Simple Summary
Euphorbiaceae is a large family of flowering plants that includes a wide spectrum of useful plants, from edible plants to toxic and medicinal plants. They are cosmopolitan plants that have very different shapes, from little herbaceous plants to big trees and cactus-like forms. This review article focuses on the potential anticancer activity of extracts, isolated compounds, and nanoparticles generated from the plants of the Euphobiaceae family based on in vitro and in vivo experiments. Possible mechanisms of action are also discussed.
Abstract
The number of cancer cases will reach 24 million in 2040, according to the International Agency for Research on Cancer. Current treatments for cancer are not effective and selective for most patients; for this reason, new anticancer drugs need to be developed and researched enough. There are potentially useful drugs for cancer isolated from plants that are being used in the clinic. Available information about phytochemistry, traditional uses, in vitro and in vivo experiments with plants, and pure compounds isolated from the Euphorbiaceae family indicates that this family of plants has the potential to develop anticancer drugs. This review examines selected species from the Euphorbiaceae family and their bioactive compounds that could have potential against different types of cancer cells. It reviews the activity of crude extracts, isolated compounds, and nanoparticles and the potential underlying mechanisms of action.
Keywords:
Euphorbiaceae family; in vitro; in vivo; anticancer; extracts; pure compounds; nanoparticles 1. Introduction
Nowadays, cancer is a serious health problem that represents a great cost to national health systems around the world. Usually cells live, repair errors, divide, and die, and new cells replace the old ones. But sometimes, a mutation in the DNA of a cell that can not repair itself through reparation mechanisms leads to multiple abnormal divisions. These multiple divisions could activate oncogenes (inducing cell growth) or/and deactivate tumor suppressor genes (repressing cell growth), leading to an uncontrolled cell cycle. All these fast divisions, without control, accumulate different mutations that could imply fast growth and cancer cell death evasion. These mutations could also favor the loss of adhesion of tumor cells, which would facilitate their movement to other areas of the body through epithelial-mesenchymal transition, promoting tumor invasion and metastasis. In addition, it has been established that tumors release some angiogenic cytokines that affect vessel formation, tumor development, invasion, and metastasis. The most important angiogenic cytokines are vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), which are also poor markers of the prognostic and aggressiveness of the illness in patients [1,2].
More than 19 million new cases of cancer and 10 million deaths from cancer were reported in 2020 [3]. The most frequently diagnosed cancer was breast cancer (11.7%), lung cancer (11.6%), colorectal cancer (10%), and prostate cancer (7.3%). Furthermore, estimates made by experts expect that in 2040 there will be 24.8 million cases of cancer (47% more than in 2020) [4]. With this future perspective, new treatments and the development of new drugs will be difficult to achieve if governments do not increase the share devoted to cancer, and this lack of financing may be a problem in receiving quality care [5]. New therapies for cancer are usually directed toward developing more effective drugs and trying to avoid the side effects of the actual anticancer drugs. But all these strategies are usually very complex or hard and expensive to achieve, using new molecular targets, nanomedicine, and bioengineering [6]. However, the plant kingdom is still being unexplored; nonetheless, several very useful anticancer drugs are of plant origin, such as Paclitaxel, Vincristine, Vinblastine, Irinotecan, Topotecan, and Etoposide [7,8,9].
The Euphorbiaceae family of plants is a group of plants that has gained the interest of the scientific community due to their traditional uses in ethnomedicine, their high diversity of compounds, the potential toxicity of these compounds, and their easy access to these cosmopolitan plants [10]. Different plants from the genus of the family, such as Euphorbia, Croton, Jatropha, and Cnidoscolus, have been tested in vitro for their anticancer activity with excellent results [11,12,13]. This gives us an opportunity to continue delving deeper into the compounds present in these plants and their mechanisms of action as potential anticancer drugs. Several secondary metabolites have been described in the Euphorbiaceae family; for example, there is a high diversity of terpenoids with different types of original skeleton configurations [14,15,16]. Some common flavonoids, like quercetin and apigenin, have been isolated from this plant family [17,18,19,20]. Additionally, it has been documented that these plants contain certain tryptamine-derived alkaloids [21].
The main purpose of this review is to bring together all the information available about the anticancer effect in vitro or in vivo of plant extracts or pure compounds isolated from the Euphorbiaceae family published in the last ten years.
2. Criteria for the Selection of Experimental Papers
This review is based on primary literature published between 2012 and 2023 (to date). The papers were selected from different electronic databases: PubMed, Google Scholar, Scopus, and Web of Science. The following terms were used to achieve the search: Euphorbiaceae with: family, anticancer, antiproliferative, cytotoxic, plant extract, pure compound, in vitro, in vivo, and nanoparticles. The articles reporting extracts or pure compounds from the Euphorbiaceae family with some anticancer, antiproliferative, or cytotoxic activity in vitro or in vivo were included. Other articles reporting different types of reviews, articles in different languages than English, articles without full text access, lacking specific plant names, without reports of clear objectives and methodology, published more than ten years ago, using plant species other than Euphorbiaceae, were excluded. Duplicate articles from different database searching results were also excluded. All the inclusion/exclusion criteria were checked again after the removal of these articles. Each selected research paper was examined, and the following data were selected and presented in the tables: scientific plant name, parts of the plants used for extract preparation or pure compound isolation, type of extract, class of compounds or different compounds identified in the extract, cancer cell lines used or animal model/cell line inoculated with cancer-inducing compounds, activity or mechanism of action, and reference. Articles explaining the mechanisms of action of Euphorbiaceae plant extracts or isolated compounds were discussed before the tables in the main text.
3. The Euphorbiaceae Family of Plants
The Euphorbiaceae family of plants has about 228 plant genera accepted according to Plants of the World Online by Royal Botanic Garden Kew [22] and more than 7000 species of plants according to the Global Biodiversity Information Facility [23]. This family of green plants has a very different overall shape. There are habits from herbaceous plants (for example, Euphorbia peplus L.) to shrubs (like Ricinus communis L.) or woody trees (Hevea brasiliensis (Willd. ex A.Juss.) Müll.Arg.) and cactus-like shapes (Euphorbia ingens (E.Mey. ex Boiss.)). These plants can be annuals or perennials, monoics or dioics, and usually present some type of latex. The leaves are oppositive (sometimes alternatives) and have stipules that could be transformed into spines or glands. The flowers gather in an inflorescence called cyathium, and sometimes they are inconspicuous flowers that lack a corolla. Sometimes the cyathium contains several masculine flowers and only one feminine flower. Androecium with one or numerous stamens, and gynoecium with two or three styles and carpels. The fruits are usually contained in capsules, and they separate easily from each other [24,25,26]. Among the best-known genera of this family, we find Euphorbia, Jatropha, Croton, Acalypha, Cnidoscolus, and Ricinus (Figure 1) [22]. Some plants in the family are very important for humans; for example, Mannihot sculenta L. is an edible plant that is cultivated in many countries and is considered a basic food. This crop is easy to grow in a variety of environments, is undemanding, and has very good nutritional properties [27,28]. Mannihot sculenta L. was grown as a crop on more than one million hectares in countries such as Ghana, Angola, Cote d’Ivoire, Brazil, Thailand, the Democratic Republic of the Congo, and Nigeria in 2021, according to the Food and Agriculture Organization of the United Nations [29]. Other plants are considered toxic to be consumed but still useful; for example, in the proper dosage, Ricinus communis L., commonly known as Castor oil, has been used in the past as a purgative [30] and is approved by the US Food and Drug Administration as an antisticking additive agent to produce hard candy [31]. Many other uses of castor’s oil have been reported, including uterine contraction [32], antiviral [33], antibacterial [34], and antinflammatory [30]. In addition, the oils extracted from the seeds are very useful for industrial applications [31,35].
Figure 1.
The Euphorbiaceae family of green plants. (created by BioRender).
Plants of the most numerous genera, Euphorbia, have been used traditionally around the world for different purposes, for example, to feed animals, as additives in food, as fuels, or for environmental uses, but the two main purposes have been to poison different animals and as folk medicines [10]. These two main purposes take advantage of the toxicological properties of the diverse phytochemistry present in these plants. For example, it is well known that different species of Euphorbiaceae, for example, Euphorbia tirucalli L., have been used in Africa to poison fish. This is a very common way of fishing with the help of plant metabolites that spread through water and poison animals [36]. On the other hand, when used as folk medicines, they are known to be used mainly for digestive disorders, skin or subcutaneous tissue disorders, infections, inflammation, or respiratory disorders [10]. For example, for digestive disorders, decoctions of Euhprobia hirta L. are recorded to be used in distant places such as Burundi, the Philippines, and China as antidiarrheal [37,38,39]. Euphorbia lathyris L. is recorded as being used as a purgative in different countries in Europe. For skin disorders, Euphorbia maculata L. is well known to be used in folk medicine against warts [40]. To treat infections, for example, Euphorbia hirta L. had been used in the past for gonorrhoea in Africa [41]. The same plant has been reported to be used in Australia for bronchitis (inflammation) [42]. For respiratory disorders, it is used in Nepal for the treatment of asthma [43].
3.1. Phytochemistry of the Euphorbiaceae Family
This family of plants is very diverse and rich in secondary metabolites. Different compounds have been isolated, for example, terpenoids and flavonoids. The first class of compounds in Euphorbiaceae are terpenoids, classified into diterpenoids, triterpenoids, and sesquiterpenoids. These metabolites have been studied in this family for a long time for their different biological activities. The diterpenoids are cyclized from the precursor geranyl-geranyl-phosphate to the corresponding diterpenoid skeleton. In the Euphorbiaceae family, compounds have been isolated with different types of diterpenoid skeletons [44,45,46].
For example, in the genus Euphorbia, different types of diterpenoids have been described, such as labdane, abietane, atisane, kaurane, isopimarane, rosane, dolabrane, casbane, cembrane, rharnnofolane, gaditanone, ingenane, ingol, jatrophane, jatropholane, lathyrane, cyclomyrsinol, myrsinol, premyrsinol, paraliane, pepluane, presegetane, segetane, tigliane, cyclojatrophane, and expoxyjatropholane [44,45]. Due to the high diversity of plants and metabolites in this genus, every year new diterpenoids are discovered. Zhu et al. [47] using the roots Euphorbia fischeriana L. described two new ent-abietane diterpenoids, euphonoids H and I. Zhang et al. [48] using the same plant species have reported for the first time the presence of a new ent-rosane diterpene, named ebracteolatas D. On the other hand, in the Croton genus, labdanes, clerodanes, trachylobane, kaurene, cembrane, and isopimarane diterpenoids have been identified [46]. Wang et al. [49] reported the isolation of a new ent-abietane diterpenoid (7b,13a,15-tri-hydroxy-ent-abieta-8(14)-en-3-one) from the leaves of Croton lachnocarpus Benth. In the genus Jatropha, different diterpenoids’ skeletons have also been described, such as tigliane, casbene, daphnane, lathyrane, jatrophane, podocarpane, and rhamnofolane [50]. For example, the team led by Yuan et al. [51] isolated jatropodagins A, a new lathyrane-type diterpenoid.
Triterpenoids are also very common and diverse in the family. In the genus Euphorbia, different types of skeletons have been described, for example, tirucallane, euphane, lanostane, cycloartanes, lupane, oleanane, taraxarane, friedoursane, friedelane, and ursane [52]. And still, nowadays, new compounds are being discovered. A new cycloartane-type triterpene (23 R/S-3b-hydroxycycloart-24-ene-23-methyl ether) was isolated from the aerial parts of Euphorbia dendroides L [53]. In the genus Croton, several triterpenoids have been described [46]. For example, lupeol has been isolated from different parts of the species Croton sylvaticus Hochst. and Croton zambezicus Müll. Arg. [54,55]. β-Sitosterol has been isolated from Croton zambezicus Müll. Arg. and Croton steenkampianus Gerstner [15,56]. Betulinic acid, betulin, and lupenone have been isolated from the fruits of Croton zambezicus [57]. Jatrogrossidione, a triterpenoid isolated from the branches and leaves of Jatropha gossypiifolia L., was isolated by Zhan et al. [58].
Also, a few sesquiterpenoids have been reported from Euphorbia, Croton, Jatropha, etc. [46,59,60]. Aryanin, a new sesquiterpene lactone, was isolated from the aerial parts of Euphorbia microsphaera Boiss by Azizi et al. [61]. Another sesquiterpene, caryophyllene, was reported to be present in Croton species as a volatile constituent [46].
The presence of flavonoids in this family is widespread. These compounds could have an impact on health, for example, as potential anticancer agents that have been previously reported [62]. For example, quercitrin has been isolated from Euphorbia hirta L. [63], and apigenin has been isolated from Croton betulaster Mull, Jatropha gossypiifolia L., and Macaranga gigantifolia Merr. [17,20,64].
Some alkaloids have been isolated from this family also, from latex, roots, or other parts of the plants. Alkaloids are important chemicals; some of them have been isolated or developed for cancer treatment, such as the alkaloids of vinca [65]. For example, Novello et al. [66] have isolated, from Croton echioides Baill, a new alkaloid (N-trans-feruloyl-3,5-dihydroxyindolin-2-one) as a mixture with other already known alkaloids (N-trans-p-coumaroyl-tryptamine, N-trans-p-coumaroyl-5-hydroxytryptamine, N-trans-4-methoxy-cinnamoyl-5-hydroxytryptamine, and N-trans-feruloyl-5-hydroxytryptamine.
