Unplanned Resections of Soft Tissue Sarcomas—Necessity of Re-Resection?
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Surgery
2.2. Radiotherapy
2.3. Chemotherapy
2.4. Statistical Analysis
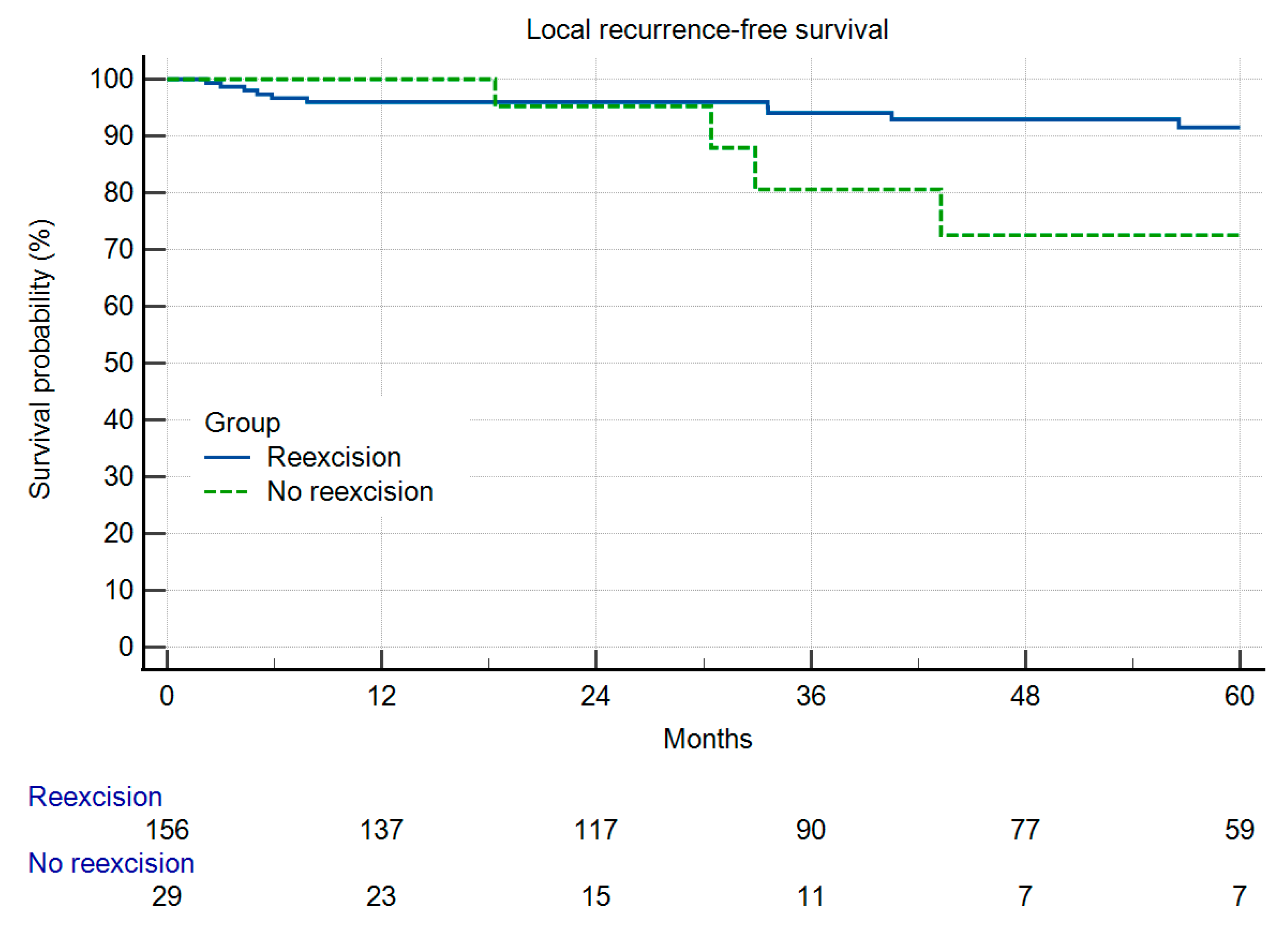
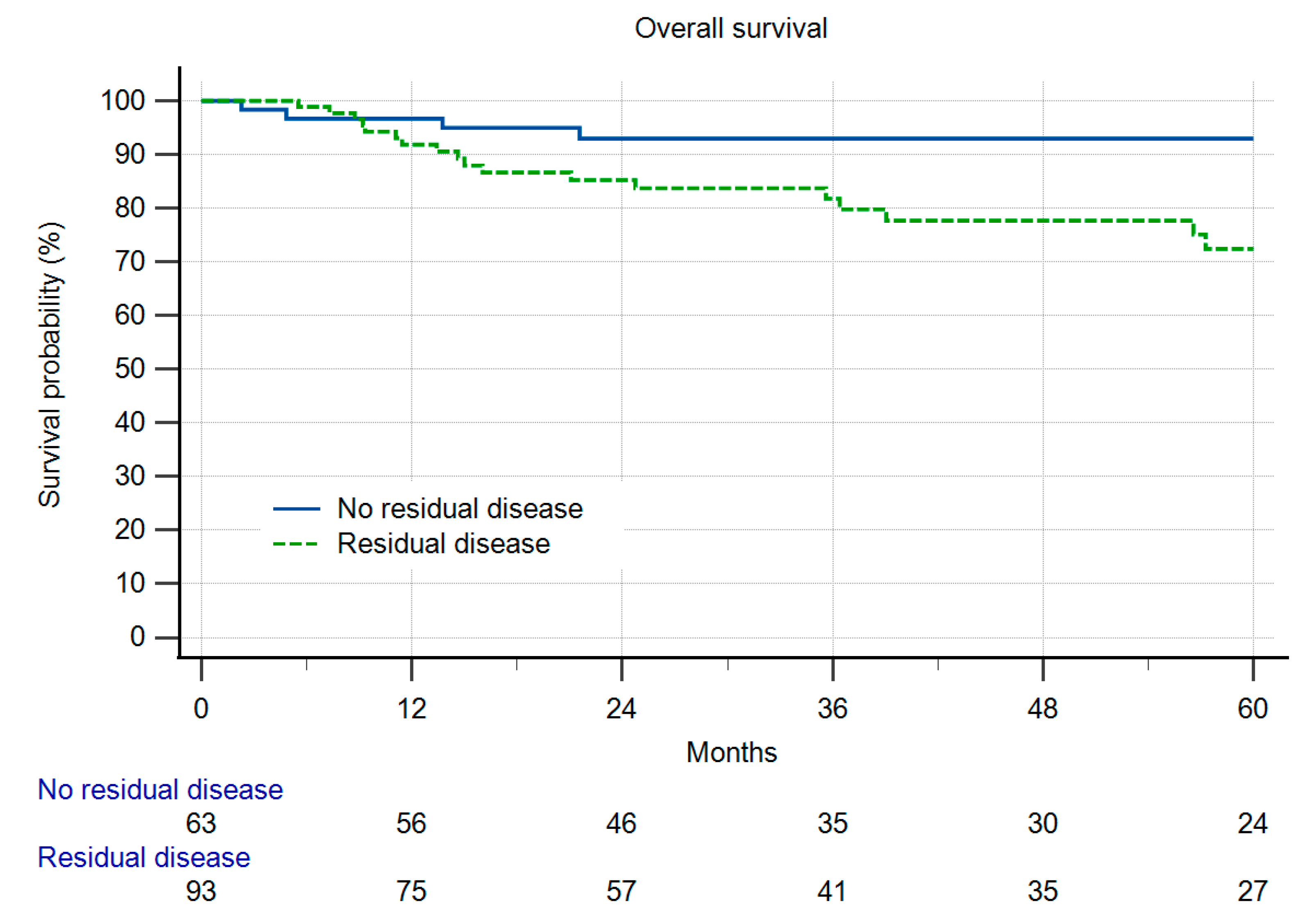
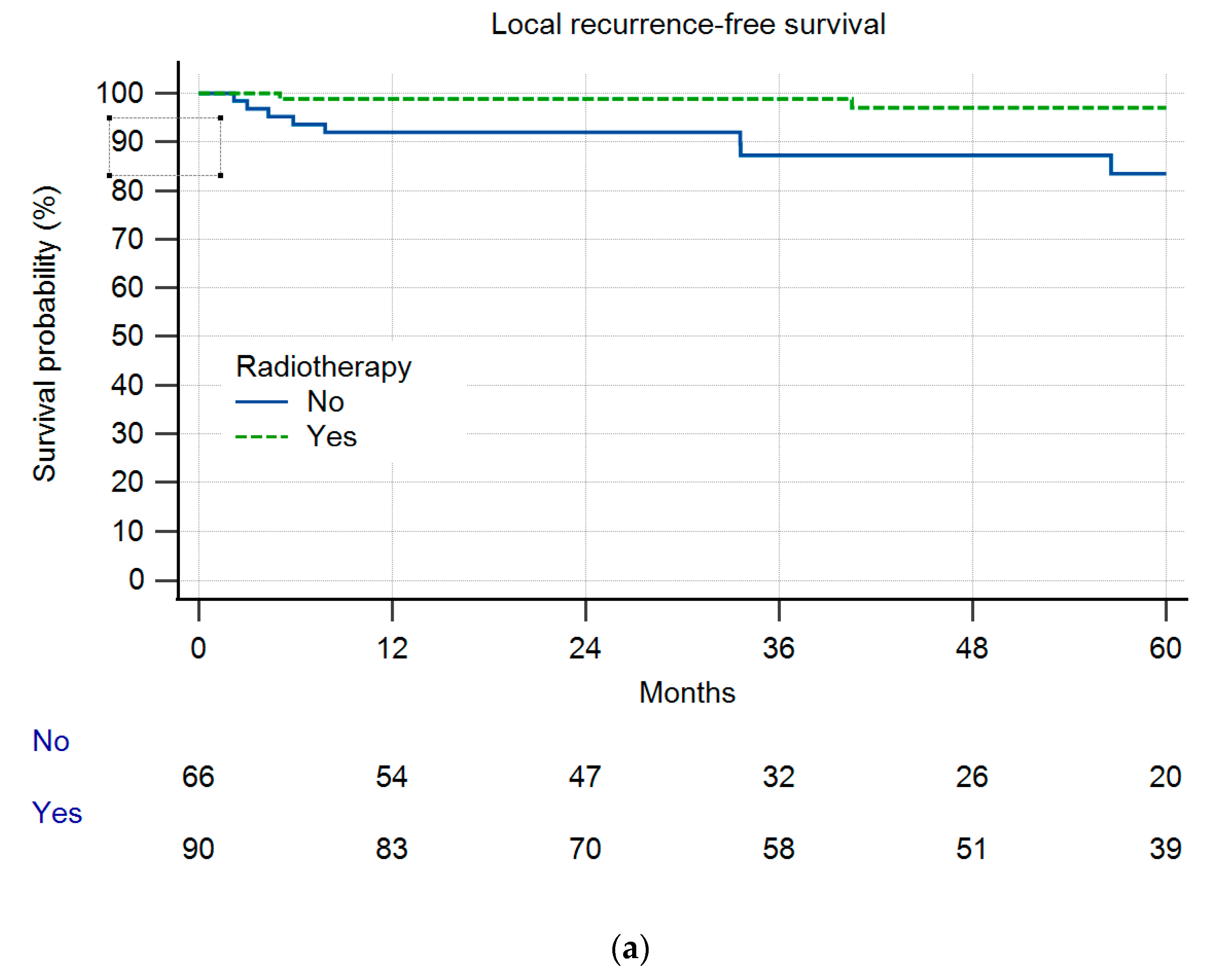
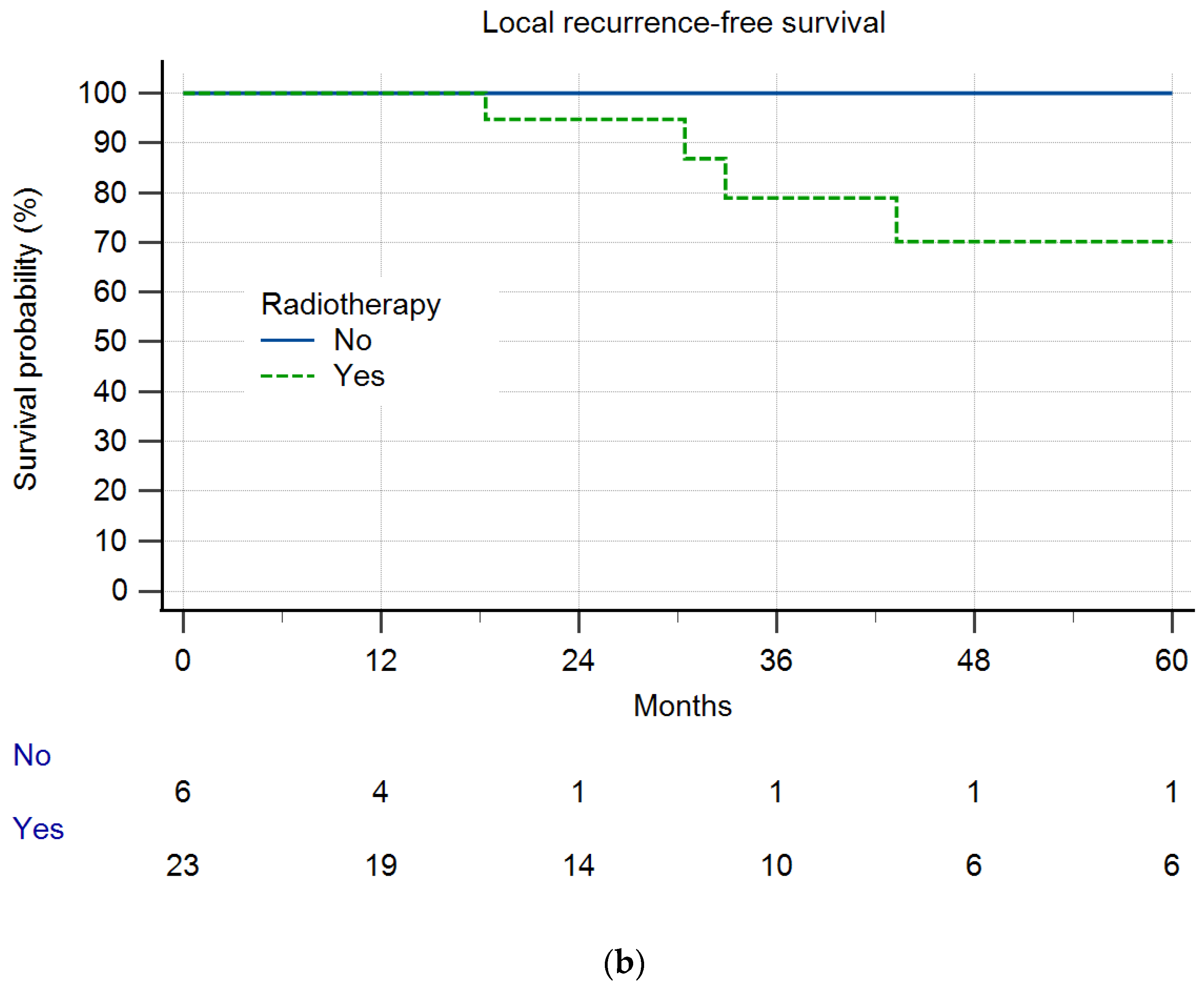
3. Results
Local Recurrence and Survival
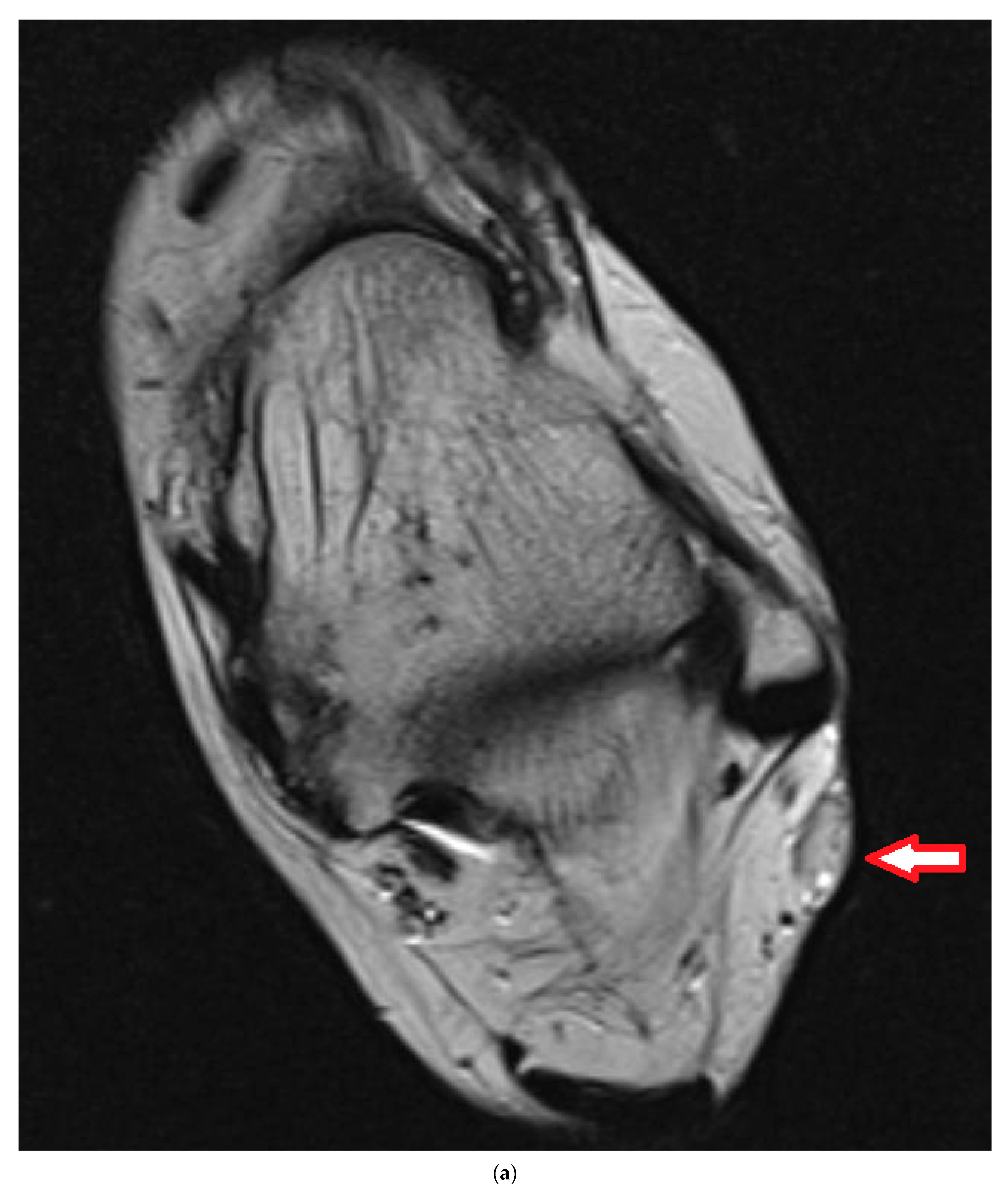
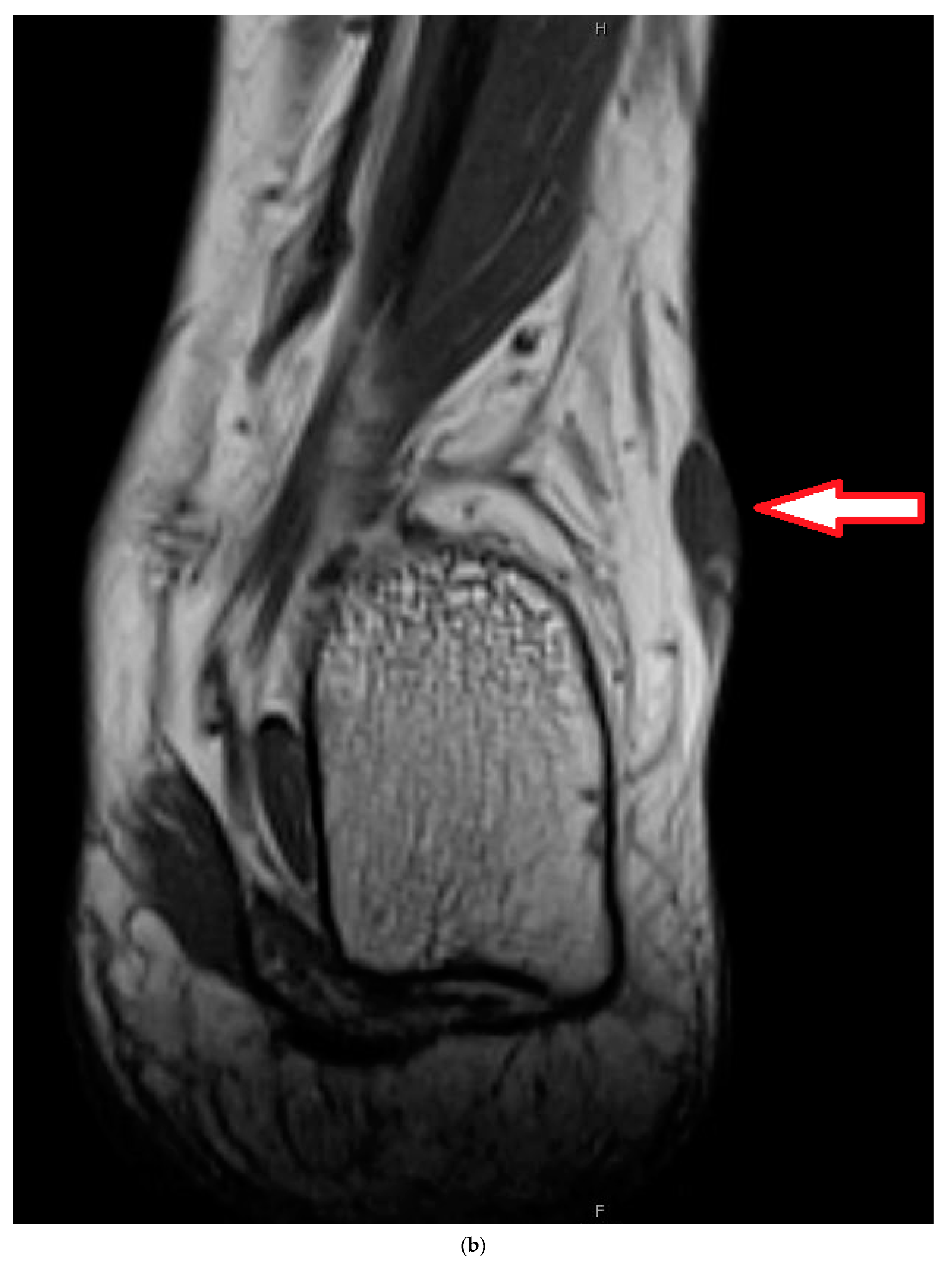
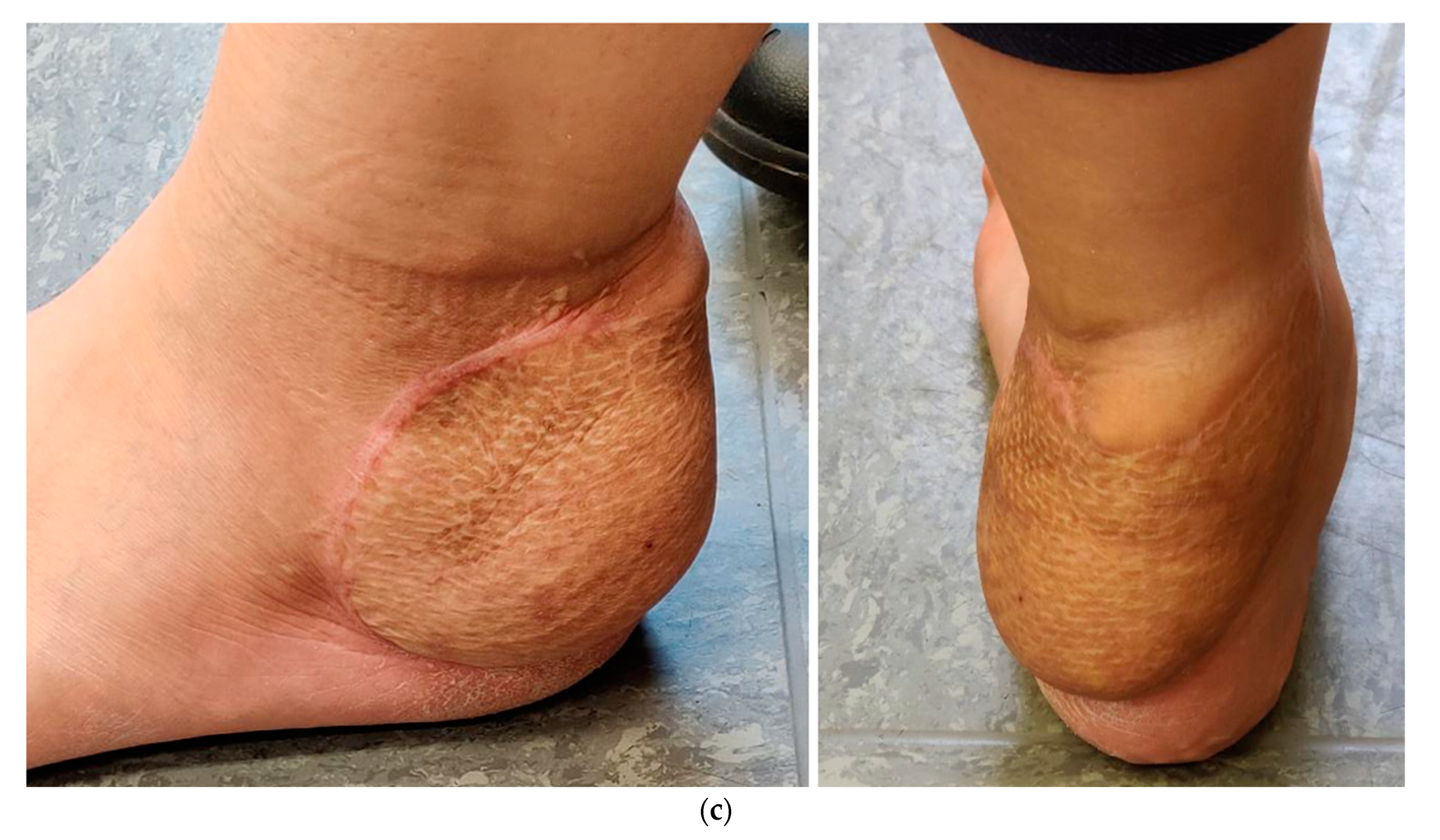
4. Illustrating Case
5. Discussion
6. Limitations of the Study
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Board, E. Soft Tissue and Bone Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2020; Volume 3. [Google Scholar]
- Arifi, S.; Belbaraka, R.; Rahhali, R.; Ismaili, N. Treatment of Adult Soft Tissue Sarcomas: An Overview. Rare Cancers Ther. 2015, 3, 69–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gronchi, A.; Miah, A.B.; Dei Tos, A.P.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Blay, J.Y.; Bolle, S.; et al. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1348–1365. [Google Scholar] [CrossRef]
- Melis, A.S.; Vos, M.; Schuurman, M.S.; van Dalen, T.; van Houdt, W.J.; van der Hage, J.A.; Schrage, Y.M.; Been, L.B.; Bonenkamp, J.B.; Bemelmans, M.H.A.; et al. Incidence of unplanned excisions of soft tissue sarcomas in the Netherlands: A population-based study. Eur. J. Surg. Oncol. 