The Perfect Timing—Immediate versus Delayed Microvascular Reconstruction of the Mandible
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistics
3. Results
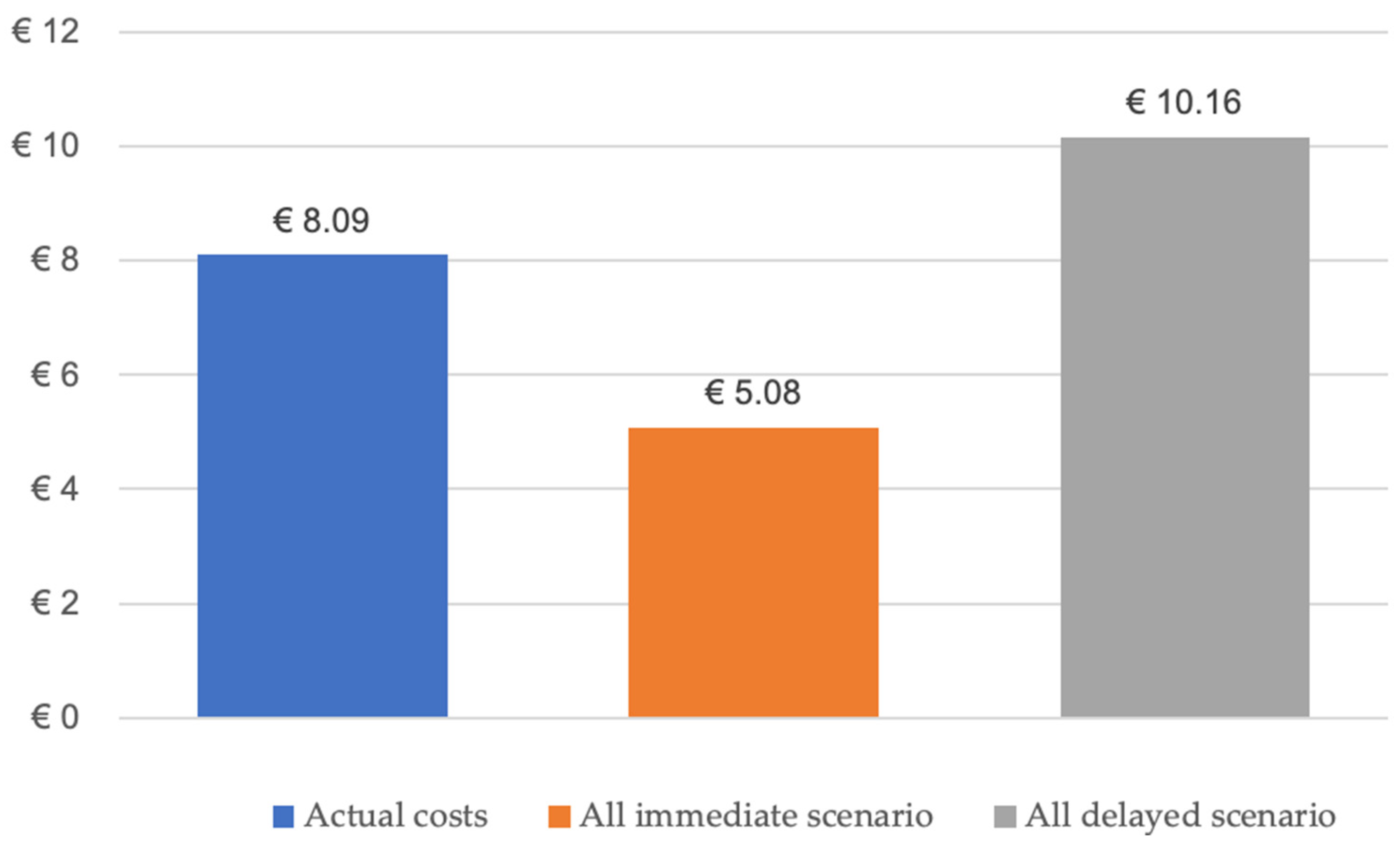
Two-Department Economic Analysis of Mandibular Reconstructions from 2010 to 2023
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kansy, K.; Mueller, A.A.; Mucke, T.; Koersgen, F.; Wolff, K.D.; Zeilhofer, H.F.; Holzle, F.; Pradel, W.; Schneider, M.; Kolk, A.; et al. Microsurgical reconstruction of the head and neck region: Current concepts of maxillofacial surgery units worldwide. J. Craniomaxillofac. Surg. 2015, 43, 1364–1368. [Google Scholar] [CrossRef]
- Thiem, D.G.E.; Siegberg, F.; Romer, P.; Blatt, S.; Pabst, A.; Heimes, D.; Al-Nawas, B.; Kammerer, P.W. Long-term donor site morbidity and flap perfusion following radial versus ulnar forearm free flap-a randomized controlled prospective clinical trial. J. Clin. Med. 2022, 11, 3601. [Google Scholar] [CrossRef] [PubMed]
- Sweeny, L.; Rosenthal, E.L.; Light, T.; Grayson, J.; Petrisor, D.; Troob, S.H.; Greene, B.J.; Carroll, W.R.; Wax, M.K. Outcomes and cost implications of microvascular reconstructions of the head and neck. Head Neck 2019, 41, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, P.W.; Al-Nawas, B. Bone reconstruction of extensive maxillomandibular defects in adults. Periodontol. 2000 2023, 93, 340–357. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, P.W.; Klein, M.O.; Moergel, M.; Gemmel, M.; Draenert, G.F. Local and systemic risk factors influencing the long-term success of angular stable alloplastic reconstruction plates of the mandible. J. Craniomaxillofac. Surg. 2014, 42, e271–e276. [Google Scholar] [CrossRef]