3.2. Cytotoxic and Anticancer Effects of Euphorbiaceae Extracts In Vitro Studies
Testing plant extracts is one of the first steps in the search for new compounds with potential biological properties, including an anticancer effect. Many articles confirm the preliminary cytotoxic properties of extracts from many medicinal plant families, which can induce apoptosis in many cancer lines. The Euphorbiaceae family is a source of many interesting compounds with broad medicinal applications. The first step of our analysis was to analyze the effect of plant extracts on cytotoxic effects in in vitro studies. Mesas et al. [67] showed that an ethanolic extract of Euphorbia lathyris seeds rich in polyphenols such as esculetin, euphorbetin, gaultherin, and kaempferol-3-rutinoside had an antiproliferative effect against colon cancer cell lines (T84 and HCT-15) and glioblastoma multiforme. The authors demonstrated that the induction of apoptosis is associated with overexpression of caspase-9 (casp-9), caspase-3 (casp-3), and caspase-8 (casp-8) and activation of autophagy. In another study, Sultana et al. [68] showed that an aqueous leaf extract of Excoecaria agallocha (L.), where the head compound was bergenin, effectively reduced the proliferation of SiHa cervical cancer cells by inducing autophagy and apoptosis in a coordinated manner, with simultaneous stimulation of mitophagy and cell cycle arrest in the G2/M phase. In contrast, Kwan et al. [69] showed that a methanolic extract of Euphorbia hirta exhibited significant inhibition of MCF-7 breast cancer cell survival through induction of apoptosis via a casp-3-independent pathway, activation of caspase-2, caspase-6, casp-8, and casp-9, accumulation of cells in the S and G2/M phases, and DNA fragmentation. Similarly, Mfotie Njoya et al. [70] showed that Croton gratissimus leaf extract exhibited cytotoxic effects on various cancer lines (A549, Caco-2, HeLa, MCF-7) and inhibited cancer cell growth through induction of caspase-3 (casp-3)/caspase-7 (casp-7) activation, with the highest induction (1.83-fold change) obtained on HeLa cells. Vargas-Madriz et al. [71] presented that Cnidoscolus aconitifolius and Porophyllum ruderale, rich in polyphenols, reduced the metabolic activity of human SW480 colon adenocarcinoma cells. In addition, both extracts increased the total number of apoptotic cells and arrested the cell cycle in the G0/G1 phases. Other studies are presented in Table 1.

Table 1.
Cytotoxic properties of selected extracts from the Euphorbiaceae family against cancer cells.
3.3. Cytotoxic and Anticancer Effects of Euphorbiaceae Pure Compounds In Vitro Studies
Many studies have focused on investigating the induction of apoptosis through various signaling pathways in cancer cells as a result of isolated plant compounds. In their study, Wisniewski et al. [145] showed that, of the compounds derived from Euphorbia sp., latilagascene B is an effective P-glycoprotein inhibitor capable of increasing doxorubicin accumulation in resistant cells (human colon carcinoma LoVo cells). In contrast, Li et al. [146] showed that Trigothysoid N, a natural diterpenoid isolated from Trigonostemon thyrsoideus, revealed a strong ability to inhibit the proliferation of A549 lung cancer cells through cell cycle arrest. In addition, the compound can inhibit tumor proliferation and migration by targeting mitochondria, regulating the signal transducer and activator of transcription 3/focal adhesion quinase) signaling pathway (STAT3/FAK), and inhibiting angiogenesis. In another study, Lin et al. [147] showed that Euphorbia L2, a lathyrane diterpenoid isolated from the seeds of Euphorbia lathyris L., possessed potent cytotoxicity against A549 lung cancer cells and induced apoptosis through the mitochondrial pathway via an increase in reactive oxygen species (ROS), loss of mitochondrial potential, release of cytochrome c, activation of casp-9 and casp-3, and cleavage of poly(ADP-ribose) polymerase. In turn, Fan et al. [148] showed that the 8,9-seco-ent-kaurane diterpenoid isolated from Croton kongensis induced apoptosis, autophagy, and metastasis suppression in triple-negative breast cancer (TNBC) cells by inhibiting Akt. Additionally, in in vivo studies, it significantly inhibited TNBC tumor growth without causing side effects. Wongprayoon et al. [149] noted that Euphorbia lactea triterpenoid friedelan-3β-ol was cytotoxic to several cancer cell lines, including HN22, HepG2, HCT116, and HeLa. Furthermore, the authors showed that the compound induced S-phase cell cycle arrest in HN22 cells without inducing apoptosis at the same concentration and exposure time. Studies about isolated compounds are presented in Table 2 below.

Table 2.
Cytotoxic properties of isolated compounds from the Euphorbiaceae family of plants against cancer cells.
3.4. Anticancer Effects of Euphorbiaceae Extracts and Pure Compounds In Vivo Studies
The anticancer effects of both extracts and pure compounds have been tested in vivo using various animal models. Many studies are available on in vivo experiments on a number of plants from the Euphorbiaceae family. de Abrantes et al. [209] showed that Tonantzitlolone B (TNZ-B), a diterpene from Stillingia loranthacea, exhibits antitumor activity (1.5 or 3 mg/kg i.p.) in a mouse model of Ehrlich ascites carcinoma. The LD50 was estimated to be approximately 25 mg/kg (i.p.). TNZ-B reduced Ehrlich tumor volume and the total number of viable tumor cells. In addition, TNZ-B reduced the density of peri-tumor microvessels, suggesting an anti-angiogenic effect. Gowrav Adiga et al. [210] investigated the anticancer activity of a methanol extract of the stem bark of Croton oblongifolius in Sprague-Dawley rats. The rats were tumor-induced with dimethylbenz(a)anthracene (DMBA), administered orally and intramammarily, and with a plant extract. After obtaining the appropriate tumor mass, the extract was administered to the rats by gavage at 200, 500, and 800 mg/kg, which showed a dose-dependent reduction in mammary tumor volume, as confirmed by histopathological observations in the treated groups. In other studies, using a mouse model of breast cancer induced by 4T1 cells, Majumder et al. [211] demonstrated the effect of Ricinus communis fruit extract, rich in ricinine, p-coumaric acid, epigallocatechin, and ricinoleic acid, on breast cancer progression in vivo. Tumors were induced in female Balb/c mice by injecting 4T1 cells subcutaneously into the mammary fat pad. After 10 days, one group of animals received 4 i.p. doses of the extract (5 mg/kg body weight), while the other group received only the vehicle (0.9% saline). The tumor in the control group continued to grow, and animals treated with the extract showed a significant reduction in tumor volume over time. Tumors removed from the animals after 22 days showed a reduction in tumor volume of over 88% compared to the control group. Table 3 shows in vivo studies.

Table 3.
Cytotoxic properties of selected extracts or pure compounds from the Euphorbiaceae family against in vivo models.
3.5. Anticancer and Cytotoxic Effects of Nanoparticles Prepared from Euphobiaceae Extracts and Pure Compounds
Nanoparticles (NP) combined with extracts or pure compounds of plant origin are currently a well-known method for biological research and enhancing the effects of various substances in in vitro or in vivo models. In the research by Ghramh et al. [218], an ethanolic leaf extract of Ricinus communis with gold nanoparticles (AuNPs) demonstrated a cytotoxic effect. Studies have shown that the extract in combination with AuNPs has a stronger effect on HeLa and HepG2 lines than the Ricinus communis extract alone. In turn, Alqahtani et al. [219] also demonstrated a stronger cytotoxic effect of Jatropha pelargoniifolia extract in combination with chitosan nanoparticles against human lung adenocarcinoma (A549) than the extract alone. The same author revealed that Euphorbia retusa with a combination of zinc oxide NPs (ZnONPs) exhibited cytotoxic activity against A549 (IC50 = 22.3 µg/mL), HepG2 (IC50 = 25.6), Huh-7 (IC50 = 25.7), MCF-7 (IC50 = 37.7), and MDA-MB-231 (IC50 = 37) [220]. Similarly, Aboulthana et al. [221] showed that the cytotoxic activity of Croton tiglium seed extract increased after the incorporation of ZnONPs against human colon cancer cells (CACO-2). Additionally, the extract combined with ZnONPs stopped the increase of CACO-2 in G2/M and increased the percentage of total apoptotic cells and necrosis. A novel approach using Excoecaria agallocha leaf extract for the synthesis of silver NPs (AgONPs) was demonstrated by Banerjee et al. [222] AgONPs exerted initial cytotoxicity specifically against all experimental malignant cells (murine melanoma (B16F10), murine colon cancer (CT26), murine lung adenocarcinoma (3LL), and murine Ehrlich ascites carcinoma (EAC)), while sparing normal cell lines. Furthermore, both in vitro and ex vivo, AgONPs are equally effective in inducing apoptosis in all cancer cells. Studies on nanoparticles are presented in Table 4 below.

Table 4.
Nanoparticles made using constituents from the plants of the Euphobiaceae family.
4. Potential Anticancer Mechanism of Action of Euphorbiaceae Extracts and Isolated Compounds
The cytotoxic activity reported by several authors in cancer cell lines treated with Euphorbiaceae extracts or isolated compounds may be due to different mechanisms of action. It is known that these extracts and compounds can activate the intrinsic pathway and the extrinsic pathway of apoptosis. The chemical structures of the main isolated compounds with anticancer activity and their underlying mechanisms of action are presented in Figure 2 below.

Figure 2.
Chemical structure of isolated compounds from Euphorbiaceae plants with anticancer potential.
To activate the intrinsic pathway, the extract or compound could act as a ROS inductor. For example, Croton gratissimus Burch, Drypetes sepiaria (Wight & Arn.) Pax & K. Hoffm., Euphorbia cactus Ehrenb. ex Boiss., Euphorbia hirta L., Euphorbia ingens E. Mey. ex Boiss., Euphorbia lathyrism L., Excoecaria agallocha L., Euphorbia helioscopia L., and Ricinus communis L. extracts [67,68,70,94,95,115,211,215], and the pure compounds Euphorbia Factor L2, Ebracteolatain A, and Ebracteolatain B [147,232], activate the intrinsic pathway at some point. DNA damage promotes the liberation of cytochrome C by mitochondria. Then, cytochrome c and the APAF-1 (Apoptotic Protease Activating Factor-1) protein join together to activate casp-9. In this stage, it becomes a cascade release of different caspases activating each other from an inactive form to an active form and, at the end, activating casp-3, leading, finally, to apoptosis [67,70,94,136]. During this process, mitochondria also release SMAC/DIABLO (Second Mitochondria-Derived Activator of Caspase), an inhibitor of the XIAP (X-linked inhibitor of apoptosis) protein [233,234]. The caspases also cleave PARP (Poly ADP-Ribose Polymerase); this process is considered a hallmark of apoptosis [233].
The extrinsic pathway could be activated too, increasing the expression of casp-2/ casp-8. For example, some authors have reported this activity with Croton gratissimus Burch, Drypetes sepiaria (Wight & Arn.) Pax & K.Hoffm, and Euphorbia lathyrism L extracts [67,70,94].
The expression of p53 could also be affected by this family of plants. Several authors have reported higher levels of p53 after treatment with Euphorbia pulcherrima Willd. ex Klotzsch, Euphorbia heterophylla L., Euhporbia ingens E.Mey. ex Boiss, and Excoecaria agallocha L. in cancer cell lines [68,105,115]. p53 is a tumor-suppressive protein with the ability to induce cell death, including through apoptotic mechanisms and other transcription-dependent mechanisms or independent mechanisms [235]. Zhou et al. [232] also found that the activity of the pure compounds Ebracteolatain A and Ebracteolatain B increased p53 levels and decreased survivin levels. Survivin is a survival protein that inhibits caspases and blocks cell death [236]. Sultana et al. [68] found an altered expression on the levels of p21, the main target protein of p53. This protein, also known as CDKN1A (cyclin-dependent kinase inhibitor 1A), regulates the progression of the cell cycle with cyclin B. The balance of Bax/Bcl family protein expression can also be regulated through p53.
Changing the Bcl2/Bax (antiapoptotic/proapoptotic) protein balance could also be regulated by Euphorbiaceae plants through the STAT3 gene transcription pathway [146]. This balance is reported to be higher for Bax proteins than Bcl2 proteins when cancer cells are treated with Croton tiglium L., Euphorbia cactus Ehrenb. ex Boiss., Euphorbia pulcherrima Willd. ex Klotzsch, Euphorbia heterophylla L., Euphorbia helioscopia L., Euphorbia hierosolymitana Boiss., and Ricinus communis L. extracts [13,95,105,106,211,215]. Proapoptotic protein Bax is higher when cancer cells receive treatment with Trigothysoid N, Ebracteolatain A, and Ebracteolatain B also [146,232].
Li et al. [146] reported that cell invasion and migration could be regulated by Trigothysoid N through the FAK pathway. Once the compound inhibits FAK, the transcription of metalloproteinase (MMP)-2 and MMP-9 is downregulated, and the cells are not able to invade or migrate to other tissues.
