Proton or Carbon Ion Therapy for Skull Base Chordoma: Rationale and First Analysis of a Mono-Institutional Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Characteristics
2.2. Patient Simulation and Immobilization
2.3. Volume Definition
2.4. Treatment Planning
2.5. Dose Prescription
2.6. Follow-Up
2.7. Statistical Analysis
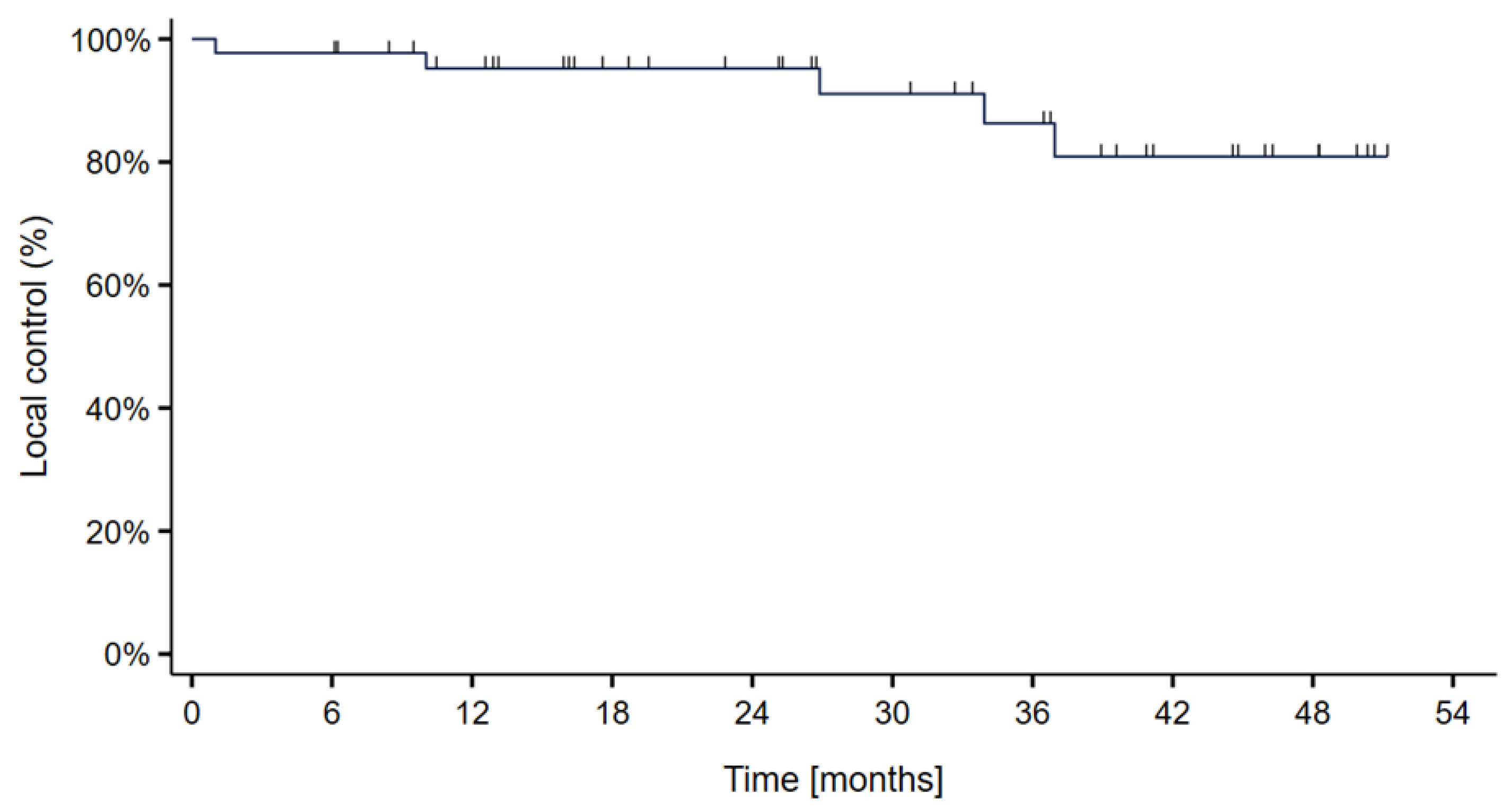
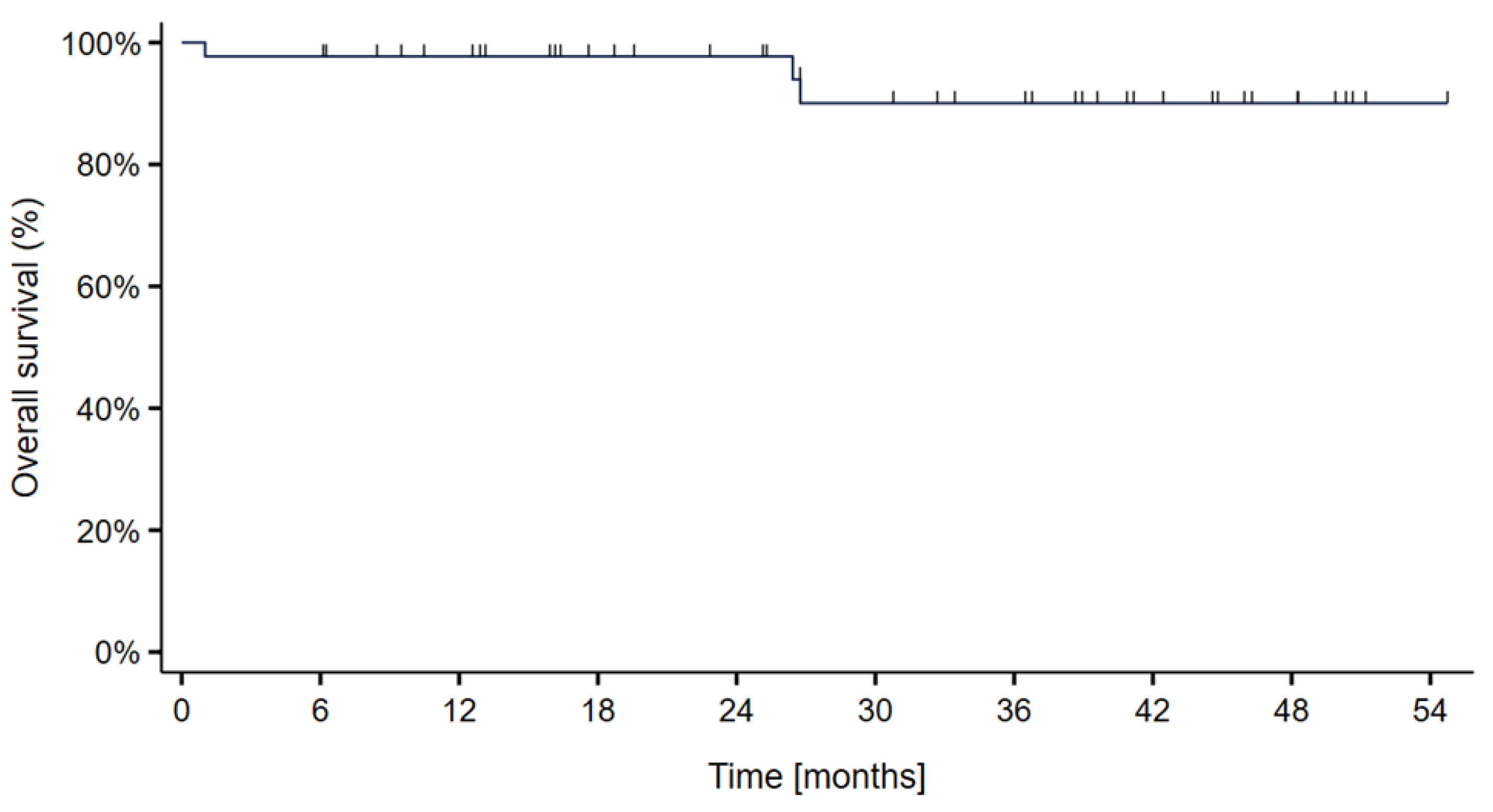
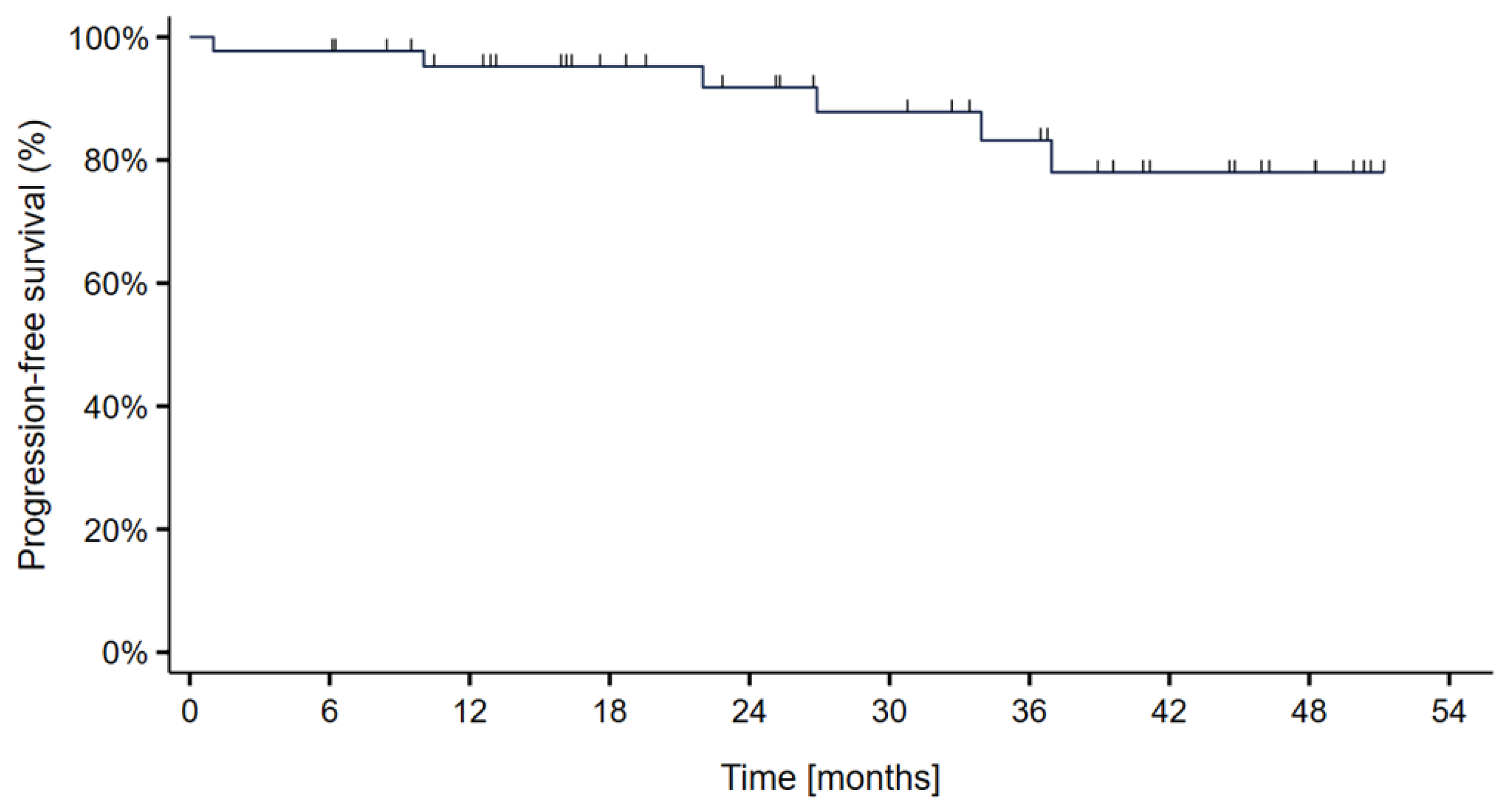
3. Results
4. Discussion
- (i.)
- Dose escalation with an inhomogeneous boost to the portion of GTV not abutting the brainstem or optic chiasm, and,
- (ii.)
- Escalation of the dose constraint to the brainstem without increased risk of brainstem toxicity. This could be achieved with LET (linear energy transfer) painting.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakker, S.H.; Jacobs, W.C.H.; Pondaag, W.; Gelderblom, H.; Nout, R.A.; Dijkstra, P.D.S.; Peul, W.C.; Vleggeert-Lankamp, C.L.A. Chordoma: A systematic review of the epidemiology and clinical prognostic factors predicting progression-free and overall survival. Eur. Spine J. 2018, 27, 3043–3058. [Google Scholar] [CrossRef]
- Gladstone, H.B.; Bailet, J.W.; Rowland, J.P. Chordoma of the oropharynx: An unusual presentation and review of the literature. Otolaryngol.–Head Neck Surg. 1998, 118, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.Z.; Chen, S.M.; Liu, J.F.; Huang, X.L.; Zhou, L. Paranasal sinuses chordoma in pediatric patient: A case report and literature review. Int. J. Pediatr. Otorhinolaryngol. 2005, 69, 1415–1418. [Google Scholar] [CrossRef]
- Lynn-Macrae, A.; Haines, G.K.; Altman, K.W., 3rd. Primary chordoma of the lateral nasal wall: Case report and review. Ear Nose Throat J. 2005, 84, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Khurram, S.A.; Biswas, D.; Fernando, M. A parapharyngeal soft tissue chordoma presenting with synchronous cervical lymph node metastasis: An unusual presentation. Head Neck Pathol. 