Naringin–Dextrin Nanocomposite Abates Diethylnitrosamine/Acetylaminofluorene-Induced Lung Carcinogenesis by Modulating Oxidative Stress, Inflammation, Apoptosis, and Cell Proliferation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Nar-Dx-NCs
2.3. Animal Experimentation
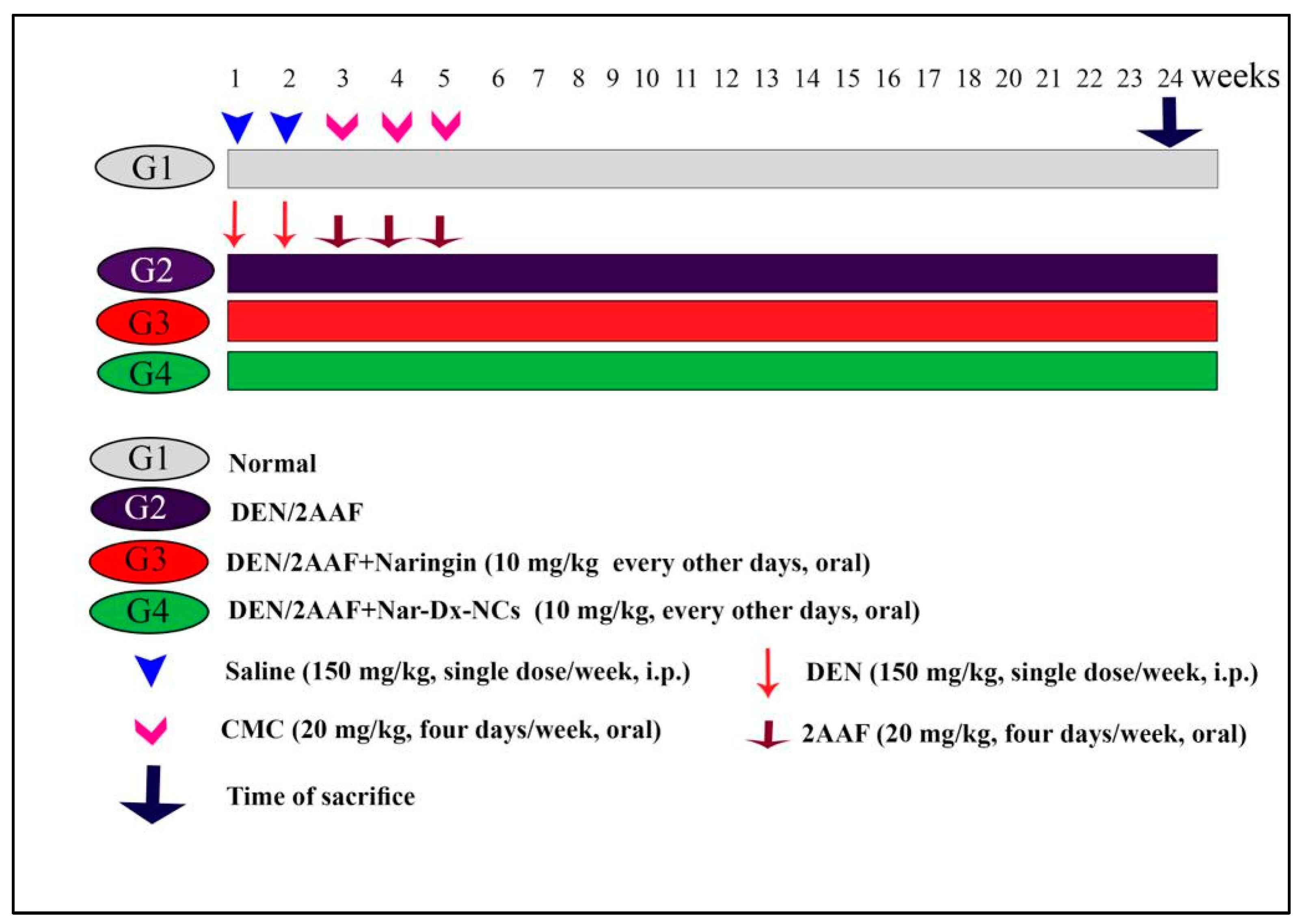
2.4. Experimental Design
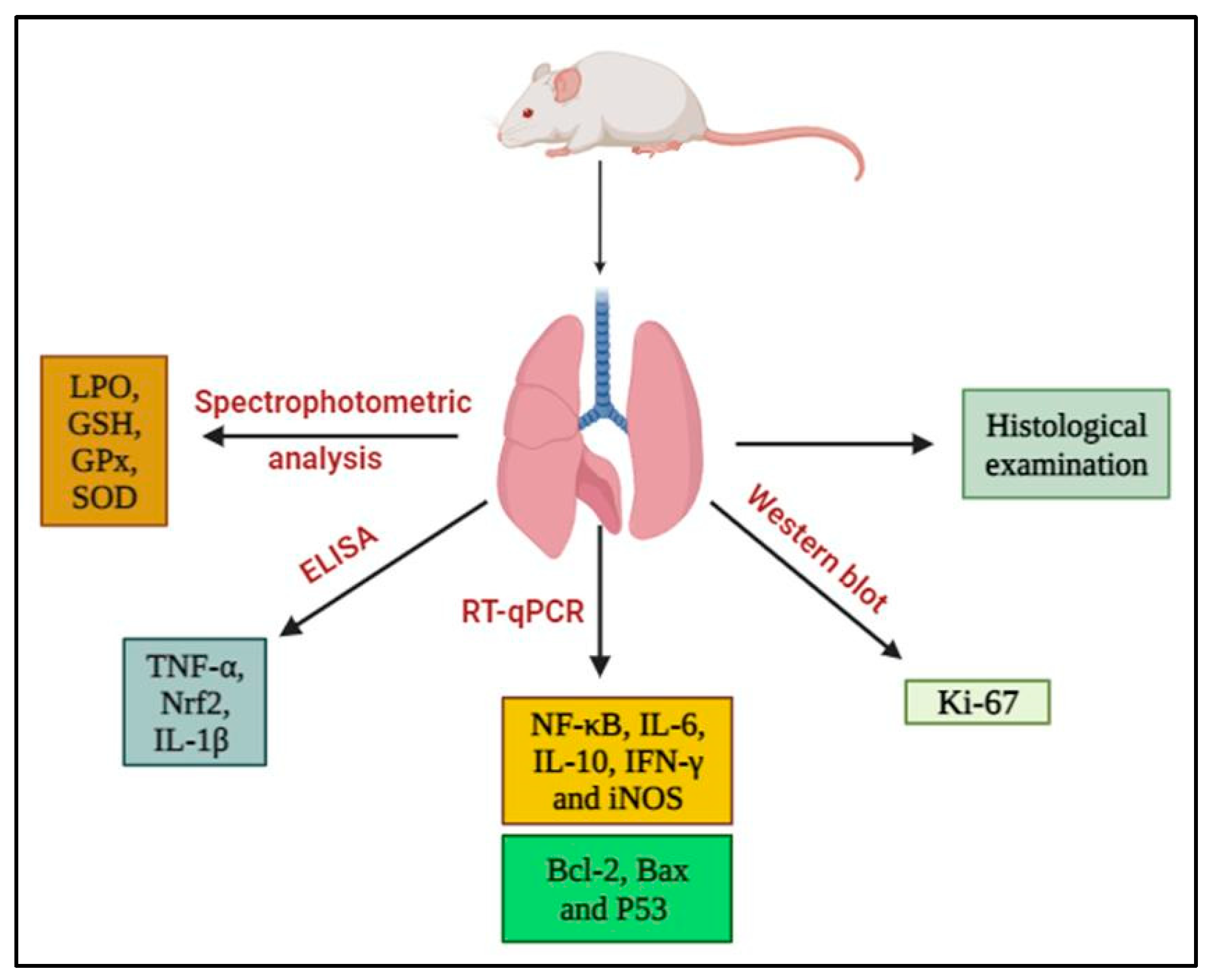
2.5. Lung Sampling and Analysis
2.6. Histopathological Examination
2.7. Biochemical Assays
2.8. Isolation of Total RNA and Reverse Transcription–Quantitative PCR (RT-qPCR)
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
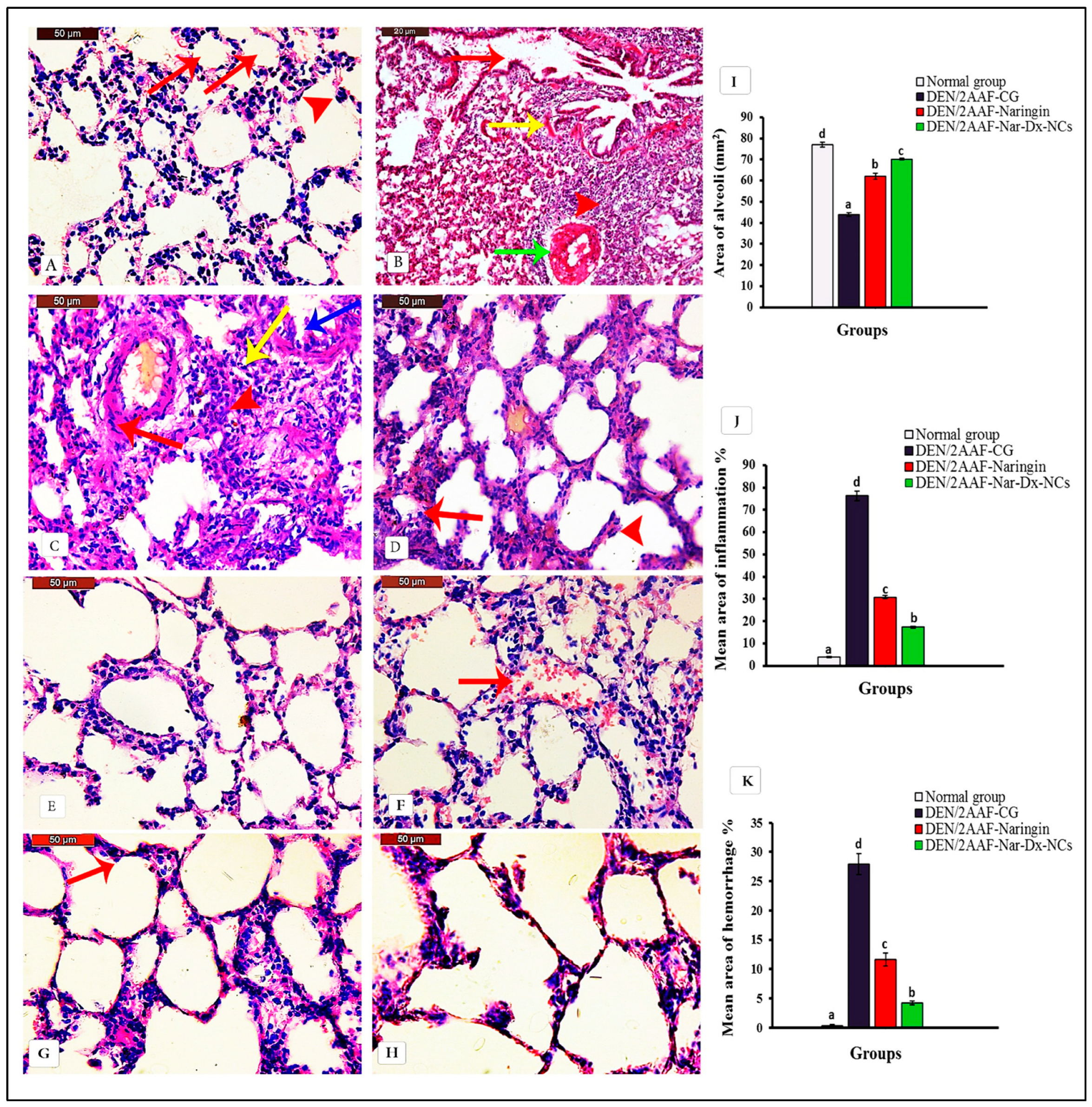
3.1. Naringin and Nar-Dx-NCs Abrogated Histopathological Changes in Lung Parenchyma of Rats Administered DEN/2AAF
3.2. Effect of Naringin and Nar-Dx-NCs on Oxidative Stress and Antioxidant Defense System in Lung Tissues
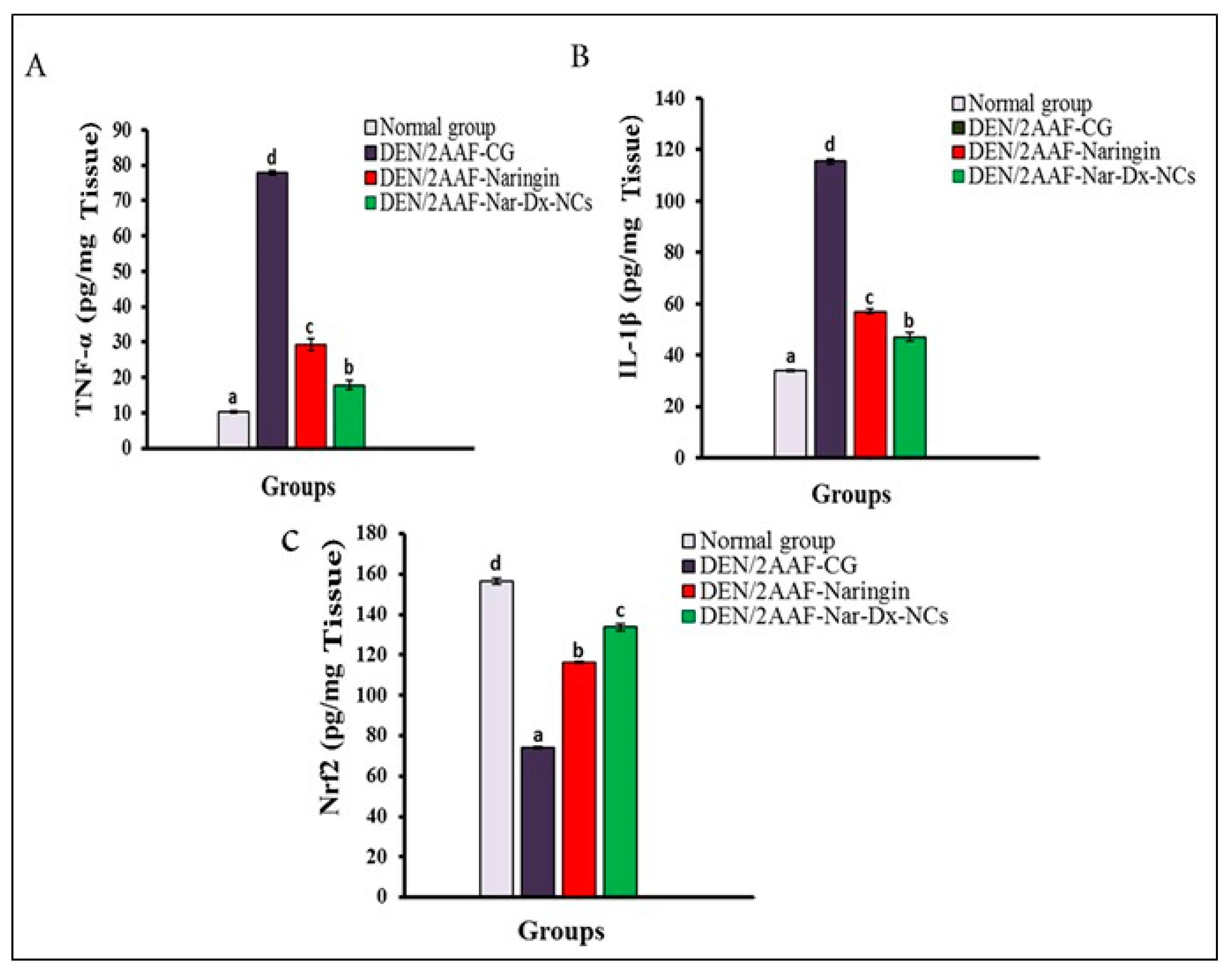
3.3. Effects of Naringin and Nar-Dx-NCs on TNF-α, IL-1β, and Nrf2 Levels in Lung Tissues
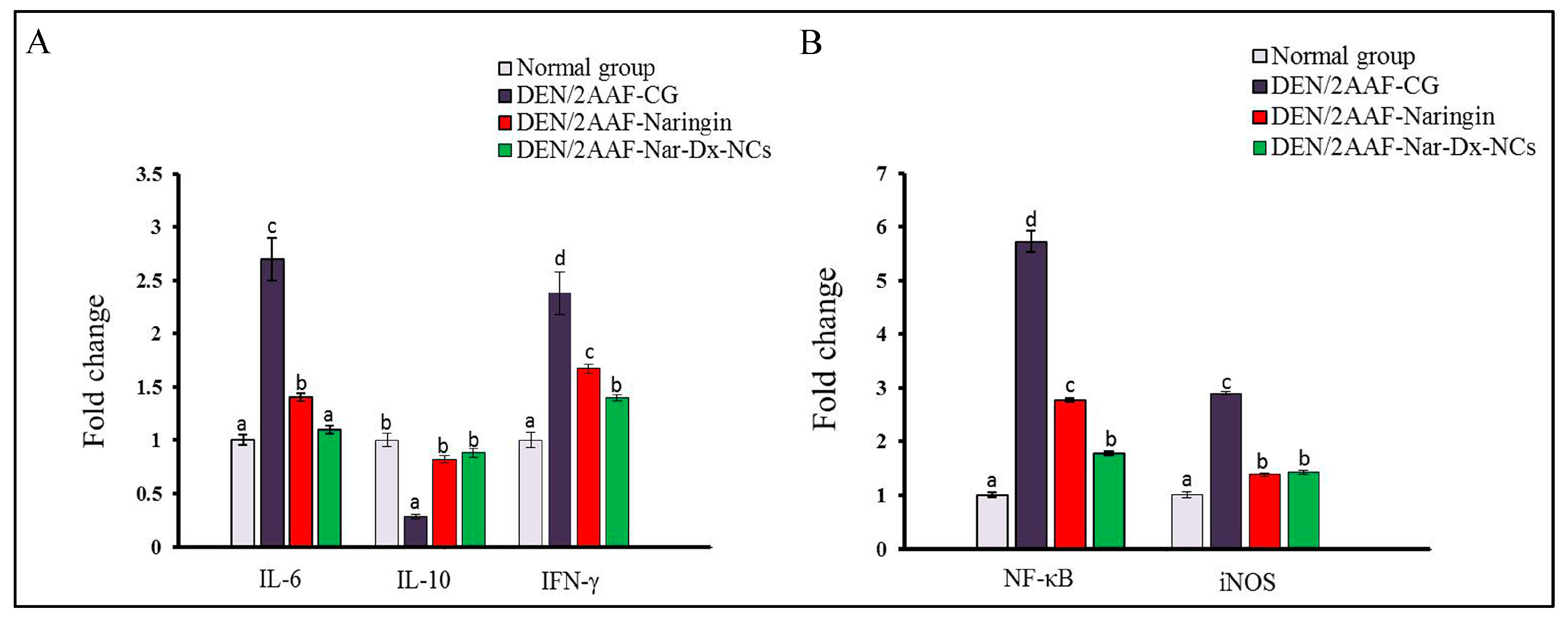
3.4. Effects of Naringin and Nar-Dx-NCs on IL-6, IL-10, NF-κB, IFN-γ, and iNOS mRNA Expression in Lung Tissues
3.5. Effects of Naringin and Nar-Dx-NCs on mRNA Expression of Antiapoptotic and Proapoptotic Biomarkers
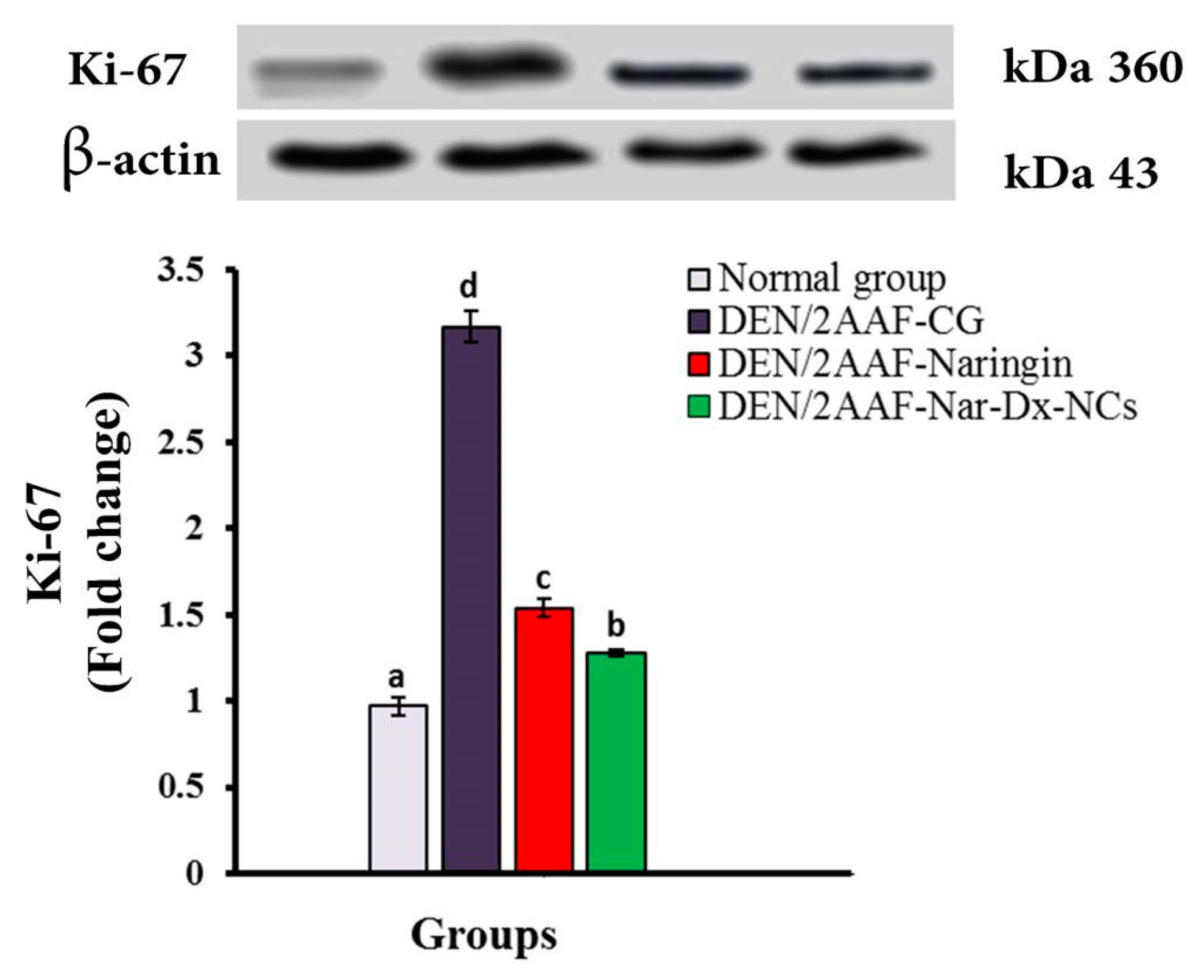
3.6. Effects of Naringin and Nar-Dx-NCs on Ki-67 Protein Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2AAF | 2-acetylaminofluorene |
| 3-NT | 3-nitrotyrosine |
| ARE | antioxidant response element |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma-2 |
| CMC | carboxymethyl cellulose |
| CYP450 | cytochrome P450 |
| DEN | diethylnitrosamine |
| EE | entrapment effectiveness |
| ELISA | enzyme-linked immunosorbent assay |
| FTIR | Fourier transformation infrared |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| H&E | hematoxylin and eosin |
| HRP | horseradish peroxidase |
| i.p. | Intraperitoneally |
| IFN-γ | interferon-γ |
| IGs | inflammatory genes |
| IL-10 | interleukin-10 |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| iNOS | inducible nitric oxide synthase |
| LPO | lipid peroxidation |
| Nar-Dx-NCs | naringin-dextrin nanocomposite |
| NF-κB | nuclear factor-κB |
| NO | nitric oxide |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| O2 | superoxide anion |
| ONOO− | peroxynitrite |
| P53 | tumor suppressor protein 53 |
| PCR | polymerase chain reaction |
| RIPA | radioimmunoprecipitation assay |
| ROS | reactive oxygen species |
| RT-qPCR | reverse transcription–quantitative polymerase chain reaction |
| SDS–PAGE | sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| SE | standard error |
| SOD | superoxide dismutase |
| TBST | tris-buffered saline with Tween 20 |
| TEM | transmission electron microscopy |
| TNF-α | tumor necrosis factor-α |
| UV-Vis | ultraviolet-visible |
| XRD | X-ray diffraction |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Saber, S.; Mahmoud, A.; Helal, N.; El-Ahwany, E.; Abdelghany, R. Liver protective effects of renin-angiotensin system inhibition have no survival benefits in hepatocellular carcinoma induced by repetitive administration of diethylnitrosamine in mice. Maced. J. Med. Sci. 2018, 6, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.E.; Henderson, J.M.; Gorrell, M.D. Animal models for hepatocellular carcinoma. Biochim. Biophy. Acta-Mol. Basis Dis. 2019, 1865, 993–1002. [Google Scholar] [CrossRef]
