Polymorphous Adenocarcinoma: A Systematic Review of the Literature and Presentation of Two Cases in a Less-Considered Anatomical Site
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
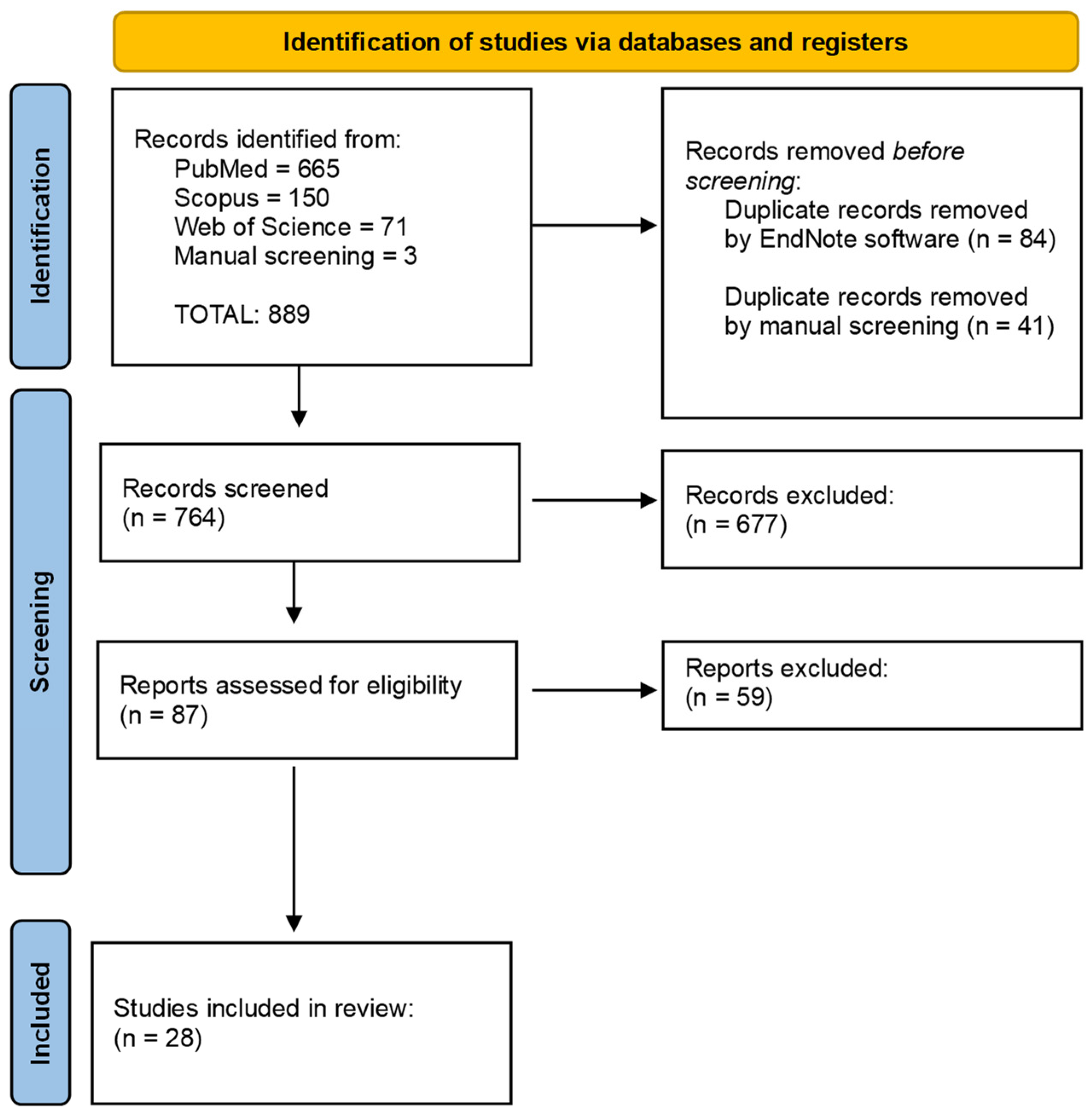
2.1. Systematic Review
2.1.1. Protocol
2.1.2. PICo and Research Question
2.1.3. Data Sources and Search Strategy
2.1.4. Eligibility Criteria
- Human studies
- English language
- Studies reporting a histologically confirmed diagnosis of PAC
2.1.5. Study Selection and Data Collection Processes
2.1.6. Statistical Analysis
- i.