2016, 10, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Walcott, B.P.; Nahed, B.V.; Mohyeldin, A.; Coumans, J.V.; Kahle, K.T.; Ferreira, M.J. Chordoma: Current concepts, management, and future directions. Lancet Oncol. 2012, 13, e69–e76. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lim, Y.C.; Song, M.H.; Seok, J.Y.; Lee, W.S.; Choi, E.C. Chondrosarcoma of the head and neck. Yonsei Med. J. 2005, 46, 228–232. [Google Scholar] [CrossRef]
- Bohman, L.E.; Koch, M.; Bailey, R.L.; Alonso-Basanta, M.; Lee, J.Y. Skull base chordoma and chondrosarcoma: Influence of clinical and demographic factors on prognosis: A SEER analysis. World Neurosurg. 2014, 82, 806–814. [Google Scholar] [CrossRef]
- Wasserman, J.K.; Gravel, D.; Purgina, B. Chordoma of the Head and Neck: A Review. Head Neck Pathol. 2018, 12, 261–268. [Google Scholar] [CrossRef]
- Fuchs, B.; Dickey, I.D.; Yaszemski, M.J.; Inwards, C.Y.; Sim, F.H. Operative management of sacral chordoma. J. Bone Jt. Surg. 2005, 87, 2211–2216. [Google Scholar]
- Bai, R.; Zhao, Z.; Wang, Y.; Zhao, W.; Wu, L.; Cui, S.; Guo, S.; Liang, C. Sacral and thoracic chordoma with pulmonary metastases: A case report and review of the literature. Mol. Clin. Oncol. 2021, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Labidi, M.; Watanabe, K.; Bouazza, S.; Bresson, D.; Bernat, A.L.; George, B.; Froelich, S. Clivus chordomas: A systematic review and meta-analysis of contemporary surgical management. J. Neurosurg. Sci. 2016, 60, 476–484. [Google Scholar] [PubMed]
- Makhdoomi, R.; Ramzan, A.; Khursheed, N.; Bhat, S.; Baba, K.; Mohsin, R.; Basharat, M.; Yameen, B.; Ahmad, R.; Iqbal, L.; et al. Clinicopathological characteristics of chordoma: An institutional experience and a review of the literature. Turk. Neurosurg. 