- Farombi, E.O.; Shrotriya, S.; Surh, Y.J. Kolaviron inhibits dimethyl nitrosamine-induced liver injury by suppressing COX-2 and iNOS expression via NF-kappaB and AP-1. Life Sci. 2009, 8, 149–155. [Google Scholar] [CrossRef]
- Pradeep, K.; Raj Mohan, C.V.; Gobianand, K.; Karthikeyan, S. Protective effect of Cassia fistula Linn. on diethylnitrosamine induced hepatocellular damage and oxidative stress in ethanol pretreated rats. Biol. Res. 2010, 43, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Mervai, Z.; Egedi, K.; Kovalszky, I.; Baghy, K. Diethylnitrosamine induces lung adenocarcinoma in FVB/N mouse. BMC Cancer 2018, 18, 157. [Google Scholar] [CrossRef]
- Geiger-Maor, A.; Guedj, A.; Even-Ram, S. Macrophages regulate the systemic response to DNA damage by a cell nonautonomous mechaism. Cancer Res. 2015, 75, 2663–2673. [Google Scholar] [CrossRef]
- Kishida, N.; Matsuda, S.; Itano, O.; Shinoda, M.; Kitago, M.; Yagi, H.; Abe, Y.; Hibi, T.; Masugi, Y.; Aiura, K.; et al. Development of a novel mouse model of hepatocellular carcinoma with nonalcoholic steatohepatitis using a high-fat, choline-defcient diet and intraperitoneal injection of diethylnitrosamine. BMC Gastroenterol. 2016, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Downs, T.R.; Arlt, V.M.; Barnett, B.C.; Posgai, R.; Pfuhler, S. Effect of 2-acetylaminofluorene and its genotoxic metabolites on DNA adduct formation and DNA damage in 3D reconstructed human skin tissue models. Mutagenesis 2021, 36, 63–74. [Google Scholar] [CrossRef]
- Tu, X.; Ma, S.; Gao, Z.; Wang, J.; Huang, S.; Chen, W. One-Step Extraction and Hydrolysis of Flavonoid Glycosides in Rape Bee Pollen Based on Soxhlet-Assisted Matrix Solid Phase Dispersion. Phytochem. Anal. 2017, 28, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, F.; Chen, H.; Cheng, K.W.; Zykova, T.; Oi, N.; Lubet, R.A.; Bode, A.M.; Wang, M.; Dong, Z. 6-C-(E-phenylethenyl)-Naringenin Suppresses Colorectal Cancer Growth by Inhibiting Cyclooxygenase-1. Cancer Res. 2014, 74, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From clinical significance to quantification. Adv. Sci. 2021, 8, 2004433. [Google Scholar] [CrossRef] [PubMed]
- Van Gorp, H.; Lamkanfi, M. The emerging roles of inflammasome-dependent cytokines in cancer development. EMBO Rep. 2019, 20, e47575. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, M.; Montero-Montero, L.; Renart, J.; Martin-Villar, E. Podoplanin in inflammation and cancer. Int. J. Mol. Sci. 2019, 20, 707. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Golubnitschaja, O.; Zhan, X. Chronic inflammation: Key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. Epma J. 2019, 10, 365–381. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Y.; Ci, X. Role of Nrf2 and its activators in respiratory diseases. Oxidative Med. Cell. Longev. 2019, 2019, 7090534. [Google Scholar] [CrossRef] [PubMed]
- Abouzed, T.K.; Althobaiti, F.; Omran, A.F.; Eldomany, E.B.; El-Shazly, S.A.; Alharthi, F.; Elkattawy, A.M.; Kahilo, K.A.; Dorghamm, D.A. The chemoprevention of spirulina platensis and garlic against diethylnitrosamine induced liver cancer in rats via amelioration of inflammatory cytokines expression and oxidative stress. Toxicol. Res. 2022, 11, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, V.; Guleria, P. Naringin: Biosynthesis and pharmaceutical applications. Indian J. Pharm. Sci. 2019, 81, 988–999. [Google Scholar] [CrossRef]
