Stereotactic Magnetic Resonance-Guided Adaptive and Non-Adaptive Radiotherapy on Combination MR-Linear Accelerators: Current Practice and Future Directions
Simple Summary
Abstract
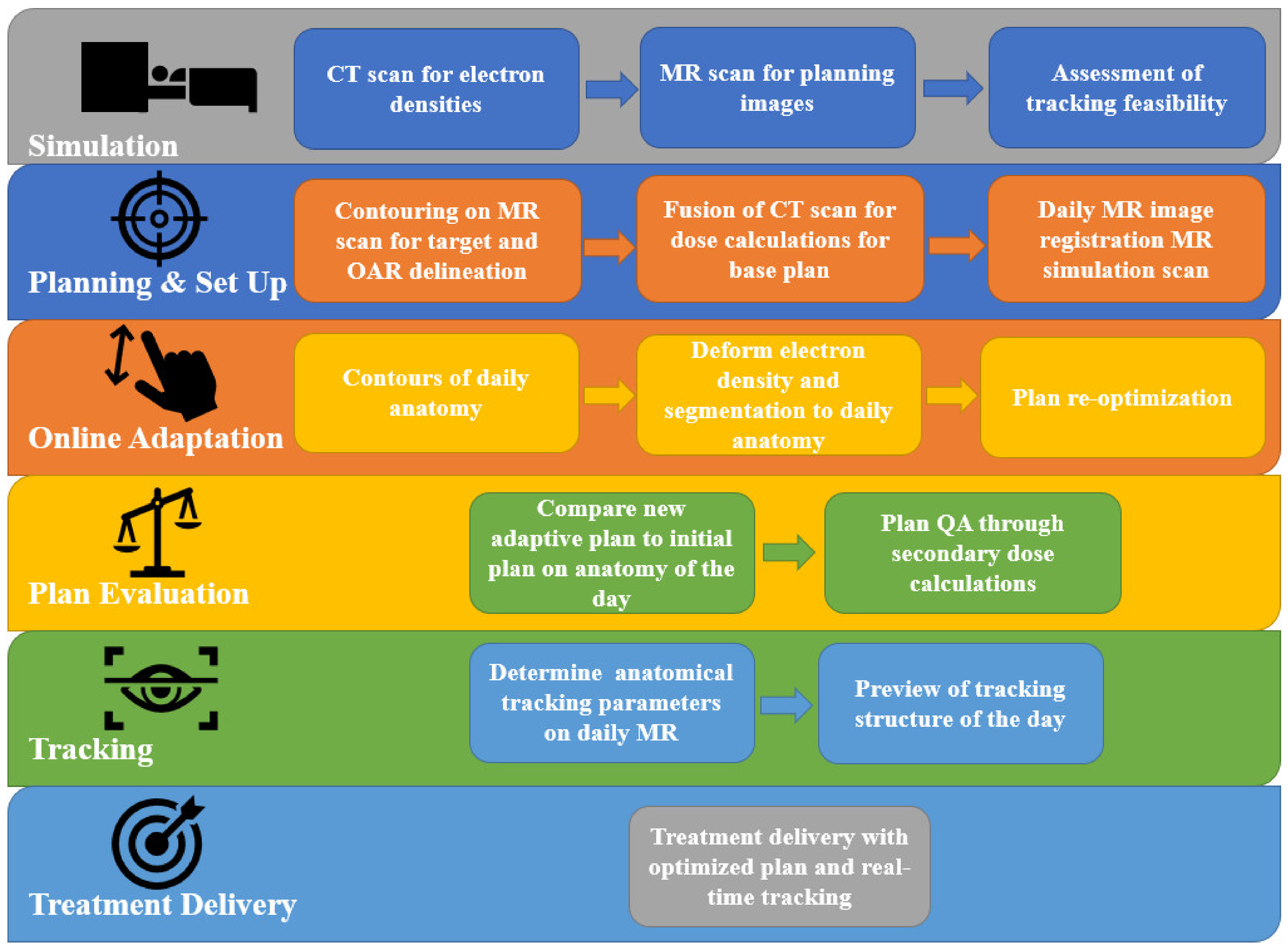
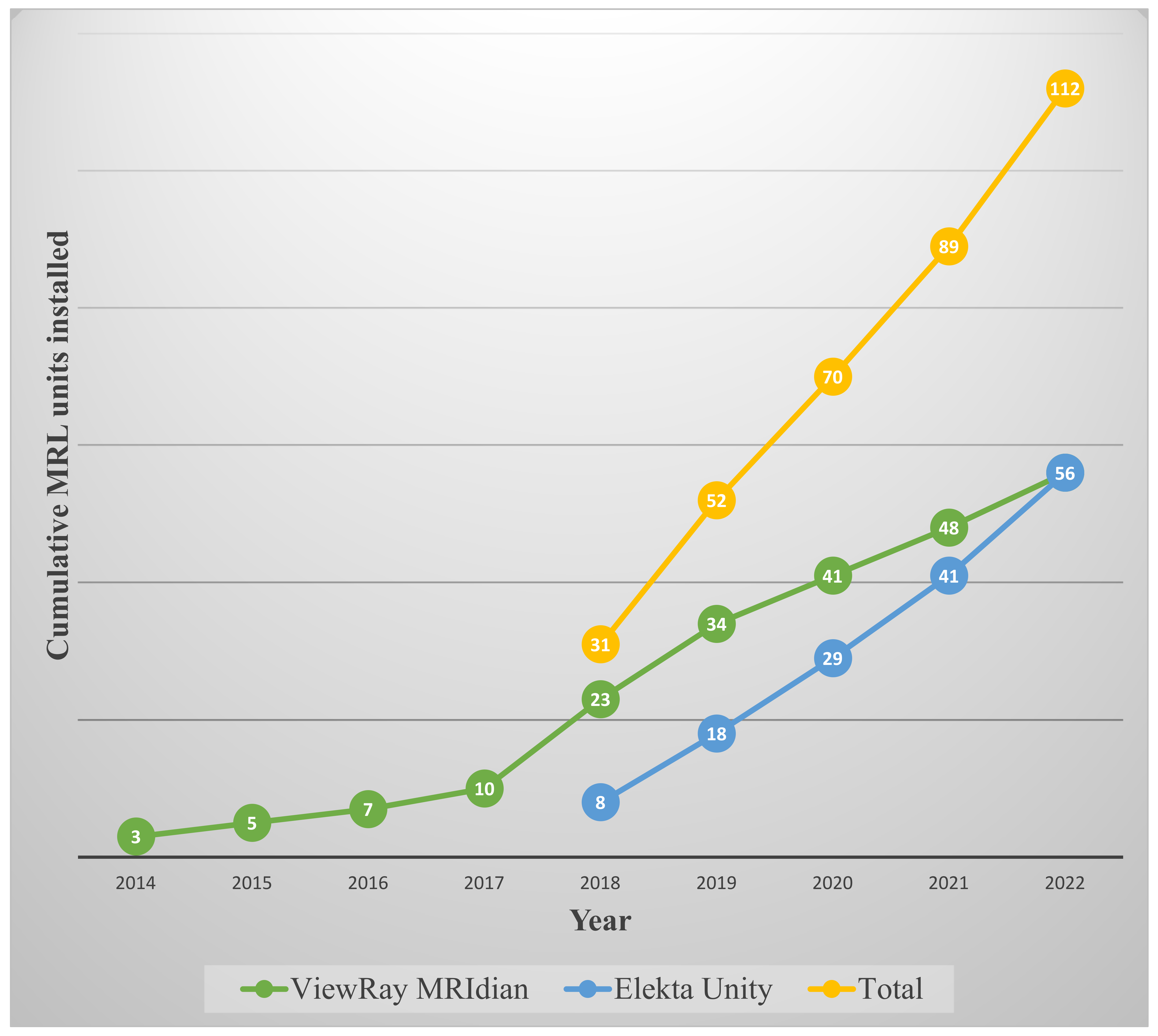
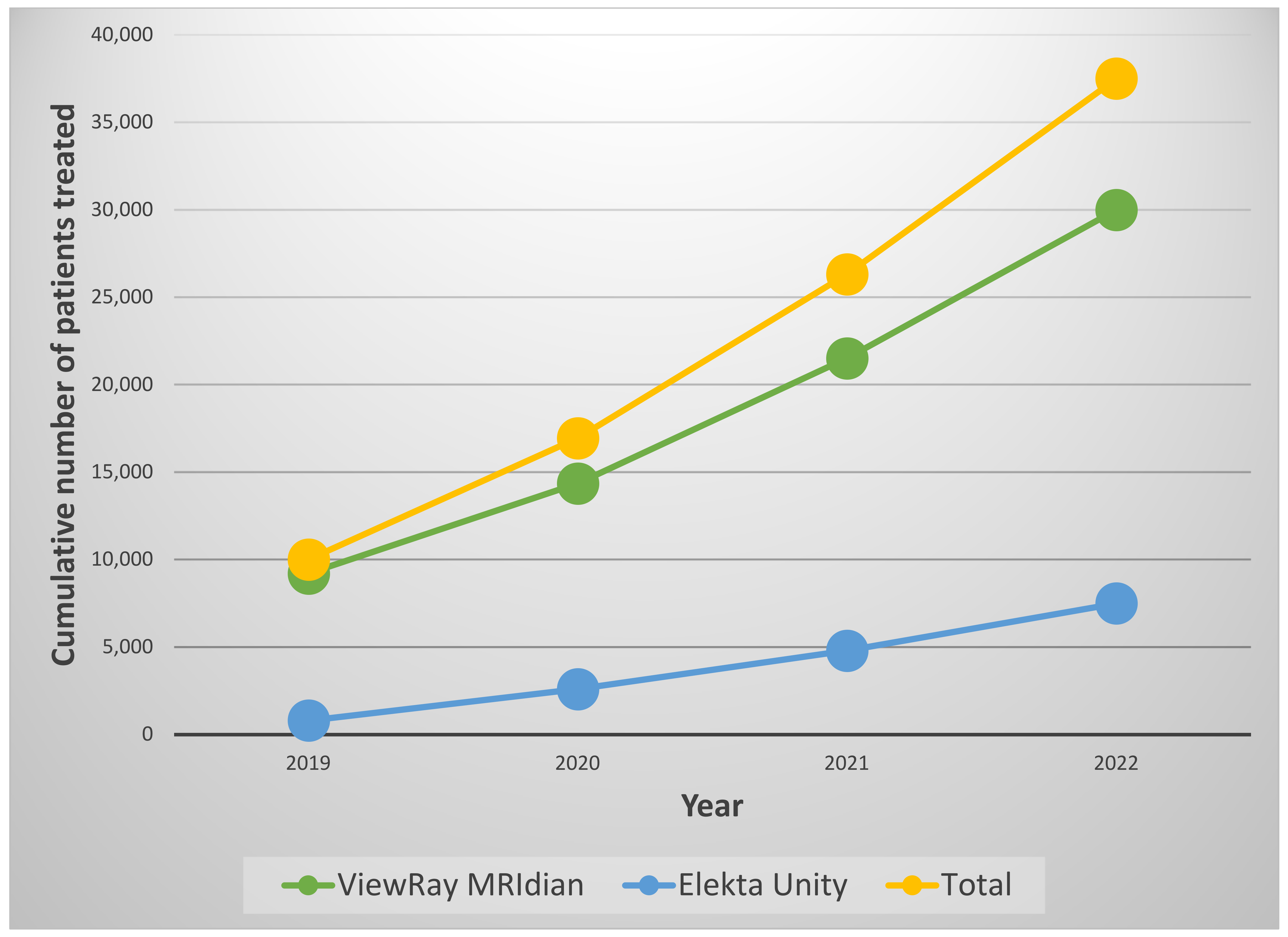
1. Introduction
2. SMART Clinical Applications
2.1. Head and Neck Cancer
2.2. Central and Ultra-Central Lung Tumors
2.3. Cardiac Metastases
2.4. Pancreatic Cancer
2.5. Liver Tumors
2.6. Adrenal Metastases
2.7. Kidney Cancer
2.8. Breast Cancer
2.9. Prostate Cancer
2.10. Spinal Metastases
2.11. Oligometastatic Cancer
2.12. Ablative Dose Re-Irradiation
3. Future Directions
MRL-Based Multiparametric MRI
4. Barriers and Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, M.; Gondhowiardjo, S.S.; Rosa, A.A.; Lievens, Y.; El-Haj, N.; Polo Rubio, J.A.; Prajogi, G.B.; Helgadottir, H.; Zubizarreta, E.; Meghzifene, A.; et al. Global Radiotherapy: Current Status and Future Directions-White Paper. JCO Glob. Oncol. 2021, 7, 827–842. [Google Scholar] [CrossRef] [PubMed]
- Atun, R.; Jaffray, D.A.; Barton, M.B.; Bray, F.; Baumann, M.; Vikram, B.; Hanna, T.P.; Knaul, F.M.; Lievens, Y.; Lui, T.Y.; et al. Expanding global access to radiotherapy. Lancet Oncol. 2015, 16, 1153–1186. [Google Scholar] [CrossRef]
- Onishi, H.; Shirato, H.; Nagata, Y.; Hiraoka, M.; Fujino, M.; Gomi, K.; Niibe, Y.; Karasawa, K.; Hayakawa, K.; Takai, Y.; et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: Updated results of 257 patients in a Japanese multi-institutional study. J. Thorac. Oncol. 2007, 2, S94–S100. [Google Scholar] [CrossRef]
- Jaffray, D.A. Image-guided radiotherapy: From current concept to future perspectives. Nat. Rev. Clin. Oncol. 2012, 9, 688–699. [Google Scholar] [CrossRef]
- Letourneau, D.; Martinez, A.A.; Lockman, D.; Yan, D.; Vargas, C.; Ivaldi, G.; Wong, J. Assessment of residual error for online cone-beam CT-guided treatment of prostate cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1239–1246. [Google Scholar] [CrossRef]
- Thomas, D.H.; Santhanam, A.; Kishan, A.U.; Cao, M.; Lamb, J.; Min, Y.; O’Connell, D.; Yang, Y.; Agazaryan, N.; Lee, P.; et al. Initial clinical observations of intra- and interfractional motion variation in MR-guided lung SBRT. Br. J. Radiol. 2018, 91, 20170522. [Google Scholar] [CrossRef] [PubMed]
- Byun, D.J.; Gorovets, D.J.; Jacobs, L.M.; Happersett, L.; Zhang, P.; Pei, X.; Burleson, S.; Zhang, Z.; Hunt, M.; McBride, S.; et al. Strict bladder filling and rectal emptying during prostate SBRT: Does it make a dosimetric or clinical difference? Radiat. Oncol. 2020, 15, 239. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Magallon-Baro, A.; Suker, M.; van Eijck, C.; Sharma, A.; Hoogeman, M.; Nuyttens, J. Pancreatic cancer treated with SBRT: Effect of anatomical interfraction variations on dose to organs at risk. Radiother. Oncol. 2019, 134, 67–73. [Google Scholar] [CrossRef]
- Noel, C.E.; Parikh, P.J.; Spencer, C.R.; Green, O.L.; Hu, Y.; Mutic, S.; Olsen, J.R. Comparison of onboard low-field magnetic resonance imaging versus onboard computed tomography for anatomy visualization in radiotherapy. Acta Oncol. 2015, 54, 1474–1482. [Google Scholar] [CrossRef]
- Casamassima, F.; Cavedon, C.; Francescon, P.; Stancanello, J.; Avanzo, M.; Cora, S.; Scalchi, P. Use of motion tracking in stereotactic body radiotherapy: Evaluation of uncertainty in off-target dose distribution and optimization strategies. Acta Oncol. 2006, 45, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, T.; Dervenoulas, G.; Politis, M. Advances in MRI Methodology. Int. Rev. Neurobiol. 2018, 141, 31–76. [Google Scholar] [CrossRef]
