Surgical Resection in Colorectal Liver Metastasis: An Umbrella Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction and Statistical Analysis
2.4. Data Analysis
2.5. Quality Assessment
3. Results
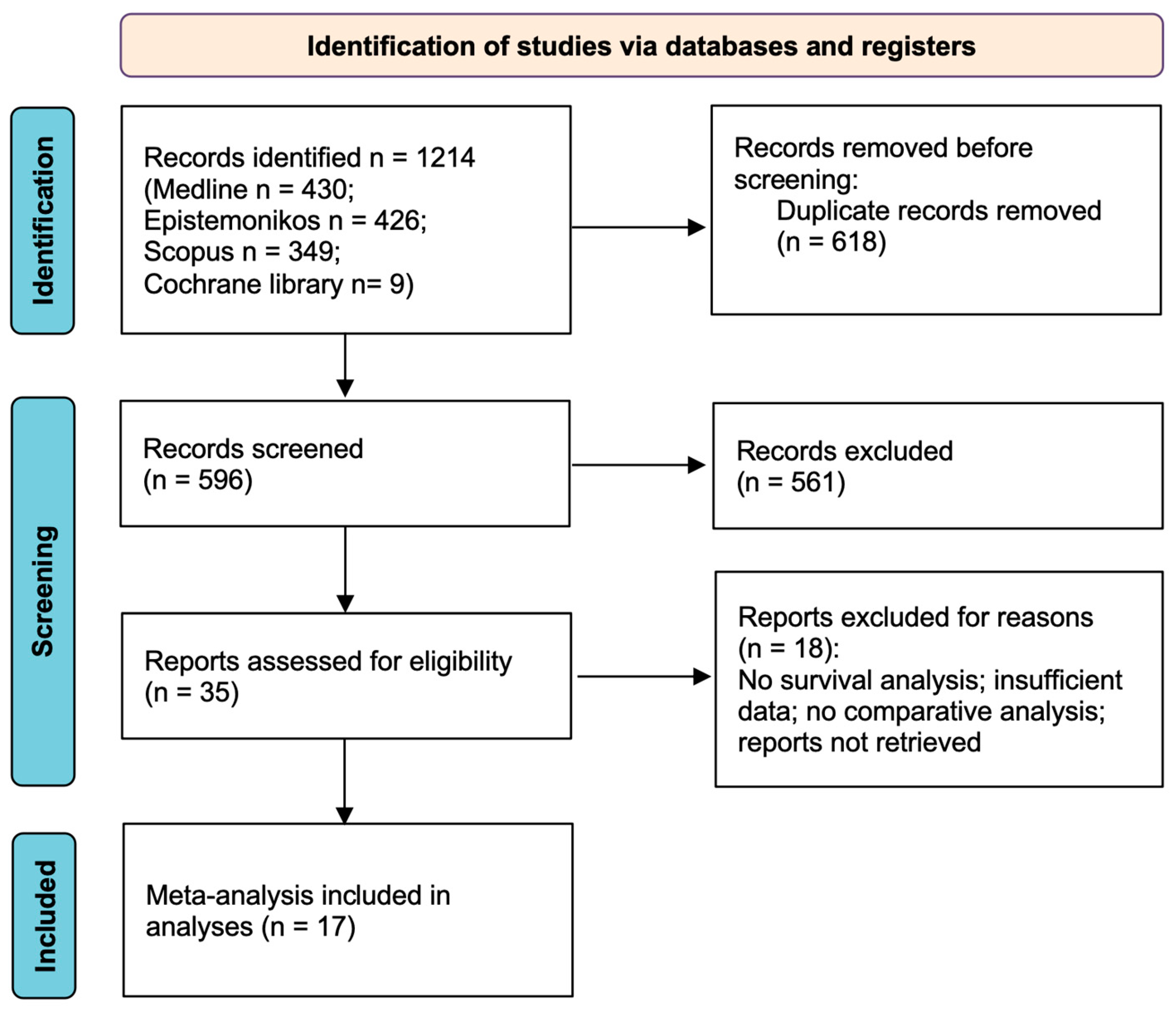
3.1. Study Selection
3.2. Quality Assessment Results
3.3. Study Characteristics
3.4. Simultaneous Resection vs. Classic Staged (“Bowel-First”) Resection
3.5. Simultaneous Resection vs. “Liver-First” Surgery
3.6. Liver-First Surgery vs. Bowel-First Surgery
3.7. Anatomic vs. Non-Anatomic Surgery
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, M.; Carioli, G.; Bertuccio, P.; Boffetta, P.; Levi, F.; La Vecchia, C.; Negri, E. European Cancer Mortality Predictions for the Year 2018 with Focus on Colorectal Cancer. Ann. Oncol. 2018, 29, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic Colorectal Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up☆. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Hajibandeh, S.; Hajibandeh, S.; Sultana, A.; Ferris, G.; Mwendwa, J.; Mohamedahmed, A.Y.Y.; Zaman, S.; Peravali, R. Simultaneous versus Staged Colorectal and Hepatic Resections for Colorectal Cancer with Synchronous Hepatic Metastases: A Meta-Analysis of Outcomes and Clinical Characteristics. Int. J. Color. Dis. 2020, 35, 1629–1650. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C.; McArdle, C.S. Epidemiology of Colorectal Liver Metastases. Surg. Oncol. 2007, 16, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2019 for the Treatment of Colorectal Cancer. Int J Clin Oncol 2020, 25, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Messersmith, W.A. NCCN Guidelines Updates: Management of Metastatic Colorectal Cancer. J. Natl. Compr. Canc Netw. 2019, 17, 599–601. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.; Fortner, J.; Sun, R.L.; Brennan, M.F.; Blumgart, L.H. Clinical Score for Predicting Recurrence after Hepatic Resection for Metastatic Colorectal Cancer: Analysis of 1001 Consecutive Cases. Ann. Surg. 1999, 230, 309–318; discussion 318–321. [Google Scholar] [CrossRef] [PubMed]
- Yaqub, S.; Margonis, G.A.; Søreide, K. Staged or Simultaneous Surgery for Colon or Rectal Cancer with Synchronous Liver Metastases: Implications for Study Design and Clinical Endpoints. Cancers 2023, 15, 2177. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Hillingsø, J.G.; Wille-Jørgensen, P. Staged or Simultaneous Resection of Synchronous Liver Metastases from Colorectal Cancer—A Systematic Review. Color. Dis. 2009, 11, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Q.; Wang, C.; Zhu, H.; Shi, Y.; Zhao, G. Simultaneous vs. Staged Resection for Synchronous Colorectal Liver Metastases: A Metaanalysis. Int. J. Color. Dis. 2011, 26, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Sui, C.-J.; Cao, L.; Li, B.; Yang, J.-M.; Wang, S.-J.; Su, X.; Zhou, Y.