- Ghanbari-Movahed, M.; Jackson, G.; Farzaei, M.H.; Bishayee, A.A. systematic review of the preventive and therapeutic effects of naringin against human malignancies. Front. Pharmacol. 2021, 12, 639840. [Google Scholar] [CrossRef]
- Mintz, K.J.; Leblanc, R.M. The use of nanotechnology to combat liver cancer: Progress and perspectives. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188621. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, S.; Bayda, S.; Hadla, M.; Caligiuri, I.; Corona, G.; Toffoli, G.; Rizzolio, F. The clinical translation of organic nanomaterials for cancer therapy: A focus on polymeric nanoparticles, micelles, liposomes and exosomes. Curr. Med. Chem. 2018, 25, 4224–4268. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shi, J.; Xie, J.; Wang, Y.; Sun, J.; Liu, T.; Zhao, Y.; Zhao, X.; Wang, X.; Ma, Y.; et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat. Biomed. Eng. 2020, 4, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ma, X.; Ma, Y.; Yang, Z.; Yuan, Y.; Liu, C. Core/Shell PEGS/HA hybrid nanoparticle via micelle-coordinated mineralization for tumor-specific therapy. ACS Appl. Mater. Interfaces 2020, 12, 12109–12119. [Google Scholar] [CrossRef]
- Milewska, S.; Niemirowicz-Laskowska, K.; Siemiaszko, G.; Nowicki, P.; Wilczewska, A.Z.; Car, H. Current trends and challenges in pharmacoeconomic aspects of nanocarriers as drug delivery systems for cancer treatment. Int. J. Nanomed. 2021, 16, 659344. [Google Scholar] [CrossRef]
- Ghanbari-Movahed, M.; Mondal, A.; Farzaei, M.H.; Bishayee, A. Quercetin-and rutin-based nano-formulations for cancer treatment: A systematic review of improved efficacy and molecular mechanisms. Phytomedicine 2022, 97, 153909. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari-Movahed, M.; Kaceli, T.; Mondal, A.; Farzaei, M.H.; Bishayee, A. Recent advances in improved anticancer efficacies of camptothecin nano-formulations: A systematic review. Biomedicines 2021, 9, 480. [Google Scholar] [CrossRef]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 2021, 69, 5–23. [Google Scholar] [CrossRef]
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in phytochemical delivery systems for improved anticancer activity. Biotech. Adv. 2020, 38, 107382. [Google Scholar] [CrossRef]
- Mohamed, E.E.; Abdel-Moneim, A.; Ahmed, O.M.; Zoheir, K.M.; Eldin, Z.E.; El-Shahawy, A.A. Anticancer activity of a novel naringin‒dextrin nanoformula: Preparation, characterization, and in vitro induction of apoptosis in human hepatocellular carcinoma cells by inducing ROS generation, DNA fragmentation, and cell cycle arrest. J. Drug Deliv. Sci. Technol. 2022, 75, 103677. [Google Scholar] [CrossRef]
- Mohamed, E.E.; Ahmed, O.M.; Abdel-Moneim, A.; Zoheir, K.M.; Elesawy, B.H.; Al Askary, A.; El-Shahawy, A.A. Protective Effects of Naringin–Dextrin Nanoformula against Chemically Induced Hepatocellular Carcinoma in Wistar Rats: Roles of Oxidative Stress, Inflammation, Cell Apoptosis, and Proliferation. Pharmaceuticals 2022, 15, 1558. [Google Scholar] [CrossRef]
