Evaluation of Bortezomib-BeEAM (2BeEAM) as Chemotherapy Regimen Prior to ASCT in Patients with Mantle Cell Lymphoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical Characteristics of the Patients
3.2. Clinical Status at the Time of HDCT/ASCT
3.3. Toxicities and Adverse Events Following HDCT/ASCT
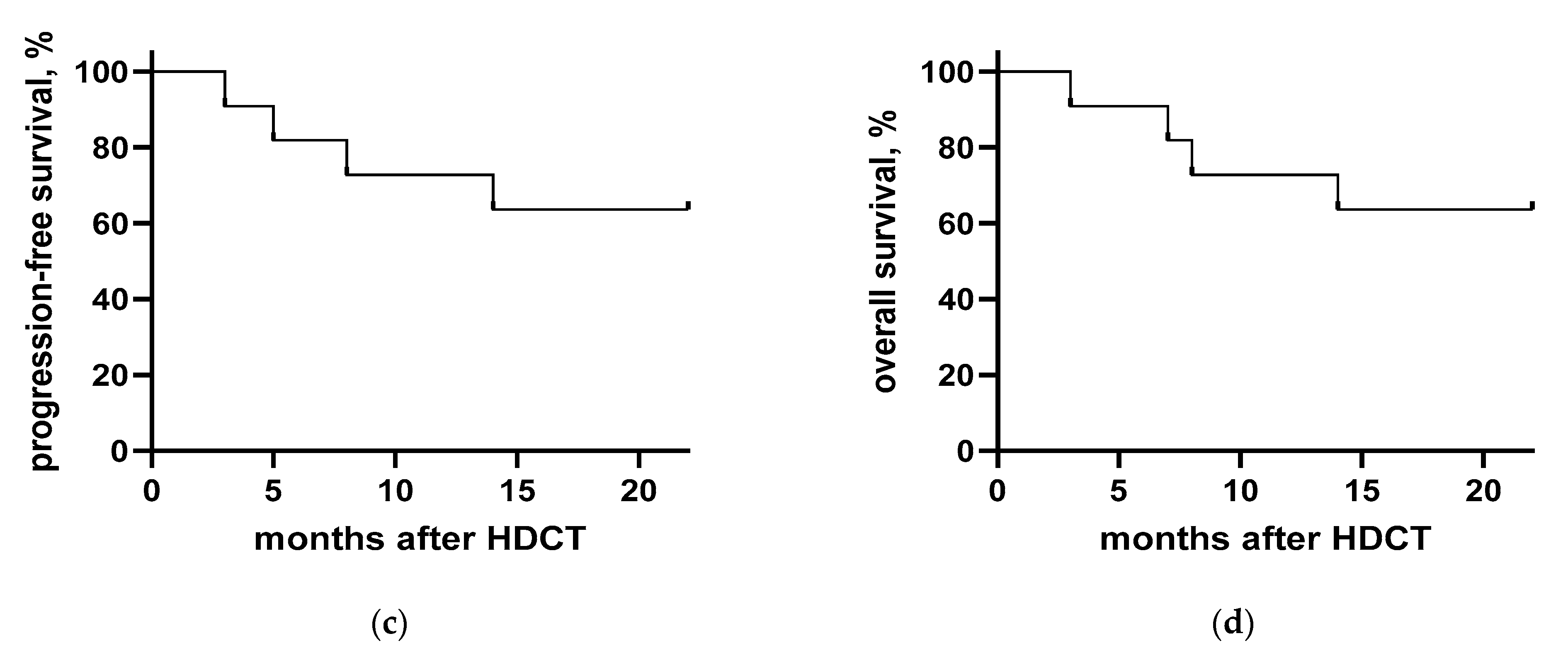
3.4. Clinical Course after HDCT/ASCT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dreyling, M.; Campo, E.; Hermine, O.; Jerkeman, M.; Le Gouill, S.; Rule, S.; Shpilberg, O.; Walewski, J.; Ladetto, M. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv62–iv71, Erratum in Am. J. Hematol. 2018, 93, E134. [Google Scholar] [CrossRef]
- Vose, J.M. Mantle cell lymphoma: 2017 update on diagnosis, risk-stratification, and clinical management. Am. J. Hematol. 2017, 92, 806–813. [Google Scholar] [CrossRef]
- Ghielmini, M.; Zucca, E. How I treat mantle cell lymphoma. Blood 2009, 114, 1469–1476. [Google Scholar] [CrossRef]
- Hermine, O.; Hoster, E.; Walewski, J.; Ribrag, V.; Brousse, N.; Thieblemont, C.; Bouabdallah, R.; Stilgenbauer, M.S.; Feugier, P.; Forstpointner, R.; et al. Alternating Courses of 3x CHOP and 3x DHAP Plus Rituximab Followed by a High Dose ARA-C Containing Myeloablative Regimen and Autologous Stem Cell Transplantation (ASCT) Is Superior to 6 Courses CHOP Plus Rituximab Followed by Myeloablative Radiochemotherapy and ASCT In Mantle Cell Lymphoma: Results of the MCL Younger Trial of the European Mantle Cell Lymphoma Network (MCL net). Blood 2010, 116, 110. [Google Scholar] [CrossRef]
- Damon, L.E.; Johnson, J.L.; Niedzwiecki, D.; Cheson, B.D.; Hurd, D.D.; Bartlett, N.L.; LaCasce, A.S.; Blum, K.A.; Byrd, J.C.; Kelly, M.; et al. Immunochemotherapy and Autologous Stem-Cell Transplantation for Untreated Patients With Mantle-Cell Lymphoma: CALGB 59909. J. Clin. Oncol. 2009, 27, 6101–6108. [Google Scholar] [CrossRef]
- Mathys, A.; Bacher, U.; Banz, Y.; Legros, M.; Taleghani, B.M.; Novak, U.; Pabst, T. Outcome of patients with mantle cell lymphoma after autologous stem cell transplantation in the pre-CAR T-cell era. Hematol. Oncol. 2021, 40, 292–296. [Google Scholar] [CrossRef]
- Ribrag, V.; Saleh, K.; Danu, A.; Koscielny, S.; Pilorge, S.; Castilla-Lorente, C.; Ghez, D.; Lazarovici, J.; Michot, J.-M.; Lapierre, V.; et al. BEAM or BeEAM High-Dose Chemotherapy Followed By ASCT: A Single Center Comparative Analysis of Toxicity. Blood 2016, 128, 4648. [Google Scholar] [CrossRef]
- Hueso, T.; Gastinne, T.; Garciaz, S.; Tchernonog, E.; Delette, C.; Casasnovas, R.-O.; Durot, E.; Houot, R.; Tessoulin, B.; Tournilhac, O.; et al. Bendamustine-EAM versus BEAM regimen in patients with mantle cell lymphoma undergoing autologous stem cell transplantation in the frontline setting: A multicenter retrospective study from Lymphoma Study Association (LYSA) centers. Bone Marrow Transplant. 2020, 55, 1076–1084. [Google Scholar] [CrossRef]