-M. Anatomical versus Nonanatomical Resection of Colorectal Liver Metastases: A Meta-Analysis. Int. J. Color. Dis. 2012, 27, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, K.; Duan, J.; Li, Z.; Su, C.; Yang, J. Meta-Analysis of Simultaneous versus Staged Resection for Synchronous Colorectal Liver Metastases. Hepatol. Res. 2013, 43, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Liu, C.; Chen, Y.; Bai, Y.; Shang, C.; Yin, R.; Yin, D.; Wang, J. Timing of Hepatectomy in Resectable Synchronous Colorectal Liver Metastases (SCRLM): Simultaneous or Delayed? Hepatology 2013, 57, 2346–2357. [Google Scholar] [CrossRef] [PubMed]
- Bijukchhe, S.M.; Heping, L.; Tao, L. Comparison between Simultaneous Resection and Staged Resection of Synchronous Colorectal Cancer with Resectable Liver Metastases: A Meta-Analysis. Eur. Surg. 2014, 46, 216–225. [Google Scholar] [CrossRef]
- Feng, Q.; Wei, Y.; Zhu, D.; Ye, L.; Lin, Q.; Li, W.; Qin, X.; Lyu, M.; Xu, J. Timing of Hepatectomy for Resectable Synchronous Colorectal Liver Metastases: For Whom Simultaneous Resection Is More Suitable—A Meta-Analysis. PLoS ONE 2014, 9, e104348. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.; Spolverato, G.; Le, G.; Mavros, M.; Doyle, F.; Pawlik, T.; Winter, D.C. Synchronous Colorectal Liver Metastasis: A Network Meta-Analysis Review Comparing Classical, Combined, and Liver-First Surgical Strategies. J. Surg. Oncol. 2014, 111. [Google Scholar] [CrossRef]
- Tang, H.; Li, B.; Zhang, H.; Dong, J.; Lu, W. Comparison of Anatomical and Nonanatomical Hepatectomy for Colorectal Liver Metastasis: A Meta-Analysis of 5207 Patients. Sci. Rep. 2016, 6, 32304. [Google Scholar] [CrossRef]
- Gavriilidis, P.; Sutcliffe, R.P.; Hodson, J.; Marudanayagam, R.; Isaac, J.; Azoulay, D.; Roberts, K.J. Simultaneous versus Delayed Hepatectomy for Synchronous Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. HPB 2018, 20, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Li, H.; Jia, G.; Fang, D.; Tang, Y.; Xie, J.; Chen, K.; Chen, Z. Parenchymal-Sparing versus Extended Hepatectomy for Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cancer Med. 2019, 8, 6165–6175. [Google Scholar] [CrossRef] [PubMed]
- Ghiasloo, M.; Pavlenko, D.; Verhaeghe, M.; Van Langenhove, Z.; Uyttebroek, O.; Berardi, G.; Troisi, R.I.; Ceelen, W. Surgical Treatment of Stage IV Colorectal Cancer with Synchronous Liver Metastases: A Systematic Review and Network Meta-Analysis. Eur. J. Surg. Oncol. 2020, 46, 1203–1213. [Google Scholar] [CrossRef]
- Magouliotis, D.E.; Tzovaras, G.; Diamantis, A.; Tasiopoulou, V.S.; Zacharoulis, D. A Meta-Analysis of Liver-First versus Classical Strategy for Synchronous Colorectal Liver Metastases. Int. J. Color. Dis. 2020, 35, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xia, Y.; Pan, X.; Yan, Z.; Zhang, L.; Yang, Z.; Wu, Y.; Xue, H.; Bai, S.; Shen, F.; et al. Simultaneous versus Staged Major Hepatectomy (≥3 Liver Segments) for Outcomes of Synchronous Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cancer Rep. 2022, 5, e1617. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, L.; Tang, J.; Sun, W.; Li, Z. Safety and Long-Term Prognosis of Simultaneous versus Staged Resection in Synchronous Colorectal Cancer with Liver Metastasis: A Systematic Review and Meta-Analysis. Eur. J. Med. Res. 2022, 27, 297. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, Y.; Hao, M.; Li, H.; Liang, X.; Yuan, D.; Ding, L. Clinical Outcomes of Parenchymal-Sparing versus Anatomic Resection for Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. World J. Surg. Oncol. 2023, 21, 241. [Google Scholar] [CrossRef]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal Cancer Liver Metastases—A Population-Based Study on Incidence, Management and Survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef]
- Kopetz, S.; Chang, G.J.; Overman, M.J.; Eng, C.; Sargent, D.J.; Larson, D.W.; Grothey, A.; Vauthey, J.-N.; Nagorney, D.M.; McWilliams, R.R. Improved Survival in Metastatic Colorectal Cancer Is Associated wth Adoption of Hepatic Resection and Improved Chemotherapy. J. Clin. Oncol. 2009, 27, 3677–3683. [Google Scholar] [CrossRef]
- Brouquet, A.; Abdalla, E.K.; Kopetz, S.; Garrett, C.R.; Overman, M.J.; Eng, C.; Andreou, A.; Loyer, E.M.; Madoff, D.C.; Curley, S.A.; et al. High Survival Rate After Two-Stage Resection of Advanced Colorectal Liver Metastases: Response-Based Selection and Complete Resection Define Outcome. J. Clin. Oncol. 2011, 29, 1083–1090. [Google Scholar] [CrossRef]
- de Ridder, J.A.M.; van der Stok, E.P.; Mekenkamp, L.J.; Wiering, B.; Koopman, M.; Punt, C.J.A.; Verhoef, C.; de Wilt, J.H. Management of Liver Metastases in Colorectal Cancer Patients: A Retrospective Case-Control Study of Systemic Therapy versus Liver Resection. Eur. J. Cancer 2016, 59, 13–21. [Google Scholar] [CrossRef] [PubMed]
- House, M.G.; Ito, H.; Gönen, M.; Fong, Y.; Allen, P.J.; DeMatteo, R.P.; Brennan, M.F.; Blumgart, L.H.; Jarnagin, W.R.; D’Angelica, M.I. Survival after Hepatic Resection for Metastatic Colorectal Cancer: Trends in Outcomes for 1600 Patients during Two Decades at a Single Institution. J. Am. Coll. Surg. 2010, 210, 744–752, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Wells, K.O.; Senagore, A. Minimally Invasive Colon Cancer Surgery. Surg. Oncol. Clin. N. Am. 2019, 28, 285–296. [Google Scholar] [CrossRef] [PubMed]
- The COlon cancer Laparoscopic or Open Resection Study Group. Laparoscopic Surgery versus Open Surgery for Colon Cancer: Short-Term Outcomes of a Randomised Trial. Lancet Oncol. 2005, 6, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Polat, F.; Willems, L.H.; Dogan, K.; Rosman, C. The Oncological and Surgical Safety of Robot-Assisted Surgery in Colorectal Cancer: Outcomes of a Longitudinal Prospective Cohort Study. Surg. Endosc. 2019, 33, 3644–3655. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, H.; Guan, X.