- Gielisch, M.W.; Siegberg, F.; Thiem, D.G.E.; Blatt, S.; Heimes, D.; Kammerer, P.W. A novel alloplastic grid reconstruction plate for the mandible—Retrospective comparative clinical analysis of failure rates and specific complications. J. Craniomaxillofac. Surg. 2023, 51, 448–453. [Google Scholar] [CrossRef]
- Hanken, H.; Wilkens, R.; Riecke, B.; Al-Dam, A.; Tribius, S.; Kluwe, L.; Smeets, R.; Heiland, M.; Eichhorn, W.; Grobe, A. Is immediate bony microsurgical reconstruction after head and neck tumor ablation associated with a higher rate of local recurrence? J. Craniomaxillofac. Surg. 2015, 43, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Goetze, E.; Moergel, M.; Gielisch, M.; Kammerer, P.W. Safety of resection margins in cad/cam-guided primarily reconstructed oral squamous cell carcinoma-a retrospective case series. Oral. Maxillofac. Surg. 2019, 23, 459–464. [Google Scholar] [CrossRef]
- Bauer, E.; Mazul, A.; Zenga, J.; Graboyes, E.M.; Jackson, R.; Puram, S.V.; Doering, M.; Pipkorn, P. Complications after soft tissue with plate vs bony mandibular reconstruction: A systematic review and meta-analysis. Otolaryngol. Head Neck Surg. 2021, 164, 501–511. [Google Scholar] [CrossRef]
- Boyd, J.B.; Mulholland, R.S.; Davidson, J.; Gullane, P.J.; Rotstein, L.E.; Brown, D.H.; Freeman, J.E.; Irish, J.C. The free flap and plate in oromandibular reconstruction: Long-term review and indications. Plast. Reconstr. Surg. 1995, 95, 1018–1028. [Google Scholar] [CrossRef]
- Baker, A.; McMahon, J.; Parmar, S. Immediate reconstruction of continuity defects of the mandible after tumor surgery. J. Oral Maxillofac. Surg. 2001, 59, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Netscher, D.T.; Meade, R.A.; Goodman, C.M.; Alford, E.L.; Stewart, M.G. Quality of life and disease-specific functional status following microvascular reconstruction for advanced (t3 and t4) oropharyngeal cancers. Plast. Reconstr. Surg. 2000, 105, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Davudov, M.M.; Harirchi, I.; Arabkheradmand, A.; Garajei, A.; Mahmudzadeh, H.; Shirkhoda, M.; Motiee-Langroudi, M.; Mirzajani, Z.; Zebardast, J.; Montazeri, A. Evaluation of quality of life in patients with oral cancer after mandibular resection: Comparing no reconstruction, reconstruction with plate, and reconstruction with flap. Medicine 2019, 98, e17431. [Google Scholar] [CrossRef]
- Awad, M.E.; Altman, A.; Elrefai, R.; Shipman, P.; Looney, S.; Elsalanty, M. The use of vascularized fibula flap in mandibular reconstruction; a comprehensive systematic review and meta-analysis of the observational studies. J. Craniomaxillofac. Surg. 2019, 47, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.M.; Turrini, O.; Parikh, P.; House, M.G.; Zyromski, N.J.; Nakeeb, A.; Howard, T.J.; Pitt, H.A.; Lillemoe, K.D. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: A single-institution experience. Arch. Surg. 2010, 145, 634–640. [Google Scholar] [CrossRef]
- Rendenbach, C.; Holterhoff, N.; Hischke, S.; Kreutzer, K.; Smeets, R.; Assaf, A.T.; Heiland, M.; Wikner, J. Free flap surgery in europe: An interdisciplinary survey. Int. J. Oral Maxillofac. Surg. 2018, 47, 676–682. [Google Scholar] [CrossRef]
- Lin, C.H.; Kang, C.J.; Tsao, C.K.; Wallace, C.G.; Lee, L.Y.; Lin, C.Y.; Wang, H.M.; Ng, S.H.; Yen, T.C.; Liao, C.T. Priority of fibular reconstruction in patients with oral cavity cancer undergoing segmental mandibulectomy. PLoS ONE 2014, 9, e94315. [Google Scholar] [CrossRef]
- Mucke, T.; Wolff, K.D.; Wagenpfeil, S.; Mitchell, D.A.; Holzle, F. Immediate microsurgical reconstruction after tumor ablation predicts survival among patients with head and neck carcinoma. Ann. Surg. Oncol. 2010, 17, 287–295. [Google Scholar] [CrossRef]