- Berger, M.D.; Branger, G.; Klaeser, B.; Taleghani, B.M.; Novak, U.; Banz, Y.; Mueller, B.U.; Pabst, T. Zevalin and BEAM (Z-BEAM) versus rituximab and BEAM (R-BEAM) conditioning chemotherapy prior to autologous stem cell transplantation in patients with mantle cell lymphoma. Hematol. Oncol. 2015, 34, 133–139. [Google Scholar] [CrossRef]
- Belch, A.; Kouroukis, C.; Crump, M.; Sehn, L.; Gascoyne, R.; Klasa, R.; Powers, J.; Wright, J.; Eisenhauer, E. A phase II study of bortezomib in mantle cell lymphoma: The National Cancer Institute of Canada Clinical Trials Group trial IND.150. Ann. Oncol. 2006, 18, 116–121. [Google Scholar] [CrossRef]
- Fisher, R.I.; Bernstein, S.H.; Kahl, B.S.; Djulbegovic, B.; Robertson, M.J.; de Vos, S.; Epner, E.; Krishnan, A.; Leonard, J.P.; Lonial, S.; et al. Multicenter Phase II Study of Bortezomib in Patients With Relapsed or Refractory Mantle Cell Lymphoma. J. Clin. Oncol. 2006, 24, 4867–4874. [Google Scholar] [CrossRef] [PubMed]
- Robak, T.; Huang, H.; Jin, J.; Zhu, J.; Liu, T.; Samoilova, O.; Pylypenko, H.; Verhoef, G.; Siritanaratkul, N.; Osmanov, E.; et al. Bortezomib-Based Therapy for Newly Diagnosed Mantle-Cell Lymphoma. N. Engl. J. Med. 2015, 372, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Robak, T.; Jin, J.; Pylypenko, H.; Verhoef, G.; Siritanaratkul, N.; Drach, J.; Raderer, M.; Mayer, J.; Pereira, J.; Tumyan, G.; et al. Frontline bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in transplantation-ineligible patients with newly diagnosed mantle cell lymphoma: Final overall survival results of a randomised, open-label, phase 3 study. Lancet Oncol. 2018, 19, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, L.D.; Maurer, M.J.; Stock, W.; Bartlett, N.; Fulton, N.; Pettinger, A.; Byrd, J.C.; Blum, K.A.; LaCasce, A.S.; Hsi, E.D.; et al. Bortezomib consolidation or maintenance following immunochemotherapy and autologous stem cell transplantation for mantle cell lymphoma: CALGB /Alliance 50403. Am. J. Hematol. 2020, 95, 583–593. [Google Scholar] [CrossRef]
- Prediletto, I.; Farag, S.A.; Bacher, U.; Jeker, B.; Taleghani, B.M.; Brégy, R.; Zander, T.; Betticher, D.; Egger, T.; Novak, U.; et al. High incidence of reversible renal toxicity of dose-intensified bendamustine-based high-dose chemotherapy in lymphoma and myeloma patients. Bone Marrow Transplant. 2019, 54, 1923–1925. [Google Scholar] [CrossRef]
- Philip, T.; Guglielmi, C.; Hagenbeek, A.; Somers, R.; Van Der Lelie, H.; Bron, D.; Sonneveld, P.; Gisselbrecht, C.; Cahn, J.-Y.; Harousseau, J.-L.; et al. Autologous Bone Marrow Transplantation as Compared with Salvage Chemotherapy in Relapses of Chemotherapy-Sensitive Non-Hodgkin's Lymphoma. N. Engl. J. Med. 1995, 333, 1540–1545. [Google Scholar] [CrossRef]
- Kumar, A.; Sha, F.; Toure, A.; Dogan, A.; Ni, A.; Batlevi, C.L.; Palomba, M.L.M.; Portlock, C.; Straus, D.J.; Noy, A.; et al. Patterns of survival in patients with recurrent mantle cell lymphoma in the modern era: Progressive shortening in response duration and survival after each relapse. Blood Cancer J. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Berger, M.D.; Branger, G.; Leibundgut, K.; Baerlocher, G.M.; Seipel, K.; Mueller, B.U.; Gregor, M.; Ruefer, A.; Pabst, T. CD34+ selected versus unselected autologous stem cell transplantation in patients with advanced-stage mantle cell and diffuse large B-cell lymphoma. Leuk. Res. 2015, 39, 561–567. [Google Scholar] [CrossRef]