The Euphorbiaceae extracts and isolated compounds could modulate the level of citoplasmatic proteins, acting as inhibitors or downregulating/upregulating them. For example, Okpako et al. [115] reported a downregulation in androgen receptors after the treatment of prostate cancer cells DU-145 with Euphorbia ingens E.Mey. ex Boiss extract. Lei et al. [206] reported inhibited tubulin polymerization after treatment with Methyltrewiasine and N-methyltreflorine. Tubulin polymerization/despolimerization plays a critical role in the cell cycle, and some important anticancer drugs are microtubule stabilizers of vegetal origin, for example, paclitaxel and docetaxel. Another example of protein regulation was reported by Shi et al. [237]. Using 8,9-seco-ent-kaurane diterpenoid isolated from Croton kongensis Gagnep in TNBC cells, the compound acted like an Akt inhibitor, which could induce apoptosis, autophagy, cell G2/M circle arrest, and inhibit cell migration. Latilagascene B was reported to be a p-glycoprotein inhibitor (Multidrug-resistant receptor) [145]. The cell cycle is regulated differently depending on the extract or pure compound used in cancer cells. In such a way that we can find stops in phase G1 [58,146], in phase G2/M [68,85], or in phase S [58] after the treatment with Euphorbiaceae. The anticancer potential mechanism of action of extracts and compounds from the Euphorbiaceae family is shown in Figure 3 below.
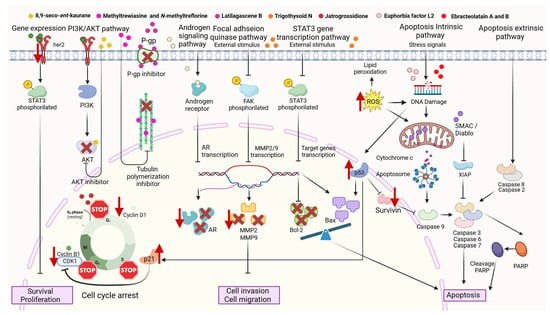
Figure 3.
Potential anticancer mechanisms of action of Euphorbiaceae extracts and isolated compounds (created by BioRender).
5. Conclusions and Future Perspectives
Research into the anticancer properties of plants in the Euphorbiaceae family shows promising potential. Cancer continues to be a global health problem, both in terms of health and economy. The lack of effectiveness and selectivity of the currently most used anticancer drugs makes it necessary to continue the search for new drugs. Nature continues to be an inexhaustible source of useful molecules to cure or alleviate diseases. It is for this reason that it is necessary to continue investigating the molecules present in living beings that surround us, including plants, algae, fungi, and marine organisms. In this review, we emphasize the usefulness of the various compounds present in the Euphorbiaceae family. This family of vascular plants is a very diverse family, both in genera and species and in secondary metabolites. The presence of different types of terpenoids, flavonoids, and alkaloids with cytotoxic activity against cancer makes us think about the number of possibilities that these compounds could offer alone or through the synthesis of different derivatives from them. All these active compounds represent an opportunity for the treatment of cancer; however, from a future perspective, it is necessary to continue researching all these molecules. Greater funding is necessary for the research of new molecules to be able to advance them more quickly from the process of discovery to the regulation and approval process for the market and patients. In this context, preclinical and clinical studies in animal models, followed by human clinical trials to evaluate their efficacy, safety, and dosage in different types of cancer, seem to play a very important role in this field.
Author Contributions
Conceptualization, V.J.-G. and P.S.; methodology, V.J.-G., P.S. and T.K.; investigation, V.J.-G., P.S. and T.K.; writing—original draft preparation, V.J.-G., P.S., T.K., J.S. and J.P.; writing—review and editing, V.J.-G., P.S., T.K., J.P., J.S. and P.R.; visualization, V.J.-G.; supervision, P.S. and P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to express their gratitude to Alexander Harrison for reviewing scientific English.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AgONPs | Silver oxide nanoparticles |
| Akt | Protein kinase B |
| APAF | Apoptotic Protease-Activating Factor-1 |
| AuNPs | Gold nanoparticles |
| Bax | Apoptosis regulator or bcl-2-like protein 4 |
| Bcl-2 | B-cell lymphoma 2 protein |
| Casp-3 | Caspase 3 |
| Casp-6 | Caspase 6 |
| Casp-7 | Caspase 7 |
| Casp-8 | Caspase 8 |
| Casp-9 | Caspase 9 |
| CuONPs | Cupper oxide nanoparticles |
| MAPK | Mitogen-activated protein kinase |
| MMP-2 | Metalloproteinase 2 |
| MMP-9 | Metalloproteinase 9 |
| NP | Nanoparticles |
| P53 | Tumor protein P53 |
| PARP | Poly ADP-Ribose Polymerase |
| PI3K | Phosphoinositide 3-kinases |
| ROS | Reactive oxygen species |
| SMAC/DIABLO | Second Mitochondria-Derived Activator of Caspase |
| STAT3 | Signal transducer and activator of transcription 3 |
| TNBC | Triple negative breast cancer |
| TNB-Z | Tonantzitlolone B |
| XIAP | X-linked inhibitor of apoptosis |
| ZnONPs | Zinc oxide nanoparticles |
References
- Sarkar, S.; Horn, G.; Moulton, K.; Oza, A.; Byler, S.; Kokolus, S.; Longacre, M. Cancer Development, Progression, and Therapy: An Epigenetic Overview. Int. J. Mol. Sci. 2013, 14, 21087–21113. [Google Scholar] [CrossRef]
- Bremnes, R.M.; Camps, C.; Sirera, R. Angiogenesis in Non-Small Cell Lung Cancer: The Prognostic Impact of Neoangiogenesis and the Cytokines VEGF and BFGF in Tumours and Blood. Lung Cancer 2006, 51, 143–158. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO-Cancer Report-2020-Global Profile; World Health Organization (WHO): Geneva, Switzerland, 2020. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Voda, A.I.; Bostan, I. Public Health Care Financing and the Costs of Cancer Care: A Cross-National Analysis. Cancers 2018, 10, 117. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative Approaches for Cancer Treatment: Current Perspectives and New Challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Plants as a Source of Anti-Cancer Agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Kingston, D.G.I.; Newman, D.J. The Search for Novel Drug Leads for Predominately Antitumor Therapies by Utilizing Mother Nature’s Pharmacophoric Libraries. Curr. Opin. Drug Discov. Dev. 2005, 8, 207–227. [Google Scholar]
- Ernst, M.; Grace, O.M.; Saslis-Lagoudakis, C.H.; Nilsson, N.; Simonsen, H.T.; Rønsted, N. Global Medicinal Uses of Euphorbia L. (Euphorbiaceae). J. Ethnopharmacol. 2015, 176, 90–101. [Google Scholar] [CrossRef]
- Saleh, Z.M.; Abdel Azeiz, A.Z.; Mehany, A.B.M.; El-Swaify, Z.A.S. Anticancer and Antimicrobial Activity of Jatropha’s Leaves Extracts. Egypt. J. Bot. 2023, 63, 621–634. [Google Scholar] [CrossRef]
- Prakash, E.; Gupta, D.K. Cytotoxic Activities of Extracts of Medicinal Plants of Euphorbiacae Family Studied on Seven Human Cancer Cell Lines. Univ. J. Plant Sci. 2013, 1, 113–117. [Google Scholar] [CrossRef]
- Li, C.; Wu, X.; Sun, R.; Zhao, P.; Liu, F.; Zhang, C. Croton tiglium Extract Induces Apoptosis via Bax/Bcl-2 Pathways in Human Lung Cancer A549 Cells. Asian Pac. J. Cancer Prev. 2016, 17, 4893–4898. [Google Scholar] [CrossRef]
- Vasas, A.; Hohmann, J. Euphorbia Diterpenes: Isolation, Structure, Biological Activity, and Synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef]
- Block, S.; Baccelli, C.; Tinant, B.; Van Meervelt, L.; Rozenberg, R.; Habib Jiwan, J.-L.; Llabrès, G.; De Pauw-Gillet, M.-C.; Quetin-Leclercq, J. Diterpenes from the Leaves of Croton zambesicus. Phytochemistry 2004, 65, 1165–1171. [Google Scholar] [CrossRef]
- Kemboi, D.; Siwe-noundou, X.; Krause, R.W.M.; Langat, M.K.; Tembu, V.J. Euphorbia Diterpenes: An Update of Isolation, Structure, Pharmacological Activities and Structure—Activity Relationship. Molecules 2021, 26, 5055. [Google Scholar] [CrossRef]
- Subramanian, S.S.; Nagarajan, S.; Sulochana, N. Flavonoids of the Leaves of Jatropha gossypifolia. Phytochemistry 1971, 10, 1690. [Google Scholar] [CrossRef]
- Šmejkal, K. Cytotoxic Potential of C-Prenylated Flavonoids. Phytochem. Rev. 2014, 13, 245–275. [Google Scholar] [CrossRef]
- Megawati, M.; Saepudin, E.; Hanafi, M.; Darmawan, A.; Lotulung, P.D.N. Identification and Bioactivity Studies of Flavonoid Compounds from Macaranga hispida (Blume) Mull.Arg. Makara J. Sci. 2015, 19, 96–100. [Google Scholar] [CrossRef]
- Coelho, P.L.C.; Oliveira, M.N.; da Silva, A.B.; Pitanga, B.P.S.; Silva, V.D.A.; Faria, G.P.; Sampaio, G.P.; Costa, M.d.F.D.; Braga-de-Souza, S.; Costa, S.L. The Flavonoid Apigenin from Croton betulaster Mull Inhibits Proliferation, Induces Differentiation and Regulates the Inflammatory Profile of Glioma Cells. Anticancer Drugs 2016, 27, 960–969. [Google Scholar] [CrossRef]
- Novello, C.R.; Marques, L.C.; Pires, M.E.; Kutschenco, A.P.; Nakamura, C.V.; Nocchi, S.; Sarragiotto, M.H.; Mello, J.C.P. Bioactive Indole Alkaloids from Croton echioides. J. Braz. Chem. Soc. 2016, 27, 2203–2209. [Google Scholar] [CrossRef]
- Euphorbiaceae. Plants of the World Online; Kew Royal Botanical Garden: Richmond, UK, 2023. [Google Scholar]
- GBIF. Euphorbiaceae in Global Biodiversity Information Facility; GBIF: Copenhagen, Denmark, 2023. [Google Scholar] [CrossRef]
- Valdés, B.; Talavera, S.; Fernández-Galiano, E. (Eds.) Flora Vascular de Andalucía Occidental; Flora Vascular: Barcelona, Spain, 1987. [Google Scholar]
- Blanca, G.; Cabezudo, B.; Cueto, M.; Salazar, C.; Morales Torres, C. (Eds.) Flora Vascular de Andalucía Oriental, 2nd ed.; Corregida y Aumentada; Conserjería de Medio Ambiente, Junta de Andalucía: Granada, Spain, 2011. [Google Scholar]
- Cueto, M.; Melendo, M.; Giménez, E.; Fuentes, J.; Carrique, E.L.; Blanca, G. First Updated Checklist of the Vascular Flora of Andalusia (S of Spain), One of the Main; Biodiversity Centres in the Mediterranean Basin: Auckland, New Zealand, 2018; Volume 339, ISBN 9781776703104. [Google Scholar]
- FAO. Save and Grow: Cassava; FAO: Rome, Italy, 2013. [Google Scholar]
- Mohidin, S.R.N.S.P.; Moshawih, S.; Hermansyah, A.; Asmuni, M.I.; Shafqat, N.; Ming, L.C. Cassava (Manihot esculenta Crantz): A Systematic Review for the Pharmacological Activities, Traditional Uses, Nutritional Values, and Phytochemistry. J. Evid.-Based Integr. Med. 2023, 28, 2515690X231206227. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT Cassava. Available online: https://www.fao.org/faostat/es/#data/qv (accessed on 15 September 2023).
- Marwat, S.K.; Rehman, F.; Khan, E.A.; Baloch, M.S.; Sci, P.J.P.; Sadiq, M.; Ullah, I.; Javaria, S.; Shaheen, S. Ricinus communis: Ethnomedicinal Uses and Pharmacological Activities. Pak. J. Pharm. Sci. 2017, 30, 1815–1827. [Google Scholar] [PubMed]
- Johnson, W. Final Report on the Safety Assessment of Ricinus communis (Castor) Seed Oil, Hydrogenated Castor Oil, Glyceryl Ricinoleate, Glyceryl Ricinoleate SE, Ricinoleic Acid, Potassium Ricinoleate, Sodium Ricinoleate, Zinc Ricinoleate, Cetyl Ricinoleate, Ethyl Ricinoleate, Glycol Ricinoleate, Isopropyl Ricinoleate, Methyl Ricinoleate, and Octyldodecyl Ricinoleate. Int. J. Toxicol. 2007, 26, 31–77. [Google Scholar]
- Kelly, A.J.; Kavanagh, J.; Thomas, J. Castor Oil, Bath and/or Enema for Cervical Priming and Induction of Labour. Cochrane Database Syst. Rev. 2013, 2013, CD0030992013. [Google Scholar] [CrossRef] [PubMed]
- Elkousy, R.H.; Said, Z.N.A.; El-Baseer, M.A.A.; El Wafa, S.A.A. Antiviral Activity of Castor Oil Plant (Ricinus communis) Leaf Extracts. J. Ethnopharmacol. 2021, 271, 113878. [Google Scholar] [CrossRef] [PubMed]
- Fitranda, M.I.; Sutrisno; Marfu’ah, S. Physicochemical Properties and Antibacterial Activity of Castor Oil and Its Derivatives. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Malang, Indonesia, 2–3 November 2019; Institute of Physics Publishing: Bristol, UK, 2020; Volume 833. [Google Scholar]
- Ogunniyi, D.S. Castor Oil: A Vital Industrial Raw Material. Bioresour. Technol. 2006, 97, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Neuwinger, H.D. Plants Used for Poison Fishing in Tropical Africa. Toxicon 2004, 44, 417–430. [Google Scholar] [CrossRef]
- Lai, X.Z.; Yang, Y.B.; Shan, X.L. The Investigation of Euphorbiaceous Medicinal Plants in Southern China. Econ. Bot. 2004, 58, S307–S320. [Google Scholar] [CrossRef]
- Polygenis-bigendako, M.J.; Lejoly, J. Plantes employées dans le traitement des diarrhées en médecine traditionnelle au burundi occidental. Bull. Soc. R. Bot. Belg./Bull. Van K. Belg. Bot. Ver. 1989, 122, 87–97. [Google Scholar]
- Gaioni, D.T. Medical Choices in a Philippine Highland Community. Ethnomedical and Biomedical Dimensions of Bauko Clinical Reality. Anthropos 2002, 97, 505–518. [Google Scholar]
- Bard, C.L. A Contribution to the History of Medicine in Southern California; Hansebooks: Norderstedt, Germany, 1894. [Google Scholar]
- Hargreaves, B.J. The Spurges of Botswana. Botsw. Notes Rec. 1991, 23, 115–130. [Google Scholar]
- Safford, W.E. The Useful Plants of the Island of Guam; Bulletin (United States National Museum); U.S. Government Printing Office: Washington, DC, USA, 1905; ISBN 9780598582195.
