DiSipio, T.; Hayes, S.C. Prevalence of breast cancer treatment sequelae over 6 years of follow-up: The Pulling Through Study. Cancer 2012, 118 (Suppl. S8), 2217–2225. [Google Scholar] [CrossRef]
- Casla, S.; Hojman, P.; Márquez-Rodas, I.; López-Tarruella, S.; Jerez, Y.; Barakat, R.; Martín, M. Running away from side effects: Physical exercise as a complementary intervention for breast cancer patients. Transl. Oncol. 2015, 17, 180–196. [Google Scholar] [CrossRef]
- Ficarra, S.; Thomas, E.; Bianco, A.; Gentile, A.; Thaller, P.; Grassadonio, F.; Papakonstantinou, S.; Schulz, T.; Olson, N.; Martin, A.; et al. Impact of exercise interventions on physical fitness in breast cancer patients and survivors: A systematic review. Breast Cancer 2022, 29, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Driver, H.S.; Taylor, S.R. Exercise and sleep. Sleep Med. Rev. 2000, 4, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Eisenhut, L.; Sadeghi-Bahmani, D.; Gerber, M.; Saemann, A.; Staub, L.; Brand, S.; Cordier, D. Effects of two types of exercise training on psychological well-being, sleep and physical fitness in patients with high-grade glioma (WHO III and IV). J. Psychiatr. Res. 2022, 151, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Mercier, J.; Ivers, H.; Savard, J. A non-inferiority randomized controlled trial comparing a home-based aerobic exercise program to a self-administered cognitive-behavioral therapy for insomnia in cancer patients. Sleep 2018, 41, zsy149. [Google Scholar] [CrossRef]
- Piraux, E.; Reychler, G.; Vancraeynest, D.; Geets, X.; Leonard, D.; Caty, G. High-intensity aerobic interval training and resistance training are feasible in rectal cancer patients undergoing chemoradiotherapy: A feasibility randomized controlled study. Rep. Pract. Oncol. Radiother. 2022, 27, 198–208. [Google Scholar] [CrossRef]
- Piraux, E.; Caty, G.; Renard, L.; Vancraeynest, D.; Tombal, B.; Geets, X.; Reychler, G. Effects of high-intensity interval training compared with resistance training in prostate cancer patients undergoing radiotherapy: A randomized controlled trial. Prostate Cancer Prostatic Dis. 2021, 24, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, P.; Denieffe, S.; Murphy, N.M.; Harrison, M. Exercise is more effective than health education in reducing fatigue in fatigued cancer survivors. Support. Care Cancer 2020, 28, 4953–4962. [Google Scholar] [CrossRef]
- Yamada, P.M.; Teranishi-Hashimoto, C.; Bantum, E.O. Paired exercise has superior effects on psychosocial health compared to individual exercise in female cancer patients. Support. Care Cancer 2021, 29, 6305–6314. [Google Scholar] [CrossRef]
- Colledge, F.; Brand, S.; Pühse, U.; Holsboer-Trachsler, E.; Zimmerer, S.; Schleith, R.; Gerber, M. A Twelve-Week Moderate Exercise Programme Improved Symptoms of Depression, Insomnia, and Verbal Learning in Post-Aneurysmal Subarachnoid Haemorrhage Patients: A Comparison with Meningioma Patients and Healthy Controls. Neuropsychobiology 2018, 76, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Kozik, T.M.; Hickman, M.C.; Schmidt, S.; Connolly, T.F.; Paustenbach, K.; Vosti, P.; Bhattacharyya, M. An exerciSe program to improve depression And sleep Disorders in oncology patients: The SAD study. Eur. J. Oncol. Nurs. 2018, 37, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Charles, C.; Bardet, A.; Ibrahimi, N.; Aromatario, O.; Cambon, L.; Imbert, A.; Pons, M.; Raynard, B.; Sauveplane, D.; Pouchepadass, C.; et al. Delivering adapted physical activity by videoconference to patients with fatigue under immune checkpoint inhibitors: Lessons learned from the PACTIMe-FEAS feasibility study. J. Telemed. Telecare. 