- Chen, Y.-B.; Lane, A.A.; Logan, B.R.; Zhu, X.; Akpek, G.; Aljurf, M.D.; Artz, A.S.; Bredeson, C.N.; Cooke, K.R.; Ho, V.T.; et al. Impact of Conditioning Regimen on Outcomes for Patients with Lymphoma Undergoing High-Dose Therapy with Autologous Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2015, 21, 1046–1053. [Google Scholar] [CrossRef]
- Flowers, C.R.; Costa, L.J.; Pasquini, M.C.; Le-Rademacher, J.; Lill, M.; Shore, T.B.; Vaughan, W.; Craig, M.; Freytes, C.O.; Shea, T.C.; et al. Efficacy of Pharmacokinetics-Directed Busulfan, Cyclophosphamide, and Etoposide Conditioning and Autologous Stem Cell Transplantation for Lymphoma: Comparison of a Multicenter Phase II Study and CIBMTR Outcomes. Biol. Blood Marrow Transplant. 2016, 22, 1197–1205. [Google Scholar] [CrossRef]
- Sellner, L.; Boumendil, A.; Finel, H.; Choquet, S.; de Rosa, G.; Falzetti, F.; Scime, R.; Kobbe, G.; Ferrara, F.; Delmer, A.; et al. Thiotepa-based high-dose therapy for autologous stem cell transplantation in lymphoma: A retrospective study from the EBMT. Bone Marrow Transplant. 2015, 51, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Visani, G.; Malerba, L.; Stefani, P.M.; Capria, S.; Galieni, P.; Gaudio, F.; Specchia, G.; Meloni, G.; Gherlinzoni, F.; Giardini, C.; et al. BeEAM (bendamustine, etoposide, cytarabine, melphalan) before autologous stem cell transplantation is safe and effective for resistant/relapsed lymphoma patients. Blood 2011, 118, 3419–3425. [Google Scholar] [CrossRef] [PubMed]
- Visani, G.; Stefani, P.M.; Capria, S.; Malerba, L.; Galieni, P.; Gaudio, F.; Specchia, G.; Meloni, G.; Gherlinzoni, F.; Gonella, R.; et al. Bendamustine, etoposide, cytarabine, melphalan, and autologous stem cell rescue produce a 72% 3-year PFS in resistant lymphoma. Blood 2014, 124, 3029–3031. [Google Scholar] [CrossRef]
- Gilli, S.; Novak, U.; Taleghani, B.M.; Baerlocher, G.M.; Leibundgut, K.; Banz, Y.; Zander, T.; Betticher, D.; Egger, T.; Rauch, D.; et al. BeEAM conditioning with bendamustine-replacing BCNU before autologous transplantation is safe and effective in lymphoma patients. Ann. Hematol. 2016, 96, 421–429. [Google Scholar] [CrossRef]
- Furtado, M.; Johnson, R.; Kruger, A.; Turner, D.; Rule, S. Addition of bortezomib to standard dose chop chemotherapy improves response and survival in relapsed mantle cell lymphoma. Br. J. Haematol. 2014, 168, 55–62. [Google Scholar] [CrossRef] [PubMed]
- William, B.M.; Allen, M.S.; Loberiza, F.R.; Bociek, R.G.; Bierman, P.J.; Armitage, J.O.; Vose, J.M. Phase I/II Study of Bortezomib-BEAM and Autologous Hematopoietic Stem Cell Transplantation for Relapsed Indolent Non-Hodgkin Lymphoma, Transformed, or Mantle Cell Lymphoma. Biol. Blood Marrow Transplant. 2014, 20, 536–542. [Google Scholar] [CrossRef]