- Manandhar, N.P. An Inventory of Some Herbal Drugs of Myagdi District, Nepal. Econ. Bot. 1995, 49, 371–379. [Google Scholar] [CrossRef]
- Zhan, Z.J.; Li, S.; Chu, W.; Yin, S. Euphorbia Diterpenoids: Isolation, Structure, Bioactivity, Biosynthesis, and Synthesis (2013–2021). Nat. Prod. Rep. 2022, 39, 2132–2174. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tang, P.; Zhu, M.; Wang, Y.; Sun, D.; Li, H.; Chen, L. Diterpenoids from the Genus Euphorbia: Structure and Biological Activity (2013–2019). Phytochemistry 2021, 190, 112846. [Google Scholar] [CrossRef] [PubMed]
- Moremi, M.P.; Makolo, F.; Viljoen, A.M.; Kamatou, G.P. A Review of Biological Activities and Phytochemistry of Six Ethnomedicinally Important South African Croton Species. J. Ethnopharmacol. 2021, 280, 114416. [Google Scholar] [CrossRef]
- Zhu, Q.F.; Xu, G.B.; Liao, S.G.; Yan, X.L. Ent-Abietane Diterpenoids from Euphorbia fischeriana and Their Cytotoxic Activities. Molecules 2022, 27, 7258. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, P.; Ma, J.; Li, X.; Yuan, W. A New Diterpenoid with Cytotoxic Activities from the Roots of Euphorbia fischeriana. Chem. Nat. Compd. 2023, 59, 701–705. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.J. Cytotoxic Ent-Abietane Diterpenoids from the Leaves of Croton lachnocarpus Benth. J. Asian Nat. Prod. Res. 2023, 25, 309–315. [Google Scholar] [CrossRef]
- Sabandar, C.W.; Ahmat, N.; Jaafar, F.M.; Sahidin, I. Medicinal Property, Phytochemistry and Pharmacology of Several Jatropha Species (Euphorbiaceae): A Review. Phytochemistry 2013, 85, 7–29. [Google Scholar] [CrossRef]
- Yuan, H.-T.; Li, Q.-F.; Tian, T.; Zhang, C.-Y.; Huang, Z.-Q.; Fan, C.-X.; Mei, K.; Zhou, J.; Zhai, X.-X.; Li, S.-B.; et al. Lathyrane Diterpenoids from Jatropha podagrica and Their Antitumor Activities in Human Osteosarcoma Cells. Nat. Prod. Res. 2021, 35, 5089–5095. [Google Scholar] [CrossRef]
- Kemboi, D.; Peter, X.; Langat, M.; Tembu, J. A Review of the Ethnomedicinal Uses, Biological Activities, and Triterpenoids of Euphorbia Species. Molecules 2020, 25, 4019. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.R.; Ashour, A.; Amen, Y.; Nagata, M.; El-Toumy, S.A.; Shimizu, K. A New Cycloartane Triterpene and Other Phytoconstituents from the Aerial Parts of Euphorbia dendroides. Nat. Prod. Res. 2022, 36, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Ngadjui, B.T.; Folefoc, G.G.; Keumedjio, F.; Dongo, E.; Sondengam, B.L.; Connolly, J.D. Crotonadiol, a Labdane Diterpenoid from the Stem Barkof Croton zambesicus. Phytochemistry 1999, 51, 171–174. [Google Scholar] [CrossRef]
- Kapingu, M.C.; Mbwambo, Z.H.; Moshi, M.J.; Magadula, J.J. Brine Shrimp Lethality of Alkaloids from Croton sylvaticus Hoechst. East Cent. Afr. J. Pharm. Sci. 2012, 15, 35–37. [Google Scholar]
- Prozesky, E.A. Antiplasmodial-and Chloroquine Resistance Reversal Properties of a New Diterpene from Croton steenkampianus; University of Pretoria (South Africa) ProQuest Dissertations Publishing: Ann Arbor, MI, USA, 2005. [Google Scholar]
- Mohamed, I.E.; El Nur, E.B.E.; Choudhary, M.I.; Khan, S.N. Bioactive Natural Products from Two Sudanese Medicinal Plants Diospyros mespiliformis and Croton zambesicus. Rec. Nat. Prod. 2009, 3, 198–203. [Google Scholar]
- Zhang, C.-Y.; Zhang, R.-R.; Zhu, J.Y. New Tetracyclic Triterpenoids from Jatropha gossypiifolia Induce Cell-Cycle Arrest and Apoptosis in RKO Cells. Fitoterapia 2018, 130, 145–151. [Google Scholar] [CrossRef]
- Cavalcante, N.B.; da Conceição Santos, A.D.; da Silva Almeida, J.R.G. The Genus Jatropha (Euphorbiaceae): A Review on Secondary Chemical Metabolites and Biological Aspects. Chem. Biol. Interact. 2020, 318, 108976. [Google Scholar] [CrossRef]
- Salehi, B.; Iriti, M.; Vitalini, S.; Antolak, H.; Pawlikowska, E.; Kręgiel, D.; Sharifi-Rad, J.; Oyeleye, S.I.; Ademiluyi, A.O.; Czopek, K.; et al. Euphorbia-Derived Natural Products with Potential for Use in Health Maintenance. Biomolecules 2019, 9, 337. [Google Scholar] [CrossRef]
- Azizi, K.; Hamedi, A.; Azarpira, N.; Hamedi, A.; Shahini, M.; Pasdaran, A. A New Cytotoxic Sesquiterpene Lactone from Euphorbia microsphaera Boiss against Human Breast Cancer (MCF-7) and Human Fibrosarcoma (HT1080) Cells. Toxicon 2021, 202, 60–66. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Gálvez, J.; Crespo, M.E.; Jiménez, J.; Suárez, A.; Zarzuelo, A. Antidiarrhoeic Activity of Quercitrin in Mice and Rats. J. Pharm. Pharmacol. 1993, 45, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Fajriah, S.; Darmawan, A. Apigenin, an Anticancer Isolated from Macaranga gigantifolia Leaves. J. Trop. Life Sci. 2016, 6, 7–9. [Google Scholar]
- Mondal, A.; Gandhi, A.; Fimognari, C.; Atanasov, A.G.; Bishayee, A. Alkaloids for Cancer Prevention and Therapy: Current Progress and Future Perspectives. Eur. J. Pharmacol. 2019, 858, 172472. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, I.; Venturella, S.R.T.; Bressiani, P.A.; Marco, I.G.; Novello, C.R.; Almeida, I.V.; Vicentini, V.E.P.; Mello, J.C.P.; Düsman, E. Cytotoxicity of Extracts and Compounds Isolated from Croton echioides in Animal Tumor Cell (HTC). Braz. J. Biol. 2022, 82, e264356. [Google Scholar] [CrossRef]
- Mesas, C.; Martínez, R.; Ortíz, R.; Galisteo, M.; López-Jurado, M.; Cabeza, L.; Perazzoli, G.; Melguizo, C.; Porres, J.M.; Prados, J. Antitumor Effect of the Ethanolic Extract from Seeds of Euphorbia lathyris in Colorectal Cancer. Nutrients 2021, 13, 566. [Google Scholar] [CrossRef]
- Sultana, T.; Mitra, A.K.; Das, S. Evaluation of Anti-Cancer Potential of Excoecaria agallocha (L.) Leaf Extract on Human Cervical Cancer (SiHa) Cell Line and Assessing the Underlying Mechanism of Action. Futur. J. Pharm. Sci. 2022, 8, 3. [Google Scholar] [CrossRef]
- Kwan, Y.P.; Saito, T.; Ibrahim, D.; Al-Hassan, F.M.S.; Oon, C.E.; Chen, Y.; Jothy, S.L.; Kanwar, J.R.; Sasidharan, S. Evaluation of the Cytotoxicity, Cell-Cycle Arrest, and Apoptotic Induction by Euphorbia hirta in MCF-7 Breast Cancer Cells. Pharm. Biol. 2016, 54, 1223–1236. [Google Scholar] [CrossRef]
- Njoya, E.M.; Eloff, J.N.; McGaw, L.J. Croton gratissimus Leaf Extracts Inhibit Cancer Cell Growth by Inducing Caspase 3/7 Activation with Additional Anti-Inflammatory and Antioxidant Activities. BMC Complement. Altern. Med. 2018, 18, 305. [Google Scholar] [CrossRef]
- Vargas-Madriz, Á.F.; Luzardo-Ocampo, I.; Moreno-Celis, U.; Roldán-Padrón, O.; Chávez-Servín, J.L.; Vergara-Castañeda, H.A.; Martínez-Pacheco, M.; Mejía, C.; García-Gasca, T.; Kuri-García, A. Comparison of Phytochemical Composition and Untargeted Metabolomic Analysis of an Extract from Cnidoscolus aconitifolius (Mill.) I. I. Johnst and Porophyllum ruderale (Jacq.) Cass. and Biological Cytotoxic and Antiproliferative Activity In Vitro. Plants 2023, 12, 1987. [Google Scholar] [CrossRef]
- Al-Massarani, S.; El-Sayed, M.-I.K.; El-Shaibany, A. Antioxidant and Anti-Proliferative Activities of Acalypha fruticosa: Possible Elucidated Mechanism. Pak. J. Pharm. Sci. 2019, 32, 2041–2050. [Google Scholar]
- Chekuri, S.; Panjala, S.; Anupalli, R.R. Cytotoxic Activity of Acalypha Indica L. Hexane Extract on Breast Cancer Cell Lines (MCF-7). J. Phytopharm. 2017, 6, 264–268. [Google Scholar] [CrossRef]
- Guillén-Meléndez, G.A.; Villa-Cedillo, S.A.; Pérez-Hernández, R.A.; Castillo-Velázquez, U.; Salas-Treviño, D.; Saucedo-Cárdenas, O.; Montes-De-oca-luna, R.; Gómez-Tristán, C.A.; Garza-Arredondo, A.J.; Zamora-ávila, D.E.; et al. Cytotoxic Effect in Vitro of Acalypha monostachya Extracts over Human Tumor Cell Lines. Plants 2021, 10, 2326. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishna, S. In-Vitro Anticancer Activity of Baliospermum montanum on T Cell Leukemia-Jurkat Cell Line and Human Breast Cancer-MCF-7. IAETSD J. Adv. Sci. Appl. Sci. 2018, 5, 173–179. [Google Scholar]
- Pipatrattanaseree, W.; Itharat, A.; Mukkasombut, N.; Saesiw, U. Potential in Vitro Anti-Allergic, Anti-Inflammatory and Cytotoxic Activities of Ethanolic Extract of Baliospermum montanum Root, Its Major Components and a Validated HPLC Method. BMC Complement. Altern. Med. 2019, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Adi, A.S.; Elya, B.; Hanafi, M. Antioxidant and Cytotoxic Bioassay on Blumeodendron toxbrai (Blume.) Stem Bark Hexane, Dichloromethane, and Methanolic Ekstract. Pharmacogn. J. 2021, 13, 139–141. [Google Scholar] [CrossRef]
- Ramadan Kamel, M.; Nafady, A.; Hassanein, A.; Ragaey, R.; Haggag, E. Phytochemical Investigation and Assessment of Antioxidant, Antimicrobial and Cytotoxic Activities of the Root Bark Chrozophora oblongifolia (Delile) Spreng. (Euphorbiaceae). J. Adv. Pharm. Res. 2019, 3, 200–208. [Google Scholar] [CrossRef]
- Sunil Kumar, K.; Rao, A.S. Evaluation of Hepatoprotective and Invitro Cytotoxic Activity of Leaves of Chrozophora plicata Linn. Res. J. Pharmacol. Pharmacodyn. 2017, 9, 19–26. [Google Scholar] [CrossRef]