- Manchun, S.; Dass, C.R.; Sriamornsak, P. Designing nanoemulsion templates for fabrication of dextrin nanoparticles via emulsion cross-linking technique. Carbohydr. Polym. 2014, 101, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Morsy, H.M.; Ahmed, O.M.; Zoheir, K.M.A.; Abdel-Moneim, A. The anticarcinogenic effect of eugenol on lung cancer induced by diethylnitrosamine/2-acetylaminofluorene in Wistar rats: Insight on the mechanisms of action. Apoptosis 2023, 28, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.; Ahmed, O.M.; El-Twab, A.; Sanaa, M.; Zaky, M.Y.; Bakry, L.N. Prophylactic effects of Cynara scolymus L. leaf and flower hydroethanolic extracts against diethylnitrosamine/acetylaminoflourene-induced lung cancer in Wistar rats. Environ. Sci. Pollut. Res. 2021, 28, 43515–43527. [Google Scholar] [CrossRef]
- Camargo, C.A.; Gomes-Marcondes, M.C.; Wutzki, N.C.; Aoyama, H. Naringin inhibits tumor growth and reduces interleukin-6 and tumor necrosis factor α levels in rats with Walker 256 carcinosarcoma. Anticancer Res. 2012, 32, 129–133. [Google Scholar] [PubMed]
- Bancroft, J.D.; Gamble, M. (Eds.) Theory and Practice of Histological Techniques, 6th ed.; Elsevier Health Sciences; Churchill Livingstone: Beijing, China, 2008. [Google Scholar]
- Abràmoff, M.D.; Magalhes, P.J.; Ram, S.J. Image processing with imageJ. Biophoton Int. 2004, 11, 36–42. [Google Scholar]
- Sthoeger, Z.; Zinger, H.; Sharabi, A.; Asher, I.; Mozes, E. The tolerogenic peptide, hCDR1, down-regulates the expression of interferon-α in murine and human systemic lupus erythematosus. PLoS ONE 2013, 8, e60394. [Google Scholar] [CrossRef]
- Sivalingam, K.; Amirthalingam, V.; Ganasan, K.; Huang, C.Y.; Viswanadha, V.P. Neferine suppresses diethylnitrosamine-induced lung carcinogenesis in Wistar rats. Food Chem. Toxicol. 2019, 123, 385–398. [Google Scholar] [CrossRef]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and naringenin: Their mechanisms of action and the potential anticancer activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Fahim, H.I.; Mohamed, E.E.; Abdel-Moneim, A. Protective effects of Persea americana fruit and seed extracts against chemically induced liver cancer in rats by enhancing their antioxidant, anti-inflammatory, and apoptotic activities. Environ. Sci. Pollut. Res. 2022, 4, 43858–43873. [Google Scholar] [CrossRef]
- Barrera, G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. Int. Sch. Res. Not. 2012, 2012, 137289. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, J.H.; Ku, H.J.; Kim, S.H.; Lim, Y.J.; Park, J.W.; Lee, J.H. Naringin protects acrolein-induced pulmonary injuries through modulating apoptotic signaling and inflammation signaling pathways in mice. J. Nutr. Biochem. 2018, 59, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Akintunde, J.K.; Abioye, J.B.; Ebinama, O.N. Potential protective effects of naringin on oculo-pulmonary injury induced by PM10 (wood smoke) exposure by modulation of oxidative damage and acetylcholine esterase activity in a rat model. Curr. Ther. Res. Clin. Exp. 2020, 92, 100586. [Google Scholar] [CrossRef]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muniz, P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: A comparative study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef]