- Hoster, E.; Klapper, W.; Hermine, O.; Kluin-Nelemans, J.C.; Walewski, J.; Van Hoof, A.; Trneny, M.; Geisler, C.H.; Di Raimondo, F.; Szymczyk, M.; et al. Confirmation of the Mantle-Cell Lymphoma International Prognostic Index in Randomized Trials of the European Mantle-Cell Lymphoma Network. J. Clin. Oncol. 2014, 32, 1338–1346. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). FDA Approves First Cell-Based Gene Therapy for Adult Patients with Relapsed or Refractory MCL. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-cell-based-gene-therapy-adult-patients-relapsed-or-refractory-mcl (accessed on 24 July 2020).
- European Medicines Agency (EMA). Tecartus (Autologous Anti-CD19-Transduced CD3+ Cells): An Overview of Tecartus and Why It Is Authorized in the EU. Available online: https://www.ema.europa.eu/en/documents/overview/tecartus-epar-medicine-overview_en.pdf (accessed on 14 July 2020).
- Schweizerisches Heilmittelinstitut Swissmedic. Tecartus, Infusionsdispersion (Autologe Anti-CD19-Transduzierte CD3+ Zellen). Available online: https://www.swissmedic.ch/swissmedic/de/home/humanarzneimittel/authorisations/new-medicines/tecartus-infusiondispersion.html (accessed on 1 February 2023).
- Heini, A.D.; Bacher, U.; Kronig, M.-N.; Wiedemann, G.; Novak, U.; Zeerleder, S.; Taleghani, B.M.; Daskalakis, M.; Pabst, T. Chimeric antigen receptor T-cell therapy for relapsed mantle cell lymphoma: Real-world experience from a single tertiary care center. Bone Marrow Transplant. 2022, 57, 1010–1012. [Google Scholar] [CrossRef]

| General Parameters | Result |
|---|---|
| Patients, number | 11 |
| Demographic characteristics | |
| Males:females (ratio) | 8:3 (2.7) |
| Median age at the time of diagnosis (years; range) | 66 (46–70) |
| Disease Parameters at Initial Diagnosis | |
| Mantle cell lymphoma | 11 (100%) |
| Ann Arbor stage | |
| I/II | 0 (0%) |
| III | 3 (27%) |
| IV | 8 (73%) |
| B-symptoms at lymphoma diagnosis 1 | 7 (64%) |
| MIPI risk category 1 | |
| Low risk | 2 (18%) |
| Intermediate/low risk | 1 (9%) |
| Intermediate risk | 2 (18%) |
| High risk | 4 (36%) |
| Morphologic grading 2 | |
| Classical type | 8 (73%) |
| Blastoid variant | 2 (18%) |
| Average Ki-67 index 1 (SD) | 36% (16.6) |
| Remission Status | Number (%) |
|---|---|
| CR | 5 (45%) |
| PR | 6 (55%) |
| Prior chemotherapy | |
| First line induction chemotherapy | 11 (100%) |
| R-CHOP/R-DHAP | 5 (45%) |
| R-CHOP/R-DHAO | 6 (55%) |
| Second line therapy | 1 (9%) |
| Median number of therapy lines (range) | 1 (1–2) |
| MIPI risk category | |
| Low risk | 1 (9%) |
| Intermediate risk | 5 (45%) |
| High risk | 5 (45%) |
| Comorbidities and ECOG status | |
| Clinically important comorbidities | 6 (55%) |
| ECOG status 1 (range) | 0 (0) |
| Hematologic recovery | |
| Median time (days) until ANC >0.5 G/l (range) | 11 (9–13) |
| Median time (days) until Tc >20 G/l (range) | 22.5 (13–97) |
| Parameter | Number (%) |