2021, 29, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. Athens Insomnia Scale: Validation of an instrument based on ICD-10 criteria. J. Psychosom. Res. 2000, 48, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Michaud, A.L.; Zhou, E.S.; Chang, G.; Recklitis, C.J. Validation of the Insomnia Severity Index (ISI) for identifying insomnia in young adult cancer survivors: Comparison with a structured clinical diagnostic interview of the DSM-5 (SCID-5). Sleep Med. 2021, 81, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Morin, C.M.; Schaefer, K.; Wallenstein, G.V. Interpreting score differences in the Insomnia Severity Index: Using health-related outcomes to define the minimally important difference. Curr. Med. Res. Opin. 2009, 25, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.L.C.; De Moor, C.; Basen-Engquist, K.; Smith, M.A.; Dunn, A.L.; Badr, H.; Pettaway, C.; Gritz, E.R. Moderator analyses of participants in the Active for Life after cancer trial: Implications for physical activity group intervention studies. Ann. Behav. Med. 2007, 33, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; Segal, R.J.; Mackey, J.R.; Gelmon, K.; Friedenreich, C.M.; Yasui, Y.; Reid, R.D.; Jespersen, D.; Cook, D.; Proulx, C.; et al. Effects of exercise dose and type on sleep quality in breast cancer patients receiving chemotherapy: A multicenter randomized trial. Breast Cancer Res. Treat. 2014, 144, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Momayyezi, M.; Fallahzadeh, H.; Farzaneh, F.; Momayyezi, M. Sleep Quality and Cancer-Related Fatigue in Patients with Cancer. J. Caring. Sci. 2021, 10, 145–152. [Google Scholar] [CrossRef]
- Gehrman, P.R.; Garland, S.N.; Matura, L.A.; Mao, J. Insomnia in breast cancer: Independent symptom or symptom cluster? Palliat. Support. Care 2017, 15, 369–375. [Google Scholar] [CrossRef]
- Hall, D.L.; Mishel, M.H.; Germino, B.B. Living with cancer-related uncertainty: Associations with fatigue, insomnia, and affect in younger breast cancer survivors. Support. Care Cancer 2014, 22, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Reinsel, R.A.; Starr, T.D.; O’Sullivan, B.; Passik, S.D.; Kavey, N.B. Polysomnographic study of sleep in survivors of breast cancer. J. Clin. Sleep Med. 2015, 11, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- McCrae, C.S.; Rowe, M.A.; Tierney, C.G.; Dautovich, N.D.; DeFinis, A.L.; McNamara, J.P. Sleep complaints, subjective and objective sleep patterns, health, psychological adjustment, and daytime functioning in community-dwelling older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2005, 60, P182–P189. [Google Scholar] [CrossRef]
- Takemura, N.; Cheung, D.S.T.; Smith, R.; Deng, W.; Ho, K.Y.; Lin, J.; Kwok, J.Y.Y.; Lam, T.-C.; Lin, C.-C. Effectiveness of aerobic exercise and mind-body exercise in cancer patients with poor sleep quality: A systematic review and meta-analysis of randomized controlled trials. Sleep Med. Rev. 2020, 53, 101334. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.E.; Janssen, D.W.; Djalilova, D.M.; Berger, A.M. Effects of Exercise on Sleep in Women with Breast Cancer: A Systematic Review. Sleep Med. Clin. 2018, 13, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, C.; Schmidt, M.E.; Steindorf, K. Effects of physical and mind-body exercise on sleep problems during and after breast cancer treatment: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2019, 176, 1–15. [Google Scholar] [CrossRef]
- Fang, Y.-Y.; Hung, C.-T.; Chan, J.-C.; Huang, S.-M.; Lee, Y.-H. Meta-analysis: Exercise intervention for sleep problems in cancer patients. Eur. J. Cancer Care 2019, 28, e13131. [Google Scholar] [CrossRef] [PubMed]
- Mercier, J.; Savard, J.; Bernard, P. Exercise interventions to improve sleep in cancer patients: A systematic review and meta-analysis. Sleep Med. Rev. 2017, 36, 43–56. [Google Scholar] [CrossRef]
- Gururaj, R.; Samuel, S.R.; Kumar, K.V.; Nagaraja, R.; Keogh, J.W. Effect of exercise based interventions on sleep and circadian rhythm in cancer survivors—A systematic review and meta-analysis. PeerJ 2024, 12, e17053. [Google Scholar] [CrossRef]
- Drozd, C.; Curtit, E.; Jacquinot, Q.; Marquine, C.; Mansi, L.; Chaigneau, L.; Dobi, E.; Viot, J.; Meynard, G.; Paillard, M.J.; et al. A randomized trial to evaluate the effects of a supervised exercise program on insomnia in patients with non-metastatic breast cancer undergoing chemotherapy: Design of the FATSOMCAN study. BMC Cancer 2023, 23, 449. [Google Scholar] [CrossRef]