- Kwok, A.C.; Agarwal, J.P. An analysis of free flap failure using the acs nsqip database. Does flap site and flap type matter? Microsurgery 2017, 37, 531–538. [Google Scholar] [CrossRef]
- Ishimaru, M.; Ono, S.; Suzuki, S.; Matsui, H.; Fushimi, K.; Yasunaga, H. Risk factors for free flap failure in 2,846 patients with head and neck cancer: A national database study in japan. J. Oral Maxillofac. Surg. 2016, 74, 1265–1270. [Google Scholar] [CrossRef]
- Wong, A.K.; Joanna Nguyen, T.; Peric, M.; Shahabi, A.; Vidar, E.N.; Hwang, B.H.; Niknam Leilabadi, S.; Chan, L.S.; Urata, M.M. Analysis of risk factors associated with microvascular free flap failure using a multi-institutional database. Microsurgery 2015, 35, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Offodile, A.C., 2nd; Aherrera, A.; Wenger, J.; Rajab, T.K.; Guo, L. Impact of increasing operative time on the incidence of early failure and complications following free tissue transfer? A risk factor analysis of 2,008 patients from the acs-nsqip database. Microsurgery 2017, 37, 12–20. [Google Scholar] [CrossRef]
- Thiem, D.G.E.; Romer, P.; Blatt, S.; Al-Nawas, B.; Kammerer, P.W. New approach to the old challenge of free flap monitoring-hyperspectral imaging outperforms clinical assessment by earlier detection of perfusion failure. J. Pers. Med. 2021, 11, 1101. [Google Scholar] [CrossRef] [PubMed]
- DeSerres, J.J.; Barber, B.R.; Seikaly, H.; Harris, J.R.; O’Connell, D.A. Free flap elevation times in head and neck reconstruction using the harmonic scalpel shears. Plast. Reconstr. Surg. Glob. Open 2016, 4, e718. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, T.; Harii, K.; Asato, H.; Takushima, A.; Ebihara, S.; Kimata, Y.; Yamada, A.; Ueda, K.; Ichioka, S. Analytic review of 2372 free flap transfers for head and neck reconstruction following cancer resection. J. Reconstr. Microsurg. 2003, 19, 363–368; discussion 369. [Google Scholar] [CrossRef]
