Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Peritoneal Carcinomatosis: Additional Information Helps to Optimize Patient Selection before Surgery
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
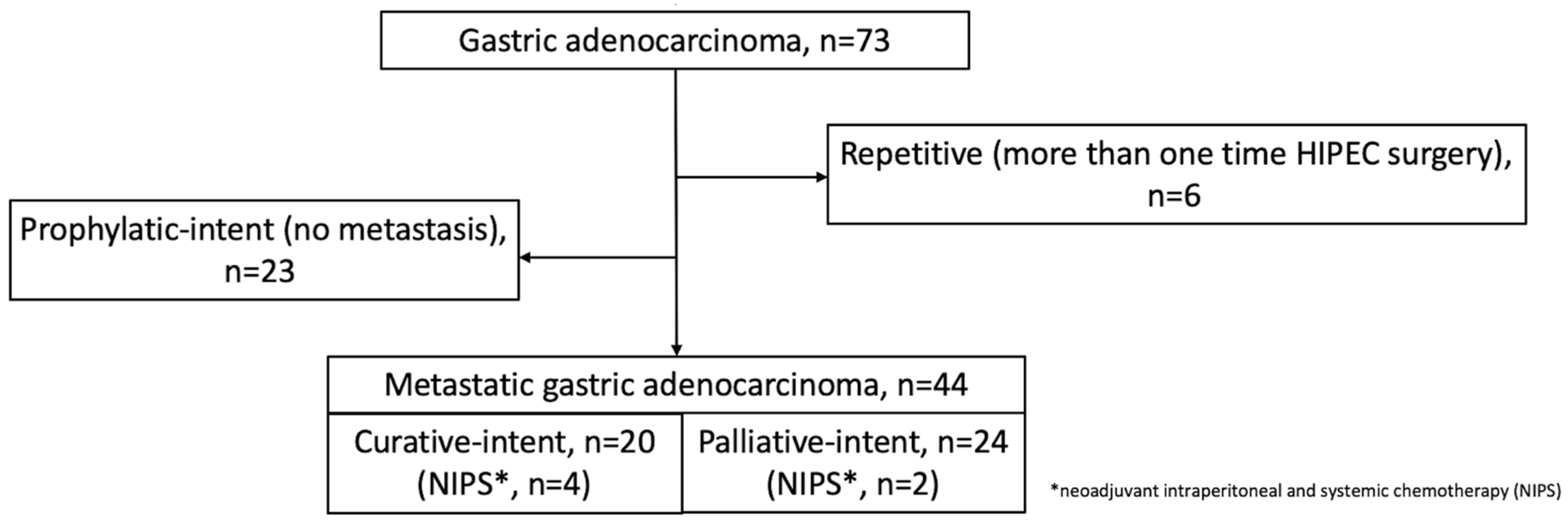
2.1. Study Population
2.2. Procedure Protocol and Patient Surveillance
2.3. Data Collection, Data Forms, and Statistical Analysis
3. Results
3.1. Population Composition
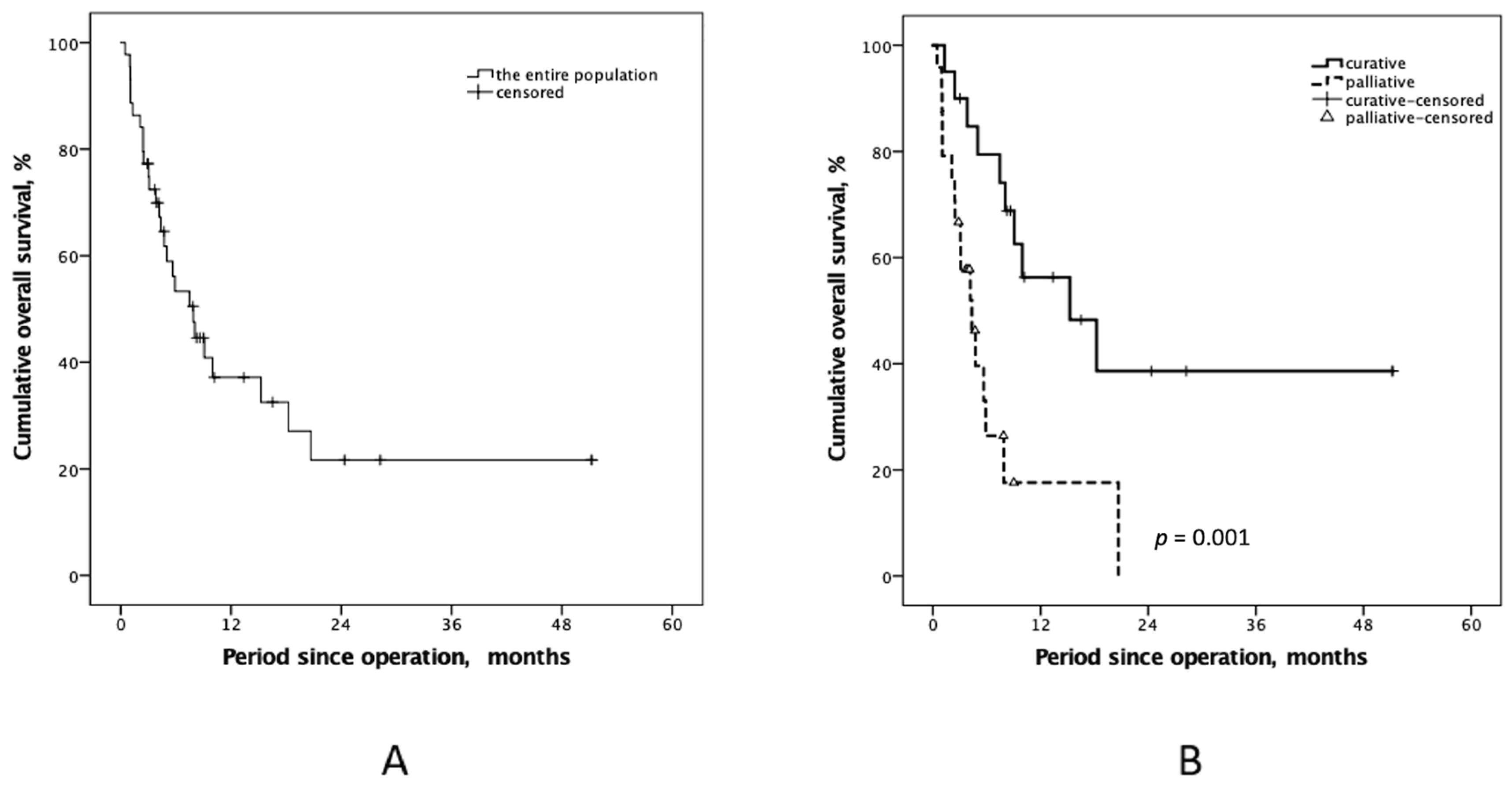
3.2. CRS-HIPEC Operation Intents and Outcomes
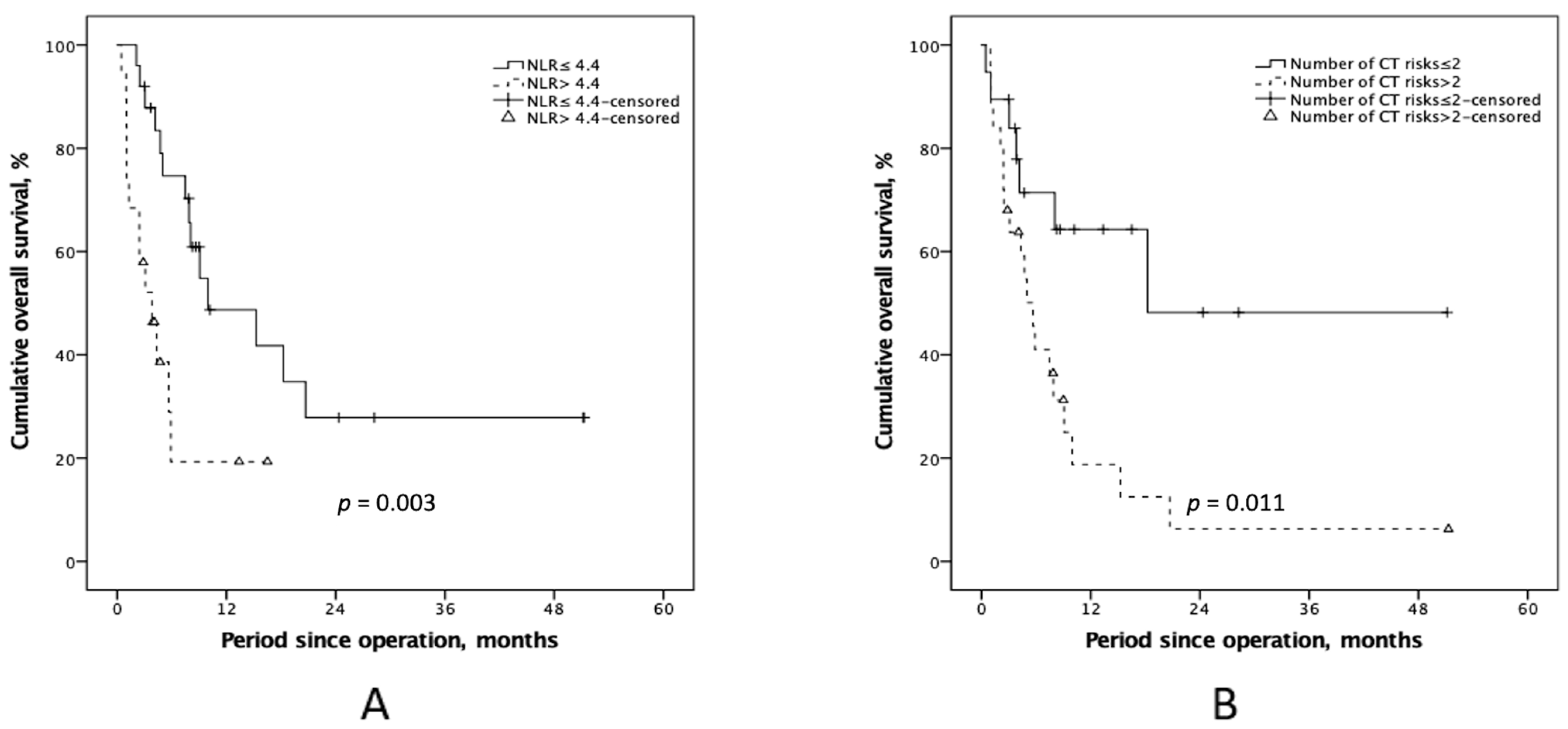
3.3. Univariate and Multivariate Analyses of Preoperative Survival Predictors
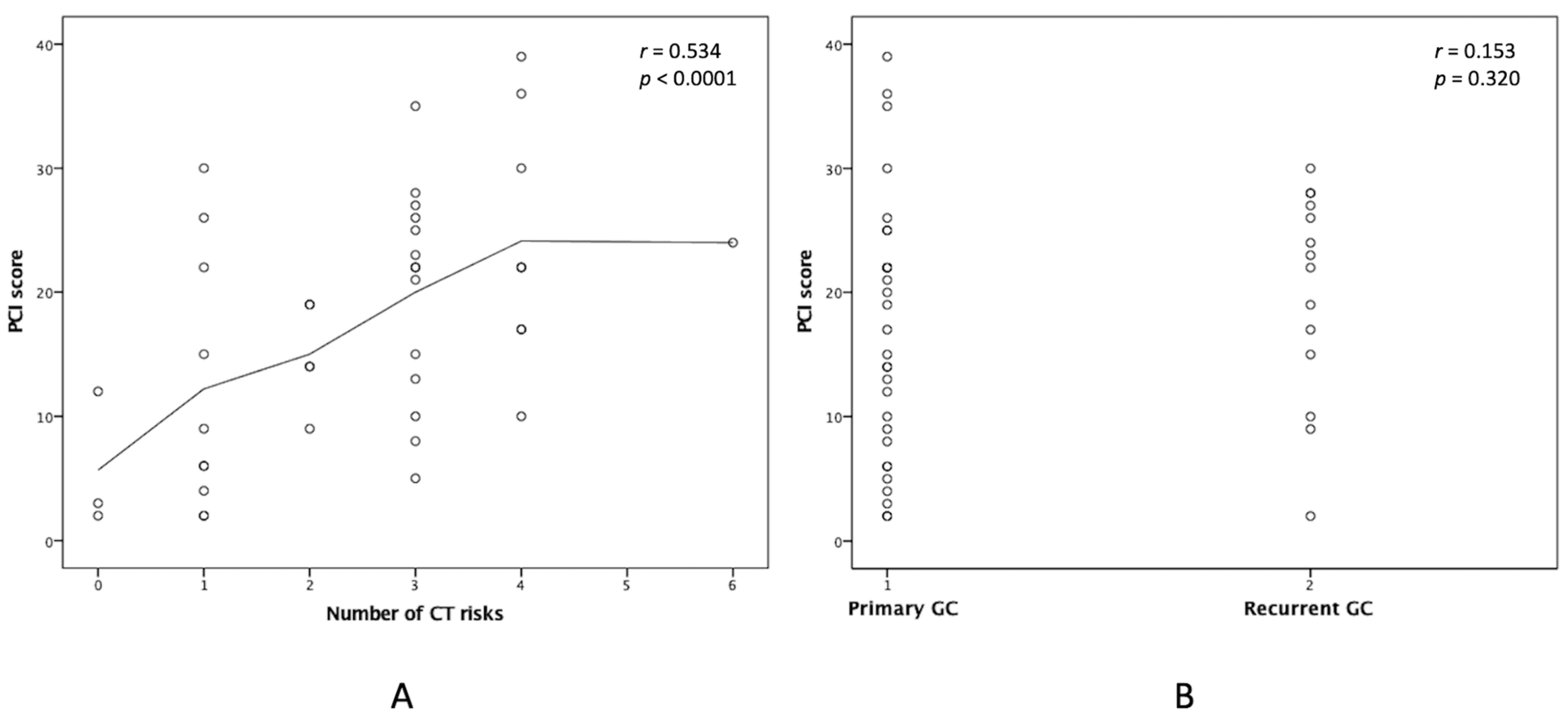
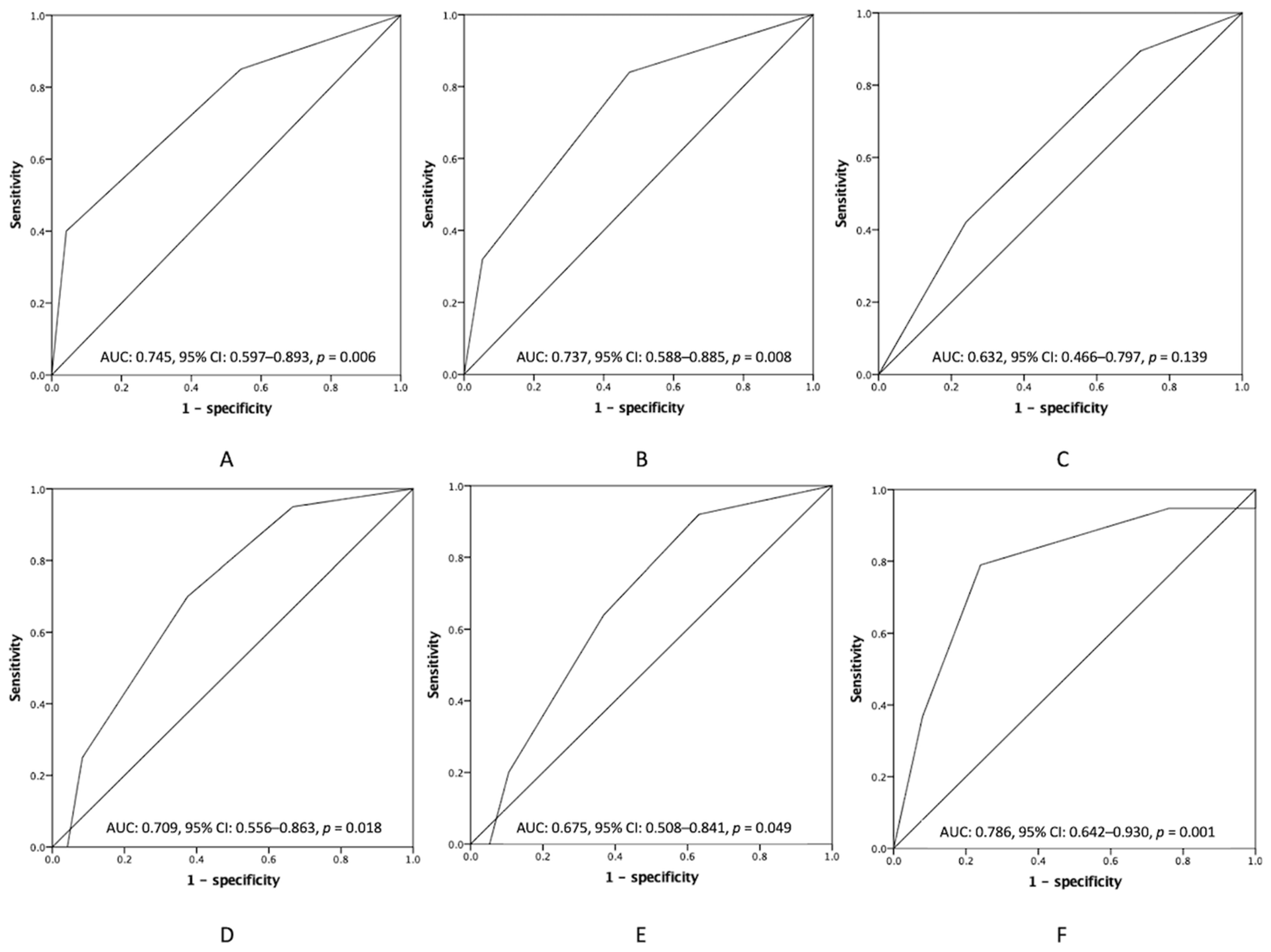
3.4. Additional Preoperative Information to the PCI in Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brandl, A.; Pachmayr, E.; Gül-Klein, S.; Alberto, M.; Thuss-Patience, P.; Rau, B. Surgical treatment of peritoneal metastases of gastric cancer. Der Chir. 2018, 89, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, I.; van Gestel, Y.R.; van Ramshorst, B.; Luyer, M.D.; Bosscha, K.; Nienhuijs, S.W.; Lemmens, V.E.; de Hingh, I.H. Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, survival and risk factors. Int. J. Cancer 2014, 134, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.Y.; El Taani, H.; Saad, A.; Badheeb, A. Advanced gastric cancer in jordan from 2004 to 2008: A study of epidemiology and out-comes. Gastrointest. Cancer Res. 2011, 4, 122–127. [Google Scholar]
- Spolverato, G.; Ejaz, A.; Kim, Y.; Squires, M.H.; Poultsides, G.A.; Fields, R.C.; Schmidt, C.; Weber, S.M.; Votanopoulos, K.; Maithel, S.K.; et al. Rates and Patterns of Recurrence after Curative Intent Resection for Gastric Cancer: A United States Multi-Institutional Analysis. J. Am. Coll. Surg. 2014, 219, 664–675. [Google Scholar] [CrossRef]