- Study characteristics: the name of the first author, the year of publication, the name of the country where the study was performed, and the design of the study.
- ii.
- Main characteristics of the included patients: mean age, sex, and anatomical site of PAC.
2.2. Case Series
2.2.1. Data Collection and Clinical Examination
2.2.2. Sample Collection
2.2.3. Histological Examination and Immunohistochemistry
3. Results
3.1. Systematic Review
3.2. Case Reports
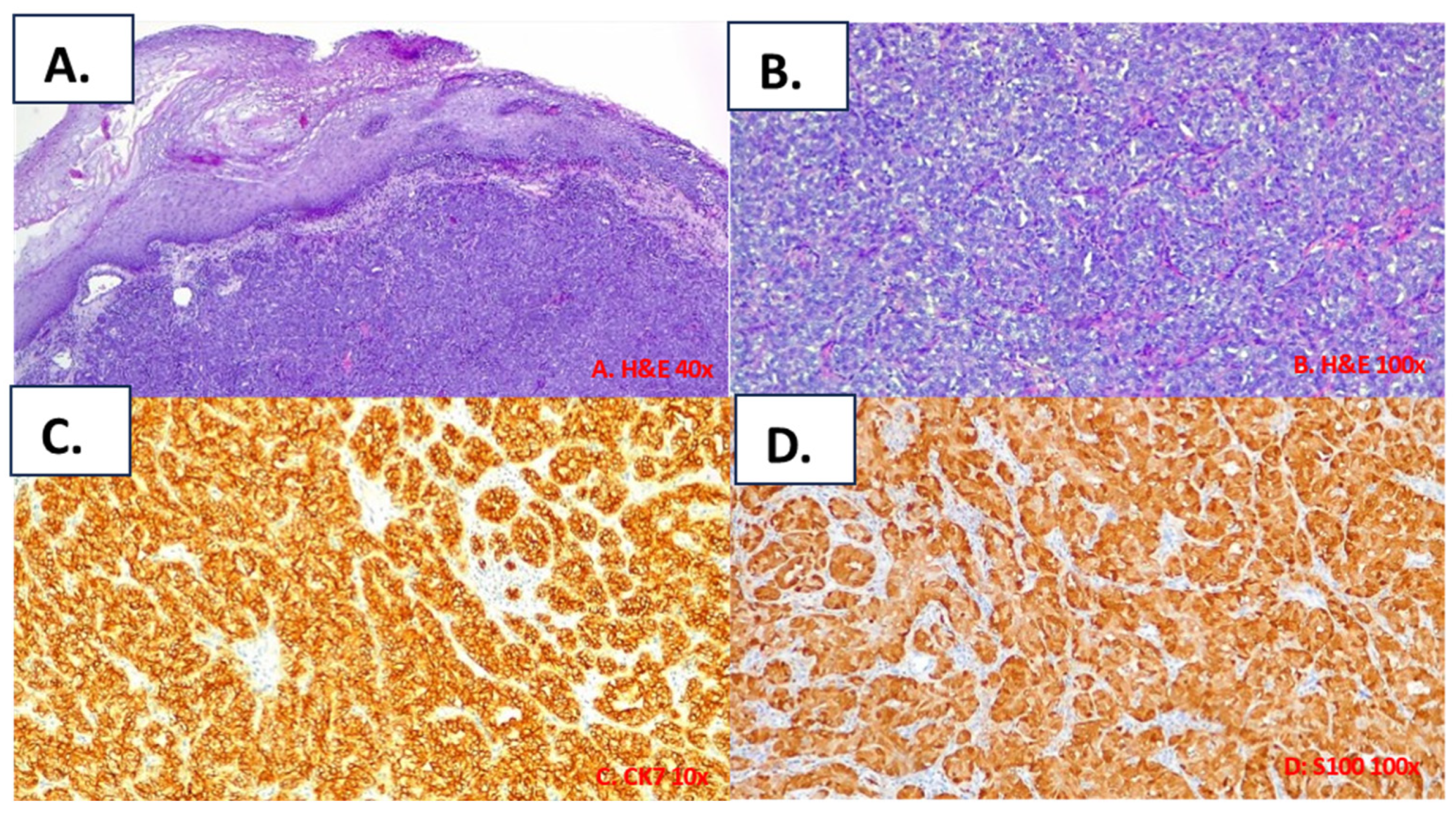
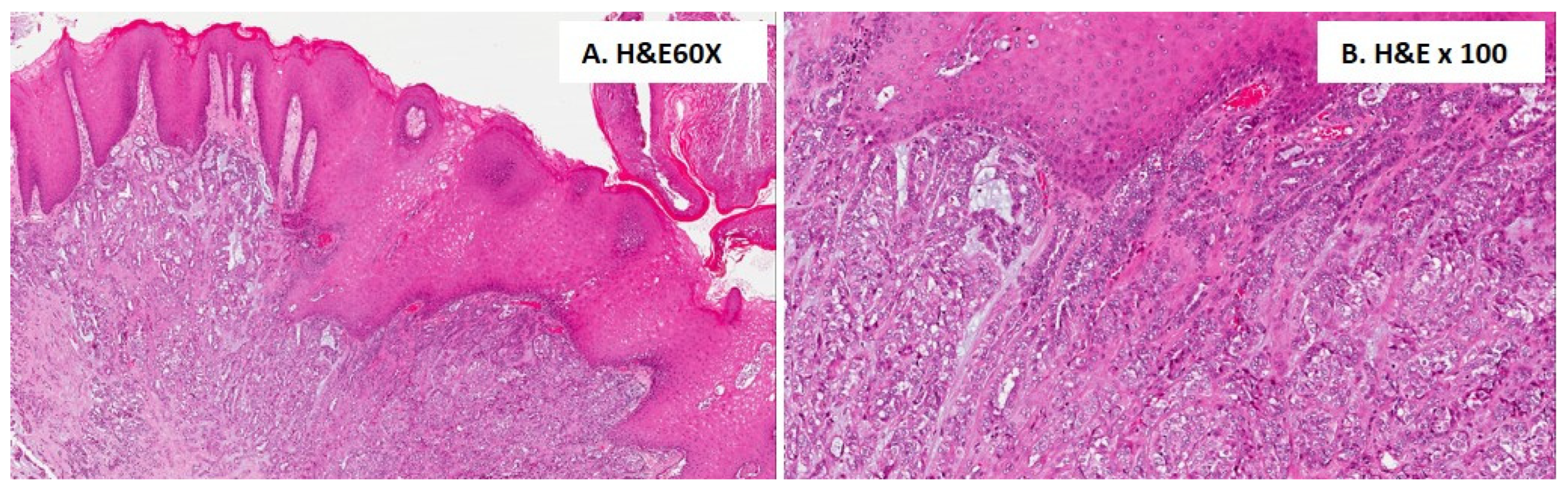
3.3. Histological Findings for the Included Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wysocki, P.T.; Westra, W.H.; Sidransky, D.; Brait, M. Advancing toward a Molecular Characterization of Polymorphous Low Grade Adenocarcinoma. Oral Oncol. 2017, 74, 192–193. [Google Scholar] [CrossRef] [PubMed]
- Batsakis, J.G.; Pinkston, G.R.; Luna, M.A.; Byers, R.M.; Sciubba, J.J.; Tillery, G.W. Adenocarcinomas of the Oral Cavity: A Clinicopathologic Study of Terminal Duct Carcinomas. J. Laryngol. Otol. 1983, 97, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Freedman, P.D.; Lumerman, H. Lobular Carcinoma of Intraoral Minor Salivary Gland Origin. Oral Surg. Oral Med. Oral Pathol. 1983, 56, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.L.; Batsakis, J.G. Polymorphous Low-Grade Adenocarcinoma of Minor Salivary Glands a Study of 14 Cases of a Distinctive Neoplasm. Cancer 1984, 53, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Seethala, R.R.; Stenman, G. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Tumors of the Salivary Gland. Head Neck Pathol. 2017, 11, 55–67. [Google Scholar] [CrossRef]
- Sankar Vinod, V.; Mani, V.; George, A.; Sivaprasad, K.K. Polymorphous Low-Grade Adenocarcinoma––Management and Reconstruction with Temporalis Myofacial Flap. J. Maxillofac. Oral Surg. 2013, 12, 105–108. [Google Scholar] [CrossRef][Green Version]
- Tomar, R.; Garg, N.; Agarwal, S. Polymorphous Low Grade Adenocarcinoma of Lip Clinically Mimicking Squamous Cell Carcinoma: An Unusual Presentation. J. Cytol. 2015, 32, 59. [Google Scholar] [CrossRef]
- Verma, V.; Mendenhall, W.M.; Werning, J.W. Polymorphous Low-Grade Adenocarcinoma of the Head and Neck. Am. J. Clin. Oncol. 2014, 37, 624–626. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Colmenero, C.M.; Patron, M.; Burgueño, M.; Sierra, I. Polymorphous Low-Grade Adenocarcinoma of the Oral Cavity: A Report of 14 Cases. J. Oral Maxillofac. Surg. 1992, 50, 595–600. [Google Scholar] [CrossRef]