|---|---|
| Median days in hospital (range) | 27 (19–89) |
| Febrile neutropenia | 11 (100%) |
| Median febrile neutropenia CTCAE grade 1 (range) | 4 (4) |
| Anemia | 11 (100%) |
| Preexisting anemia | 11 (100%) |
| Median anemia CTCAE grade 1 (range) | 3 (2–3) |
| Thrombocytopenia | 11 (100%) |
| Median thrombocytopenia CTCAE grade 1 (range) | 4 (4) |
| Preexisting thrombocytopenia | 4 (36%) |
| New/intensified neuropathy at hospitalization | 6 (55%) |
| New/intensified peripheral sensory neuropathy | 5 (45%) |
| Median peripheral sensory neuropathy CTCAE grade (range) | 2 (1–3) |
| New/intensified peripheral motoric neuropathy | 2 (18%) |
| Median peripheral motoric neuropathy CTCAE grade (range) | 2.5 (2–3) |
| Diarrhea | 11 (100%) |
| Weight loss 1 | 10 (91%) |
| Rash | 10 (91%) |
| Xerostomy | 10 (91%) |
| Hypotension | 9 (82%) |
| Nausea | 9 (82%) |
| Mucositis | 9 (82%) |
| Decline in renal function | 7 (64%) |
| Fatigue | 7 (64%) |
| Constipation | 6 (55%) |
| Dyspnea | 6 (55%) |
| Severe infections 2 | 3 (27%) |
| Infections with detected microbial pathogen | 10 (91%) |
| Infections with detected bacterial pathogen | 10 (91%) |
| Infections with detected fungal pathogen | 6 (55%) |
| Infections with detected viral pathogen | 2 (18%) |
| Best Response Rate | Number (%) |
|---|---|
| 100-day follow-up | |
| Complete remission | 7 (64%) |
| Partial remission | 3 (27%) |
| Death between HDCT and follow-up | 1 (9%) |
| Last follow-up | |
| Complete remission | 6 (55%) |
| Partial remission | 1 (9%) |
| Relapse following HDCT/ASCT | 1 (9%) |
| Death after partial remission | 2 (18%) |
| Death after relapse after 100-day follow-up | 1 (9%) |
| Patients with further MCL therapy after HDCT/ASCT | 9 (82%) |
| Overall mortality | 4 (36%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huwyler, F.; Kunz, R.; Bacher, U.; Hoffmann, M.; Novak, U.; Daskalakis, M.; Banz, Y.; Pabst, T. Evaluation of Bortezomib-BeEAM (2BeEAM) as Chemotherapy Regimen Prior to ASCT in Patients with Mantle Cell Lymphoma. Cancers 2023, 15, 2091. https://doi.org/10.3390/cancers15072091
Huwyler F, Kunz R, Bacher U, Hoffmann M, Novak U, Daskalakis M, Banz Y, Pabst T. Evaluation of Bortezomib-BeEAM (2BeEAM) as Chemotherapy Regimen Prior to ASCT in Patients with Mantle Cell Lymphoma. Cancers. 2023; 15(7):2091. https://doi.org/10.3390/cancers15072091
Chicago/Turabian StyleHuwyler, Fabrizio, Rebekka Kunz, Ulrike Bacher, Michèle Hoffmann, Urban Novak, Michael Daskalakis, Yara Banz, and Thomas Pabst. 2023. "Evaluation of Bortezomib-BeEAM (2BeEAM) as Chemotherapy Regimen Prior to ASCT in Patients with Mantle Cell Lymphoma" Cancers 15, no. 7: 2091. https://doi.org/10.3390/cancers15072091
APA StyleHuwyler, F., Kunz, R., Bacher, U., Hoffmann, M., Novak, U., Daskalakis, M., Banz, Y., & Pabst, T. (2023). Evaluation of Bortezomib-BeEAM (2BeEAM) as Chemotherapy Regimen Prior to ASCT in Patients with Mantle Cell Lymphoma. Cancers, 15(7), 2091. https://doi.org/10.3390/cancers15072091