- Weygand, J.; Fuller, C.D.; Ibbott, G.S.; Mohamed, A.S.; Ding, Y.; Yang, J.; Hwang, K.P.; Wang, J. Spatial Precision in Magnetic Resonance Imaging-Guided Radiation Therapy: The Role of Geometric Distortion. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Lim Joon, D.; Nguyen, B.T.; Hiew, C.Y.; Esler, S.; Angus, D.; Chao, M.; Wada, M.; Quong, G.; Khoo, V. MRI scans significantly change target coverage decisions in radical radiotherapy for prostate cancer. J. Med. Imaging Radiat. Oncol. 2014, 58, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Dhermain, F. Radiotherapy of high-grade gliomas: Current standards and new concepts, innovations in imaging and radiotherapy, and new therapeutic approaches. Chin. J. Cancer 2014, 33, 16–24. [Google Scholar] [CrossRef]
- Lagendijk, J.J.; Raaymakers, B.W.; van Vulpen, M. The magnetic resonance imaging-linac system. Semin. Radiat. Oncol. 2014, 24, 207–209. [Google Scholar] [CrossRef]
- Acharya, S.; Fischer-Valuck, B.W.; Kashani, R.; Parikh, P.; Yang, D.; Zhao, T.; Green, O.; Wooten, O.; Li, H.H.; Hu, Y.; et al. Online Magnetic Resonance Image Guided Adaptive Radiation Therapy: First Clinical Applications. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Carr, H.Y. Steady-State Free Precession in Nuclear Magnetic Resonance. Phys. Rev. 1958, 112, 1693–1701. [Google Scholar] [CrossRef]
- De Mol van Otterloo, S.R.; Christodouleas, J.P.; Blezer, E.L.A.; Akhiat, H.; Brown, K.; Choudhury, A.; Eggert, D.; Erickson, B.A.; Daamen, L.A.; Faivre-Finn, C.; et al. Patterns of Care, Tolerability, and Safety of the First Cohort of Patients Treated on a Novel High-Field MR-Linac within the MOMENTUM Study: Initial Results from a Prospective Multi-Institutional Registry. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 867–875. [Google Scholar] [CrossRef] [PubMed]
- De Leon, J.; Woods, A.; Twentyman, T.; Meade, M.; Sproule, V.; Chandran, S.; Christiansen, J.; Kennedy, N.; Marney, M.; Barooshian, K.; et al. Analysis of data to Advance Personalised Therapy with MR-Linac (ADAPT-MRL). Clin. Transl. Radiat. Oncol. 2021, 31, 64–70. [Google Scholar] [CrossRef]
- Menard, C.; van der Heide, U.A. Introduction: Magnetic resonance imaging comes of age in radiation oncology. Semin. Radiat. Oncol. 2014, 24, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Mutic, S.; Dempsey, J.F. The ViewRay system: Magnetic resonance-guided and controlled radiotherapy. Semin. Radiat. Oncol. 2014, 24, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Wachowicz, K.; De Zanche, N.; Yip, E.; Volotovskyy, V.; Fallone, B.G. CNR considerations for rapid real-time MRI tumor tracking in radiotherapy hybrid devices: Effects of B0 field strength. Med. Phys. 2016, 43, 4903. [Google Scholar] [CrossRef]
- Hori, M.; Hagiwara, A.; Goto, M.; Wada, A.; Aoki, S. Low-Field Magnetic Resonance Imaging: Its History and Renaissance. Investig. Radiol. 2021, 56, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Shultz, D.C. High Field MR Guided Using the Unity Platform. In Proceedings of the 9th MR in RT Symposium, Los Angeles, CA, USA, 7 February 2023. [Google Scholar]
- Gillies, R.J.; Bhujwalla, Z.M.; Evelhoch, J.; Garwood, M.; Neeman, M.; Robinson, S.P.; Sotak, C.H.; Van Der Sanden, B. Applications of magnetic resonance in model systems: Tumor biology and physiology. Neoplasia 2000, 2, 139–151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tomaszewski, M.R.; Gillies, R.J. The Biological Meaning of Radiomic Features. Radiology 2021, 299, E256. [Google Scholar] [CrossRef]