- Corbitt, C.; Skoracki, R.J.; Yu, P.; Hanasono, M.M. Free flap failure in head and neck reconstruction. Head Neck 2014, 36, 1440–1445. [Google Scholar] [CrossRef]
- Rosado, P.; Cheng, H.T.; Wu, C.M.; Wei, F.C. Influence of diabetes mellitus on postoperative complications and failure in head and neck free flap reconstruction: A systematic review and meta-analysis. Head Neck 2015, 37, 615–618. [Google Scholar] [CrossRef]
- Pattani, K.M.; Byrne, P.; Boahene, K.; Richmon, J. What makes a good flap go bad? A critical analysis of the literature of intraoperative factors related to free flap failure. Laryngoscope 2010, 120, 717–723. [Google Scholar] [CrossRef]
- Yu, P.; Chang, D.W.; Miller, M.J.; Reece, G.; Robb, G.L. Analysis of 49 cases of flap compromise in 1310 free flaps for head and neck reconstruction. Head Neck 2009, 31, 45–51. [Google Scholar] [CrossRef]
- Crawley, M.B.; Sweeny, L.; Ravipati, P.; Heffelfinger, R.; Krein, H.; Luginbuhl, A.; Goldman, R.; Curry, J. Factors associated with free flap failures in head and neck reconstruction. Otolaryngol. Head Neck Surg. 2019, 161, 598–604. [Google Scholar] [CrossRef]
- Ritschl, L.M.; Mucke, T.; Hart, D.; Unterhuber, T.; Kehl, V.; Wolff, K.D.; Fichter, A.M. Retrospective analysis of complications in 190 mandibular resections and simultaneous reconstructions with free fibula flap, iliac crest flap or reconstruction plate: A comparative single centre study. Clin. Oral Investig. 2021, 25, 2905–2914. [Google Scholar] [CrossRef]