2022, 48, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Smolle, M.A.; Tunn, P.U.; Goldenitsch, E.; Posch, F.; Szkandera, J.; Bergovec, M.; Liegl-Atzwanger, B.; Leithner, A. The Prognostic Impact of Unplanned Excisions in a Cohort of 728 Soft Tissue Sarcoma Patients: A Multicentre Study. Ann. Surg. Oncol. 2017, 24, 1596–1605. [Google Scholar] [CrossRef]
- Lewis, J.J.; Leung, D.; Espat, J.; Woodruff, J.M.; Brennan, M.F. Effect of reresection in extremity soft tissue sarcoma. Ann. Surg. 2000, 231, 655–663. [Google Scholar] [CrossRef]
- Danieli, M.; Barretta, F.; Fiore, M.; Radaelli, S.; Sangalli, C.; Barisella, M.; Stacchiotti, S.; Palassini, E.; Miceli, R.; Callegaro, D.; et al. Unplanned Excision of Extremity and Trunk Wall Soft Tissue Sarcoma: To Re-resect or Not to Re-resect? Ann. Surg. Oncol. 2021, 28, 4706–4717. [Google Scholar] [CrossRef]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wust, P.; Reichardt, P.; Schem, B.C.; Abdel-Rahman, S.; Daugaard, S.; Salat, C.; Wendtner, C.M.; et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol. 2010, 11, 561–570. [Google Scholar] [CrossRef]
- Bianchi, G.; Sambri, A.; Cammelli, S.; Galuppi, A.; Cortesi, A.; Righi, A.; Caldari, E.; Ferrari, S.; Donati, D. Impact of residual disease after “unplanned excision” of primary localized adult soft tissue sarcoma of the extremities: Evaluation of 452 cases at a single Institution. Musculoskelet. Surg. 2017, 101, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Casali, P.G.; Miceli, R.; Mariani, L.; Bertulli, R.; Lozza, L.; Collini, P.; Olmi, P.; Mussi, C.; Gronchi, A. Prognostic effect of re-excision in adult soft tissue sarcoma of the extremity. Ann. Surg. Oncol. 