- Alicia Dean, M. Polymorphous Low-Grade Adenocarcinomaof the Oral Cavity: Report of Two Cases. J. Oral Maxillofac. Surg. 1994, 52, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Crean, S.J.; Bryant, C.; Bennett, J.; Harris, M. Four Cases of Polymorphous Low-Grade Adenocarcinoma. Int. J. Oral Maxillofac. Surg. 1996, 25, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Gaffey, T.A.; Kay, P.A.; Minato, H.; Serizawa, H.; Lewis, J.E. Polymorphous Low-Grade Adenocarcinoma of the Major Salivary Glands: Report of Three Cases in an Unusual Location. Histopathology 2004, 44, 164–171. [Google Scholar] [CrossRef] [PubMed]
- González-García, R.; Rodríguez-Campo, F.J.; Muñoz-Guerra, M.F.; Nam-Cha, S.H.; Sastre-Pérez, J.; Naval-Gías, L. Polymorphous Low-Grade Adenocarcinoma of the Palate: Report of Cases. Auris Nasus Larynx 2005, 32, 275–280. [Google Scholar] [CrossRef]
- Fife, T.A.; Smith, B.; Sullivan, C.A.; Browne, J.D.; Waltonen, J.D. Polymorphous Low-Grade Adenocarcinoma: A 17 Patient Case Series. Am. J. Otolaryngol. 2013, 34, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Elhakim, M.T.; Breinholt, H.; Godballe, C.; Andersen, L.J.; Primdahl, H.; Kristensen, C.A.; Bjørndal, K. Polymorphous Low-Grade Adenocarcinoma: A Danish National Study. Oral Oncol. 2016, 55, 6–10. [Google Scholar] [CrossRef]
- Kennedy, L.K.S. Polymorphous Low-Grade Adenocarcinoma of the Tongue. Laryngoscope 1987, 97, 533–536. [Google Scholar] [CrossRef]
- Scally, C.M.; Irwin, S.T.; Nirodi, N. Low Grade Polymorphous Adenocarcinoma of a Minor Salivary Gland. J. Laryngol. Otol. 1988, 102, 284–287. [Google Scholar] [CrossRef]
- Nicolatou, O.; Kakarantza-Angelopoulou, E.; Angelopoulos, A.P.; Anagnostopoulou, S. Polymorphous Low-Grade Adenocarcinoma of the Palate: Report of a Case with Electron Microscopy. J. Oral Maxillofac. Surg. 1988, 46, 1008–1013. [Google Scholar] [CrossRef]
- Fliss, D.M.; Zirkin, H.; Puterman, M.; Tovi, F. Low-Grade Papillary Adenocarcinoma of Buccal Mucosa Salivary Gland Origin. Head Neck 1989, 11, 237–241. [Google Scholar] [CrossRef]
- De Diego, J.I. Polymorphous Low-Grade Adenocarcinoma of the Tongue. J. Laryngol. Otol. 1996, 110, 700–703. [Google Scholar] [CrossRef] [PubMed]
- Mincione, G.P.; Nesi, G.; Amorosi, A. Polymorphous Low-Grade Adenocarcinoma of the Soft Palate: Fine Needle Aspiration (FNA) Cytology: Polymorphous Low-Grade Adenocarcinoma. Cytopathology 1999, 10, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Tincani, A.J.; Altemani, A.; Martins, A.S.; Barreto, G.; Valério, J.B.; Del Negro, A.; Araújo, P.P.C. Polymorphous Low-Grade Adenocarcinoma at the Base of the Tongue: An Unusual Location. Ear Nose Throat J. 2005, 84, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Pintor, M.F.; Figueroa, L.; Martínez, B. Polymorphous Low Grade Adenocarcinoma: Review and Case Report. Med. Oral Patol. Oral Cir Bucal 2007, 12, 549–551. [Google Scholar]