- Taghizadeh, R.; Moustaki, M.; Harris, S.; Roblin, P.; Farhadi, J. Does post-mastectomy radiotherapy affect the outcome and prevalence of complications in immediate diep breast reconstruction? A prospective cohort study. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 1379–1385. [Google Scholar] [CrossRef]
- Miller, H.; Bush, K.; Delancy, M.; Leo, N.; Joshi, H.; Saracco, B.; Adams, A.; Gaughan, J.; Bonawitz, S. Effect of preoperative radiation on free flap outcomes for head and neck reconstruction: An updated systematic review and meta-analysis. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 743–752. [Google Scholar] [CrossRef]
- Mijiti, A.; Kuerbantayi, N.; Zhang, Z.Q.; Su, M.Y.; Zhang, X.H.; Huojia, M. Influence of preoperative radiotherapy on head and neck free-flap reconstruction: Systematic review and meta-analysis. Head Neck 2020, 42, 2165–2180. [Google Scholar] [CrossRef] [PubMed]
- Benatar, M.J.; Dassonville, O.; Chamorey, E.; Poissonnet, G.; Ettaiche, M.; Pierre, C.S.; Benezery, K.; Hechema, R.; Demard, F.; Santini, J.; et al. Impact of preoperative radiotherapy on head and neck free flap reconstruction: A report on 429 cases. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Tall, J.; Bjorklund, T.C.; Skogh, A.C.; Arnander, C.; Halle, M. Vascular complications after radiotherapy in head and neck free flap reconstruction: Clinical outcome related to vascular biology. Ann. Plast. Surg. 2015, 75, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Kiener, J.L.; Hoffman, W.Y.; Mathes, S.J. Influence of radiotherapy on microvascular reconstruction in the head and neck region. Am. J. Surg. 1991, 162, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Sams, A. Histological changes in the larger blood vessels of the hind limb of the mouse after x-irradiation. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1965, 9, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Guelinckx, P.J.; Boeckx, W.D.; Fossion, E.; Gruwez, J.A. Scanning electron microscopy of irradiated recipient blood vessels in head and neck free flaps. Plast. Reconstr. Surg. 1984, 74, 217–226. [Google Scholar] [CrossRef]
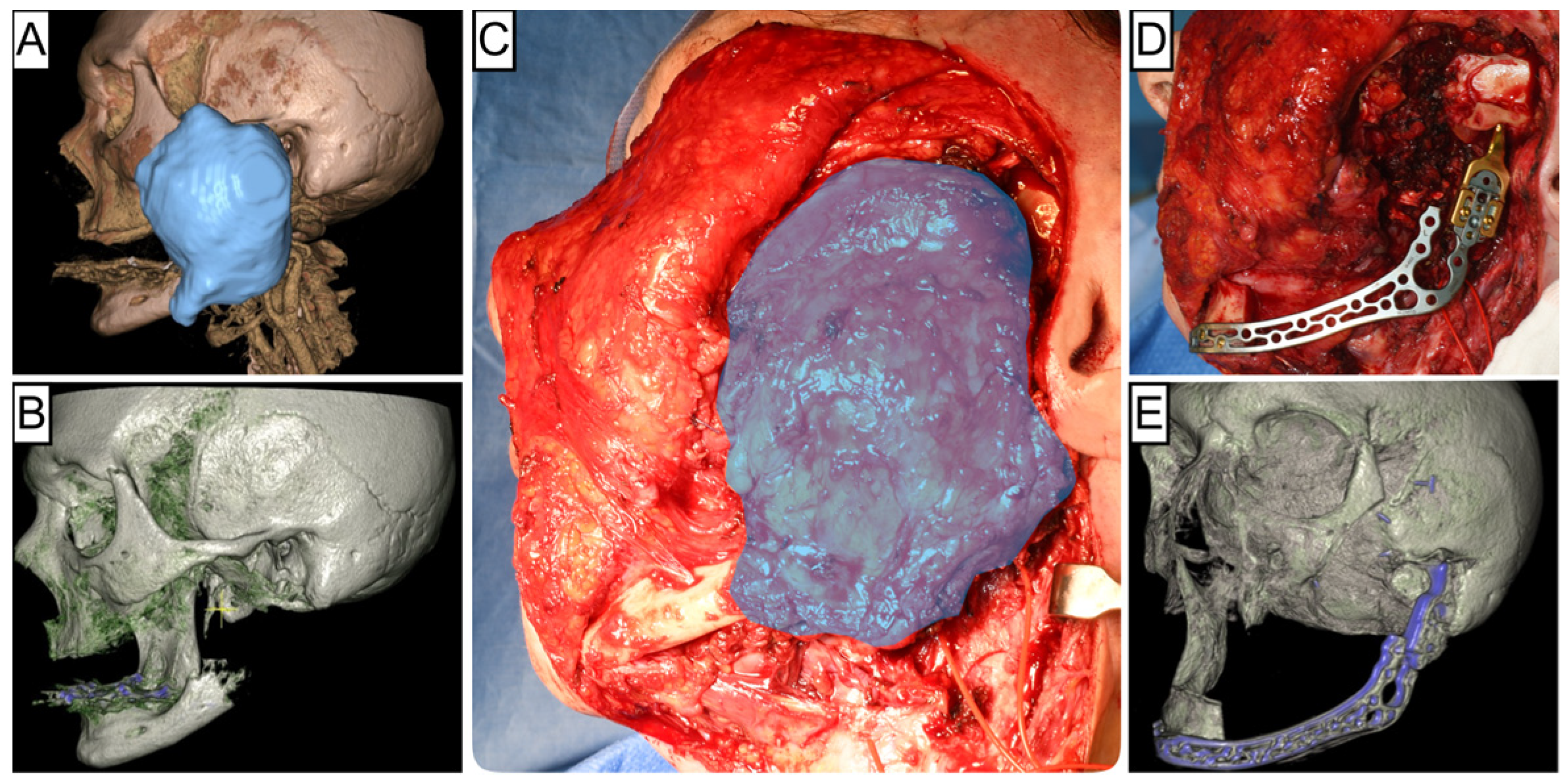
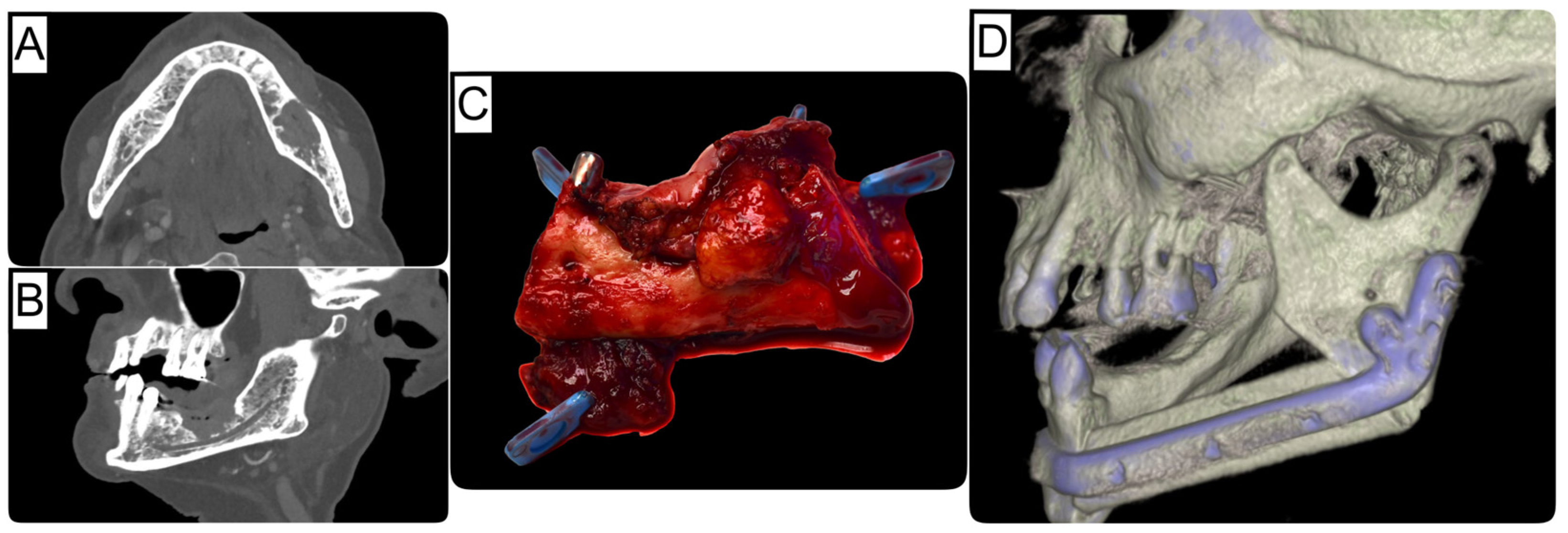
| Immediate Reconstruction (n = 72) | Delayed Reconstruction (n = 105) | ||||
|---|---|---|---|---|---|
| Mean (min/max) | N (%) | Mean (min/max) | N (%) | ||
| Age at reconstruction | 59 (14/83) | 59 (18/87) | |||
| Gender | male | 47 (65.3) | 84 (80.0) | ||
| female | 25 (34.7) | 21 (20.0) | |||
| Flap type | fibula flap | 55 (76.4) | 71 (67.6) | ||
| scapula | 9 (12.5) | 20 (19.0) | |||
| DCIAF | 7 (9.7) | 14 (13.3) | |||
| MFCF | 1 (1.4) | 0 | |||
| Adjuvant XRT | no | 17 (23.6) | 16 (15.2) | ||
| yes | 55 (76.4) | 89 (84.8) | |||
| Adjuvant chemotherapy | no | 38 (52.8) | 48 (45.7) | ||
| yes | 34 (47.2) | 57 (54.3) | |||
| Reconstruction on XRT | no | 68 (94.4) | 16 (15.2) | ||
| yes | 4 (5.6) | 89 (84.8) | |||
| Local recurrence | no | 43 (76.8) | 55 (75.3) | ||
| yes | 13 (23.2) | 18 (24.7) | |||
| Leading Diagnosis | Immediate Reconstruction, N (%) | Delayed Reconstruction, N (%) | Total, N (%) |
|---|---|---|---|
| OSCC | 57 (79.2) | 76 (72.4) | 133 (75.1) |
| Ameloblastoma | 1 (1.4) | 4 (3.8) | 5 (2.8) |
| ACC | 0 | 3 (2.9) | 3 (1.7) |
| Giant cell granuloma | 1 (1.4) | 0 | 1 (0.6) |
| Osteosarcoma | 0 | 1 (1.0) | 1 (0.6) |
| Ossifying fibroma | 1 (1.4) | 1 (1.0) | 2 (1.1) |
| Keratocyst | 0 | 1 (1.0) | 1 (0.6) |
| ORN | 8 (11.1) | 11 (10.5) | 19 (10.7) |
| Pseudarthrosis | 1 (1.4) | 0 | 1 (0.6) |
| Defect | 1 (1.4) | 5 (4.8) | 6 (3.4) |
| ARONJ | 2 (2.8) | 3 (2.9) | 5 (2.8) |
| Total | 72 (100) | 105 (100) |