2013, 23, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Hug, E.B.; Loredo, L.N.; Slater, J.D.; Devries, A.; Grove, R.I.; Schaefer, R.A.; Rosenberg, A.E.; Slater, J.M. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J. Neurosurg. 1999, 91, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, J.; Zhang, L.; Jia, G.; Tang, J.; Wang, L.; Wang, Z. Prognostic factors for long-term outcome of patients with surgical resection of skull base chordomas—106 cases review in one institution. Neurosurg. Rev. 2010, 33, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J.; McTiernan, A.; Cooper, N.; Wong, Y.K.; Francis, M.; Vernon, S.; Strauss, S.J. Incidence and survival of malignant bone sarcomas in England 1979–2007. Int. J. Cancer 2012, 131, E508–E517. [Google Scholar] [CrossRef]
- Jian, B.J.; Bloch, O.G.; Yang, I.; Han, S.J.; Aranda, D.; Parsa, A.T. A comprehensive analysis of intracranial chordoma and survival: A systematic review. Br. J. Neurosurg. 2011, 25, 446–453. [Google Scholar] [CrossRef]
- McDonald, M.W.; Linton, O.R.; Moore, M.G.; Ting, J.Y.; Cohen-Gadol, A.A.; Shah, M.V. Influence of residual tumor volume and radiation dose coverage in outcomes for clival chordoma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 304–311. [Google Scholar] [CrossRef]
- Weber, D.C.; Malyapa, R.; Albertini, F.; Bolsi, A.; Kliebsch, U.; Walser, M.; Pica, A.; Combescure, C.; Lomax, A.J.; Schneider, R. Long term outcomes of patients with skull-base low-grade chondrosarcoma and chordoma patients treated with pencil beam scanning proton therapy. Radiother. Oncol. 2016, 120, 169–174. [Google Scholar] [CrossRef]
- Munzenrider, J.E.; Liebsch, N.J. Proton therapy for tumors of the skull base. Strahlenther. Onkol. 1999, 175, 57–63. [Google Scholar] [CrossRef]
- Uhl, M.; Mattke, M.; Welzel, T.; Roeder, F.; Oelmann, J.; Habl, G.; Jensen, A.; Ellerbrock, M.; Jäkel, O.; Haberer, T.; et al. Highly effective treatment of skull base chordoma with carbon ion irradiation using a raster scan technique in 155 patients: First long-term results. Cancer 2014, 120, 3410–3417. [Google Scholar] [CrossRef] [PubMed]