- Tomaszewski, M.R.; Latifi, K.; Boyer, E.; Palm, R.F.; El Naqa, I.; Moros, E.G.; Hoffe, S.E.; Rosenberg, S.A.; Frakes, J.M.; Gillies, R.J. Delta radiomics analysis of Magnetic Resonance guided radiotherapy imaging data can enable treatment response prediction in pancreatic cancer. Radiat. Oncol. 2021, 16, 237. [Google Scholar] [CrossRef]
- Park, S.I.; Guenette, J.P.; Suh, C.H.; Hanna, G.J.; Chung, S.R.; Baek, J.H.; Lee, J.H.; Choi, Y.J. The diagnostic performance of CT and MRI for detecting extranodal extension in patients with head and neck squamous cell carcinoma: A systematic review and diagnostic meta-analysis. Eur. Radiol. 2021, 31, 2048–2061. [Google Scholar] [CrossRef] [PubMed]
- Sumi, M.; Nakamura, T. Extranodal spread in the neck: MRI detection on the basis of pixel-based time-signal intensity curve analysis. J. Magn. Reson. Imaging 2011, 33, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Boeke, S.; Monnich, D.; van Timmeren, J.E.; Balermpas, P. MR-Guided Radiotherapy for Head and Neck Cancer: Current Developments, Perspectives, and Challenges. Front. Oncol. 2021, 11, 616156. [Google Scholar] [CrossRef] [PubMed]
- Chuter, R.W.; Pollitt, A.; Whitehurst, P.; MacKay, R.I.; van Herk, M.; McWilliam, A. Assessing MR-linac radiotherapy robustness for anatomical changes in head and neck cancer. Phys. Med. Biol. 2018, 63, 125020. [Google Scholar] [CrossRef]
- Fischer-Valuck, B.W.; Henke, L.; Green, O.; Kashani, R.; Acharya, S.; Bradley, J.D.; Robinson, C.G.; Thomas, M.; Zoberi, I.; Thorstad, W.; et al. Two-and-a-half-year clinical experience with the world’s first magnetic resonance image guided radiation therapy system. Adv. Radiat. Oncol. 2017, 2, 485–493. [Google Scholar] [CrossRef]
- Chen, A.M.; Cao, M.; Hsu, S.; Lamb, J.; Mikaeilian, A.; Yang, Y.; Agazaryan, N.; Low, D.A.; Steinberg, M.L. Magnetic resonance imaging guided reirradiation of recurrent and second primary head and neck cancer. Adv. Radiat. Oncol. 2017, 2, 167–175. [Google Scholar] [CrossRef]
- McDonald, B.A.; Vedam, S.; Yang, J.; Wang, J.; Castillo, P.; Lee, B.; Sobremonte, A.; Ahmed, S.; Ding, Y.; Mohamed, A.S.R.; et al. Initial Feasibility and Clinical Implementation of Daily MR-Guided Adaptive Head and Neck Cancer Radiation Therapy on a 1.5T MR-Linac System: Prospective R-IDEAL 2a/2b Systematic Clinical Evaluation of Technical Innovation. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1606–1618. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Hsu, S.; Lamb, J.; Yang, Y.; Agazaryan, N.; Steinberg, M.L.; Low, D.A.; Cao, M. MRI-guided radiotherapy for head and neck cancer: Initial clinical experience. Clin. Transl. Oncol. 2018, 20, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.H.; Kim, M.S.; Chen, H.; Poon, I.; Husain, Z.; Eskander, A.; Boldt, G.; Louie, A.V.; Karam, I. Stereotactic Radiation Therapy for De Novo Head and Neck Cancers: A Systematic Review and Meta-Analysis. Adv. Radiat. Oncol. 2021, 6, 100628. [Google Scholar] [CrossRef]
- Strom, T.; Wishka, C.; Caudell, J.J. Stereotactic Body Radiotherapy for Recurrent Unresectable Head and Neck Cancers. Cancer Control 2016, 23, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, N.T.; Xu-Welliver, M.; Williams, T.M. Stereotactic body radiation therapy (SBRT) for early stage non-small cell lung cancer (NSCLC): Contemporary insights and advances. J. Thorac. Dis. 2018, 10, S2451–S2464. [Google Scholar] [CrossRef]