- Arora, S.K.; Sreedharanunni, S.; Dey, P. Cytomorphological Features of an Aggressive Variant of Polymorphous Low-Grade Adenocarcinoma in Adolescence with Lymph Node Metastasis. Diagn. Cytopathol. 2013, 41, 186–188. [Google Scholar] [CrossRef]
- Kawahara, A.; Harada, H.; Abe, H.; Yamaguchi, T.; Taira, T.; Mihashi, H.; Naito, Y.; Akiba, J.; Kage, M. Fine Needle Aspiration Cytology of Metastatic Polymorphous Low-Grade Adenocarcinoma of the Palate in a Cervical Lymph Node. Cytopathology 2013, 24, 63–65. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, C.; Raghav, N. Polymorphous Low-Grade Adenocarcinoma of the Palate: Report of a Case. Indian J. Cancer 2011, 48, 518. [Google Scholar] [CrossRef]
- Andreu-Barasoain, M.; Vicente-Martin, J.; Gomez De La Fuente, E.; Salamanca-Santamaria, J.; Pampin-Franco, A.; Lopez-Estebaranz, J.L. Polymorphous Low-Grade Adenocarcinoma in the Upper Lip: A Well-Described but Infrequently Recognized Tumor. Dermatol. Online J. 2013, 19, 19265. [Google Scholar] [CrossRef]
- Lee, D.H.; Yoon, T.M.; Lee, J.K.; Lim, S.C. Polymorphous Low-Grade Adenocarcinoma of the Maxillary Sinus. J. Craniofac. Surg. 2013, 24, e213–e214. [Google Scholar] [CrossRef]
- Radhika, T. Polymorphous Low Grade Adenocarcinoma of Retromolar Region—A Rare Case Report with Distinct Clinical Manifestations. J. Clin. Diagn. Res. 2015, 9, ZD11. [Google Scholar] [CrossRef]
- Sathyanarayanan, R.; Suresh, V.; Therese Thomas, B.A. Polymorphous Low-Grade Adenocarcinoma of the Palate: A Rare Case Report. Iran. J. Cancer Prev. 2015; in press. [Google Scholar] [CrossRef]
- Khosla, D.; Verma, S.; Gupta, N.; Punia, R.-S.; Kaur, G.; Pandey, A.-K.; Dimri, K. Polymorphous Low-Grade Adenocarcinoma of the Parotid in a Teenager. Iran J. Otorhinolaryngol. 2017, 29, 299–302. [Google Scholar] [PubMed]
- Vadla, P.; Pathipaka, S.; Madala, J.; Guttikonda, V. Polymorphous Adenocarcinoma of the Oral Cavity: A Skeptical Case Mimicking Lobular Carcinoma of Breast and Gastric Carcinoma. J. Oral Maxillofac. Pathol. 2018, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Muniswammappa, S.; Bavle, R.; Makarla, S.; Venugopal, R. Polymorphous Adenocarcinoma: High-Grade Transformation With Immunohistochemical Workup. Cureus 2022, 14, e23639. [Google Scholar] [CrossRef] [PubMed]
- Nakasone, T.; Matsuzaki, A.; Tamasiro, K.; Sunagawa, N.; Goto, S.; Hirano, F.; Makishi, S.; Matayoshi, A. Polymorphous Adenocarcinoma of the Sublingual Gland: A Case Report and Literature Review. J. Oral Maxillofac. Surg. Med. Pathol. 2021, 33, 561–567. [Google Scholar] [CrossRef]