- Hug, E.B. Review of skull base chordomas: Prognostic factors and long-term results of proton-beam radiotherapy. Neurosurg. Focus 2001, 10, E11. [Google Scholar] [CrossRef] [PubMed]
- Grün, R.; Friedrich, T.; Elsässer, T.; Krämer, M.; Zink, K.; Karger, C.P.; Durante, M.; Engenhart-Cabillic, R.; Scholz, M. Impact of enhancements in the local effect model (LEM) on the predicted RBE-weighted target dose distribution in carbon ion therapy. Phys. Med. Biol. 2012, 57, 7261–7274. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- US Department of Health and Human Services; National Institutes of Health National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010−06-14_QuickReference_5x7.pdf (accessed on 27 September 2022).
- Brito da Silva, H.; Straus, D.; Barber, J.K.; Rostomily, R.C.; Ferreira, M.; Sekhar, L.N., Jr. Cranial Chordoma: A New Preoperative Grading System. Neurosurgery 2018, 83, 403–415. [Google Scholar] [CrossRef]
- Hottinger, A.L.; Bojaxhiu, B.; Ahlhelm, F.; Walser, M.; Bachtiary, B.; Zepter, S.; Lomax, T.; Pica, A.; Weber, D.C. Prognostic impact of the “Sekhar grading system for cranial Chordomas” in patients treated with pencil beam scanning proton therapy: An institutional analysis. Radiat. Oncol. 2020, 15, 96. [Google Scholar] [CrossRef]
- Leah, P.; Dower, A.; Vescovi, C.; Mulcahy, M.; Al, K.D. Clinical experience of intracranial chordoma—A systematic review and meta-analysis of the literature. J. Clin. Neurosci. 2018, 53, 6–12. [Google Scholar] [CrossRef]
- Basler, L.; Poel, R.; Schröder, C.; Bolsi, A.; Lomax, A.; Tanadini-Lang, S.; Guckenberger, M.; Weber, D.C. Dosimetric analysis of local failures in skull-base chordoma and chondrosarcoma following pencil beam scanning proton therapy. Radiat. Oncol. 2020, 15, 266. [Google Scholar] [CrossRef]