- Askari, V.R.; Shafiee-Nick, R. Promising neuroprotective effects of β-caryophyllene against LPS-induced oligodendrocyte toxicity: A mechanistic study. Biochem. Pharmacol. 2018, 159, 154–171. [Google Scholar] [CrossRef] [PubMed]
- Jawa, R.S.; Anillo, S.; Huntoon, K.; Baumann, H.; Kulaylat, M. Interleukin-6 in surgery, trauma, and critical care part II: Clinical implications. J. Intensive Care Med. 2011, 26, 73–87. [Google Scholar] [CrossRef]
- Janakiram, N.B.; Valerio, M.S.; Goldman, S.M.; Dearth, C.L. The role of the inflammatory response in mediating functional recovery following composite tissue injuries. Int. J. Mol. Sci. 2021, 22, 13552. [Google Scholar] [CrossRef]
- Man, S.; Li, J.; Qiu, P.; Liu, J.; Liu, Z.; Ma, L.; Gao, W. Inhibition of lung cancer in diethylnitrosamine-induced mice by Rhizoma paridis saponins. Mol. Carcinog. 2017, 56, 1405–1413. [Google Scholar] [CrossRef]
- Wu, Y.; Sreeharsha, N.; Sharma, S.; Mishra, A.; Singh, A.K.; Gubbiyappa, S.K. Anticancer effect of rosiglitazone, a PPAR-γ agonist against Diethylnitrosamine-induced lung carcinogenesis. ACS Omega 2020, 5, 5334–5339. [Google Scholar] [CrossRef]
- Cicek, B.; Hayme, S.; Kuzucu, M.; Cetin, A.; Yeni, Y.; Genc, S.; Yildirim, S.; Bolat, I.; Kantarci, M.; Gul, M.; et al. Sorafenib Alleviates Inflammatory Signaling of Tumor Microenvironment in Lung Cancer. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Lin, X.; Ju, Y.N.; Gao, W.; Li, D.M.; Guo, C.C. Desflurane attenuates ventilator-induced lung injury in rats with acute respiratory distress syndrome. BioMed. Res. Int. 2018, 2018, 7507314. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, K.; Zhang, S.; Shan, L.; Tang, J. Tetramethylpyrazine showed therapeutic effects on sepsis-induced acute lung injury in rats by inhibiting endoplasmic reticulum stress protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling-induced apoptosis of pulmonary microvascular endothelial cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 1225–1231. [Google Scholar]
- Yang, Y.; Huang, Z.; Li, J.; Mo, Z.; Huang, Y.; Ma, C.; Wang, W.; Pan, X.; Wu, C. PLGA porous microspheres dry powders for codelivery of afatinib-loaded solid lipid nanoparticles and paclitaxel: Novel therapy for EGFR tyrosine kinase inhibitors resistant nonsmall cell lung cancer. Adv. Healthc. Mater. 2019, 8, 1900965. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S.; Sandur, S.K.; Pandey, M.K.; Sethi, G. Inflammation and cancer: How hot is the link? Biochem. Pharmacol. 2006, 72, 1605–1621. [Google Scholar] [CrossRef]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef]
- Sánchez-Ortega, M.; Carrera, A.C.; Garrido, A. Role of NRF2 in lung cancer. Cells 2021, 10, 1879. [Google Scholar] [CrossRef]
- Hamzavi, M.; Tadbir, A.A.; Rezvani, G.; Ashraf, M.J.; Fattahi, M.J.; Khademi, B.; Sardari, Y.; Jeirudi, N. Tissue expression, serum and salivary levels of IL-10 in patients with head and neck squamous cell carcinoma. Asian Pac. J. Cancer Prev. 2013, 14, 1681–1685. [Google Scholar] [CrossRef]