- Ikpefan, E.O.; Ayinde, B.A.; Mudassir, A.; Farooq, A.D. Comparative in Vitro Assessment of the Methanol Extracts of the Leaf, Stem, and Root Barks of Cnidoscolus aconitifolius on Lung and Breast Cancer Cell Lines. Turk. J. Pharm. Sci. 2019, 16, 375–379. [Google Scholar] [CrossRef]
- Kumarasamy, K.P.; Nallaperumal, N.; Natarajan, C.; Nallamadan, J. An in vitro cytotoxicity study of Cnidoscolus chayamansa McVaugh on selected cell lines. World J. Pharm. Pharm. Sci. 2014, 3, 1110–1116. [Google Scholar]
- Sánchez-Aguirre, O.A.; Juárez-Aguilar, E.; Montoya-Hernández, E.L.; Vázquez-Hernández, M.; Colorado-Peralta, R.; Sánchez-Medina, A.; Márquez-López, M.E.; Hernández-Romero, D. Antioxidant Potential of Cnidoscolus multilobus (Pax) I.M. Johnst and Its Antiproliferative and Cytotoxic Effect on Cervical Cancer Cells. Eur. J. Integr. Med. 2022, 53, 102134. [Google Scholar] [CrossRef]
- Paredes, P.F.M.; De Morais, S.M.; Brito, F.C.R.; Moura, L.F.W.G.; De Rodrigues, P.A.; Benjamin, S.R.; Magalhães, F.E.A.; Florean, E.O.P.T.; Guedes, M.I.F. Characterization of Cnidoscolus quercifolius Pohl Bark Root Extract and Evaluation of Cytotoxic Effect on Human Tumor Cell Lines. Asian Pac. J. Trop. Biomed. 2018, 8, 345–351. [Google Scholar] [CrossRef]
- Worarat, C.; Pompimon, W.; Udomputtimekakul, P.; Sukdee, S.; Sombutsiri, P.; Kuanmuang, N.; Suwan, I.; Khamyong, Y.; Suksabai, C.; Artkla, W.; et al. In Vitro Screening for Cytotoxic, Anti-Bacterial, Anti-HIV1-RT Activities and Chemical Constituents of Croton fluviatilis, Croton acutifolius, and Croton thorelii. Nat. Prod. J. 2021, 12, 92. [Google Scholar] [CrossRef]
- Bhavana, J.; Mk, K.; Sumathy, A. Cytotoxic and Pro-Apoptotic Activities of Leaf Extract of Croton bonplandianus Baill. against Lung Cancer Cell Line A549; NISCAIR-CSIR: New Delhi, India, 2016; Volume 54. [Google Scholar]
- Shantabi, L.; Jagetia, G.C.; Moirangthem, D.S.; Nongalleima, K. Anticancer Activity of an Ehnomedicinal Plant Croton caudatus Geiseler, Kam Sabut in Cultured HeLa Cells. Biocatal. Agric. Biotechnol. 2020, 23, 101500. [Google Scholar] [CrossRef]
- Rosangkima, G.; Jagetia, G. Anticancer, Antioxidant and Analgesic Properties of Croton caudatus. Int. J. Curr. Res. 2015, 7, 20640–20646. [Google Scholar]
- De, B.; Mitra, I.; Choudhury, M.D.; Paul, S.B.; Deb, L.; De, A.; Nath, R. In Vitro Cytotoxic Study of Croton caudatus Geiseler—A Traditionally Known Anticancerous Plant in North-East India. Pleione 2018, 12, 165. [Google Scholar] [CrossRef]
- Jéssica, d.A.G.S.; Gibbelly, C.d.S.; Marilia, G.d.F.S.; Vanessa, F.d.S.; Jaciana, d.S.A.; Teresinha, G.d.S.; Sônia, P.L. Physicochemical Characteristics and Cytotoxic Effect of the Methanolic Extract of Croton heliotropiifolius Kunth (Euphorbiaceae). Afr. J. Pharm. Pharmacol. 2017, 11, 321–326. [Google Scholar] [CrossRef]
- Ernandes, P.A.D.S.; Silva, J.C.P.D.; Sales, D.L.; Ribeiro, P.R.V.; de Brito, E.S.; Kerntopf, M.R.; Delmondes, G.D.A.; Pinheiro, J.C.A.; Salazar, G.J.T.; Batista, F.L.A.; et al. Chemical Constituents and Biological Activities of Croton heliotropiifolius Kunth. Antibiotics 2021, 10, 1074. [Google Scholar] [CrossRef]
- Yeboah, K.O.; Emosivbe, M.; Ntim, E.A.; Sam, G.H.; Ainooson, G. The Antiproliferative, Antimigratory and Anticlonogenic Effects of Croton membranaceus Mϋll. Arg. (Euphorbiaceae) Hydroethanolic Root Extract in Human 22Rv1 Castration-Resistant Prostate Cancer Cells. Acta Pharm. Sci. 2023, 61, 121–140. [Google Scholar] [CrossRef]
- Motta, L.B.; Furlan, C.M.; Santos, D.Y.A.C.; Salatino, M.L.F.; Negri, G.; de Carvalho, J.E.; Monteiro, P.A.; Ruiz, A.L.T.G.; Caruzo, M.B.; Salatino, A. Antiproliferative Activity and Constituents of Leaf Extracts of Croton sphaerogynus Baill. (Euphorbiaceae). Ind. Crops Prod. 2013, 50, 661–665. [Google Scholar] [CrossRef]
- Vieira, G.T.; Oliveira, T.T.d; Monteiro, L.P.; Kanashiro, M.M.; Costa, M.R.d; Pereira, W.L. Atividade citotóxica do extrato de Croton urucurana Baill contra linhagens de células leucêmicas humanas U937 e THP1. Ciênc. Nat. 2017, 39, 512. [Google Scholar] [CrossRef]
- Gadamsetty, G.; Maru, S.; Tyagi, A.; Chakravarthula, S.N. Anti-Inflammatory, Cytotoxic and Antioxidant Effects of Methanolic Extracts of Drypetes sepiaria (Euphorbiaceae). Afr. J. Tradit. Complement. Altern. Med. AJTCAM/Afr. Netw. Ethnomed. 2013, 10, 274–282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Hamoud, G.A.; Fantoukh, O.I.; Amina, M.; Nasr, F.A.; Al Musayeib, N.M.; Ahmed, M.Z.; Noman, O.M.; Al-Sharidah, R.E.; Alasmari, F.; Alqahtani, A.S. Unprecedented Insights on Chemical and Biological Significance of Euphorbia cactus Growing in Saudi Arabia. Plants 2022, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Siddiqui, B.S.; Farooq, A.D.; Begum, S.; Siddiqui, F.; Kashif, M. In vitro growth inhibition and cytotoxicity of Euphorbia caducifolia against four human cancer cell lines and its phytochemical characterisation. Nat. Prod. Res. 2017, 31, 2936–2940. [Google Scholar] [CrossRef] [PubMed]
- Rédei, D.; Kúsz, N.; Szabó, M.; Pinke, G.; Zupkó, I.; Hohmann, J. First Phytochemical Investigation of Secondary Metabolites of Euphorbia davidii Subils. and Antiproliferative Activity of Its Extracts Short Communication. Acta Biol. Hung. 2015, 66, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.T.; Yousef, A.K.; Ahmed, F.A.; Elgneady, F.M.; El-Adl, K.; Elhady, M.M. In Silico ADMET, Docking, Anti-Proliferative and Antimicrobial Evaluations of Ethanolic Extract of Euphorbia dendroides L. S. Afr. J. Bot. 2022, 150, 607–620. [Google Scholar] [CrossRef]
- Ikpefan, E.O.; Ayinde, B.A.; Mudassar, A.; Farooq, A.D. In Vitro Antiproliferative and Antioxidant Assessment of the Extract and Partitioned Fractions of Leaves of Euphorbia graminea Jacq. (Euphorbiaceae). Niger. J. Pharm. 2021, 55, 26–31. [Google Scholar] [CrossRef]
- Kemboi, D.; Langat, M.K.; Siwe-Noundou, X.; Krause, R.W.M.; Isaacs, M.L.; Tembu, V.J. In Vitro Antibacterial and Cytotoxic Effects of Euphorbia grandicornis Blanc Chemical Constituents. BMC Complement. Med. Ther. 2022, 22, 90. [Google Scholar] [CrossRef]
- Magozwi, D.K.; Peter, X.; Langat, M.K.; Mhlanga, R.; Vukea, N.; Mare, J.A.d.L.; Siwe-Noundou, X.; Krause, R.W.M.; Tembu, V.J. In Vitro Cytotoxic Effects of Chemical Constituents of Euphorbia grandicornis Blanc against Breast Cancer Cells. Sci. Afr. 2021, 14, e01002. [Google Scholar] [CrossRef]
- Radi, M.H.; El-Shiekh, R.A.; El-Halawany, A.M.; Al-Abd, A.M.; Abdel-Sattar, E. In Vitro Cytotoxic Study of Euphorbia grantii Oliv. Aerial Parts against MCF-7 and MCF-7ADRBreast Cancer Cell Lines: A Bioactivity-Guided Isolation. ACS Omega 2023, 8, 18299–18305. [Google Scholar] [CrossRef]
- Al-Robai, S.A.; Ahmed, A.A.; Ahmed, A.A.E.; Zabin, S.A.; Mohamed, H.A.; Alghamdi, A.A.A. Phenols, Antioxidant and Anticancer Properties of Tagetes minuta, Euphorbia granulata and Galinsoga parviflora: In Vitro and in Silico Evaluation. J. Umm Al-Qura Univ. Appl. Sci. 2023, 9, 15–28. [Google Scholar] [CrossRef]
- Deveci, E.; Çayan, G.T.; Karakurt, S.; Duru, M.E. Anti-Colorectal Cancer Effects of Medicinal Plants: Euphorbia helioscopia, Ferula elaeochytris, and Sideritis albiflora. Commagene J. Biol. 2021, 5, 73–77. [Google Scholar] [CrossRef]
- El-Hallouty, S.M.; Gohar, L.H.; Elgazzar, E.M. Enhancement of Radiation-Induced Cytotoxicity in Hepatocellular Carcinoma Cells by Some Plant Extracts In Vitro. Egypt. J. Radiat. Sci. Appl. 2022. [Google Scholar] [CrossRef]
- EL-Hallouty, S.; Batawi, A.; Shafi, M.; Rashwan, E.; Elhawary, E.; Abdelaal, N. Cytotoxicity of five plant extracts against different human cancer cell lines and their molecular mechanism. Asian J. Pharm. Clin. Res. 2020, 13, 194–198. [Google Scholar] [CrossRef]
- Al-Saraireh, Y.M.; Youssef, A.M.M.; Za’al Alsarayreh, A.; Al Hujran, T.A.; Al-Sarayreh, S.; Al-Shuneigat, J.M.; Alrawashdeh, H.M. Phytochemical and Anti-Cancer Properties of Euphorbia hierosolymitana Boiss. Crude Extracts. J. Pharm. Pharmacogn. Res. 2021, 9, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Anitha, P.; Geegi, P.G.; Yogeswari, J.; Anthoni, S.A. In Vitro Anticancer Activity of Ethanolic Extract of Euphorbia hirta (L.). Sci. Technol. Arts Res. J. 2014, 3, 1. [Google Scholar] [CrossRef]
- Rashmi; Gupta, P. In Vitro Anti-Inflammatory and Anti-Proliferative Potential of Roots Extract of Euphorbia hirta Linn. J. Phytopharm. 2021, 10, 7–9. [Google Scholar] [CrossRef]
- Sharma, N.; Samarakoon, K.W.; Gyawali, R.; Park, Y.H.; Lee, S.J.; Oh, S.J.; Lee, T.H.; Jeong, D.K. Evaluation of the Antioxidant, Anti-Inflammatory, and Anticancer Activities of Euphorbia hirta Ethanolic Extract. Molecules 2014, 19, 14567–14581. [Google Scholar] [CrossRef]
- Senniappan, P.; Srinivas, K.; Venkata, M.; Rao, B. In-Vitro Antioxidant and Cytotoxicity Activity of Euphorbia hirta Petroleum Ether, Chloroform Extracts. Eur. Chem. Bull. 2023, 12, 2949–2960. [Google Scholar]