- Ouyang, T.; Zhang, N.; Zhang, Y.; Jiao, J.; Ren, J.; Huang, T.; Chen, J. Clinical characteristics, immunohistochemistry, and outcomes of 77 patients with skull base chordomas. World Neurosurg. 2014, 81, 790–797. [Google Scholar] [CrossRef]
- Menezes, A.H. Clival and craniovertebral junction chordomas. World Neurosurg. 2014, 81, 690–692. [Google Scholar] [CrossRef]
- Chugh, R.; Tawbi, H.; Lucas, D.R.; Biermann, J.S.; Schuetze, S.M.; Baker, L.H. Chordoma: The nonsarcoma primary bone tumor. Oncologist 2007, 12, 1344–1350. [Google Scholar] [CrossRef]
- Casali, P.G.; Stacchiotti, S.; Sangalli, C.; Olmi, P.; Gronchi, A. Chordoma. Curr. Opin. Oncol. 2007, 19, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Koto, M.; Ikawa, H.; Kaneko, T.; Hagiwara, Y.; Hayashi, K.; Tsuji, H. Long-term outcomes of skull base chordoma treated with high-dose carbon-ion radiotherapy. Head Neck 2020, 42, 2607–2613. [Google Scholar] [CrossRef] [PubMed]
- Shirai, K.; Fukata, K.; Adachi, A.; Saitoh, J.I.; Musha, A.; Abe, T.; Kanai, T.; Kobayashi, D.; Shigeta, Y.; Yokoo, S.; et al. Dose-volume histogram analysis of brainstem necrosis in head and neck tumors treated using carbon-ion radiotherapy. Radiother. Oncol. 2017, 125, 36–40. [Google Scholar] [CrossRef]
- Fossati, P.; Perpar, A.; Stock, M.; Georg, P.; Carlino, A.; Gora, J.; Martino, G.; Hug, E.B. Carbon Ion Dose Constraints in the Head and Neck and Skull Base: Review of MedAustron Institutional Protocols. Int. J. Part. Ther. 2021, 8, 25–35. [Google Scholar] [CrossRef]
- Schulz-Ertner, D.; Nikoghosyan, A.; Didinger, B.; Debus, J. Carbon ion radiation therapy for chordomas and low grade chondrosarcomas—Current status of the clinical trials at GSI-. Radiother. Oncol. 2004, 73, S53–S56. [Google Scholar] [CrossRef] [PubMed]