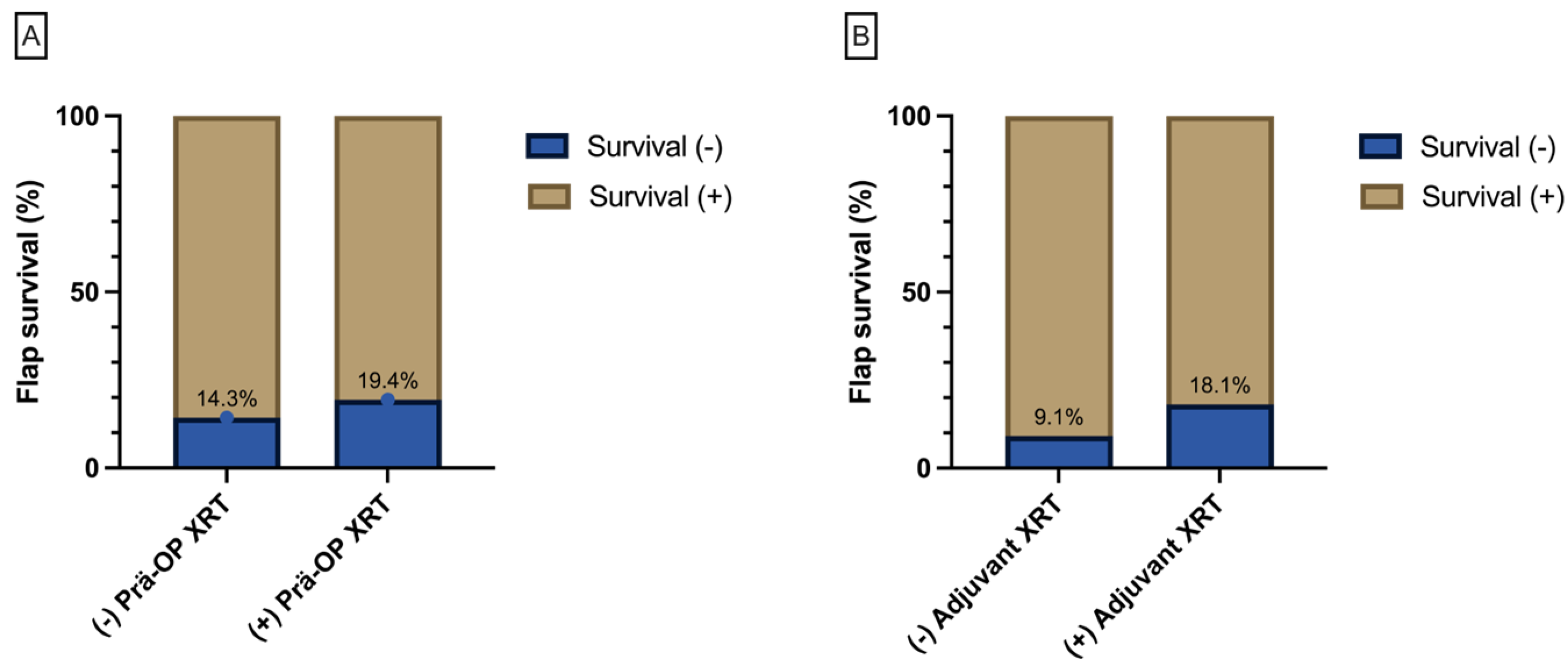
| Reconstruction Timing | Flap Survival | Total | Chi-Square (P) | |||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| University | A | Immediate Reconstruction | 6 (11.3%) | 47 (88.7%) | 53 (100%) (55%) | 0.74 |
| Delayed Reconstruction | 8 (18.2%) | 36 (81.8%) | 44 (100%) (45%) | |||
| University | B | Immediate Reconstruction | 5 (26.3%) | 14 (73.7%) | 19 (100%) | |
| Delayed Reconstruction | 10 (16.4%) | 51 (83.6%) | 61 (100%) | |||
| Total | 29 (16.4%) | 148 (83.6%) | 177 (100%) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiem, D.G.E.; Siegberg, F.; Vinayahalingam, S.; Blatt, S.; Krüger, M.; Lethaus, B.; Al-Nawas, B.; Zimmerer, R.; Kämmerer, P.W. The Perfect Timing—Immediate versus Delayed Microvascular Reconstruction of the Mandible. Cancers 2024, 16, 974. https://doi.org/10.3390/cancers16050974
Thiem DGE, Siegberg F, Vinayahalingam S, Blatt S, Krüger M, Lethaus B, Al-Nawas B, Zimmerer R, Kämmerer PW. The Perfect Timing—Immediate versus Delayed Microvascular Reconstruction of the Mandible. Cancers. 2024; 16(5):974. https://doi.org/10.3390/cancers16050974
Chicago/Turabian StyleThiem, Daniel G. E., Fabia Siegberg, Shankeeth Vinayahalingam, Sebastian Blatt, Maximilian Krüger, Bernd Lethaus, Bilal Al-Nawas, Rüdiger Zimmerer, and Peer W. Kämmerer. 2024. "The Perfect Timing—Immediate versus Delayed Microvascular Reconstruction of the Mandible" Cancers 16, no. 5: 974. https://doi.org/10.3390/cancers16050974
APA StyleThiem, D. G. E., Siegberg, F., Vinayahalingam, S., Blatt, S., Krüger, M., Lethaus, B., Al-Nawas, B., Zimmerer, R., & Kämmerer, P. W. (2024). The Perfect Timing—Immediate versus Delayed Microvascular Reconstruction of the Mandible. Cancers, 16(5), 974. https://doi.org/10.3390/cancers16050974