- Mauceri, R.; Bazzano, M.; Coppini, M.; Tozzo, P.; Panzarella, V.; Campisi, G. Diagnostic Delay of Oral Squamous Cell Carcinoma and the Fear of Diagnosis: A Scoping Review. Front. Psychol. 2022, 13, 1009080. [Google Scholar] [CrossRef]
- Akpeh, J.; Okechi, U.; Ezeanolue, B. Primary Minor Salivary Gland Tumors: A Retrospective Review of Cases Seen in a Tertiary Institution in South East Nigeria. Niger. J. Clin. Pract. 2022, 25, 368. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.L.; Ismaila, N.; Beadle, B.; Caudell, J.J.; Chau, N.; Deschler, D.; Glastonbury, C.; Kaufman, M.; Lamarre, E.; Lau, H.Y.; et al. Management of Salivary Gland Malignancy: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1909–1941. [Google Scholar] [CrossRef]
- Lawal, A.O.; Adisa, A.O.; Kolude, B.; Adeyemi, B. Malignant Salivary Gland Tumours of the Head and Neck Region: A Single Institutions Review. Pan Afr. Med. J. 2015, 20, 121. [Google Scholar] [CrossRef]
- Walvekar, R.; Phalke, N.P. The Evaluation and Management of Carcinoma of the Minor Salivary Glands. Otolaryngol. Clin. N. Am. 2021, 54, 629–639. [Google Scholar] [CrossRef]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef]
- Mauceri, R.; Coppini, M.; Vacca, D.; Bertolazzi, G.; Panzarella, V.; Fede, O.D.; Tripodo, C.; Campisi, G. Salivary Microbiota Composition in Patients with Oral Squamous Cell Carcinoma: A Systematic Review. Cancers 2022, 14, 5441. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, H.; Rautava, J. Oral Microbiota and Cancer Development. Pathobiology 2021, 88, 116–126. [Google Scholar] [CrossRef]
- Kumar, M.; Stivaros, N.; Barrett, A.W.; Thomas, G.J.; Bounds, G.; Newman, L. Polymorphous Low-Grade Adenocarcinoma—A Rare and Aggressive Entity in Adolescence. Br. J. Oral Maxillofac. Surg. 2004, 42, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Khurram, S.A.; Barrett, A.W.; Speight, P.M. Diagnostic Difficulties in Lesions of the Minor Salivary Glands. Diagn. Histopathol. 2017, 23, 250–259. [Google Scholar] [CrossRef][Green Version]
- Beltran, D.; Faquin, W.C.; Gallagher, G.; August, M. Selective Immunohistochemical Comparison of Polymorphous Low-Grade Adenocarcinoma and Adenoid Cystic Carcinoma. J. Oral Maxillofac. Surg. 2006, 64, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Katabi, N.; Xu, B. Polymorphous Adenocarcinoma. Surg. Pathol. Clin. 2021, 14, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, T.; Takei, H. Immunohistochemical Profile of Polymorphous Adenocarcinoma of Minor Salivary Gland: A Systematic Review and Meta-Analysis. Head Neck Pathol. 2022, 16, 980–990. [Google Scholar] [CrossRef]