- Mali, P. Evaluation of Cytotoxicity of Aqueous Extract of Euphorbia hirta Using Human Colon Adenocarcinoma and Vero Cell Line. Toxicol. Int. 2017, 24, 17–21. [Google Scholar] [CrossRef]
- De Sousa, A.S.; Fernandes, T.C.C.; Cardona, Y.T.; Almeida, P.M.D.; Marin-Morales, M.A.; Dos Santos, A.V.; Randau, K.P.; Benko-Iseppon, A.M.; Brasileiro-Vidal, A.C. Cytotoxic and Genotoxic Effects of Ethanolic Extract of Euphorbia hyssopifolia L. on HepG2 Cells. J. Ethnopharmacol. 2015, 170, 16–19. [Google Scholar] [CrossRef]
- Alghamdi, A.A.A.; Ali, N.M.; Alam, M.M.; Nazreen, S.; Mahzari, A. HPLC Profile, Anticancer, Anti-Inflammatory and Antioxidant Activities of Euphorbia inarticulata Ethanolic Extract. Adv. Pharmacol. Pharm. 2023, 11, 156–167. [Google Scholar] [CrossRef]
- Okpako, I.O.; Ng, F.A.; Kyama, C.M.; Oyem, J.C.; Njeru, S.N. Antiproliferative Activity of Euphorbia ingens Extract against Prostate Cancer Cell Line: An in Silico and in Vitro Analysis. Preprints 2023. [Google Scholar] [CrossRef]
- Wongprayoon, P.; Charoensuksai, P. Cytotoxic and anti-migratory activities from hydroalcoholic extract of Euphorbia lactea Haw. Against HN22 cell line. Thai Bull. Pharm. Sci. 2018, 13, 69–77. [Google Scholar]
- Taş, A.; Şahin-Bölükbaşı, S.; Çevik, E.; Özmen, E.; Gümüş, E.; Siliğ, Y. An in Vitro Study of Cytotoxic Activity of Euphorbia macroclada Boiss on MCF–7 Cells. Indian J. Pharm. Educ. Res. 2018, 52, S119–S123. [Google Scholar] [CrossRef]
- Ebrahim, H.Y.; Osman, S.A.; Haffez, H.R.; Hassan, Z.A. In-vitro screening of some plant extracts for their potential anticancer activity. Afr. J. Tradit. Complement. Altern. Med. 2020, 17, 1–8. [Google Scholar] [CrossRef]
- Younus, M.; Hasan, M.M.; Hanif, M.; Sarwar, G.; Ahmad, K.; Ur Rehman, S.M.; Shirazi, J.H.; Jamil, Q.A.; Khan, K.R.; Syed, S.K.; et al. Botanical Features and Anti-Cancerous Potential of Euphorbia nivulia Buch.-Ham. Trop. J. Nat. Product. Res. 2021, 5, 1591–1596. [Google Scholar] [CrossRef]
- Al-Yousef, H.M.; Alqahtani, A.S.; Ghani, A.S.A.; El-Toumy, S.A.; El-Dougdoug, W.I.A.; Hassan, W.H.B.; Hassan, H.M. Nephroprotective, Cytotoxic and Antioxidant Activities of Euphorbia paralias. Saudi J. Biol. Sci. 2021, 28, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Aslantürk, Ö.S.; Aşkin Çelik, T. Antioxidant, Cytotoxic and Apoptotic Activities of Extracts from Medicinal Plant Euphorbia platyphyllos L. J. Med. Plants Res. 2013, 7, 1293–1304. [Google Scholar] [CrossRef]
- Aslantürk, Ö.S.; Yılmaz, E.Ş.; Aşkın Çelik, T.; Güzel, Y. Evaluation of the Antioxidant and Cytotoxic Potency of Euphorbia rigida and Arbutus andrachne Methanol Extracts in Human Hepatocellular Carcinoma Cell Lines In Vitro. Beni Suef Univ. J. Basic. Appl. Sci. 2021, 10, 51. [Google Scholar] [CrossRef]
- de Souza, L.S.; Tosta, C.L.; de Oliveira, B.J.R.P.; Varricchio, M.C.B.N.; Kitagawa, R.R.; Filgueiras, P.R.; Kuster, R.M. Chemical Profile and Cytotoxic Evaluation of Aerial Parts of Euphorbia tirucalli L. on Gastric Adenocarcinoma (AGS Cells). Nat. Prod. Res. 2023, 37, 4267–4273. [Google Scholar] [CrossRef]
- Archanjo, A.B.; De, F.; Careta, P.; Costa, A.V.; Nunes, C. Evaluation of cytotoxicity and expression of caspase-3 and p53 in HCT-116 cells of lineage treated with different extracts of Euphorbia tirucalli L. Arch. Vet. Sci. 2016, 21, 35–45. [Google Scholar] [CrossRef]
- Al-Faifi, Z.I.A.; Masrahi, Y.S.; Aly, M.S.; Al-Turki, T.A.; Dardeer, T. Evaluation of Cytotoxic and Genotoxic Effects of Euphorbia triaculeata Forssk. Extract. Asian Pac. J. Cancer Prev. 2017, 18, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Aliomrani, M.; Jafarian, A.; Zolfaghari, B. Phytochemical Screening and Cytotoxic Evaluation of Euphorbia turcomanica on Hela and HT-29 Tumor Cell Lines. Adv. Biomed. Res. 2017, 6, 68. [Google Scholar] [CrossRef]
- Campos, A.; Vendramini-Costa, D.B.; Longato, G.B.; Zermiani, T.; Ruiz, A.L.T.G.; De Carvalho, J.E.; Pandiella, A.; Filho, V.C. Antiproliferative Effect of Synadenium grantii Hook f. Stems (Euphorbiaceae) and a Rare Phorbol Diterpene Ester. Int. J. Toxicol. 2016, 35, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Kanunfre, C.C.; Leffers, T.; Cruz, L.S.; Luz, L.E.C.; Crisma, A.R.; Wang, M.; Avula, B.; Khan, I.A.; Beltrame, F.L. Euphorbia umbellata Bark Extracts—An in Vitro Cytotoxic Study. Rev. Bras. Farmacogn. 2017, 27, 206–213. [Google Scholar] [CrossRef]
- Ali, M.M.; Khattab, M.; Mohamed, M.A.; Abdelsalam, R.M.; Mahmoud, K.; Soliman, S.; Barghash, M.F. The Cytotoxic Effect of Synadenium grantii Extract against Human Lung Carcinoma A549 Cells and Its Role in Improvement of Histopathological and Biomarkers Changes in Benzo(a)Pyrene-Induced Lung Cancer in Rats. Int. J. Res. Pharm. Sci. 2020, 11, 962–971. [Google Scholar] [CrossRef]
- Periyasamy, K.; Pharm, I.J.; Sci, B.; Batsa, A.J.S. Anticancer activity of Excoecaria agallocha leaf extract in cell line model. Int. J. Pharm. Biol. Sci. 2013, 3, 392–398. [Google Scholar]
- Mohapatra, R. The in Vitro Anti-Tumor Efficacy of Methanolic Leaf Extract of Exoecaria agallocha in Cancer Cell Lines of Different Tissue Origin. Int. J. Innov. Res. Technol. 2021, 8, 1348. [Google Scholar]
- Hettihewa, L.M.; Munasinghe, M.M.A.B.; Bulugahapitiya, V.B.; Kihara, N. Dose dependent anti proliferative and cytotoxic effects of Flueggea leucopyrus Willd against human ovarian carcinoma; MTS and human telomerase enzyme inhibition. EJBPS 2015, 2, 14–18. [Google Scholar]
- Vassallo, A.; Armentano, M.F.; Miglionico, R.; Caddeo, C.; Chirollo, C.; Gualtieri, M.J.; Ostuni, A.; Bisaccia, F.; Faraone, I.; Milella, L. Hura crepitans L. Extract: Phytochemical Characterization, Antioxidant Activity, and Nanoformulation. Pharmaceutics 2020, 12, 553. [Google Scholar] [CrossRef]
- Mongalo, N.; Soyingbe, O.; Makhafola, T. Antimicrobial, Cytotoxicity, Anticancer and Antioxidant Activities of Jatropha zeyheri Sond. Roots (Euphorbiaceae). Asian Pac. J. Trop. Biomed. 2019, 9, 307–314. [Google Scholar] [CrossRef]
- Cruz, A.J.D.P.; Raymundo, J.K.G.; Jacinto, S.D.; Tolentino, J.E.; Ipulan-Colet, L.A.D.G. Combining in vitro and in ovo assays to screen for anti-cancer and anti-angiogenic effects of the leaf extracts of Mallotus cumingii Müll.Arg. (Euphorbiaceae). Malays. Appl. Biol. 2022, 51, 73–82. [Google Scholar] [CrossRef]
- Benny, B.; Krishna, A.S.; Samraj, S.; John, P.; Radhakrishnan, U. Cytotoxic and Antiproliferative Potential of Methanolic Extract of Mallotus phillippensis in MCF-7 Cell Line. J. Phytopharm. 2022, 11, 60–63. [Google Scholar] [CrossRef]
- Chen, X.-M.; Zhang, Z.-J.; Liu, A.-L.; Lv, F.-J.; Li, S.-F. Cholinesterase and Human Lung Cancer Cells (A-549) Inhibitory Activity of the Cassava Peel of Euphorbiaceae In Vitro. In International Conference on Food Science and Engineering (ICFSE 2016); Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2016; ISBN 978-1-60595-390-8. [Google Scholar]
- Al-Douri, N.; Al-Jaidi, B.A.; Hammad, H.M.; Shakya, A.K.; Alkhalailah, T.J.M.; Shilbayeh, S.A.R.; Ibrahim, A.A.; Venugopala, K.N.; Kamal, Y.T. Biological Evaluation of Mercurialis annua Extracts for Possible Antioxidant, Antiproliferative and Cytotoxic Activity. Indian. J. Pharm. Educ. Res. 2022, 56, S479–S486. [Google Scholar] [CrossRef]
- Nascimento, A.K.L.; Melo-Silveira, R.F.; Dantas-Santos, N.; Fernandes, J.M.; Zucolotto, S.M.; Rocha, H.A.O.; Scortecci, K.C. Antioxidant and Antiproliferative Activities of Leaf Extracts from Plukenetia volubilis Linneo (Euphorbiaceae). Evid.-Based Complement. Altern. Med. 2013, 2013, 950272. [Google Scholar] [CrossRef] [PubMed]
- Islam Shah, T.; Sharma, E.; Shah, G.A. Inhibitory Property of Aqueous Extract of Ricinus communis Leaves on Proliferation of Melanoma Treated against A375 Cell Lines. World J. Pharm. Sci. 2015, 3, 758–761. [Google Scholar]
- Prakash, E.; Gupta, D.K. In Vitro Study of Extracts of Ricinus communis Linn on Human Cancer Cell Lines. J. Med. Sci. Public Health 2014, 2, 15–20. [Google Scholar]
- Albino, R.C.; Antoniassi, R.; de Faria-Machado, A.F.; Ferraris, F.K.; Amendoeira, F.C.; Ramos, D.F.; Silva, P.E.A.; Leitão, S.G.; Oliveira, D.R. Traditional Detoxification of Jatropha curcas L. Seeds. J. Ethnopharmacol. 2019, 241, 111970. [Google Scholar] [CrossRef]
- Florence, D. An Investigation into The Antineoplastic Properties of Schinziophyton rautanenii and Colophospermum mopane . Ph.D. Thesis, The University of Namibia, Windhuk, Namibia, 2008. [Google Scholar]
- Menon, M.; Varghese, L. Evaluation of the Phytochemical Constituents and Tumor Reduction Potentials of Tragia involucrata Linn. J. Appl. Biol. Biotechnol. 2023, 11, 84–91. [Google Scholar] [CrossRef]
- Euphorbia Species-Derived Diterpenes and Coumarins as Multidrug Resistance Modulators in Human Colon Carcinoma Cells. Anticancer. Res. 2016, 36, 2259–2264.