- Iannalfi, A.; D’Ippolito, E.; Riva, G.; Molinelli, S.; Gandini, S.; Viselner, G.; Fiore, M.R.; Vischioni, B.; Vitolo, V.; Bonora, M.; et al. Proton and carbon ion radiotherapy in skull base chordomas: A prospective study based on a dual particle and a patient-customized treatment strategy. Neuro-Oncology 2020, 22, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
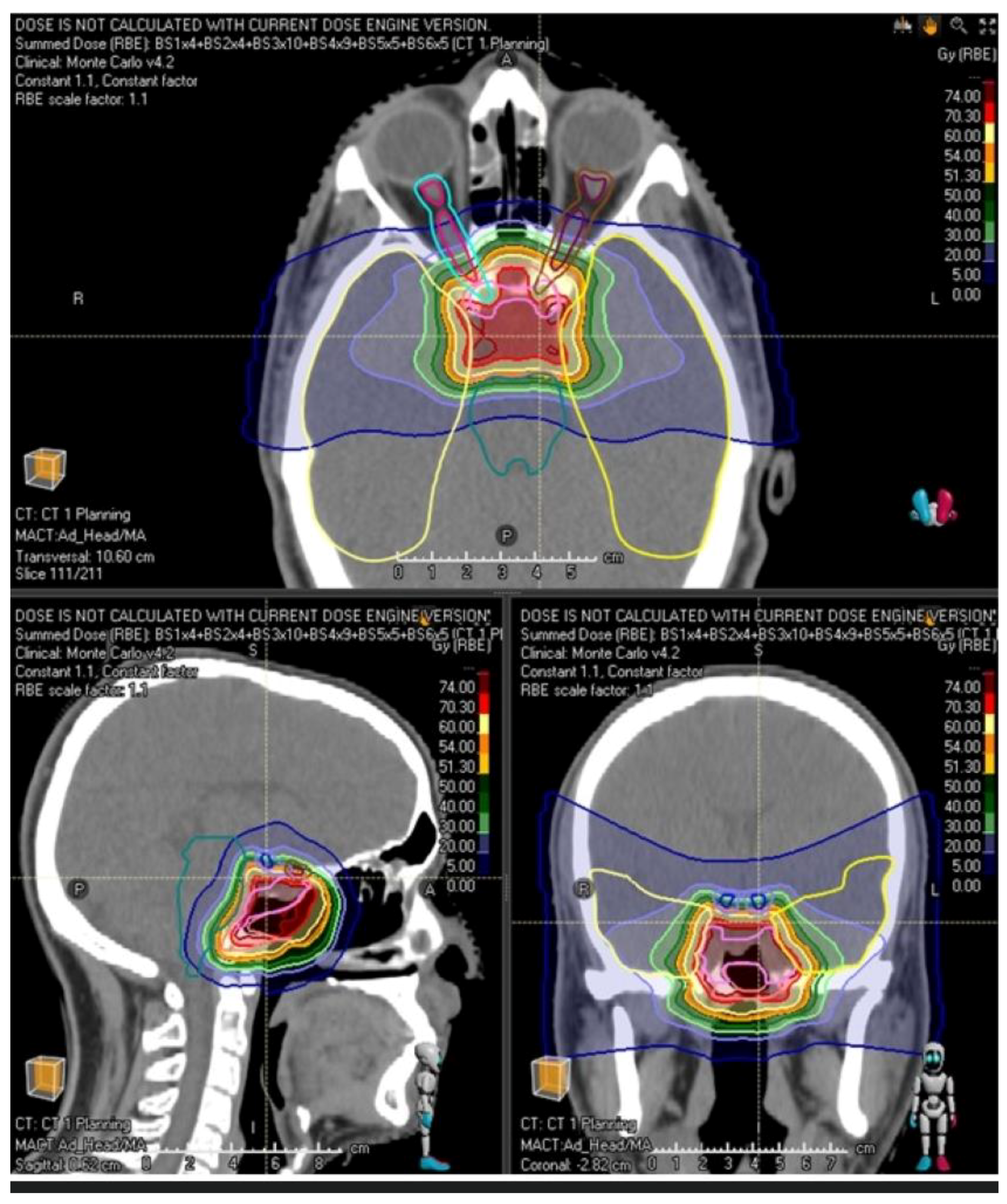
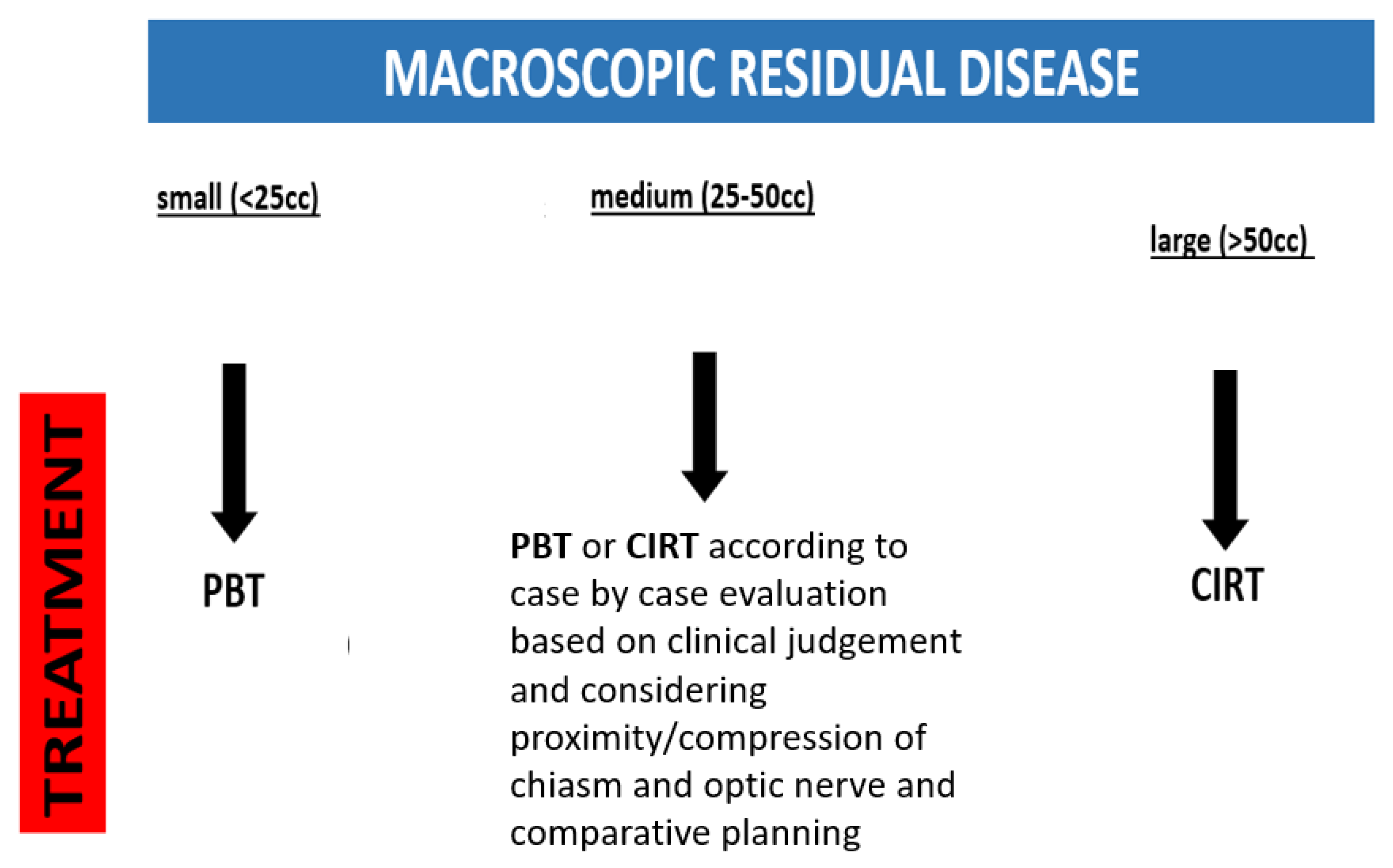
| Sex: | Total Patients: 44 |
|---|---|
| Male | 24/55% |
| Female | 20/45% |
| AGE: | Years |
| Average (range) | 47 (19–87) |
| KPS: Median (range) | 90% (60–100) |
| TUMOR SITE | |
| Upper clivus | 30/68% |
| Lower clivus | 14/32% |
| SURGERY: | |
| Subtotal tumor resection | 25/57% |
| Gross total resection | 9/21% |
| Biopsy | 5/12% |
| Decompression | 4/9% |
| TUMOR-RELATED SYMPTOMS: | 41/93% |
| Cranial nerve deficit: | 27/61% |
| - High: III, IV, VI (diplopia, ptosis) | 22/50% |
| - Middle: V, VII (trigeminal neuralgia, facial paralysis/weakness) | 3/7% |
| - Low: IX, X, XII (dysphagia and tongue lateral deviation) | 8/18% |
| GROSS TUMOR VOLUME median (range) cm3 | 28.1 (1.4–218.9) |
| Brainstem or optic pathway compression/abutment | |
| - yes | 25/57% |
| - no | 19/43% |
| Vascular involvement (A. carotis/A. basilaris) | |
| - yes | 2/5% |
| - no | 42/95% |
| Intradural invasion | |
| - yes | 3/7% |
| - no | 41/93% |
| Local Failures | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Tumor location | lower clivus | lower clivus | lower clivus | upper clivus | upper clivus |
| Tumor volume (ccm) | 76 | 49 | 101 | 242 | 80 |
| Vascular involvement | NO | NO | NO | NO | NO |
| Intradural invasion | NO | YES | NO | NO | NO |
| Brainstem/optic compression/abutment | YES | YES | NO | NO | YES |
| Type of particles | protons | protons | protons | protons | carbon |
| Radiation prescription dose (Gy RBE) | 76 | 75.6 | 78.2 | 78 | 66 |
| Dose to CTV1 95% | 63.39 | 61.44 | 68.69 | 69.26 | 59.93 |