- Atta, I.S.; Al Qahtani, F.N. DOG1, Alpha-Amylase and P63 Expression in Acinic Cell Carcinoma of Salivary Gland; Immunohistochemical, Clinical and Radiological Study. J. Histol. Histopathol. 2015, 2, 13. [Google Scholar] [CrossRef][Green Version]
- Sivakumar, N.; Narwal, A.; Pandiar, D.; Devi, A.; Anand, R.; Bansal, D.; Kamboj, M. Diagnostic Utility of P63/P40 in the Histologic Differentiation of Salivary Gland Tumors: A Systematic Review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 133, 189–198. [Google Scholar] [CrossRef]
- Inoue, K.; Fry, E.A. Alterations of P63 and P73 in Human Cancers. In Mutant p53 and MDM2 in Cancer; Deb, S.P., Deb, S., Eds.; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2014; Volume 85, pp. 17–40. ISBN 978-94-017-9210-3. [Google Scholar]
- Seethala, R.R.; LiVolsi, V.A.; Zhang, P.J.; Pasha, T.L.; Baloch, Z.W. Comparison of P63 and P73 Expression in Benign and Malignant Salivary Gland Lesions. Head Neck 2005, 27, 696–702. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Head and Neck Tumours, 5th ed.; WHO Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2022; Volume 9. [Google Scholar]
- Xu, B.; Barbieri, A.L.; Bishop, J.A.; Chiosea, S.I.; Dogan, S.; Di Palma, S.; Faquin, W.C.; Ghossein, R.; Hyrcza, M.; Jo, V.Y.; et al. Histologic Classification and Molecular Signature of Polymorphous Adenocarcinoma (PAC) and Cribriform Adenocarcinoma of Salivary Gland (CASG): An International Interobserver Study. Am. J. Surg. Pathol. 2020, 44, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Khurana, A.; Gupta, S.; Chauhan, A. Polymorphous Low-Grade Adenocarcinoma of Base of the Tongue: A Case Report and Review of Literature of an Orphan Disease. Int. J. Head Neck Surg. 2020, 11, 71–74. [Google Scholar] [CrossRef]
- Paleri, V.; Robinson, M.; Bradley, P. Polymorphous Low-Grade Adenocarcinoma of the Head and Neck. Curr. Opin. Otolaryngol. Head Neck Surg. 2008, 6, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Vander Poorten, V.; Triantafyllou, A.; Skálová, A.; Stenman, G.; Bishop, J.A.; Hauben, E.; Hunt, J.L.; Hellquist, H.; Feys, S.; De Bree, R.; et al. Polymorphous Adenocarcinoma of the Salivary Glands: Reappraisal and Update. Eur. Arch. Otorhinolaryngol. 2018, 275, 1681–1695. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.D.; Vazquez, A.; Marchiano, E.; Park, R.C.; Baredes, S.; Eloy, J.A. Polymorphous Low-Grade Adenocarcinoma of the Head and Neck: A Population-Based Study of 460 Cases: SEER Head and Neck PLGA. Laryngoscope 2015, 125, 1644–1649. [Google Scholar] [CrossRef]
- Mifsud, M.J.; Tanvetyanon, T.; Mccaffrey, J.C.; Otto, K.J.; Padhya, T.A.; Kish, J.; Trotti, A.M.; Harrison, L.B.; Caudell, J.J. Adjuvant Radiotherapy versus Concurrent Chemoradiotherapy for the Management of High-Risk Salivary Gland Carcinomas: Adjuvant RT Versus Chemoradiotherapy for Salivary Carcinoma. Head Neck 2016, 38, 1628–1633. [Google Scholar] [CrossRef]