- Chen, M.; Chen, K.; Hou, H.; Li, W.; Wang, X.; Dao, Q.; Wang, Z. Incidence and mortality trends in gastric cancer in the United States, 1992–2019. Int. J. Cancer 2023, 152, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, B.; Arvieux, C.; Glehen, O.; Beaujard, A.C.; Rivoire, M.; Baulieux, J.; Fontaumard, E.; Brachet, A.; Caillot, J.L.; Faure, J.L.; et al. Peritoneal carcinomatosis from non-gynecologic malignancies: Results of the EVOCAPE 1 multicentric prospective study. Cancer 2000, 88, 358–363. [Google Scholar] [CrossRef]
- Bilici, A. Treatment options in patients with metastatic gastric cancer: Current status and future perspectives. World J. Gastroenterol. 2014, 20, 3905–3915. [Google Scholar] [CrossRef]
- Koizumi, W.; Narahara, H.; Hara, T.; Takagane, A.; Akiya, T.; Takagi, M.; Miyashita, K.; Nishizaki, T.; Kobayashi, O.; Takiyama, W.; et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol. 2008, 9, 215–221. [Google Scholar] [CrossRef]
- Los, G.; Mutsaers, P.H.; Van Der Vijgh, W.J.; Baldew, G.S.; De Graaf, P.W.; McVie, J.G. Direct diffusion of cis-diamminedichloroplatinum(II) in intraperitoneal rat tumors after intraperitoneal chemotherapy: A comparison with systemic chemotherapy. Cancer Res. 1989, 49, 3380–3384. [Google Scholar]
- Sugarbaker, P.H. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: Progress toward a new standard of care. Cancer Treat. Rev. 2016, 48, 42–49. [Google Scholar] [CrossRef]