- Li, Y.; Liu, Y.; Li, Y.; Liu, F.; Zhao, Y.; Xu, J.; Guo, Y. Trigothysoid, N. Inhibits Tumor Proliferation and Migration by Targeting Mitochondria and the STAT3/FAK Pathway. Arab. J. Chem. 2023, 16, 104930. [Google Scholar] [CrossRef]
- Lin, M.; Tang, S.; Zhang, C.; Chen, H.; Huang, W.; Liu, Y.; Zhang, J. Euphorbia Factor L2 Induces Apoptosis in A549 Cells through the Mitochondrial Pathway. Acta Pharm. Sin. B 2017, 7, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.Z.; Chen, L.; Su, T.; Li, W.; Huang, J.L.; Sang, J.; Tang, G.H.; Yin, S. Discovery of 8,9-Seco-Ent-Kaurane Diterpenoids as Potential Leads for the Treatment of Triple-Negative Breast Cancer. J. Med. Chem. 2021, 64, 9926–9942. [Google Scholar] [CrossRef] [PubMed]
- Wongprayoon, P.; Leelasart, S.; Jantham, J.; Pootaeng-on, Y.; Oekchuae, S.; Limpachayaporn, P.; Rayanil, K.O.; Charoensuksai, P. A Triterpenoid Friedelan-3β-Ol Isolated from Euphorbia lactea Exhibited Cytotoxic Activity against HN22 Cells by Inducing an S-Phase Cell Cycle Arrest. J. Appl. Pharm. Sci. 2022, 12, 31–48. [Google Scholar] [CrossRef]
- Li, H.H.; Qi, F.M.; Dong, L.L.; Fan, G.X.; Che, J.M.; Guo, D.D.; Zhang, Z.X.; Fei, D.Q. Cytotoxic and Antibacterial Pyran-2-One Derivatives from Croton crassifolius. Phytochem. Lett. 2014, 10, 304–308. [Google Scholar] [CrossRef]
- Cui, J.J.; Ji, K.L.; Liu, H.C.; Zhou, B.; Liu, Q.F.; Xu, C.H.; Ding, J.; Zhao, J.X.; Yue, J.M. Cytotoxic Tigliane Diterpenoids from Croton damayeshu. J. Nat. Prod. 2019, 82, 1550–1557. [Google Scholar] [CrossRef]
- Karina, P.; Uchôa, S.; Nunes Da Silva, J.; Silveira, R.; Anne, M.; Lima, S.; Braz-Filho, R. Trachylobane and kaurane diterpenes from Croton floribundus Spreng. Quim. Nova 2013, 36, 778–782. [Google Scholar]
- Liu, C.-P.; Xu, J.-B.; Zhao, J.-X.; Xu, C.-H.; Dong, L.; Ding, J.; Yue, J.-M. Diterpenoids from Croton laui and Their Cytotoxic and Antimicrobial Activities. J. Nat. Prod. 2014, 77, 1013–1020. [Google Scholar] [CrossRef]
- Zhao, B.Q.; Peng, S.; He, W.J.; Liu, Y.H.; Wang, J.F.; Zhou, X.J. Antitubercular and Cytotoxic Tigliane-Type Diterpenoids from Croton tiglium. Bioorg Med. Chem. Lett. 2016, 26, 4996–4999. [Google Scholar] [CrossRef]
- Zhang, X.L.; Wang, L.; Li, F.; Yu, K.; Wang, M.K. Cytotoxic Phorbol Esters of Croton tiglium. J. Nat. Prod. 2013, 76, 858–864. [Google Scholar] [CrossRef]
- Wang, J.F.; Yang, S.H.; Liu, Y.Q.; Li, D.X.; He, W.J.; Zhang, X.X.; Liu, Y.H.; Zhou, X.J. Five New Phorbol Esters with Cytotoxic and Selective Anti-Inflammatory Activities from Croton tiglium. Bioorg Med. Chem. Lett. 2015, 25, 1986–1989. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Zhao, Y.; Liu, H.; Tang, C.; Zhang, M.; Ke, C.; Ye, Y. Isolation and Structure Characterization of Cytotoxic Phorbol Esters from the Seeds of Croton tiglium. Planta Med. 2017, 83, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Cândido-Bacani, P.D.M.; Figueiredo, P.D.O.; Matos, M.D.F.C.; Garcez, F.R.; Garcez, W.S. Cytotoxic Orbitide from the Latex of Croton urucurana. J. Nat. Prod. 2015, 78, 2754–2760. [Google Scholar] [CrossRef] [PubMed]
- Abreu, L.S.; do Nascimento, Y.M.; do Espirito-Santo, R.F.; Meira, C.S.; Santos, I.P.; Brandão, R.B.; Souto, A.L.; Guedes, M.L.S.; Soares, M.B.P.; Villarreal, C.F.; et al. Phenylpropanoids from Croton velutinus with Cytotoxic, Trypanocidal and Anti-Inflammatory Activities. Fitoterapia 2020, 145, 104632. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cheng, X.; Sun, Y.; Liu, P. A new friedelane triterpenoid possessing cytotoxicity from the leaves and stems of Drypetes hainanensis. Chem. Nat. Compd. 2014, 50, 93–96. [Google Scholar] [CrossRef]
- Abdel-Sattar, E.; Abou-Hussein, D.; Petereit, F. Chemical Constituents from the Leaves of Euphorbia Ammak Growing in Saudi Arabia. Pharmacogn. Res. 2015, 7, 14–17. [Google Scholar] [CrossRef]
- Aljubiri, S.M.; Mahgoub, S.A.; Almansour, A.I.; Shaaban, M.; Shaker, K.H. Isolation of Diverse Bioactive Compounds from Euphorbia balsamifera: Cytotoxicity and Antibacterial Activity Studies. Saudi J. Biol. Sci. 2021, 28, 417–426. [Google Scholar] [CrossRef]
- Shadi, S.; Saeidi, H.; Ghanadian, M.; Rahimnejad, M.R.; Aghaei, M.; Ayatollahi, S.M.; Iqbal Choudhary, M. New Macrocyclic Diterpenes from Euphorbia connata Boiss. with Cytotoxic Activities on Human Breast Cancer Cell Lines. Nat. Prod. Res. 2015, 29, 607–614. [Google Scholar] [CrossRef]
- Foda, S.M.; Zaghloul, M.G.; Marzouk, A.M.; Shaker Mansour, E.-S. Phytochemical Investigation and Biological Studies of Euphorbia tithymaloides L. Family Euphorbiaceae. Delta Univ. Sci. J. 2022, 5, 260–271. [Google Scholar] [CrossRef]
- Shamsabadipour, S.; Zarei, S.M.; Ghanadian, M.; Ayatollahi, S.A.; Rahimnejad, M.R.; Saeedi, H.; Aghaei, M. A New Taraxastane Triterpene from Euphorbia denticulata with Cytotoxic Activity against Prostate Cancer Cells. Iran. J. Pharm. Res. IJPR 2018, 17, 336. [Google Scholar]
- Yuan, W.J.; Yang, G.P.; Zhang, J.H.; Zhang, Y.; Chen, D.Z.; Li, S.L.; Di, Y.T.; Hao, X.J. Three New Diterpenes with Cytotoxic Activity from the Roots of Euphorbia ebracteolata Hayata. Phytochem. Lett. 2016, 18, 176–179. [Google Scholar] [CrossRef]
- Chen, G.; Ma, T.; Ma, Y.; Han, C.; Zhang, J.; Sun, Y. Chemical Composition, Anti-Breast Cancer Activity and Extraction Techniques of Ent-Abietane Diterpenoids from Euphorbia fischeriana Steud. Molecules 2022, 27, 4282. [Google Scholar] [CrossRef] [PubMed]
- Zhong, N.F.; Huang, H.H.; Wei, J.C.; Yang, Y.C.; Gao, X.X.; Wei, X.Y.; Wang, A.H.; Jia, J.M. Euphorfiatnoids A−I: Diterpenoids from the Roots of Euphorbia fischeriana with Cytotoxic Effects. Phytochemistry 2022, 203, 113372. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Li, B.T.; Sheng, G.; Zhang, A.L.; Li, X.C.; Tian, J.M. Cytotoxic Diterpenoids from Euphorbia fischeriana. Chem. Biodivers. 2021, 18, e2000919. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Yu, D.; Zhu, G.; Xu, J. Three New Abietane Diterpenoids from the Aerial Parts of Euphorbia fischeriana and Their Cytotoxic Effects. Phytochem. Lett. 2023, 55, 56–60. [Google Scholar] [CrossRef]
- Zhou, M.; Ma, Q.; He, L.; Chen, Y.H.; Zhu, B.Y.; Wang, J.H.; Yang, Q.; Liu, S.; Ma, L.M. Cytotoxic Jatrophane Diterpenoids from the Aerial Parts of Euphorbia helioscopia. J. Asian Nat. Prod. Res. 2021, 23, 731–737. [Google Scholar] [CrossRef]
- Lu, Y.B.; Luo, S.; Wang, Y.X.; Feng, Z.Y.; Gao, K.; Chen, J.J. Jatrophane Diterpenoids with Cytotoxic Activity from the Whole Plant of Euphorbia heliosocpia L. Phytochemistry 2022, 203, 113420. [Google Scholar] [CrossRef]
- Adedoyin, B.A. In Vitro Cytotoxicity of Euphorbia heterophylla against Human Cancer Cell Lines. Afr. J. Eng. Environ. Res. 2022, 3, 1. [Google Scholar]
- Hu, R.; Sang, J.; Li, W.; Tian, Y.; Zou, M.F.; Tang, G.H.; Yin, S. Structurally Diverse Triterpenoids with Cytotoxicity from Euphorbia hypericifolia. Fitoterapia 2021, 151, 104888. [Google Scholar] [CrossRef]
- Hegazy, M.E.F.; Hamed, A.R.; Ibrahim, M.A.A.; Talat, Z.; Reda, E.H.; Abdel-Azim, N.S.; Hammouda, F.M.; Nakamura, S.; Matsuda, H.; Haggag, E.G.; et al. Euphosantianane A–D: Antiproliferative Premyrsinane Diterpenoids from the Endemic Egyptian Plant Euphorbia sanctae-catharinae. Molecules 2018, 23, 2221. [Google Scholar] [CrossRef]
- Jia, H.Y.; Liao, Z.X.; Liu, F.Y.; Wu, L.; Xu, C.; Zuo, B. A New Phenylpropanoid from the Roots of Euphorbia nematocypha. Nat. Prod. Res. 2015, 29, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Jia, H.-Y.; Zuo, B.; Liao, Z.-X.; Ji, L.-J.; Sun, H.-F.; Wang, Q. Chemical Constituents of the Aerial Parts of Euphorbia nematocypha. Nat. Prod. Commun. 2016, 11, 1934578X1601100210. [Google Scholar] [CrossRef]
- Song, N.; Zheng, X.; Wang, J.; Zhu, L.; Wang, C.; Cai, L.; Ding, Z. Cytotoxicity and Molecular-Docking Approach of a New Rosane-Type Diterpenoid from the Roots of Euphorbia nematocypha. Front. Chem. 2022, 10, 912738. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, J.S.; Liu, H.C.; Zhang, Y.; Yin, W.H.; Liu, Q.F.; Wang, G.W.; Zhao, J.X.; Yue, J.M. Phonerilins A–K, Cytotoxic Ingenane and Ingol Diterpenoids from Euphorbia neriifolia. Tetrahedron 2022, 123, 132955. [Google Scholar] [CrossRef]
- Ghanadian, M.; Saeidi, H.; Aghaei, M.; Rahiminejad, M.R.; Ahmadi, E.; Ayatollahi, S.M.; Choudhary, M.I.; Bahmani, B. New Jatrophane Diterpenes from Euphorbia osyridea with Proapoptotic Effects on Ovarian Cancer Cells. Phytochem. Lett. 2015, 12, 302–307. [Google Scholar] [CrossRef]
- Wang, K.; Yu, H.; Wu, H.; Wang, X.; Pan, Y.; Chen, Y.; Liu, L.; Jin, Y.; Zhang, C. A New Casbane Diterpene from Euphorbia pekinensis. Nat. Prod. Res. 2015, 29, 1456–1460. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Zeng, Y.; Ma, B.; Bi, K.; Chen, X. A New Cytotoxic Cembrane Diterpene from the Roots of Euphorbia pekinensis Rupr. Fitoterapia 2013, 90, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Zeng, X.T.; Xu, D.Q.; Yue, S.J.; Fu, R.J.; Yang, X.; Liu, Z.X.; Tang, Y.P. Pimarane, Abietane, and Labdane Diterpenoids from Euphorbia pekinensis Rupr. and Their Anti-Tumor Activities. Phytochemistry 2022, 197, 113113. [Google Scholar] [CrossRef]
- Ismail, M.; Ahmed, M.H.; Zaki, M.; Moawad, A.; Mohammed, R. Colorectal Cytotoxic Activities of Phenolic Constituents Isolated from Euphorbia pseudocactus Cultivated in Egypt. J. Appl. Pharm. Sci. 2022, 12, 97–102. [Google Scholar] [CrossRef]
- Wu, S.; Gan, L.; Su, T.; Wei, X.; Yin, S. New Ingenane and Ingol Diterpenoids from Euphorbia royleana. Nat. Prod. Res. 2023, 37, 1130–1137. [Google Scholar] [CrossRef]
- Banjar, M.F.S.; Mohamed, G.A.; Shehata, I.A.; Abdallah, H.M.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Koshak, A.E.; Ibrahim, S.R.M. Cycloschimperols A and B, New Cytotoxic Cycloartane Triterpenoids from Euphorbia schimperi. Phytochem. Lett. 2019, 32, 90–95. [Google Scholar] [CrossRef]
- Aljubiri, S.M.; Mahmoud, K.; Mahgoub, S.A.; Almansour, A.I.; Shaker, K.H. Bioactive Compounds from Euphorbia schimperiana with Cytotoxic and Antibacterial Activities. S. Afr. J. Bot. 2021, 141, 357–366. [Google Scholar] [CrossRef]
- Zolfaghari, B.; Yazdiniapour, Z.; Ghanadian, M.; Lanzotti, V. Cyclomyrsinane and Premyrsinane Diterpenes from Euphorbia sogdiana Popov. Tetrahedron 2016, 72, 5394–5401. [Google Scholar] [CrossRef]
- Zhu, H.; Ren, X.; Huang, Y.; Su, T.; Yang, L. Chemical Constituents of Euphorbia stracheyi Boiss (Euphorbiaceae). Metabolites 2023, 13, 852. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Liu, G.H.; Dawa, D.; Ding, L.S.; Cao, Z.X.; Zhou, Y. Cytotoxic Diterpenoids from the Roots of Euphorbia stracheyi. Phytochem. Lett. 2020, 36, 183–187. [Google Scholar] [CrossRef]
- Rédei, D.; Kúsz, N.; Sátori, G.; Kincses, A.; Spengler, G.; Burián, K.; Barina, Z.; Hohmann, J. Bioactive Segetane, Ingenane, and Jatrophane Diterpenes from Euphorbia Taur. Planta Med. 2018, 84, 729–735. [Google Scholar] [CrossRef]
- Silva, V.A.O.; Rosa, M.N.; Tansini, A.; Oliveira, R.J.S.; Martinho, O.; Lima, J.P.; Pianowski, L.F.; Reis, R.M. In Vitro Screening of Cytotoxic Activity of Euphol from Euphorbia tirucalli on a Large Panel of Human Cancer-Derived Cell Lines. Exp. Ther. Med. 2018, 16, 557–566. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Z.; Wang, Y.; Li, X. Highly Oxygenated Ent-Atisane and Podocarpane Diterpenoids from Excoecaria agallocha. Nat. Prod. Res. 2022, 36, 3924–3930. [Google Scholar] [CrossRef]