| Dose to CTV1 98% | 60.27 | 59.67 | 64.95 | 61.76 | 57.25 |
| Dose to CTV2 95% | 68.78 | 73.46 | 70.96 | 74.24 | 64.88 |
| Dose to CTV2 98% | 65.01 | 70.54 | 67.58 | 71.16 | 59.03 |
| Time to recurrence (months) | 10 | 34 | 27 | 37 | 1 |
| Surgical resection | biopsy | decompression | subtotal | subtotal | biopsy |
| Radiation-induced brain tissue changes | NO | NO | NO | YES | NO |
| Alive at time of analysis: yes/no | NO | YES | YES | YES | NO |
| Follow up duration (months) | 26 | 55 | 39 | 43 | 1 |
| Tumor Volume | Local Recurrence | |
|---|---|---|
| no | yes | |
| <50 cm3 | 35 (89.7%) | 1 (20.0%) |
| >50 cm3 | 4 (10.3%) | 4 (80.0%) |
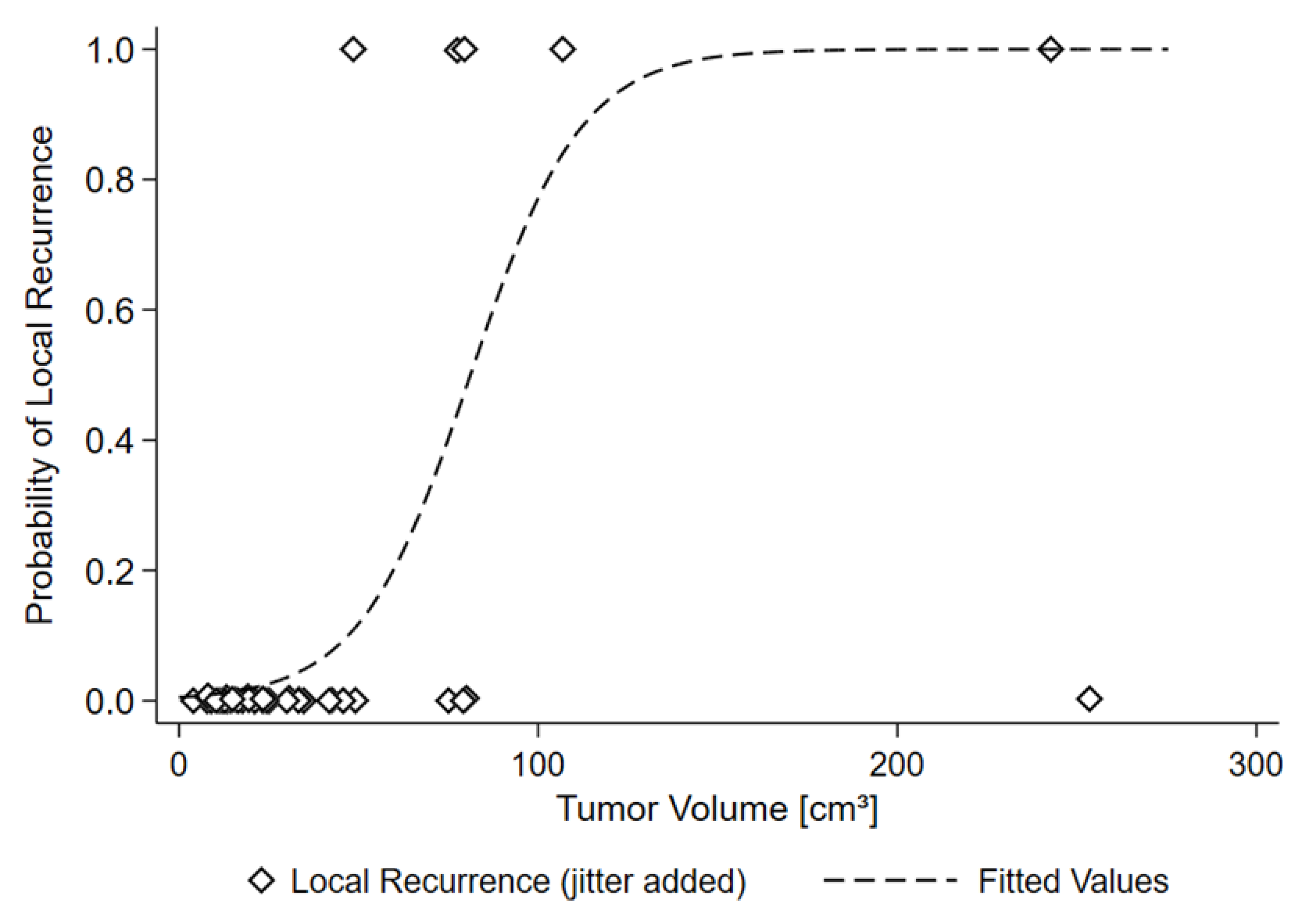
| RISK OF LOCAL RECURRENCE | ||
| 50 cm3 | 11.8% | |
| 81.1 cm3 | 50% | |
| Patient | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| GTV pre-OP volume (cc) | 48.64 | 79.63 | 8.31 | 24.64 | 80.46 |
| Surgery | biopsy | debulking | debulking | debulking | biopsy |
| Site | lower clivus | lower clivus | upperclivus | lowerclivus | upper and lower clivus |
| CTV1 95% (Gy) | 55.65 | 48.19 | 48.2 | 59.83 | 59.93 |
| CTV1 98% (Gy) | 51.84 | 45.65 | 46.3 | 58.08 | 57.25 |
| CTV2 95% (Gy) | 63.09 | 64.10 | 62.61 | 65.30 | 64.88 |
| CTV2 95% (Gy) | 59.65 | 62.41 | 58.05 | 64.39 | 59.03 |
| Abutment or compression of optic structures and/or brainstem | YES | YES | YES | NO | YES |
| Toxicity | Mucositis G1, Fatigue G1 | Tinnitus G1, Alopecia G2 | Headache G2, Vertigo G2 | Dysphagia G2, Mucositis G2 | Nausea G2, Appetite loss G2 |
| Radiation-induced brain tissue changes or brain necrosis | NO | NO | NO | NO | NO |
| Local control | YES | YES | YES | YES | NO |
| Follow up (months) | 13 | 33 | 11 | 11 | 1 |
| Pros | Cons | |
|---|---|---|
| PBT | Excellent result confirmed by multicentric series with large number of patients and long follow up | Larger spot size and less steep lateral penumbra possibly resulting in more undercoverage of target volumes in unfavorable cases |