- Ji, Z.-H.; Peng, K.-W.; Yu, Y.; Li, X.-B.; Yonemura, Y.; Liu, Y.; Sugarbaker, P.H.; Li, Y. Current status and future prospects of clinical trials on CRS + HIPEC for gastric cancer peritoneal metastases. Int. J. Hyperth. 2017, 33, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Gilly, F.N.; Arvieux, C.; Cotte, E.; Boutitie, F.; Mansvelt, B.; Bereder, J.M.; Lorimier, G.; Quenet, F.; Elias, D.; et al. Peritoneal Carcinomatosis from Gastric Cancer: A Multi-Institutional Study of 159 Patients Treated by Cytoreductive Surgery Combined with Perioperative Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2010, 17, 2370–2377. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Santos-Sousa, H.; Araujo, F.; Nogueiro, J.; Sousa-Pinto, B. Impact of Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemo-therapy in the Treatment of Gastric Cancer with Peritoneal Carcinomatosis: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2022, 29, 7528–7537. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.H.C.; Kwek, J.W.; Hosseini, R.; Chanyaputhipong, J.; Tham, C.K.; Soo, K.C.; Teo, M.C.C. Proposed radiological criteria for pre-operative determination of resectability in perito-neal-based malignancies. J. Med. Imaging Radiat. Oncol. 2016, 60, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. In Peritoneal Carcinomatosis: Principles of Management; Sugarbaker, P.H., Ed.; Springer: Boston, MA, USA, 1996; Volume 82, pp. 359–374. [Google Scholar] [CrossRef]
- Hung, H.-C.; Hsu, P.-J.; Chang, T.-C.; Chou, H.-H.; Huang, K.-G.; Lai, C.-H.; Lee, C.-W.; Yu, M.-C.; You, J.-F.; Hsu, J.-T.; et al. Neutrophil-to-lymphocyte-ratio-based perioperative prognosis prediction model on early mortality after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Asian J. Surg. 2022, 45, 2676–2685. [Google Scholar] [CrossRef]