- Rosenberg, L.; Weissler, M.; Hayes, D.N.; Shockley, W.; Zanation, A.; Rosenman, J.; Chera, B. Concurrent Chemoradiotherapy for Locoregionally Advanced Salivary Gland Malignancies. Head Neck 2012, 34, 872–876. [Google Scholar] [CrossRef]
- Thompson, L.D.R. Keratocystic odontogenic tumor. Ear Nose Throat J. 2014, 93, 386–388. [Google Scholar] [CrossRef]
- Seethala, R.R.; Johnson, J.T.; Barnes, E.L.; Myers, E.N. Polymorphous Low-Grade Adenocarcinoma. The University of Pittsburgh Experience. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 385–392. [Google Scholar] [CrossRef]
- Chiapasco, M. Manuale Illustrato Di Chirurgia Orale, 3rd ed.; EdizioniEdra: Milan, Italy, 2013. [Google Scholar]
- Villanueva-Alcojol, L. Contralateral Neck Dissection in Oral Squamous Cell Carcinoma: When It Shoud Be Done? Plast. Aesthetic Res. 2016, 3, 181. [Google Scholar] [CrossRef]
- Mourad, M.A.F.; Higazi, M.M. MRI Prognostic Factors of Tongue Cancer: Potential Predictors of Cervical Lymph Nodes Metastases. Radiol. Oncol. 2019, 53, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Aydil, U.; Kızıl, Y.; Bakkal, F.K.; Köybaşıoğlu, A.; Uslu, S. Neoplasms of the Hard Palate. J. Oral Maxillofac. Surg. 2014, 72, 619–626. [Google Scholar] [CrossRef] [PubMed]

| N. | Author, Year | Country | N. of Case | Age | Sex | Site |
|---|---|---|---|---|---|---|
| 1 | Evans HL, 1984 [4] | USA | 14 | 74 (27–76) | 8 M/6 F | 11 palate; 2 buccal mucosa; 1 posterior mandible |
| 2 | Kennedy LKS, 1987 [17] | USA | 1 | 48 | M | tongue |
| 3 | Scally CM, 1988 [18] | Ireland | 1 | 39 | F | buccal mucosa |
| 4 | Nicolatou O, 1988 [19] | Greece | 1 | 68 | M | palate |
| 5 | Fliss DM, 1989 [20] | Israel | 1 | 45 | M | buccal mucosa |
| 6 | Colmenero CM, 1992 [10] | Spain | 4 | 59.7 ± 16.3 | 1 M/3 F | 3 palate; 1 retromolar pad |
| 7 | Dean A, 1994 [11] | Spain | 2 | 53.5 ± 10.5 | M | 2 palate |
| 8 | Crean SJ, 1996 [12] | UK | 4 | 52.5 ± 10.5 | 2 M/2 F | 3 palate; 1 right retromolar area |
| 9 | De Diego JI, 1996 [21] | Spain | 1 | 60 | M | tongue |
| 10 | Mincione GP, 1999 [22] | Italy | 1 | 67 | M | soft palate |
| 11 | Nagao T, 2004 [13] | Japan | 3 | 65 ± 11.4 | 2 M/1 F | 2 parotid gland; 1 submandibular gland |
| 12 | Tincani AJ, 2005 [23] | Brazil | 1 | 69 | M | tongue |
| 13 | González-García R, 2005 [14] | Spain | 6 | 61.2 ± 9.4 | n.d. | palate |
| 14 | Pintor MF, 2007 [24] | Chile | 1 | 65 | F | hard palate and the alveolar ridge |
| 15 | Arora SK, 2013 [25] | India | 1 | 18 | M | hard palate |