- Wulf, J.; Hädinger, U.; Oppitz, U.; Thiele, W.; Ness-Dourdoumas, R.; Flentje, M. Stereotactic radiotherapy of targets in the lung and liver. Strahlenther. Onkol. 2001, 177, 645–655. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Videtic, G.M.M.; Donington, J.; Giuliani, M.; Heinzerling, J.; Karas, T.Z.; Kelsey, C.R.; Lally, B.E.; Latzka, K.; Lo, S.S.; Moghanaki, D.; et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract. Radiat. Oncol. 2017, 7, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, R.; Paulus, R.; Galvin, J.; Michalski, J.; Straube, W.; Bradley, J.; Fakiris, A.; Bezjak, A.; Videtic, G.; Johnstone, D.; et al. Stereotactic Body Radiation Therapy for Inoperable Early Stage Lung Cancer. JAMA 2010, 303, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, R.D.; Hu, C.; Michalski, J.M.; Bradley, J.C.; Galvin, J.; Johnstone, D.W.; Choy, H. Long-term Results of Stereotactic Body Radiation Therapy in Medically Inoperable Stage I Non–Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 1287–1288. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, R.; McGarry, R.; Yiannoutsos, C.; Papiez, L.; Tudor, K.; DeLuca, J.; Ewing, M.; Abdulrahman, R.; DesRosiers, C.; Williams, M.; et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J. Clin. Oncol. 2006, 24, 4833–4839. [Google Scholar] [CrossRef] [PubMed]
- Bezjak, A.; Paulus, R.; Gaspar, L.E.; Timmerman, R.D.; Straube, W.L.; Ryan, W.F.; Garces, Y.I.; Pu, A.T.; Singh, A.K.; Videtic, G.M.; et al. Safety and Efficacy of a Five-Fraction Stereotactic Body Radiotherapy Schedule for Centrally Located Non-Small-Cell Lung Cancer: NRG Oncology/RTOG 0813 Trial. J. Clin. Oncol. 2019, 37, 1316–1325. [Google Scholar] [CrossRef]
- Fakiris, A.J.; McGarry, R.C.; Yiannoutsos, C.T.; Papiez, L.; Williams, M.; Henderson, M.A.; Timmerman, R. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: Four-year results of a prospective phase II study. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.A.; Tang, C.; Binkley, M.S.; Jin, M.; Wynne, J.F.; von Eyben, R.; Hara, W.Y.; Trakul, N.; Loo, B.W., Jr.; Diehn, M. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer 2015, 89, 50–56. [Google Scholar] [CrossRef]
- Lindberg, K.; Grozman, V.; Karlsson, K.; Lindberg, S.; Lax, I.; Wersall, P.; Persson, G.F.; Josipovic, M.; Khalil, A.A.; Moeller, D.S.; et al. The HILUS-Trial-a Prospective Nordic Multicenter Phase 2 Study of Ultracentral Lung Tumors Treated With Stereotactic Body Radiotherapy. J. Thorac. Oncol. 2021, 16, 1200–1210. [Google Scholar] [CrossRef]
- Henke, L.; Kashani, R.; Yang, D.; Zhao, T.; Green, O.; Olsen, L.; Rodriguez, V.; Wooten, H.O.; Li, H.H.; Hu, Y.; et al. Simulated Online Adaptive Magnetic Resonance-Guided Stereotactic Body Radiation Therapy for the Treatment of Oligometastatic Disease of the Abdomen and Central Thorax: Characterization of Potential Advantages. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 1078–1086. [Google Scholar] [CrossRef]
- Regnery, S.; Buchele, C.; Weykamp, F.; Pohl, M.; Hoegen, P.; Eichkorn, T.; Held, T.; Ristau, J.; Rippke, C.; Konig, L.; et al. Adaptive MR-Guided Stereotactic Radiotherapy is Beneficial for Ablative Treatment of Lung Tumors in High-Risk Locations. Front. Oncol. 2021, 11, 757031. [Google Scholar] [CrossRef] [PubMed]