- Manzanedo, I.; Pereira, F.; Rihuete Caro, C.; Pérez-Viejo, E.; Serrano, Á.; Gutierrez Calvo, A.; Regueira, F.M.; Casado-Adam, Á.; Cascales-Campos, P.A.; Arteaga, X.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Gastric Cancer with Peritoneal Carcinomatosis: Multicenter Study of Spanish Group of Peritoneal Oncologic Surgery (GECOP). Ann. Surg. Oncol. 2019, 26, 2615–2621. [Google Scholar] [CrossRef]
- Coccolini, F.; Catena, F.; Glehen, O.; Yonemura, Y.; Sugarbaker, P.; Piso, P.; Montori, G.; Ansaloni, L. Complete versus incomplete cytoreduction in peritoneal carcinosis from gastric cancer, with consideration to PCI cut-off. Systematic review and meta-analysis. Eur. J. Surg. Oncol. (EJSO) 2015, 41, 911–919. [Google Scholar] [CrossRef]
- Yonemura, Y.; Elnemr, A.; Endou, Y.; Hirano, M.; Mizumoto, A.; Takao, N.; Ichinose, M.; Miura, M.; Li, Y. Multidisciplinary therapy for treatment of patients with peritoneal carcinomatosis from gastric cancer. World J. Gastrointest. Oncol. 2010, 2, 85–97. [Google Scholar] [CrossRef]
- Koh, J.-L.; Yan, T.D.; Glenn, D.; Morris, D.L. Evaluation of Preoperative Computed Tomography in Estimating Peritoneal Cancer Index in Colorectal Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2009, 16, 327–333. [Google Scholar] [CrossRef]
- Yonemura, Y.; Endou, Y.; Sasaki, T.; Hirano, M.; Mizumoto, A.; Matsuda, T.; Takao, N.; Ichinose, M.; Miura, M.; Li, Y. Surgical treatment for peritoneal carcinomatosis from gastric cancer. Eur. J. Surg. Oncol. (EJSO) 2010, 36, 1131–1138. [Google Scholar] [CrossRef]