-D.; Cai, C.-N.; Yang, L.-K.; Li, Y.-C.; Zhu, Y.-H.; Li, P.-P.; Liu, X.-L.; Yang, D.-J. The Impact of Laparoscopic Converted to Open Colectomy on Short-Term and Oncologic Outcomes for Colon Cancer. J. Gastrointest. Surg. 2015, 19, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Mentha, G.; Majno, P.; Terraz, S.; Rubbia-Brandt, L.; Gervaz, P.; Andres, A.; Allal, A.S.; Morel, P.; Roth, A.D. Treatment Strategies for the Management of Advanced Colorectal Liver Metastases Detected Synchronously with the Primary Tumour. Eur. J. Surg. Oncol. 2007, 33 (Suppl. 2), S76–S83. [Google Scholar] [CrossRef] [PubMed]
- Thelen, A.; Jonas, S.; Benckert, C.; Spinelli, A.; Lopez-Hänninen, E.; Rudolph, B.; Neumann, U.; Neuhaus, P. Simultaneous versus Staged Liver Resection of Synchronous Liver Metastases from Colorectal Cancer. Int. J. Color. Dis. 2007, 22, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Margonis, G.A.; Vauthey, J. Precision Surgery for Colorectal Liver Metastases: Current Knowledge and Future Perspectives. Ann. Gastroenterol. Surg. 2022, 6, 606–615. [Google Scholar] [CrossRef]
- DeMatteo, R.P.; Palese, C.; Jarnagin, W.R.; Sun, R.L.; Blumgart, L.H.; Fong, Y. Anatomic Segmental Hepatic Resection Is Superior to Wedge Resection as an Oncologic Operation for Colorectal Liver Metastases. J. Gastrointest. Surg. 2000, 4, 178–184. [Google Scholar] [CrossRef]
- Berger, M.F.; Mardis, E.R. The Emerging Clinical Relevance of Genomics in Cancer Medicine. Nat. Rev. Clin. Oncol. 2018, 15, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Thierry, A.; Kai-Keen, S.; Won, K.T.; Vittrup, J.B.; Henrik, J.L.; Cornelis, P.; Denis, S.; Rocio, G.-C.; Benavides, M.; Peter, G.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Jean-Yves, D.; Oliner Kelly, S.; Salvatore, S.; Josep, T.; Ronald, B.; Mario, B.; Yves, H.; Gyorgy, B.; David, C.; Jacek, J.; et al. Panitumumab–FOLFOX4 Treatment and RAS Mutations in Colorectal Cancer. N. Engl. J. Med. 2013, 369, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-Targeted Therapy with Trastuzumab and Lapatinib in Treatment-Refractory, KRAS Codon 12/13 Wild-Type, HER2-Positive Metastatic Colorectal Cancer (HERACLES): A Proof-of-Concept, Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2016, 17, 738–746. [Google Scholar] [CrossRef]
- Scott, K.; Axel, G.; Rona, Y.; Eric, V.C.; Jayesh, D.; Takayuki, Y.; Harpreet, W.; Fortunato, C.; Fotios, L.; Sang, H.Y.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef]
| Author, Year | Primary Studies Design (n) | Purpose | N° of Patients (n) | Condition | Comparison | Outcome (n Studies) | Metrics [95% CI] | p-Value | I2 (%) | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Hillingsø JG, 2008 [12] | R (16) | To determine the level of evidence for recommendations of a treatment strategy of synchronous liver metastases from colorectal cancer | 1650 | Synchronous CRLM | Simultaneous vs. staged surgery | 5-year OS (11) | OR: 0.90 [0.66,1.24] | 0.14 | 24 | Critically low |