- Ligtenberg, H.; Hackett, S.L.; Merckel, L.G.; Snoeren, L.; Kontaxis, C.; Zachiu, C.; Bol, G.H.; Verhoeff, J.J.C.; Fast, M.F. Towards mid-position based Stereotactic Body Radiation Therapy using online magnetic resonance imaging guidance for central lung tumours. Phys. Imaging Radiat. Oncol. 2022, 23, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Finazzi, T.; Haasbeek, C.J.A.; Spoelstra, F.O.B.; Palacios, M.A.; Admiraal, M.A.; Bruynzeel, A.M.E.; Slotman, B.J.; Lagerwaard, F.J.; Senan, S. Clinical Outcomes of Stereotactic MR-Guided Adaptive Radiation Therapy for High-Risk Lung Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Henke, L.E.; Olsen, J.R.; Contreras, J.A.; Curcuru, A.; DeWees, T.A.; Green, O.L.; Michalski, J.; Mutic, S.; Roach, M.C.; Bradley, J.D.; et al. Stereotactic MR-Guided Online Adaptive Radiation Therapy (SMART) for Ultracentral Thorax Malignancies: Results of a Phase 1 Trial. Adv. Radiat. Oncol. 2019, 4, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.M.; Sim, A.J.; Feygelman, V.; Latifi, K.; Rosenberg, S.A. Adaptive hypofractionted and stereotactic body radiotherapy for lung tumors with real-time MRI guidance. Front. Oncol. 2023, 13, 1061854. [Google Scholar] [CrossRef]
- Sandoval, M.L.; Sim, A.J.; Bryant, J.M.; Bhandari, M.; Wuthrick, E.J.; Perez, B.A.; Dilling, T.J.; Redler, G.; Andreozzi, J.; Nardella, L.; et al. MR-Guided SBRT/Hypofractionated RT for Metastatic and Primary Central and Ultracentral Lung Lesions. JTO Clin. Res. Rep. 2023, 100488. [Google Scholar] [CrossRef]
- Reardon, M.J.; Walkes, J.C.; Benjamin, R. Therapy insight: Malignant primary cardiac tumors. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 548–553. [Google Scholar] [CrossRef]
- Hudzik, B.; Miszalski-Jamka, K.; Glowacki, J.; Lekston, A.; Gierlotka, M.; Zembala, M.; Polonski, L.; Gasior, M. Malignant tumors of the heart. Cancer Epidemiol. 2015, 39, 665–672. [Google Scholar] [CrossRef]
- Goldberg, A.D.; Blankstein, R.; Padera, R.F. Tumors metastatic to the heart. Circulation 2013, 128, 1790–1794. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Murphy, M.C.; Sweeney, M.S.; Putnam, J.B., Jr.; Walker, W.E.; Frazier, O.H.; Ott, D.A.; Cooley, D.A. Surgical treatment of cardiac tumors: A 25-year experience. Ann. Thorac. Surg. 1990, 49, 612–617; discussion 617–618. [Google Scholar] [CrossRef]
- Cham, W.C.; Freiman, A.H.; Carstens, P.H.; Chu, F.C. Radiation therapy of cardiac and pericardial metastases. Radiology 1975, 114, 701–704. [Google Scholar] [CrossRef]
- Bonomo, P.; Livi, L.; Rampini, A.; Meattini, I.; Agresti, B.; Simontacchi, G.; Paiar, F.; Mangoni, M.; Bonucci, I.; Greto, D.; et al. Stereotactic body radiotherapy for cardiac and paracardiac metastases: University of Florence experience. Radiol. Med. 2013, 118, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Sim, A.J.; Palm, R.F.; DeLozier, K.B.; Feygelman, V.; Latifi, K.; Redler, G.; Washington, I.R.; Wuthrick, E.J.; Rosenberg, S.A. MR-guided stereotactic body radiation therapy for intracardiac and pericardial metastases. Clin. Transl. Radiat. Oncol. 2020, 25, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.H.G.; Shi, Q.; Meyers, J.P.; Herman, J.M.; Choung, M.; Wolpin, B.M.; Ahmad, S.; Marsh, R.d.W.; Schwartz, L.H.; Behr, S.; et al. Alliance A021501: Preoperative mFOLFIRINOX or mFOLFIRINOX plus hypofractionated radiation therapy (RT) for borderline resectable (BR) adenocarcinoma of the pancreas. J. Clin. Oncol. 2021, 39, 377. [Google Scholar] [CrossRef]