2006, 13, 110–117. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Eilber, F.R.; Morton, D.L. The management of locally recurrent soft-tissue sarcoma. Ann. Surg. 1982, 196, 87–91. [Google Scholar] [CrossRef]
- Potter, B.K.; Adams, S.C.; Pitcher, J.D., Jr.; Temple, H.T. Local recurrence of disease after unplanned excisions of high-grade soft tissue sarcomas. Clin. Orthop. Relat. Res. 2008, 466, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, C.R.; Wafa, H.; Grimer, R.J.; Carter, S.R.; Tillman, R.M.; Abudu, A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J. Bone Jt. Surg. Br. 2008, 90, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Rehders, A.; Stoecklein, N.H.; Poremba, C.; Alexander, A.; Knoefel, W.T.; Peiper, M. Reexcision of soft tissue sarcoma: Sufficient local control but increased rate of metastasis. World J. Surg. 2009, 33, 2599–2605. [Google Scholar] [CrossRef]
- Charoenlap, C.; Imanishi, J.; Tanaka, T.; Slavin, J.; Ngan, S.Y.; Chander, S.; Dowsey, M.M.; Goyal, C.; Choong, P.F. Outcomes of unplanned sarcoma excision: Impact of residual disease. Cancer Med. 2016, 5, 980–988. [Google Scholar] [CrossRef]