- Kobelt, D.; Zhang, C.; Clayton-Lucey, I.A.; Glauben, R.; Voss, C.; Siegmund, B.; Stein, U. Pro-inflammatory TNF-α and IFN-γ Promote Tumor Growth and Metastasis via Induction of MACC1. Front. Immunol. 2020, 11, 980. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Todoric, J.; Antonucci, L.; Karin, M. Targeting Inflammation in Cancer Prevention and Therapy. Cancer Prev. Res. 2016, 9, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.; Jain, P.; Khan, Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.A.; Heeba, G.H.; Elwy, H.M.; Murali, C.; El-Awady, R.; Amin, A. Molecular characterization of the grape seeds extract’s effect against chemically induced liver cancer: In vivo and in vitro analyses. Sci. Rep. 2018, 8, 1270–1276. [Google Scholar] [CrossRef]
- Rashid, S.; Ali, N.; Nafees, S.; Ahmad, T.; Hasan, S.K.; Sultana, S. Abrogation of 5-flourouracil induced renal toxicity by bee propolis via targeting oxidative stress and inflammation in Wistar rats. J. Pharm. Res. 2013, 7, 189–194. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Y.; Li, Y.; Xu, W.; Luo, F.; Wang, B.; Pang, Y.; Xiang, Q.; Zhou, J.; Wang, X.; et al. NF-κB-mediated inflammation leading to EMT via miR-200c is involved in cell transformation induced by cigarette smoke extract. Toxicol. Sci. 2013, 135, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Atiq, A.; Shal, B.; Naveed, M.; Khan, A.; Ali, J.; Zeeshan, S.; Al-Sharari, S.D.; Kim, Y.S.; Khan, S. Diadzein ameliorates 5-fluorouracil-induced intestinal mucositis by suppressing oxidative stress and inflammatory mediators in rodents. Eur. J. Pharmacol. 2019, 843, 292–306. [Google Scholar] [CrossRef]
- Hayaza, S.; Darmanto, W.; Wahyuningsih, S.P.A.; Susilo, R.J.K.; Husen, S.A.; Winarni, D.; Doong, R.A. Immunomodulatory activity of Okra raw polysaccharide extract by regulating TNF-A, IFN-G Levels, and cell apoptosis in DEN-induced mice. Res. J. Pharm. Technol. 2022, 15, 546–550. [Google Scholar] [CrossRef]
- Zhang, H.W.; Hu, J.J.; Fu, R.Q.; Liu, X.; Zhang, Y.H.; Li, J.; Liu, L.; Li, Y.N.; Deng, Q.; Luo, Q.S.; et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 2018, 8, 11255. [Google Scholar] [CrossRef]
- Unsal, V.; Kurutaş, E.B. Experimental Hepatic Carcinogenesis: Oxidative Stress and Natural Antioxidants. Maced. J. Med. Sci. 2017, 5, 686–691. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–324. [Google Scholar] [CrossRef]
- Bishayee, A.; Barnes, K.F.; Bhatia, D.; Darvesh, A.S.; Carroll, R.T. Resveratrol suppresses oxidative stress and inflammatory response in diethylnitrosamine-initiated rat hepatocarcinogenesis. Cancer Prev. Res. 2010, 3, 753–763. [Google Scholar] [CrossRef]
- Yu, X.; Ge, L.; Niu, L.; Lian, X.; Ma, H.; Pang, L. The dual role of inducible nitric oxide synthase in myocardial ischemia/reperfusion injury: Friend or foe? Oxidative Med. Cell. Longev. 2018, 2018, 8364848. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Attia, S.M.; Bakheet, S.A.; Zoheir, K.M.; Ansari, M.A.; Korashy, H.M.; Abdel-Hamied, H.E.; Ashour, A.E.; Abd-Allah, A.R. Naringin attenuates the development of carrageenan-induced acute lung inflammation through inhibition of NF-κb, STAT3 and pro-inflammatory mediators and enhancement of IκBα and anti-inflammatory cytokines. Inflammation 2015, 38, 846–857. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, H.; Nie, Y.C.; Chen, J.L.; Su, W.W.; Li, P.B. Naringin attenuates acute lung injury in LPS-treated mice by inhibiting NF-κB pathway. Int. Immunopharmacol. 2011, 11, 1606–1612. [Google Scholar] [CrossRef]