- Chang, D.T.; Schellenberg, D.; Shen, J.; Kim, J.; Goodman, K.A.; Fisher, G.A.; Ford, J.M.; Desser, T.; Quon, A.; Koong, A.C. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 2009, 115, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Hammel, P.; Huguet, F.; van Laethem, J.L.; Goldstein, D.; Glimelius, B.; Artru, P.; Borbath, I.; Bouche, O.; Shannon, J.; Andre, T.; et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients with Locally Advanced Pancreatic Cancer Controlled after 4 Months of Gemcitabine with or without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016, 315, 1844–1853. [Google Scholar] [CrossRef]
- Koong, A.C.; Le, Q.T.; Ho, A.; Fong, B.; Fisher, G.; Cho, C.; Ford, J.; Poen, J.; Gibbs, I.C.; Mehta, V.K.; et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 1017–1021. [Google Scholar] [CrossRef]
- Koong, A.C.; Christofferson, E.; Le, Q.T.; Goodman, K.A.; Ho, A.; Kuo, T.; Ford, J.M.; Fisher, G.A.; Greco, R.; Norton, J.; et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 320–323. [Google Scholar] [CrossRef]
- Hoyer, M.; Roed, H.; Sengelov, L.; Traberg, A.; Ohlhuis, L.; Pedersen, J.; Nellemann, H.; Kiil Berthelsen, A.; Eberholst, F.; Engelholm, S.A.; et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother. Oncol. 2005, 76, 48–53. [Google Scholar] [CrossRef]
- Schellenberg, D.; Goodman, K.A.; Lee, F.; Chang, S.; Kuo, T.; Ford, J.M.; Fisher, G.A.; Quon, A.; Desser, T.S.; Norton, J.; et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 678–686. [Google Scholar] [CrossRef]
- Schellenberg, D.; Kim, J.; Christman-Skieller, C.; Chun, C.L.; Columbo, L.A.; Ford, J.M.; Fisher, G.A.; Kunz, P.L.; Van Dam, J.; Quon, A.; et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 181–188. [Google Scholar] [CrossRef]
- Zhu, X.; Ju, X.; Cao, Y.; Shen, Y.; Cao, F.; Qing, S.; Fang, F.; Jia, Z.; Zhang, H. Patterns of Local Failure after Stereotactic Body Radiation Therapy and Sequential Chemotherapy as Initial Treatment for Pancreatic Cancer: Implications of Target Volume Design. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Bernard, V.; Herman, J.M. Pancreas SBRT: Who, What, When, Where, and How. Pract. Radiat. Oncol. 2020, 10, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Arcelli, A.; Guido, A.; Buwenge, M.; Simoni, N.; Mazzarotto, R.; Macchia, G.; Deodato, F.; Cilla, S.; Bonomo, P.; Scotti, V.; et al. Higher Biologically Effective Dose Predicts Survival in SBRT of Pancreatic Cancer: A Multicentric Analysis (PAULA-1). Anticancer Res. 2020, 40, 465–472. [Google Scholar] [CrossRef]
- Krishnan, S.; Chadha, A.S.; Suh, Y.; Chen, H.C.; Rao, A.; Das, P.; Minsky, B.D.; Mahmood, U.; Delclos, M.E.; Sawakuchi, G.O.; et al. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.J.; Prezzano, K.M.; Hermann, G.M.; Singh, A.K. Dose escalation of radiation therapy with or without induction chemotherapy for unresectable locally advanced pancreatic cancer. Radiat. Oncol. 2018, 13, 214. [Google Scholar] [CrossRef]
- Reyngold, M.; O’Reilly, E.M.; Varghese, A.M.; Fiasconaro, M.; Zinovoy, M.; Romesser, P.B.; Wu, A.; Hajj, C.; Cuaron, J.J.; Tuli, R.; et al. Association of Ablative Radiation Therapy with Survival Among Patients with Inoperable Pancreatic Cancer. JAMA Oncol. 2021, 7, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Tchelebi, L.T.; Zaorsky, N.G.; Rosenberg, J.C.; Sharma, N.K.; Tuanquin, L.C.; Mackley, H.B.; Ellis, R.J. Reducing the Toxicity of Radiotherapy for Pancreatic Cancer With Magnetic Resonance-guided Radiotherapy. Toxicol. Sci. 2020, 175, 19–23. [Google Scholar] [CrossRef]
- Bohoudi, O.; Bruynzeel, A.M.E.; Senan, S.; Cuijpers, J.P.; Slotman, B.J.; Lagerwaard, F.J.; Palacios, M.A. Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer. Radiother. Oncol. 2017, 125, 439–444. [Google Scholar] [CrossRef]