| First Author et al. Year | Sample N = Total Sample M = Mean Age Exercise Intervention, n = xx UC/Other Group, n = xx | Study Design | Gender (m:f) | Cancer Site (%) | Cancer Stage (%) | Treatment Status | Line of Treatment Mean Time Since Treatment | Use of Hypnotic/Anxiolytic Medication |
|---|---|---|---|---|---|---|---|---|
| Charles et al. 2021 [34] | N = 16 M = 54 ± 12.2 EX group: 16 | Monocentric single-arm feasibility trial | 8:8 | Melanoma (68.75%) Lung (18.75%) Other (12.5%) | NR (more than two-thirds treated for metastatic melanoma) | To be starting or undergoing immunotherapy
| Line of treatment:
Mean time since the beginning of therapy: 8 ± 8.2 years | NR |
| Colledge et al. 2018 [32] | N = 48 M = 58.5 ± 12.4 aSAH group, n = 15 Meningioma group, n = 16 Healthy control group = 17 | Exploratory intervention study | 18:30 | Meningioma | NR |
No patient from either group was undergoing RX | Line of treatment: NR Mean time to entry in the study after surgery:
| Use of antidepressants:
|
| Eisenhut et al. 2022 [26] | N = 29 M = 52.1 ± 12.5 Endurance training group, n = 10 Strength training group, n = 11 Active control group (UC + sharing experiences from their daily lives), n = 8 | RCT | 44.8%:55.2% | High grade glioma | III (24.1%), IV (75.9%) | After neurosurgical tumor resection or biopsy and undergoing RX and/or CX.
| NR | NR |
| Kozik et al. 2018 [33] | N = 75 M = 59 ± 10 EX group, n = 75 | Single-arm observational study | 13:62 | Breast (57.3%), lung (16%), other (26.7%) | NR | Ongoing chemoradiotherapy or within 6 months from completion | NR | NR |
| Mercier et al. 2018 [27] | N = 41 M = 57.1 EX group, n = 20 CBT-I group, n = 21 | RCT | 9:32 | Breast (53.7%), Gynecologic (7.3%), Lymphoma (7.3%), Prostate (14.6%), Head and neck (9.8%), Other (7.3%) | 0 (4.9%), I (39%), II (26.8%), III (14.6%) Unknown (14.6%) | Post adjuvant treatment (except HX)
| Line of treatment: NR Mean time (from last RX at baseline):
|
|
| Piraux et al. 2021 [29] | N = 72 M = None RES training group, n = 6 HIIT group, n = 6 UC, n = 6 | RCT | NR | Prostate | NR | During RX (at least 25 scheduled radiation sessions with or without HX)
| Line of treatment: NR Days between the start of HX and the start of RX:
| NR |
| Piraux et al. 2022 [28] | N = 18 M = None RES training group, n = 6 HIIT group, n = 6 UC, n = 6 | RCT | 13:5 | Rectal | II (33.3%) III (66.7%) | During neoadjuvant chemoradiotherapy followed by surgery A total dose of 45.0 Gy in 25 fractions over 5 weeks with concurrent oral capecitabine (dose of 1500 mg/m2 twice daily on days of RX) or continuous intravenous infusions of 5-fluorouracile (dose of 225 mg/m2 daily, five days per week) | NR | NR |
| Sheehan et al. 2020 [30] | N = 37 M = 55 ± 2 EX group, n = 19 Health education group, n = 18 | Non-RCT | 4:33 | Breast (81%), Prostate (11%), lung (6%) Endometrial (5%), Esophageal (5%), Multiple myeloma (6%), Cervical (6%) | NR | After surgery and CX-RX treatment, ongoing HX
| Line of treatment: NR Mean time:
| 56.7% of patients used anti-inflammatory |
| Yamada et al. 2021 [31] | N = 28 M = 58 ± 11 EX in pairs, n = 14 EX individually, n = 14 | Experiential study | 0:28 | Breast (92.9%), Ovarian (3.6%), Lymphoma (3.6%) | NR | To have completed clinical cancer treatments at least 3 months previously | NR |
| Studies | Aim * Criteria about Sleep/Fatigue | Intervention | Components and Intensity | Assessment Time | Adherence | Insomnia Outcome | Effect on Sleep |
|---|---|---|---|---|---|---|---|
| Charles et al. 2021 [34] | To evaluate the feasibility and the acceptability of a videoconference-based 6-month program promoting physical activity * To report a level of fatigue ≥ 4 on a 10-point visual analogous scale | EX group | Type: [Supervised by videoconference] articular mobilization, aerobic and resistance exercises, relaxation or stretching Frequency: 150 min/week Intensity: moderate Time: 45 to 60 min Duration: 6 months | Before (T1), 6 months at the end of the program (T2), and 3 months later (T3) | Adherence rate: 87.5% (at T2) Avg number of supervised sessions: 20.8 ± 4.8; 87% of planned sessions | ISI | Secondary outcome Descriptive statistics:
|
| Colledge et al. 2018 [32] | To compare the effects of an exercise program in aSAH population with another patient group, and a group of healthy controls | 3 EX groups | Type: [supervised once a week and unsupervised for the others] walking techniques, flexibility and motor skill learning tasks, and taught behavioral skills Frequency: 3–5 times/week Intensity: 55–65% of max HR (first 4 weeks), 65–75% (weeks 5–8), 75–85% (last 4 weeks) Time: 30 to 45 min Duration: 12 weeks | Baseline (1 week before intervention), after intervention (12 weeks), and 6-month follow-up | Adherence rate: 72% (at 12 weeks) and 67% (at 6 months follow-up) | ISI EEG | Secondary outcome Descriptive ISI scores decreased among all groups at pre-post-test, 6 months follow-up
Large ES for Time x Group founded for insomnia (ISI) Descriptively meningioma group had shorter SOL than other groups across every time point
Large ES founded for Group for the variable SOL (baseline to follow-up) |
| Eisenhut et al. 2022 [26] | To investigate the impact of endurance and strength training on symptoms of depression, feelings of stress and anxiety, fatigue, insomnia and physical fitness, compared to an active control condition | Endurance training group Strength training group | Type: [Supervised] on treadmill or bicycle Frequency: twice weekly Intensity: RPE at 11-14 Time: 35 to 45 min Duration: 6 weeks Type: [Supervised] weightlifting and resistance exercises, 3–5 sets of 10–15 rep Frequency: twice weekly Intensity: RPE at 11-15 Time: 35 to 45 min Duration: 6 weeks | Baseline, week 3 (mid-program), and week 6 (end of program) | Adherence rate: 93.16% (at 6 weeks) | ISI | Secondary outcome Descriptive statistics:
Small ES (baseline vs. week 6)
Medium ES (baseline vs. week 6) |
| Kozik et al. 2018 [33] | To determine if a structured, supervised outpatient exercise program specifically for cancer patients would be associated with improvements in insomnia and depression after attending for 10-weeks | EX group | Type: [Supervised] cardiovascular circuit training, strength training Frequency: twice weekly Intensity: None Time: 90 min Duration: 10 weeks | Baseline, after intervention (week 10) | Drop-out rate: 46.7% | Athens Insomnia Instrument | Primary outcome Significant decrease of ISI scores: 9.5 ± 3.7 at baseline to 6.3 ± 3.5, p < 0.01 |
| Mercier et al. 2018 [27] | To assess the efficacy of a 6-week home-based aerobic exercise program compared to that of a 6-week self-administered cognitive-behavioral therapy for insomnia (CBT-I) to improve sleep in cancer patients * To have insomnia symptoms, as indicated by a score of 8 or greater on the ISI | EX group | Type: [Home-based] individualized aerobic training: brisk walking, jogging, swimming or a combination of different aerobic EXs Frequency: 3–5 times/week Intensity: RPE at 3 to 5 Time: 20 to 30 min until 150 min Duration: 6 weeks | Baseline (pre-treatment), week 6 (post treatment), 3- and 6-months follow-ups | Drop-out rate: 7.3% | ISI Actigraphy | Primary outcome
NS group x time interaction founded but marginally (p = 0.06) EX intervention was significantly inferior to CBT-I at post-intervention but was non-inferior at follow-up No significant effects suggesting that both interventions had a modest impact on participants’ objective sleep. Only significant time effects from pretreatment to posttreatment were obtained in the CBT-I group only on early morning awakenings (reduction 5 min; p = 0.01) and WASO (reduction 7 min; p < 0.01). |