- Habauzit, V.; Sacco, S.M.; Gil-Izquierdo, A.; Trzeciakiewicz, A.; Morand, C.; Barron, D.; Pinaud, S.; Offord, E.; Horcajada, M.N. Differential effects of two citrus flavanones on bone quality in senescent male rats in relation to their bioavailability and metabolism. Bone 2011, 49, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Chtourou, Y.; Aouey, B.; Kebieche, M.; Fetoui, H. Protective role of naringin against cisplatin induced oxidative stress, inflammatory response and apoptosis in rat striatum via suppressing ROS-mediated NF-κB and P53 signaling pathways. Chem. Biol. Interact. 2015, 239, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Wu, W.; Ge, S.; Jia, R.; Lin, T.; Yuan, Y.; Kuang, H.; Yang, B.; Wu, L.; Wei, J.; et al. Naringin protects against perfluorooctane sulfonate-induced liver injury by modulating NRF2 and NF-κB in mice. Int. Immunopharmacol. 2018, 65, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Purvis, J.E.; Karhohs, K.W.; Mock, C.; Batchelor, E.; Loewer, A.; Lahav, G. p53 dynamics control cell fate. Science 2012, 336, 1440–1444. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Lu, Y.; Liao, L.; Li, D.; Liu, L.; Liu, H.; Xu, H. Nitidine chloride induces apoptosis in human hepatocellular carcinoma cells through a pathway involving p53, p21, Bax and Bcl-2. Oncol. Rep. 2015, 33, 1264–1274. [Google Scholar] [CrossRef]
- Campbell, K.J.; Tait, S.W. Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 2018, 8, 180002. [Google Scholar] [CrossRef]
- Kang, M.H.; Reynolds, C.P. Bcl-2 inhibitors: Targeting mitochondrial apoptotic pathways in cancer therapy. Clin. Cancer Res. 2009, 15, 1126–1132. [Google Scholar] [CrossRef]
- Zhu, L.; Han, M.B.; Gao, Y.; Wang, H.; Dai, L.; Wen, Y.; Na, L.X. Curcumin triggers apoptosis via upregulation of Bax/Bcl-2 ratio and caspase activation in SW872 human adipocytes. Mol. Med. Rep. 2015, 12, 1151–1156. [Google Scholar] [CrossRef]
- Liu, Z.; Ding, Y.; Ye, N.; Wild, C.; Chen, H.; Zhou, J. Direct activation of Bax protein for cancer therapy. Med. Res. Rev. 2016, 36, 313–341. [Google Scholar] [CrossRef]
- Liu, X.; Fan, L.; Lu, C.; Yin, S.; Hu, H. Functional role of p53 in the regulation of chemical-induced oxidative stress. Oxidative Med. Cell. Longev. 2020, 2020, 6039769. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Li, A.; Inagaki, Y. Induction of apoptosis by cinobufacini preparation through mitochondria- and Fas-mediated caspase-dependent pathways in human hepatocellular carcinoma cells. Food Chem. Toxicol. 2012, 20, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Qi, Q.L.; Wang, M.T.; Li, Q.Y. Therapeutic potential of naringin: An overview. Pharm. Biol. 2016, 54, 3203–3210. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in cancer and apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef]
- Raina, R.; Hussain, A.; Sharma, R. Molecular insight into apoptosis mediated by flavones in cancer. World Acad. Sci. J. 2020, 2, 6. [Google Scholar] [CrossRef]
- Tramm, T.; Kyndi, M.; Sørensen, F.B.; Overgaard, J.; Alsner, J. Influence of intra-tumoral heterogeneity on the evaluation of BCL2, E-cadherin, EGFR, EMMPRIN, and Ki-67 expression in tissue microarrays from breast cancer. Acta Oncol. 2018, 57, 102–106. [Google Scholar] [CrossRef]