- Asep, S.; Hening, H.; Gema, S.P.; Gigih, S.; Widya, M.C. Sahidin Anticancer Activity of Jatrophone an Isolated Compound from Jatropha gossypifolia Plant against Hepatocellular Cancer Cell Hep G2 1886. Biomed. Pharmacol. J. 2017, 10, 667–673. [Google Scholar] [CrossRef]
- Byrappa, M.; Viswanathan, G.; Doss, J.; Ananthi, J.; Rajan, N.; Raja, L.; Venkateshan, N. Cytotoxic Pyrrolidinone from Leaves of Jatropha tanjorensis. Int. J. Res. Sci. Innov. 2018, 5, 45–50. [Google Scholar]
- Segun, P.A.; Ogbole, O.O.; Ismail, F.M.D.; Nahar, L.; Evans, A.R.; Ajaiyeoba, E.O.; Sarker, S.D. Bioassay-Guided Isolation and Structure Elucidation of Cytotoxic Stilbenes and Flavonols from the Leaves of Macaranga barteri. Fitoterapia 2019, 134, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Wanandri, R.; Rewa, S.; Saputri, R.D.; Tjahjandarie, S.; Tanjung, M. Cytotoxic Activity of Flavonols from Macaranga gigantea. Pharm. J. Farm. Indones. 2020, 17, 360–367. [Google Scholar]
- Yang, D.S.; Wei, J.G.; Peng, W.B.; Wang, S.M.; Sun, C.; Yang, Y.P.; Liu, K.C.; Li, X.L. Cytotoxic Prenylated Bibenzyls and Flavonoids from Macaranga Kurzii. Fitoterapia 2014, 99, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Tanjung, M.; Tjahjandarie, T.S. Dihydroflavonols from the Leaves of Macaranga recurvata and Their Cytotoxic and Antioxidant Activities. J. Chem. Pharm. Res. 2014, 6, 90–95. [Google Scholar]
- Zhang, Y.; Zhou, D.; Liu, W.; Li, C.; Hao, L.; Zhang, G.; Deng, S.; Yang, R.; Qin, J.; Li, J.; et al. Cytotoxic Activity and Related Mechanisms of Prenylflavonoids Isolated from Mallotus conspurcatus Croizat. Chem. Biodivers. 2019, 16, e1800465. [Google Scholar] [CrossRef]
- Mbeunkeu, A.B.D.; Azebaze, A.G.B.; Tala, M.F.; Teinkela, J.E.M.; Noundou, X.S.; Krause, R.W.M.; Vardamides, J.C.; Laatsch, H. Three New Pentacyclic Triterpenoids from Twigs of Manniophyton fulvum (Euphorbiaceae). Phytochem. Lett. 2018, 27, 1–8. [Google Scholar] [CrossRef]
- Toussaint-Douhoré, G.Y.; Soro, Y.; Ouédraogo, N.; Vaca-Garcia, C.; Koffi-Attioua, B.; Carraz, M. Liver Cancer Antiproliferative Activity of a New Nor-Cucurbitacin from Mareya micrantha Müll. Arg. Fitoterapia 2023, 166, 105471. [Google Scholar] [CrossRef]
- Johnson-Ajinwo, O.R.; Richardson, A.; Li, W.W. Cytotoxic Effects of Stem Bark Extracts and Pure Compounds from Margaritaria discoidea on Human Ovarian Cancer Cell Lines. Phytomedicine 2015, 22, 1–4. [Google Scholar] [CrossRef]
- Yakubu, O.F.; Adebayo, A.H.; Dokunmu, T.M.; Zhang, Y.J.; Iweala, E.E.J. Cytotoxic Effects of Compounds Isolated from Ricinodendron heudelotii. Molecules 2019, 24, 145. [Google Scholar] [CrossRef]
- Mangisa, M.; Tembu, V.J.; Fouche, G.; Nthambeleni, R.; Peter, X.; Langat, M.K. Ent-Abietane Diterpenoids from Suregada zanzibariensis Baill. (Euphorbiaceae), Their Cytotoxic and Anticancer Properties. Nat. Prod. Res. 2019, 33, 3240–3247. [Google Scholar] [CrossRef]
- Lei, C.; Li, Y.N.; Li, J.N.; Zhou, Y.B.; Cui, M.J.; Fu, K.C.; Li, J.; Hou, A.J. Two New Cytotoxic Maytansinoids Targeting Tubulin from Trewia nudiflora. Planta Med. 2021, 88, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Hu, S.; Wen, Q.; Ma, Y.L.; Jiang, Z.H.; Tang, J.Y.; Fu, Y.H. Novel γ-Lactone Derivatives from Trigonostemon heterophyllus with Their Potential Antiproliferative Activities. Bioorg. Chem. 2018, 79, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, G.Y.; Song, X.P.; Yang, L.Q.; Han, C.R.; Wu, X.Y.; Zheng, C.J.; Ran, X.; Tang, R.F. Trigoxyphins O-T, Six New Heterodimers from Trigonostemon xyphophylloides. Tetrahedron Lett. 2013, 54, 6434–6438. [Google Scholar] [CrossRef]
- de Abrantes, R.A.; Batista, T.M.; Mangueira, V.M.; de Sousa, T.K.G.; Ferreira, R.C.; Moura, A.P.G.; Abreu, L.S.; Alves, A.F.; Velozo, E.S.; Batista, L.M.; et al. Antitumor and Antiangiogenic Effects of Tonantzitlolone B, an Uncommon Diterpene from Stillingia loranthacea. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Adiga, P.G.; Shridhar, N.B.; Sanganal, J.S.; Rao, S.; Shilpa, N.; Aparna, V.; Santhosh, I.M.; Tirumala, M.; Sindhu, B.M. In Vivo Anti-Tumor Efficacy of Croton oblongifolius in DMBA Induced Mammary Tumor in Rats. Indian J. Anim. Res. 2018, 52, 144–147. [Google Scholar] [CrossRef]
- Majumder, M.; Debnath, S.; Gajbhiye, R.L.; Saikia, R.; Gogoi, B.; Samanta, S.K.; Das, D.K.; Biswas, K.; Jaisankar, P.; Mukhopadhyay, R. Ricinus communis L. Fruit Extract Inhibits Migration/Invasion, Induces Apoptosis in Breast Cancer Cells and Arrests Tumor Progression in Vivo. Sci. Rep. 2019, 9, 14493. [Google Scholar] [CrossRef]
- De Castro, J.B.R.; Wemmenson, F.; Moura, G.; Solheiro Bezerra, A.; De, P.; Rodrigues, A.; Da Costa, H.P.S.; Do, N.; Canabrava, V.; Matheus, C.; et al. Antitumoral activity and in vivo toxicity of Cnidoscolus quercifolius pohl (euphorbiaceae). Int. J. Dev. Res. 2020, 10, 20359. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, Y.; Wang, G.; Li, Y.; Huang, W. Penduliflaworosin, a Diterpenoid from Croton crassifolius, Exerts Anti-Angiogenic Effect via VEGF Receptor-2 Signaling Pathway. Molecules 2017, 22, 126. [Google Scholar] [CrossRef]
- Ma, L.; Chen, Z.; Wang, W.; Zhang, J.; Zhang, H.; Liu, J. The Molecular Mechanism of ESZM Extract on Treatment ER(−) Breast Cancer in Vivo: Application of “Combat Poison with Poison” Theory. Preprint 2021. [Google Scholar] [CrossRef]
- Cheng, J.; Han, W.; Wang, Z.; Shao, Y.; Wang, Y.; Zhang, Y.; Li, Z.; Xu, X.; Zhang, Y. Hepatocellular Carcinoma Growth Is Inhibited by Euphorbia helioscopia L. Extract in Nude Mice Xenografts. Biomed. Res. Int. 2015, 2015, 601015. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Chen, C.H.; Hsu, C.H.; Kuo, T.Y.; Liu, C.C.; Liao, A.T.C.; Lin, C.S. Inhibiting Autophagy Potentiates the Antitumor Efficacy of Euphorbia royleana for Canine Mammary Gland Tumors. BMC Vet. Res. 2020, 16, 193. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S. Evaluation of the Antioxidant and In Vivo Anticancer Activity of Excoecaria agallocha Bark Extrac. Ph.D. Thesis, Brac University, Dhaka, Bangladesh, 2019. [Google Scholar]
- Ghramh, H.A.; Khan, K.A.; Ibrahim, E.H.; Setzer, W.N. Synthesis of Gold Nanoparticles (AuNPs) Using Ricinus communis Leaf Ethanol Extract, Their Characterization, and Biological Applications. Nanomaterials 2019, 9, 765. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.S.; Al-Yousef, H.M.; Alqahtani, A.S.; Tabish Rehman, M.; AlAjmi, M.F.; Almarfidi, O.; Amina, M.; Alshememry, A.; Syed, R. Preparation, Characterization, and in Vitro-in Silico Biological Activities of Jatropha pelargoniifolia Extract Loaded Chitosan Nanoparticles. Int. J. Pharm. 2021, 606, 120867. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.A.; Attia, G.H.; Elgamal, A.; Aleraky, M.; Youns, M.; Ibrahim, A.M.; Abdou, R.; Shaikh, I.A.; El Raey, M.A. Cytotoxic Activity of Zinc Oxide Nanoparticles Mediated by Euphorbia retusa. Crystals 2022, 12, 903. [Google Scholar] [CrossRef]
- Aboulthana, W.M.; Omar, N.I.; El-Feky, A.M.; Hasan, E.A.; Ibrahim, N.E.S.; Youssef, A.M. In Vitro Study on Effect of Zinc Oxide Nanoparticles on the Biological Activities of Croton tiglium L. Seeds Extracts. Asian Pac. J. Cancer Prev. 2022, 23, 2671–2686. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Das, S.; Choudhury, P.; Ghosh, S.; Baral, R.; Choudhuri, S.K. A Novel Approach of Synthesizing and Evaluating the Anticancer Potential of Silver Oxide Nanoparticles in Vitro. Chemotherapy 2017, 62, 279–289. [Google Scholar] [CrossRef] [PubMed]
- El Raey, M.A.; El-Hagrassi, A.M.; Osman, A.F.; Darwish, K.M.; Emam, M. Acalypha wilkesiana Flowers: Phenolic Profiling, Cytotoxic Activity of Their Biosynthesized Silver Nanoparticles and Molecular Docking Study for Its Constituents as Topoisomerase-I Inhibitors. Biocatal. Agric. Biotechnol. 2019, 20, 101243. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Singh, M. Eco-Friendly Synthesis of Copper Oxide, Zinc Oxide and Copper Oxide–Zinc Oxide Nanocomposites, and Their Anticancer Applications. J. Inorg. Organomet. Polym. Mater. 2020, 30, 400–409. [Google Scholar] [CrossRef]
- Cherian, A.M.; Snima, K.S.; Kamath, C.R.; Nair, S.V.; Lakshmanan, V.K. Effect of Baliospermum montanum Nanomedicine Apoptosis Induction and Anti-Migration of Prostate Cancer Cells. Biomed. Pharmacother. 2015, 71, 201–209. [Google Scholar] [CrossRef]
- Boomi, P.; Poorani, G.P.; Selvam, S.; Palanisamy, S.; Jegatheeswaran, S.; Anand, K.; Balakumar, C.; Premkumar, K.; Prabu, H.G. Green Biosynthesis of Gold Nanoparticles Using Croton sparsiflorus Leaves Extract and Evaluation of UV Protection, Antibacterial and Anticancer Applications. Appl. Organomet. Chem. 2020, 34, e5574. [Google Scholar] [CrossRef]
- Abd El-Moaty, H.I.; Ismail, E.H.; Abu-Khudir, R.; Soliman, N.A.; Sabry, D.Y.; Al Abdulsalam, N.K.; Sorour, W.A.; Khalil, M.M.H. Characterization and Evaluation of Multiple Biological Activities of Phytosynthesized Gold Nanoparticles Using Aqueous Extract of Euphorbia dendroides. Nanomater. Nanotechnol. 2022, 12, 18479804221141266. [Google Scholar] [CrossRef]
- Lingaraju, K.; Naika, H.R.; Nagaraju, G.; Nagabhushana, H. Biocompatible Synthesis of Reduced Graphene Oxide from Euphorbia heterophylla (L.) and Their in-Vitro Cytotoxicity against Human Cancer Cell Lines. Biotechnol. Rep. 2019, 24, e00376. [Google Scholar] [CrossRef] [PubMed]
- Ghramh, H.A.; Khan, K.A.; Ibrahim, E.H. Biological Activities of Euphorbia peplus Leaves Ethanolic Extract and the Extract Fabricated Gold Nanoparticles (AuNPs). Molecules 2019, 24, 1431. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Naveen; Dhatwalia, J.; Thakur, S.; Radhakrishnan, A.; Chauhan, A.; Chandan, G.; Choi, B.H.; Neetika; Nidhi. Antioxidant, Antimicrobial, and Cytotoxic Potential of Euphorbia royleana Extract-Mediated Silver and Copper Oxide Nanoparticles. Chem. Pap. 2023, 77, 4643–4657. [Google Scholar] [CrossRef]
- Bhuvaneswari, R.; Xavier, R.J.; Arumugam, M. Facile Synthesis of Multifunctional Silver Nanoparticles Using Mangrove Plant Excoecaria agallocha L. for Its Antibacterial, Antioxidant and Cytotoxic Effects. J. Parasit. Dis. 2017, 41, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.B.; Peng, G.; Wang, J.; Nie, S.; Li, J.; Jia, Y.C.; Zhang, Q.Y. Ebracteolatain A and Ebracteolatain B Induce Apoptosis of Human Hepatoma Cell Line (HepG2). Trop. J. Pharm. Res. 2015, 14, 1821–1828. [Google Scholar] [CrossRef]
- Chaitanya, G.V.; Alexander, J.S.; Babu, P.P. PARP-1 Cleavage Fragments: Signatures of Cell-Death Proteases in Neurodegeneration. Cell Commun. Signal. 2010, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. Mechanisms of Caspase Activation and Inhibition during Apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef]
- Hao, Q.; Chen, J.; Lu, H.; Zhou, X. The ARTS of P53-Dependent Mitochondrial Apoptosis. J. Mol. Cell Biol. 2023, 14, mjac074. [Google Scholar] [CrossRef]
- Jaiswal, P.L.; Goel, A.; Mittal, R.D. Survivin: A Molecular Biomarker in Cancer. Indian J. Med. Res. 2015, 141, 389. [Google Scholar]
- Shi, S.-Q.; Fan, Y.-Y.; Xu, C.-H.; Ding, J.; Wang, G.-W.; Yue, J.-M. Supporting Information Cytotoxic 8,9-Seco-Ent-Kaurane Diterpenoids from Croton kongensis. J. Asian Nat. Prod. Res. 2018, 20, 920–927. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).