| 16 | Kawahara A, 2013 [26] | Japan | 1 | 70 | M | palate |
| 17 | Gupta S, 2011 [27] | India | 1 | 52 | M | palate |
| 18 | Andreu-Barasoain M, 2013 [28] | Spain | 1 | 75 | F | upper lip |
| 19 | Lee DH, 2013 [29] | Korea | 1 | 59 | M | maxillary sinus |
| 20 | Fife TA, 2013 [15] | USA | 17 | 62.7 ± 14.2 | 7 M/10 F | hard palate 7/17 (41.2%) hard/soft palate junction 5/17 (29.4%) soft palate 2/17 (11.8%) lip 2/17 (11.8%) retromolar trigone 1/17 (5.9%) |
| 21 | Tomar R 2015 [7] | India | 1 | 45 | M | upper lip |
| 22 | Radhika T, 2015 [30] | India | 1 | 59 | M | retromolar region |
| 23 | Elhakim MT, 2016 [16] | Denmark | 73 | 58 (16–86) | 26 M/47 F | 3 parotid; 4 lip; 7 buccal mucosa; 2 floor of mouth; 53 palate; 1 nose; 3 pharynx |
| 24 | Sathyanarayanan R, 2015 [31] | India | 1 | 63 | F | palate |
| 25 | Khosla D, 2017 [32] | India | 1 | 16 | M | parotid gland |
| 26 | Vadla P, 2018 [33] | India | 1 | 50 | M | right upper posterior part of alveolus (upper gingiva) |
| 27 | Muniswammappa S, 2022 [34] | India | 1 | 50 | F | buccal mucosa |
| 28 | Nakasone T, 2021 [35] | Japan | 1 | 81 | F | sublingual gland |
| Case | Age | Sex | Smoker | Surgical Treatment | Radiotherapy | Follow-Up (Months) | Recurrence/ Metastases |
|---|---|---|---|---|---|---|---|
| #1 | 73 | F | No | Yes | No | 18 | Yes, lymph node metastases |
| #2 | 68 | M | Yes | Yes | No | 50 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauceri, R.; Coppini, M.; Alecci, G.; Cordova, A.; Florena, A.M.; Magro, G.; Toro, C.; Campisi, G. Polymorphous Adenocarcinoma: A Systematic Review of the Literature and Presentation of Two Cases in a Less-Considered Anatomical Site. Cancers 2024, 16, 220. https://doi.org/10.3390/cancers16010220
Mauceri R, Coppini M, Alecci G, Cordova A, Florena AM, Magro G, Toro C, Campisi G. Polymorphous Adenocarcinoma: A Systematic Review of the Literature and Presentation of Two Cases in a Less-Considered Anatomical Site. Cancers. 2024; 16(1):220. https://doi.org/10.3390/cancers16010220
Chicago/Turabian StyleMauceri, Rodolfo, Martina Coppini, Giuseppe Alecci, Adriana Cordova, Ada Maria Florena, Gaetano Magro, Corrado Toro, and Giuseppina Campisi. 2024. "Polymorphous Adenocarcinoma: A Systematic Review of the Literature and Presentation of Two Cases in a Less-Considered Anatomical Site" Cancers 16, no. 1: 220. https://doi.org/10.3390/cancers16010220
APA StyleMauceri, R., Coppini, M., Alecci, G., Cordova, A., Florena, A. M., Magro, G., Toro, C., & Campisi, G. (2024). Polymorphous Adenocarcinoma: A Systematic Review of the Literature and Presentation of Two Cases in a Less-Considered Anatomical Site. Cancers, 16(1), 220. https://doi.org/10.3390/cancers16010220