- Wen, S.; Zhou, W.; Li, C.M.; Hu, J.; Hu, X.M.; Chen, P.; Shao, G.L.; Guo, W.H. Ki-67 as a prognostic marker in early-stage non-small cell lung cancer in Asian patients: A meta-analysis of published studies involving 32 studies. BMC Cancer 2015, 15, 520. [Google Scholar] [CrossRef]
- Wang, D.; Ye, W.; Shi, Q. Prognostic Value of Ki-67 Expression in Advanced Lung Squamous Cell Carcinoma Patients Treated with Chemotherapy. Cancer Manag. Res. 2021, 13, 6429. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed]


| Genes | GenBank Accession Number | Sequence (5′–3′) |
|---|---|---|
| NF-κB | NM_001276711.1 | F: TTCAACATGGCAGACGACGA R: TGCTCTAGTATTTGAAGGTATGGG |
| Bcl-2 | NM_016993.1 | F: TAAGCTGTCACAGAGGGGCT R: TGAAGAGTTCCTCCACCACC |
| Bax | NM_007527.3 | F: CTGGATCCAAGACCAGGGTG R: CCTTTCCCCTTCCCCCATTC |
| P53 | NM_030989.3 | F: GTTTTTGTTCCTGAGCCCCG R: GAGCAAGGGGTGACTTTGGG |
| iNOS | NM_012611 | F: CTATGGCCGCTTTGATGTGC R: CAACCTTGGTGTTGAAGGCG |
| IFN-γ | NM_138880.2 | F: ACAACCCACAGATCCAGCAC R: CCAGAATCAGCACCGACTCC |
| IL-6 | NM_012589.2 | F: CACTTCACAAGTCGGAGGCT R: AGCACACTAGGTTTGCCGAG |
| IL-10 | NM_012854 | F: TTGAACCACCCGGCATCTAC R: CCAAGGAGTTGCTCCCGTTA |
| β-actin | NM_031144.3 | F: TCACTATCGGCAATGTGCGG R: GCTCAGGAGGAGCAATGATG |
| Groups | LPO (nmole MDA/100 mg Tissue/h) | GSH (nmole /100 mg Tissue) | GPx (mU/100 mg Tissue) | SOD (U/g Tissue) |
|---|---|---|---|---|
| Normal group | 65.06 ± 0.084 a | 85.86 ± 1.03 d | 214.40 ± 1.40 c | 16.76 ± 0.51 c |
| DEN/2AAF | 134.45 ± 0.83 c | 44.00 ± 0.75 a | 85.61 ± 0.77 a | 3.11 ± 0.02 a |
| DEN/2AAF + Naringin | 75.28± 1.06 b | 67.30 ± 0.98 b | 205.51 ± 0.97 b | 9.26 ± 0.12 b |
| DEN/2AAF + Nar-Dx-NCs | 62.73 ± 0.55 a | 75.73 ± 0.91 c | 212.53 ± 2.52 c | 11.03 ± 0.37 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, E.E.; Ahmed, O.M.; Zoheir, K.M.A.; El-Shahawy, A.A.G.; Tamur, S.; Shams, A.; Burcher, J.T.; Bishayee, A.; Abdel-Moneim, A. Naringin–Dextrin Nanocomposite Abates Diethylnitrosamine/Acetylaminofluorene-Induced Lung Carcinogenesis by Modulating Oxidative Stress, Inflammation, Apoptosis, and Cell Proliferation. Cancers 2023, 15, 5102. https://doi.org/10.3390/cancers15205102
Mohamed EE, Ahmed OM, Zoheir KMA, El-Shahawy AAG, Tamur S, Shams A, Burcher JT, Bishayee A, Abdel-Moneim A. Naringin–Dextrin Nanocomposite Abates Diethylnitrosamine/Acetylaminofluorene-Induced Lung Carcinogenesis by Modulating Oxidative Stress, Inflammation, Apoptosis, and Cell Proliferation. Cancers. 2023; 15(20):5102. https://doi.org/10.3390/cancers15205102
Chicago/Turabian StyleMohamed, Eman E., Osama M. Ahmed, Khairy M. A. Zoheir, Ahmed A. G. El-Shahawy, Shadi Tamur, Anwar Shams, Jack T. Burcher, Anupam Bishayee, and Adel Abdel-Moneim. 2023. "Naringin–Dextrin Nanocomposite Abates Diethylnitrosamine/Acetylaminofluorene-Induced Lung Carcinogenesis by Modulating Oxidative Stress, Inflammation, Apoptosis, and Cell Proliferation" Cancers 15, no. 20: 5102. https://doi.org/10.3390/cancers15205102
APA StyleMohamed, E. E., Ahmed, O. M., Zoheir, K. M. A., El-Shahawy, A. A. G., Tamur, S., Shams, A., Burcher, J. T., Bishayee, A., & Abdel-Moneim, A. (2023). Naringin–Dextrin Nanocomposite Abates Diethylnitrosamine/Acetylaminofluorene-Induced Lung Carcinogenesis by Modulating Oxidative Stress, Inflammation, Apoptosis, and Cell Proliferation. Cancers, 15(20), 5102. https://doi.org/10.3390/cancers15205102