- Yang, X.J.; Huang, C.-Q.; Suo, T.; Mei, L.-J.; Yang, G.-L.; Cheng, F.; Zhou, Y.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Improves Survival of Patients with Peritoneal Carcinomatosis from Gastric Cancer: Final Results of a Phase III Randomized Clinical Trial. Ann. Surg. Oncol. 2011, 18, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Königsrainer, I.; Horvath, P.; Struller, F.; Königsrainer, A.; Beckert, S. Initial clinical experience with cytoreductive surgery and hyperthermic intraperi-toneal chemotherapy in signet-ring cell gastric cancer with peritoneal metastases. J. Gastric Cancer 2014, 14, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Tanioka, H.; Okawaki, M.; Yano, S.; Yoshimitsu, T.; Tokuda, K.; Nyuya, A.; Yamaguchi, Y.; Nagasaka, T. Neutrophil-to-lymphocyte ratio before each chemotherapy line predicts clinical outcomes in patients with unresectable gastric cancer. Oncol. Lett. 2023, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Jiang, R.; Chen, W.; Liu, Y.; Wang, G.; Gong, X.; Wang, Y. Novel prognostic indicator combining inflammatory indicators and tumor markers for gastric cancer. World J. Surg. Oncol. 2023, 21, 50. [Google Scholar] [CrossRef]
- Shiroyama, T.; Nagatomo, I.; Koyama, S.; Hirata, H.; Nishida, S.; Miyake, K.; Fukushima, K.; Shirai, Y.; Mitsui, Y.; Takata, S.; et al. Impact of sarcopenia in patients with advanced non–small cell lung cancer treated with PD-1 inhibitors: A preliminary retrospective study. Sci. Rep. 2019, 9, 2447. [Google Scholar] [CrossRef]
- Hayano, K.; Ohira, G.; Kano, M.; Suito, H.; Matsumoto, Y.; Kurata, Y.; Otsuka, R.; Isozaki, T.; Toyozumi, T.; Murakami, K.; et al. Prognostic Impact of Hepatic Steatosis Evaluated by CT on Immunotherapy for Gastric Cancer: Associations with Sarcopenia, Systemic Inflammation, and Hormones. Oncology 2023, 101, 185–192. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Kaneko, M.; Nozawa, H.; Sasaki, K.; Hongo, K.; Hiyoshi, M.; Tada, N.; Murono, K.; Nirei, T.; Kawai, K.; Sunami, E.; et al. Elevated Neutrophil to Lymphocyte Ratio Predicts Poor Prognosis in Advanced Colorectal Cancer Patients Receiving Oxaliplatin-Based Chemotherapy. Oncology 2012, 82, 261–268. [Google Scholar] [CrossRef]