- Traub, F.; Griffin, A.M.; Wunder, J.S.; Ferguson, P.C. Influence of unplanned excisions on the outcomes of patients with stage III extremity soft-tissue sarcoma. Cancer 2018, 124, 3868–3875. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Trepka, S.; Ptaszynski, K.; Kolodziejczyk, M. Surgery quality and tumor status impact on survival and local control of resectable liposarcomas of extremities or the trunk wall. Clin. Orthop. Relat. Res. 2013, 471, 860–870. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, I.; Kang, H.G.; Kang, S.C.; Choi, J.R.; Kim, H.S. Does delayed reexcision affect outcome after unplanned excision for soft tissue sarcoma? Clin. Orthop. Relat. Res. 2011, 469, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Decanter, G.; Stoeckle, E.; Honore, C.; Meeus, P.; Mattei, J.C.; Dubray-Longeras, P.; Ferron, G.; Carrere, S.; Causeret, S.; Guilloit, J.M.; et al. Watch and Wait Approach for Re-excision After Unplanned Yet Macroscopically Complete Excision of Extremity and Superficial Truncal Soft Tissue Sarcoma is Safe and Does Not Affect Metastatic Risk or Amputation Rate. Ann. Surg. Oncol. 2019, 26, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Gouin, F.; Stoeckle, E.; Honoré, C.; Ropars, M.; Jafari, M.; Mattei, J.C.; Rochwerger, A.; Carrere, S.; Waast, D.; Ferron, G.; et al. Overall survival in patients with re-excision of positive microscopic margins of limb and trunk wall soft tissue sarcoma operated outside of a reference center: A nationwide cohort analysis. BMC Cancer 2022, 22, 1034. [Google Scholar] [CrossRef]