- Rudra, S.; Jiang, N.; Rosenberg, S.A.; Olsen, J.R.; Roach, M.C.; Wan, L.; Portelance, L.; Mellon, E.A.; Bruynzeel, A.; Lagerwaard, F.; et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019, 8, 2123–2132. [Google Scholar] [CrossRef]
- Chuong, M.D.; Bryant, J.; Mittauer, K.E.; Hall, M.; Kotecha, R.; Alvarez, D.; Romaguera, T.; Rubens, M.; Adamson, S.; Godley, A.; et al. Ablative 5-Fraction Stereotactic Magnetic Resonance-Guided Radiation Therapy with On-Table Adaptive Replanning and Elective Nodal Irradiation for Inoperable Pancreas Cancer. Pract. Radiat. Oncol. 2021, 11, 134–147. [Google Scholar] [CrossRef]
- Hassanzadeh, C.; Rudra, S.; Bommireddy, A.; Hawkins, W.G.; Wang-Gillam, A.; Fields, R.C.; Cai, B.; Park, J.; Green, O.; Roach, M.; et al. Ablative Five-Fraction Stereotactic Body Radiation Therapy for Inoperable Pancreatic Cancer Using Online MR-Guided Adaptation. Adv. Radiat. Oncol. 2021, 6, 100506. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.; Palm, R.F.; Herrera, R.; Rubens, M.; Hoffe, S.E.; Kim, D.W.; Kaiser, A.; Ucar, A.; Fleming, J.; De Zarraga, F.; et al. Multi-Institutional Outcomes of Patients Aged 75 years and Older with Pancreatic Ductal Adenocarcinoma Treated with 5-Fraction Ablative Stereotactic Magnetic Resonance Image-Guided Adaptive Radiation Therapy (A-SMART). Cancer Control 2023, 30, 10732748221150228. [Google Scholar] [CrossRef] [PubMed]
- Heerkens, H.D.; van Vulpen, M.; Erickson, B.; Reerink, O.; Intven, M.P.; van den Berg, C.A.; Molenaar, I.Q.; Vleggaar, F.P.; Meijer, G.J. MRI guided stereotactic radiotherapy for locally advanced pancreatic cancer. Br. J. Radiol. 2018, 91, 20170563. [Google Scholar] [CrossRef] [PubMed]
- Henke, L.; Kashani, R.; Robinson, C.; Curcuru, A.; DeWees, T.; Bradley, J.; Green, O.; Michalski, J.; Mutic, S.; Parikh, P.; et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother. Oncol. 2018, 126, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.A.; Straza, M.W.; Chen, X.; Mickevicius, N.; Erickson, B.; Schultz, C.; Awan, M.; Ahunbay, E.; Li, X.A.; Paulson, E.S. Initial clinical experience of Stereotactic Body Radiation Therapy (SBRT) for liver metastases, primary liver malignancy, and pancreatic cancer with 4D-MRI based online adaptation and real-time MRI monitoring using a 1.5 Tesla MR-Linac. PLoS ONE 2020, 15, e0236570. [Google Scholar] [CrossRef]
- Bryant, J.M.; Palm, R.F.; Liveringhouse, C.; Boyer, E.; Hodul, P.; Malafa, M.; Denbo, J.; Kim, D.; Carballido, E.; Fleming, J.B.; et al. Surgical and Pathologic Outcomes of Pancreatic Adenocarcinoma (PA) After Preoperative Ablative Stereotactic Magnetic Resonance Image Guided Adaptive Radiation Therapy (A-SMART). Adv. Radiat. Oncol. 2022, 7, 101045. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.J.; Lee, P.; Low, D.; Kim, J.; Mittauer, K.E.; Bassetti, M.F.; Glide-Hurst, C.; Raldow, A.; Yang, Y.; Portelance, L.; et al. Stereotactic MR-Guided On-Table Adaptive Radiation Therapy (SMART) for Patients with Borderline or Locally Advanced Pancreatic Cancer: Primary Endpoint Outcomes of a Prospective Phase II Multi-Center International Trial. Int. J. Radiat. Oncol. 2022, 114, 1062–1063. [Google Scholar] [CrossRef]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Borad, M.; Brown, D.; Burgoyne, A.; et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 541–565. [Google Scholar] [CrossRef]