- Jeong, J.H.; Lim, S.M.; Yun, J.Y.; Rhee, G.W.; Lim, J.Y.; Cho, J.Y.; Kim, Y.R. Comparison of two inflammation-based prognostic scores in patients with unresectable ad-vanced gastric cancer. Oncology 2012, 83, 292–299. [Google Scholar] [CrossRef]
- Hung, H.C.; Lee, J.C.; Wang, Y.C.; Cheng, C.H.; Wu, T.H.; Lee, C.F.; Wu, T.J.; Chou, H.S.; Chan, K.M.; Lee, W.C. Response Prediction in Immune Checkpoint Inhibitor Immunotherapy for Advanced Hepatocellular Carcinoma. Cancers 2021, 13, 1607. [Google Scholar] [CrossRef] [PubMed]
| Curative-Intent, n = 20 | Palliative-Intent, n = 24 | p-Value | |
|---|---|---|---|
| Basic conditions | |||
| Age, years old (≤65/>65) | 19 (95.0%)/1 (5.0%) | 19 (79.2%)/5 (20.8%) | 0.128 |
| Gender (Male/Female) | 8 (40.0%)/12 (60.0%) | 10 (41.7%)/14 (58.3%) | 0.911 |
| ECOG performance (0–1/2) | 17 (85.0%)/3 (15.0%) | 20 (83.3%)/4 (16.7%) | 0.880 |
| Co-morbidities/Histories | |||
| Smoke (No/Yes) | 17 (85.0%)/3 (15.0%) | 21 (87.5%)/3 (12.5%) | 0.810 |
| Alcohol use (No/Yes) | 18 (90.0%)/2 (10.0%) | 19 (79.2%)/5 (20.8%) | 0.328 |
| Diabetes (No/Yes) | 17 (85.0%)/3 (15.0%) | 20 (83.3%)/4 (16.7%) | 0.880 |
| Hypertension (No/Yes) | 17 (85.0%)/3 (15.0%) | 18 (75.0%)/6 (25.0%) | 0.413 |
| Viral hepatitis (No/Yes) | 19 (95.0%)/1 (5.0%) | 21 (87.5%)/3 (12.5%) | 0.389 |
| Co-malignancy (No/Yes) | 18 (90.0%)/2 (10.0%) | 21 (87.5%)/3 (12.5%) | 0.795 |
| Abdomen op Hx (No/Yes) | 13 (65.0%)7 (35.0%) | 12 (50.0%)/12 (50.0%) | 0.317 |