- Alsina, A.C.; Sacchetti, F.; Kaya, H.; Yaman, B.; Tamsel, İ.; Sabah, D. Impact of the unplanned excision on the oncological outcomes of patients with soft tissue sarcomas: A single-center retrospective review of 490 patients. Acta Orthop. Traumatol. Turc. 2022, 56, 272–277. [Google Scholar] [CrossRef]
- Sacchetti, F.; Alsina, A.C.; Morganti, R.; Innocenti, M.; Andreani, L.; Muratori, F.; Scoccianti, G.; Totti, F.; Campanacci, D.A.; Capanna, R. Re-excision after unplanned excision of soft tissue sarcoma: A systematic review and metanalysis. The rationale of systematic re-excision. J. Orthop. 2021, 25, 244–251. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, W.; Huang, W.; Yan, W.; Wang, H.; Qu, G.; Wang, K.; Qu, X.; Wang, C.; Chen, Y. Optimal timing of re-excision in synovial sarcoma patients: Immediate intervention versus waiting for local recurrence. J. Surg. Oncol. 2023. [CrossRef]
- Wang, E.H.M.; Araneta, K.T.S.; Gaston, C.L.L.; Rubio, D.A.T.; de Dios, A.M.V.; Cañal, J.P.A.; Goleta-Dy, A.N.; Alcasabas, A.P.A.; Odoño, E.G.; Atun, J.M.L.; et al. Unplanned Excision of Soft Tissue Sarcomas of the Extremities in a Low-to-Middle-Income Country. Ann. Surg. Oncol. 2023, 30, 3681–3689. [Google Scholar] [CrossRef] [PubMed]
- Thacker, M.M.; Potter, B.K.; Pitcher, J.D.; Temple, H.T. Soft tissue sarcomas of the foot and ankle: Impact of unplanned excision, limb salvage, and multimodality therapy. Foot Ankle Int. 2008, 29, 690–698. [Google Scholar] [CrossRef]