| Chen J, 2011 [13] | R (14) | To compare outcomes between simultaneous resection and staged resection from all published comparative studies in the literature | 2204 | Synchronous CRLM | Simultaneous vs. staged surgery | 5-year OS (10) | OR: 1.14 [0.86,1.50] | 0.37 | 0 | Critically low |
| Sui CJ, 2012 [14] | R (7) | To compare anatomic resection versus non-anatomic resection for colorectal liver metastases with respect to perioperative and oncological outcomes | 1662 | CRLM | Anatomic vs. non-anatomic resection | 5-year DFS (2) | OR: 1.27 [0.66,2.42] | 0.47 | 61.5 | Critically low |
| Li ZQ, 2013 [15] | R (19) | To identify the optimal timing of surgical resection for colon and liver in CRLM patients | 2724 | Synchronous CRLM | Simultaneous vs. staged surgery | 5-year OS (13) | OR: 1.12 [0.88,1.42] | 0.38 | 0 | Critically low |
| 5-year DFS (3) | OR: 0.61 [0.33,1.13] | 0.12 | 0 | |||||||
| Yin Z, 2013 [16] | R (17) | To define the safety and efficacy of simultaneous versus delayed resection of the colon and liver in CRLM patients | 2880 | Synchronous CRLM | Simultaneous vs. staged surgery | DFS (4) | HR: 1.04 [0.76,1.43] | 0.79 | 53 | Critically low |
| OS (10) | HR: 0.96 [0.81,1.14] | 0.64 | 0 | |||||||
| Bijukchhe SM, 2014 [17] | R (20) | To evaluate the efficacy between simultaneous resection and staged resection in patients with CRLM | 3194 | Synchronous CRLM | Simultaneous vs. staged surgery | 5-year OS (12) | OR: 1.08 [0.84,1.38] | 0.54 | 0 | Critically low |
| 5-year DFS (4) | OR: 0.60 [0.34,1.04] | 0.07 | 0 | |||||||
| Feng Q, 2014 [18] | R (22) | To compare simultaneous resection with staged strategy in colorectal cancer for patients with synchronous liver metastases patients, balancing baseline characteristics | 4494 | Synchronous CRLM | Simultaneous vs. staged surgery | OS (15) | HR: 0.96 [0.86,1.08] | 0.50 | 0 | Low |
| DFS (6) | HR: 0.97 [0.64,1.47] | 0.87 | 76 | |||||||
| Kelly ME, 2014 [19] | R (18) | To compare the short- and long-term outcomes for the classical colorectal-first, the liver-first, and the combined resections approaches | 3605 | Synchronous CRLM | Simultaneous vs. liver first | 5-year OS (3) | OR: 0.78 [0.44,1.40] | 0.410 | 0 | Critically low |
| Bowel first vs. liver first | 5-year OS (4) | OR: 0.85 [0.59,1.22] | 0.374 | 69.8 | ||||||
| Bowel first vs. simultaneous | 5-year OS (14) | OR: 0.99 [0.83,1.19] | 0.927 | 5.3 | ||||||
| Tang H, 2016 [20] | R (21) | To compare the efficacy of anatomic resection procedure and non-anatomic procedure for CRLM | 5207 | CRLM | Anatomic vs. non-anatomic resection | OS (12) | HR: 1.06 [0.95,1.18] | 0.18 | 27.3 | Critically low |
| DFS (5) | HR: 1.11 [0.99,1.24] | 0.76 | 0 | |||||||
| Gavriilidis P, 2018 [21] | R (30) | To assess outcomes between patients undergoing simultaneous or delayed hepatectomy for synchronous colorectal liver metastases | 5300 | Synchronous CRLM | Simultaneous vs. staged surgery | OS (17) | HR: 0.97 [0.88,1.08] | 0.601 | 0 | Critically low |