| Piraux et al. 2021 [29] | To investigate the effects of HIIT and RES training compared to UC on CTRF, QoL, depression, daytime sleepiness, insomnia, sleep quality, functional exercise capacity and executive function in prostate cancer patients during radiotherapy. | RES group HIIT group | Type: [supervised] Resistance training (i.e., 8 exercises of body wait, resistance bands, dumbbells, 1-3 sets of 8–12 rep) Frequency: 3 times/week Intensity: RPE at 4–6 Time: 30 to 40 min Duration: 5 or 8 weeks Type: [Supervised] on cycle ergometer (60-s work interval at 90–100 rev/min at ≥85% of THRmax and 60-s active rest at 50–60 rev/min) Frequency: 3 times/week Time: 26 to 40 min Duration: 5 or 8 weeks | Baseline (10 days before RX) and after last fraction of RX | Drop-out rate: 7.69% Attendance at EX sessions: 93.5% in HIIT group and 91.4% in RES group | ISI | Secondary outcome Descriptive statistics:
NS difference between 3 groups after exercise program for ISI scores |
| Piraux et al. 2022 [28] | To determine the feasibility of HIIT and RES during NACRT in rectal cancer patients. | RES group HIIT group | Type: [Supervised] resistance training (i.e., 8 exercises of body wait, resistance bands, dumbbells, 1–3 sets of 8–12 rep Frequency: 3 times/week Intensity: RPE at 4–6 Time: 30 to 40 min Duration: 5 weeks Type: [Supervised] on cycle ergometer or cross-trainer (60-s work interval at 90–100 rev/min at ≥85% of THRmax and 60-s active rest at 50–60 rev/min) Frequency: 3 times/week Time: 26 to 40 min Duration: 5 or 8 weeks | Baseline (10 days before NACRT) and after last fraction of RX | Adherence rate: 100% Attendance at EX sessions: 92% in HIIT group and 88% in RES group | ISI | Secondary outcome
NS difference between 3 groups after exercise program for insomnia |
| Sheehan et al. 2020 [30] | To determine the effects of a 10-week EX intervention compared with a health education intervention on fatigue, quality of life outcomes and functional fitness in cancer survivors with documented fatigue. * To experience ongoing fatigue (score < 45 points) on the FACT-F | Exercise group Health education group | Type: [Supervised and home-based] progressive aerobic training and stretching with majority of brisk walking for fatigued patients (RPE at 11–13, 66–85 HR max) Frequency: twice weekly Intensity: None Time: 60 min Duration: 10 weeks Group-based fatigue management sessions, emphasizing non-exercise strategies Time: 60 min Frequency: once weekly Duration: 10 weeks | Baseline, post intervention (week 10), follow-up at 16 weeks post-intervention (26 weeks) | Adherence rate: 100% (10 weeks) | ISI | Secondary outcome
|
| Yamada et al. 2021 [31] | To compare the effect of exercise (12 weeks) on psychosocial health in paired versus individually trained cancer patients. | Exercise in pairs and individually | Type: [Supervised], cardiovascular training, resistance training exercises (5–7 exercises), balance and flexibility Frequency: 3 times/week Intensity: 40–60% of HR reserve and 40–60% of 1-RM, RPE at 3-6 Time: 90 min Duration: 12 weeks | pre- (baseline), mid- (6-weeks), and post (12 weeks) intervention | Adherence rate: 78.5% for individually trained patients and 85.7% for paired patients | ISI | Secondary outcome
No time effect
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drozd, C.; Curtit, E.; Gillet, V.; Jacquinot, Q.; Meneveau, N.; Mougin, F. Exercise Intervention on Insomnia in Patients with a Cancer: A Systematic Review of the Literature. Cancers 2024, 16, 2241. https://doi.org/10.3390/cancers16122241
Drozd C, Curtit E, Gillet V, Jacquinot Q, Meneveau N, Mougin F. Exercise Intervention on Insomnia in Patients with a Cancer: A Systematic Review of the Literature. Cancers. 2024; 16(12):2241. https://doi.org/10.3390/cancers16122241
Chicago/Turabian StyleDrozd, Chloé, Elsa Curtit, Valérie Gillet, Quentin Jacquinot, Nathalie Meneveau, and Fabienne Mougin. 2024. "Exercise Intervention on Insomnia in Patients with a Cancer: A Systematic Review of the Literature" Cancers 16, no. 12: 2241. https://doi.org/10.3390/cancers16122241
APA StyleDrozd, C., Curtit, E., Gillet, V., Jacquinot, Q., Meneveau, N., & Mougin, F. (2024). Exercise Intervention on Insomnia in Patients with a Cancer: A Systematic Review of the Literature. Cancers, 16(12), 2241. https://doi.org/10.3390/cancers16122241