| Previous C/T (No/Yes) | 10 (50.0%)/10 (50.0%) | 11 (45.8%)/13 (54.2%) | 0.783 |
| Ascites (No/Yes) | 12 (60.0%)/8 (40.0%) | 11 (45.8%)/13 (54.2%) | 0.349 |
| Number of CT risks (≤2/>2) | 12 (60.0%)/8 (40.0%) | 7 (29.2%)/17 (70.8%) | 0.040 |
| Clinical symptoms (None/Mild/Severe) | 1 (5.0%)/18 (90.0%)/1 (5.0%) | 0 (0.0%)/16 (66.6%)/8 (33.3%) | 0.044 |
| Status (Primary/Recurrent) | 17 (85.0%)/3 (15.0%) | 13 (54.2%)/11 (45.8%) | 0.029 |
| Curative-Intent, n = 20 b | Palliative-Intent, n = 24 | p-Value | |
|---|---|---|---|
| Surgical characteristics | |||
| NIPS (No/Yes) | 16 (80.0%)/4 (20.0%) | 22 (91.7%)/2 (8.3%) | 0.261 |
| Number of organs resected | 5.7 ± 2.9 | 1.5 ± 1.8 | <0.001 |
| Blood transfusion (No/Yes) | 14 (70.0%)/6 (30.0%) | 16 (66.6%)/8 (33.3%) | 0.878 |
| HIPEC duration, mins | 105.9 ± 17.1 | 102.8 ± 19.7 | 0.612 |
| Inlet temperature, °C | 43.7 ± 0.9 | 43.9 ± 0.9 | 0.476 |
| Outlet temperature, °C | 41.9 ± 0.6 | 39.8 ± 0.9 | 0.375 |
| Highest intraoperative BT, °C | 38.6 ± 0.6 | 39.2 ± 1.5 | 0.180 |
| Tumor characteristics | |||
| PCI score PCI class (I/II/III/IV) | 11.0 ± 7.0 8 (40.0%)/8 (40.0%)/ 4 (20.0%)/0 (0.0%) | 23.5 ± 8.1 2 (8.3%)/4 (16.7%)/ 12 (50.0%)/6 (25.0%) | <0.001 0.002 |
| Differentiation (Well/ Moderate/Poor) a | 2 (10.0%)/1 (5.0%)/17 (85.0%) | 2 (8.3%)/1 (4.2%)/21 (87.5%) | 0.971 |
| NLR | 3.6 ± 3.2 | 12.8 ± 19.7 | <0.001 |
| NLR (≤4.4/>4.4) | 15 (75.0%)/5 (25.0%) | 10 (41.7%)/14 (58.3%) | 0.026 |
| Survival outcomes | |||
| Months after diagnosing M1 status | 17.2 ± 13.3 | 4.4 ± 4.1 | 0.023 |
| OS after, months (min-max.) | 14.7 ± 14.3 (1.3–51.2) | 4.4 ± 4.1 (0.5–20.7) | 0.001 |
| 6-month-OS rate | 79.4% | 26.4% | <0.001 |
| 12-month-OS rate | 56.3% | 17.6% | <0.001 |
| 36-month-OS rate | 38.6% | 0.0% | <0.001 |