| Deng G, 2019 [22] | R (17) P (1) | To assess the safety and efficacy of parenchymal-sparing hepatectomy as a treatment of colorectal liver metastases | 7081 | CRLM | Anatomic vs. non-anatomic resection | OS (16) | HR: 1.01 [0.94,1.08] | 0.82 | 0 | Critically low |
| DFS (11) | HR: 1.00 [0.94,1.07] | 0.92 | 0 | |||||||
| Ghiasloo M, 2020 [23] | R (43) P (1) | To compare the bowel-first approach, simultaneous resection, and the liver-first approach for patients with colorectal cancer with synchronous liver metastases | 10,848 | Synchronous CRLM | Liver first vs. bowel first | 5-year DFS | OR: 0.57 [0.16,2.06] | 0.39 | 91 | Critically low |
| 5-year OS | OR: 0.88 [0.54,1.44] | 0.61 | 44 | |||||||
| Simultaneous vs. bowel first | 5-year DFS | OR: 1.07 [0.71,1.62] | 0.74 | 31 | ||||||
| 5-year OS | OR: 0.90 [0.75,1.08] | 0.26 | 14 | |||||||
| Simultaneous vs. Liver first | 5-year OS | OR: 0.47 [0.25,0.90] | 0.02 | 0 | ||||||
| Hajibandeh S, 2020 [4] | R (41) | To evaluate the comparative outcomes and clinical characteristics of simultaneous and staged colorectal and hepatic resections for colorectal cancer with synchronous hepatic metastases | 12,081 | Synchronous CRLM | Simultaneous vs. staged surgery | 5-year OS (23) | OR: 0.88 [0.73,1.07] | 0.19 | 45 | Critically low |
| Magouliotis DE, 2020 [24] | R (8) P (2) | To compare the perioperative outcomes of liver-first and classical strategy for the management of synchronous colorectal liver metastases | 3656 | Synchronous CRLM | Bowel first vs. Liver first | 5-year DFS (4) | OR: 1.25 [0.78,1.99] | 0.36 | 9 | Critically low |
| 5-year OS (6) | OR: 1.23 [0.75,2.03] | 0.42 | 66 | |||||||
| Liu J, 2022 [25] | R (17) P (1) | To compare the difference between patients with CRLM who underwent simultaneous resection and staged resection, especially during major hepatectomy (≥3 liver segments) | 5101 | Synchronous CRLM | Simultaneous vs. staged surgery | 5-year mortality (13) | OR: 1.08 [0.93,1.25] | 0.337 | 33.5 | Critically low |
| Wang SH, 2022 [26] | R (22) RCT (1) | To evaluate the safety and long-term prognoses of simultaneous and staged resection of CRLM | 4862 | Synchronous CRLM | Simultaneous vs. staged surgery | 5-year DFS (4) | HR: 1.26 [0.96,1.66] | 0.098 | 18.1 | Critically low |
| 5-year OS (10) | HR: 1.13 [0.95,1.34] | 0.164 | 34.6 | |||||||
| Wang K, 2023 [27] | R (22) | To evaluate the safety and efficacy of parenchymal-sparing resection over anatomic resection for colorectal liver metastases | 7228 | CRLM | Anatomic vs. non-anatomic resection | OS (22) | HR: 1.08 [0.95,1.22] | 0.245 | 49.3 | Critically low |
| DFS (14) | HR: 0.84 [0.41,1.73] | 0.259 | 75.1 |
| Parameter | Meta-Analyses Included (n) | Primary Studies Included (n) | Patients in the First Group (n) | Patients in the Second Group (n) | Metrics [95% CI] Recalculated | p-Value | I2 (%) |