- Traweek, R.S.; Martin, A.N.; Rajkot, N.F.; Guadagnolo, B.A.; Bishop, A.J.; Lazar, A.J.; Keung, E.Z.; Torres, K.E.; Hunt, K.K.; Feig, B.W.; et al. Re-excision After Unplanned Excision of Soft Tissue Sarcoma is Associated with High Morbidity and Limited Pathologic Identification of Residual Disease. Ann. Surg. Oncol. 2023, 30, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Alamanda, V.K.; Delisca, G.O.; Mathis, S.L.; Archer, K.R.; Ehrenfeld, J.M.; Miller, M.W.; Homlar, K.C.; Halpern, J.L.; Schwartz, H.S.; Holt, G.E. The financial burden of reexcising incompletely excised soft tissue sarcomas: A cost analysis. Ann. Surg. Oncol. 2013, 20, 2808–2814. [Google Scholar] [CrossRef] [PubMed]
- Saeed, H.; King, D.M.; Johnstone, C.A.; Charlson, J.A.; Hackbarth, D.A.; Neilson, J.C.; Bedi, M. Preoperative Radiation Therapy Followed by Reexcision May Improve Local Control and Progression-Free Survival in Unplanned Excisions of Soft Tissue Sarcomas of the Extremity and Chest-Wall. Int. J. Surg. Oncol. 2016, 2016, 5963167. [Google Scholar] [CrossRef] [PubMed]
- Demizu, Y.; Jin, D.; Sulaiman, N.S.; Nagano, F.; Terashima, K.; Tokumaru, S.; Akagi, T.; Fujii, O.; Daimon, T.; Sasaki, R.; et al. Particle Therapy Using Protons or Carbon Ions for Unresectable or Incompletely Resected Bone and Soft Tissue Sarcomas of the Pelvis. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 367–374. [Google Scholar] [CrossRef]
- Allignet, B.; Pou, P.; Izarn, F.; Ray-Coquard, I.; Blay, J.Y.; Dufresne, A.; Brahmi, M.; Bouhamama, A.; Meeus, P.; Vaz, G.; et al. Efficacy and Safety of Adjuvant Radiotherapy in Re-excised Soft-tissue Sarcoma After Unplanned Resection. Oncologist 2023, 28, 633–639. [Google Scholar] [CrossRef]