| Univariate a | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| ECOG 0 1 2 | 1 1.48 6.09 | 0.60–3.65 2.05–18.06 | 0.395 0.001 | |||
| Preoperative NLR | ||||||
| ≤4.4 | 1 | 1 | ||||
| >4.4 | 3.22 | 1.42–7.30 | 0.005 | 3.70 | 1.55–8.79 | 0.003 |
| Histologic differentiation | ||||||
| Well | 1 | |||||
| Moderate | 2.57 | 0.93–7.09 | 0.068 | |||
| Poor | 3.07 | 1.14–8.22 | 0.026 | |||
| Number of CT risks | ||||||
| ≤2 | 1 | 1 | ||||
| >2 | 2.91 | 1.22–6.96 | 0.016 | 3.26 | 1.33–7.98 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, H.-C.; Hsu, P.-J.; Lee, C.-W.; Hsu, J.-T.; Wu, T.-J. Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Peritoneal Carcinomatosis: Additional Information Helps to Optimize Patient Selection before Surgery. Cancers 2023, 15, 2089. https://doi.org/10.3390/cancers15072089
Hung H-C, Hsu P-J, Lee C-W, Hsu J-T, Wu T-J. Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Peritoneal Carcinomatosis: Additional Information Helps to Optimize Patient Selection before Surgery. Cancers. 2023; 15(7):2089. https://doi.org/10.3390/cancers15072089
Chicago/Turabian StyleHung, Hao-Chien, Po-Jung Hsu, Chao-Wei Lee, Jun-Te Hsu, and Ting-Jung Wu. 2023. "Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Peritoneal Carcinomatosis: Additional Information Helps to Optimize Patient Selection before Surgery" Cancers 15, no. 7: 2089. https://doi.org/10.3390/cancers15072089
APA StyleHung, H.-C., Hsu, P.-J., Lee, C.-W., Hsu, J.-T., & Wu, T.-J. (2023). Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Peritoneal Carcinomatosis: Additional Information Helps to Optimize Patient Selection before Surgery. Cancers, 15(7), 2089. https://doi.org/10.3390/cancers15072089