| Well-known toxicity profile and well-validated dose constraints | Significant dependency on residual tumor volume | |
| CIRT | Smaller spot size and sharper penumbra potentially resulting in better target volume coverage also in unfavorable cases | More limited clinical experience available (fewer patients, shorter follow up) |
| Potentially less dependent on residual tumor volume | Less well-established dose constraints for OARs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tubin, S.; Fossati, P.; Mock, U.; Lütgendorf-Caucig, C.; Flechl, B.; Pelak, M.; Georg, P.; Fussl, C.; Carlino, A.; Stock, M.; et al. Proton or Carbon Ion Therapy for Skull Base Chordoma: Rationale and First Analysis of a Mono-Institutional Experience. Cancers 2023, 15, 2093. https://doi.org/10.3390/cancers15072093
Tubin S, Fossati P, Mock U, Lütgendorf-Caucig C, Flechl B, Pelak M, Georg P, Fussl C, Carlino A, Stock M, et al. Proton or Carbon Ion Therapy for Skull Base Chordoma: Rationale and First Analysis of a Mono-Institutional Experience. Cancers. 2023; 15(7):2093. https://doi.org/10.3390/cancers15072093
Chicago/Turabian StyleTubin, Slavisa, Piero Fossati, Ulrike Mock, Carola Lütgendorf-Caucig, Birgit Flechl, Maciej Pelak, Petra Georg, Christoph Fussl, Antonio Carlino, Markus Stock, and et al. 2023. "Proton or Carbon Ion Therapy for Skull Base Chordoma: Rationale and First Analysis of a Mono-Institutional Experience" Cancers 15, no. 7: 2093. https://doi.org/10.3390/cancers15072093
APA StyleTubin, S., Fossati, P., Mock, U., Lütgendorf-Caucig, C., Flechl, B., Pelak, M., Georg, P., Fussl, C., Carlino, A., Stock, M., & Hug, E. (2023). Proton or Carbon Ion Therapy for Skull Base Chordoma: Rationale and First Analysis of a Mono-Institutional Experience. Cancers, 15(7), 2093. https://doi.org/10.3390/cancers15072093