|---|---|---|---|---|---|---|---|
| Simultaneous resection vs. “bowel-first” resection | |||||||
| OS | 4 | 23 | 1750 | 2242 | HR: 0.96 [0.88, 1.05] | 0.40 | 0 |
| DFS | 2 | 7 | 544 | 397 | HR: 0.95 [0.67, 1.35] | 0.79 | 72 |
| 5-y OS | 9 | 38 | 3774 | 6211 | OR: 1.05 [0.86, 1.27] | 0.64 | 70 |
| 5-y DFS | 4 | 5 | 245 | 275 | OR: 0.94 [0.60, 1.47] | 0.78 | 47 |
| Perioperative mortality | 10 | 21 | 2259 | 3842 | RR: 1.88 [1.33, 2.65] | <0.001 | 11 |
| Postoperative complications | 9 | 44 | 4486 | 5415 | RR: 0.98 [0.90, 1.07] | 0.71 | 50 |
| Recurrence | 3 | 14 | 1318 | 1455 | RR: 1.04 [0.91, 1.19] | 0.56 | 62 |
| Blood loss, ml | 5 | 9 | 642 | 1261 | SMD: −0.35 [−0.92, 0.23] | 0.24 | 97 |
| Perioperative blood transfusion | 1 | 9 | 616 | 1213 | RR: 1.11 [0.96, 1.30] | 0.17 | 40 |
| Hospitalization, days | 8 | 26 | 3233 | 3137 | SMD: −1.08 [−1.55, −0.60] | <0.001 | 98 |
| Operative time, min | 4 | 13 | 923 | 950 | SMD: −0.58 [−1.10, −0.07] | 0.03 | 96 |
| Simultaneous resection vs. “liver-first” resection | |||||||
| 5-y OS | 2 | 3 | 88 | 86 | OR: 0.47 [0.25, 0.90] | 0.02 | 0 |
| “Liver-first” vs. “bowel-first” resection | |||||||
| 5-y OS | 3 | 8 | 525 | 1783 | OR: 1.15 [0.93, 1.42] | 0.19 | 18 |
| 5-y DFS | 3 | 5 | 232 | 1305 | OR: 1.01 [0.72, 1.42] | 0.96 | 0 |
| Parameter | Meta-Analyses Included (n) | Primary Studies Included (n) | Patients Undergoing Anatomic Resection (n) | Patients Undergoing Non-Anatomic Resection (n) | Metrics [95% CI] Recalculated | p-Value | I2 (%) |
|---|---|---|---|---|---|---|---|
| OS | 4 | 27 | 3368 | 4399 | HR: 1.04 [0.98, 1.11] | 0.16 | 31 |
| DFS | 4 | 17 | 2660 | 3763 | HR: 1.08 [0.97, 1.20] | 0.17 | 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milazzo, M.; Todeschini, L.; Caimano, M.; Mattia, A.; Cristin, L.; Martinino, A.; Bianco, G.; Spoletini, G.; Giovinazzo, F. Surgical Resection in Colorectal Liver Metastasis: An Umbrella Review. Cancers 2024, 16, 1849. https://doi.org/10.3390/cancers16101849
Milazzo M, Todeschini L, Caimano M, Mattia A, Cristin L, Martinino A, Bianco G, Spoletini G, Giovinazzo F. Surgical Resection in Colorectal Liver Metastasis: An Umbrella Review. Cancers. 2024; 16(10):1849. https://doi.org/10.3390/cancers16101849
Chicago/Turabian StyleMilazzo, Martina, Letizia Todeschini, Miriam Caimano, Amelia Mattia, Luca Cristin, Alessandro Martinino, Giuseppe Bianco, Gabriele Spoletini, and Francesco Giovinazzo. 2024. "Surgical Resection in Colorectal Liver Metastasis: An Umbrella Review" Cancers 16, no. 10: 1849. https://doi.org/10.3390/cancers16101849
APA StyleMilazzo, M., Todeschini, L., Caimano, M., Mattia, A., Cristin, L., Martinino, A., Bianco, G., Spoletini, G., & Giovinazzo, F. (2024). Surgical Resection in Colorectal Liver Metastasis: An Umbrella Review. Cancers, 16(10), 1849. https://doi.org/10.3390/cancers16101849