- Pretell-Mazzini, J.; Barton, M.D., Jr.; Conway, S.A.; Temple, H.T. Unplanned excision of soft-tissue sarcomas: Current concepts for management and prognosis. J. Bone Jt. Surg. Am. 2015, 97, 597–603. [Google Scholar] [CrossRef]
- George, A.; Grimer, R. Early symptoms of bone and soft tissue sarcomas: Could they be diagnosed earlier? Ann. R. Coll. Surg. Engl. 2012, 94, 261–266. [Google Scholar] [CrossRef] [PubMed]
- von Mehren, M.; Kane, J.M.; Bui, M.M.; Choy, E.; Connelly, M.; Dry, S.; Ganjoo, K.N.; George, S.; Gonzalez, R.J.; Heslin, M.J.; et al. NCCN Guidelines Insights: Soft Tissue Sarcoma, Version 1.2021. J. Natl. Compr. Canc Netw. 2020, 18, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Mesko, N.W.; Wilson, R.J.; Lawrenz, J.M.; Mathieu, J.L.; Ghiam, M.K.; Mathis, S.L.; Halpern, J.L.; Schwartz, H.S.; Holt, G.E. Pre-operative evaluation prior to soft tissue sarcoma excision—Why can’t we get it right? Eur. J. Surg. Oncol. 2018, 44, 243–250. [Google Scholar] [CrossRef] [PubMed]

| Reresection | No Reresection | |
|---|---|---|
| Grading | ||
| G1 | 23 (14.8%) | 6 (24%) |
| G2 | 54 (34.8%) | 8 (32%) |
| G3 | 60 (38.7%) | 11 (44%) |
| X | 19 (12.2%) | 4 (13.8%) |
| Mean age | 57 ys | 60 ys |
| Location | ||
| Superficial | 56 (35.9%) | 1 (3.4%) |
| Deep | 100 (64.1%) | 28 (96.6%) |
| Size of the lesion | ||
| Mean size (cm) | 4.5 | 10.1 |
| Entity | ||
| UPS | 43 (27.6%) | 8 (27.6%) |
| Myxofibrosarcoma | 27 (17.3%) | 2 (6.9%) |
| Leiomyosarcoma | 19 (12.2%) | 2 (6.9%) |
| Liposarcoma | 16 (10.3%) | 7 (24.1%) |
| Dermatofibrosarcoma | 13 (8.3%) | |
| Synovialsarcoma | 11 (7.1%) | 2 (6.9%) |
| MPNST | 3 (10.3%) | |
| Others | 27 (17.3%) | 5 (17.2%) |
| Radiotherapy | 90 (57.7%) | 23 (79.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fromm, J.; Klein, A.; Mentrup, F.; Lindner, L.H.; Nachbichler, S.; Holzapfel, B.M.; Goller, S.S.; Knösel, T.; Dürr, H.R. Unplanned Resections of Soft Tissue Sarcomas—Necessity of Re-Resection? Cancers 2024, 16, 1851. https://doi.org/10.3390/cancers16101851
Fromm J, Klein A, Mentrup F, Lindner LH, Nachbichler S, Holzapfel BM, Goller SS, Knösel T, Dürr HR. Unplanned Resections of Soft Tissue Sarcomas—Necessity of Re-Resection? Cancers. 2024; 16(10):1851. https://doi.org/10.3390/cancers16101851
Chicago/Turabian StyleFromm, Julian, Alexander Klein, Franziska Mentrup, Lars H. Lindner, Silke Nachbichler, Boris Michael Holzapfel, Sophia Samira Goller, Thomas Knösel, and Hans Roland Dürr. 2024. "Unplanned Resections of Soft Tissue Sarcomas—Necessity of Re-Resection?" Cancers 16, no. 10: 1851. https://doi.org/10.3390/cancers16101851
APA StyleFromm, J., Klein, A., Mentrup, F., Lindner, L. H., Nachbichler, S., Holzapfel, B. M., Goller, S. S., Knösel, T., & Dürr, H. R. (2024). Unplanned Resections of Soft Tissue Sarcomas—Necessity of Re-Resection? Cancers, 16(10), 1851. https://doi.org/10.3390/cancers16101851