- Adam, R.; Chiche, L.; Aloia, T.; Elias, D.; Salmon, R.; Rivoire, M.; Jaeck, D.; Saric, J.; Le Treut, Y.P.; Belghiti, J.; et al. Hepatic resection for noncolorectal nonendocrine liver metastases: Analysis of 1452 patients and development of a prognostic model. Ann. Surg. 2006, 244, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef]
- Smith, J.J.; D’Angelica, M.I. Surgical management of hepatic metastases of colorectal cancer. Hematol. Oncol. Clin. N. Am. 2015, 29, 61–84. [Google Scholar] [CrossRef]
- Ruers, T.; Van Coevorden, F.; Punt, C.J.; Pierie, J.E.; Borel-Rinkes, I.; Ledermann, J.A.; Poston, G.; Bechstein, W.; Lentz, M.A.; Mauer, M.; et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. JNCI J. Natl. Cancer Inst. 2017, 109, djx015. [Google Scholar] [CrossRef]
- Rim, C.H.; Lee, J.S.; Kim, S.Y.; Seong, J. Comparison of radiofrequency ablation and ablative external radiotherapy for the treatment of intrahepatic malignancies: A hybrid meta-analysis. JHEP Rep. 2023, 5, 100594. [Google Scholar] [CrossRef] [PubMed]
- Dawson, L.A.; Winter, K.A.; Knox, J.J.; Zhu, A.X.; Krishnan, S.; Guha, C.; Kachnic, L.A.; Gillin, M.; Hong, T.S.; Craig, T.; et al. NRG/RTOG 1112: Randomized Phase III Study of Sorafenib vs. Stereotactic Body Radiation Therapy (SBRT) Followed by Sorafenib in Hepatocellular Carcinoma (HCC) (NCT01730937). In Proceedings of the ASTRO’s 64th Annual Meeting, San Antonio, TX, USA, 23–26 October 2022; p. 1057. [Google Scholar]
- Ohri, N.; Tome, W.A.; Mendez Romero, A.; Miften, M.; Ten Haken, R.K.; Dawson, L.A.; Grimm, J.; Yorke, E.; Jackson, A. Local Control After Stereotactic Body Radiation Therapy for Liver Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 188–195. [Google Scholar] [CrossRef]
- Pan, C.C.; Kavanagh, B.D.; Dawson, L.A.; Li, X.A.; Das, S.K.; Miften, M.; Ten Haken, R.K. Radiation-associated liver injury. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S94–S100. [Google Scholar] [CrossRef] [PubMed]
- Sterzing, F.; Brunner, T.B.; Ernst, I.; Baus, W.W.; Greve, B.; Herfarth, K.; Guckenberger, M. Stereotactic body radiotherapy for liver tumors: Principles and practical guidelines of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther. Onkol. 2014, 190, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.M.; Modh, A.; Glide-Hurst, C.; Chetty, I.J.; Movsas, B. Real-time Magnetic Resonance-guided Liver Stereotactic Body Radiation Therapy: An Institutional Report Using a Magnetic Resonance-Linac System. Cureus 2019, 11, e5774. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Henke, L.E.; Shaverdian, N.; Mittauer, K.; Wojcieszynski, A.P.; Hullett, C.R.; Kamrava, M.; Lamb, J.; Cao, M.; Green, O.L.; et al. A Multi-Institutional Experience of MR-Guided Liver Stereotactic Body Radiation Therapy. Adv. Radiat. Oncol. 2019, 4, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, L.; Cellini, F.; Manfrida, S.; Chiloiro, G.; Teodoli, S.; Cusumano, D.; Fionda, B.; Mattiucci, G.C.; De Gaetano, A.M.; Azario, L.; et al. Use of Indirect Target Gating in Magnetic Resonance-guided Liver Stereotactic Body Radiotherapy: Case Report of an Oligometastatic Patient. Cureus 2018, 10, e2292. [Google Scholar] [CrossRef]
- Moreno, P.; de la Quintana Basarrate, A.; Musholt, T.J.; Paunovic, I.; Puccini, M.; Vidal, O.; Ortega, J.; Kraimps, J.L.; Bollo Arocena, E.; Rodriguez, J.M.; et al. Adrenalectomy for solid tumor metastases: Results of a multicenter European study. Surgery 2013, 154, 1215–1222; discussion 1222–1223. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Yaney, A.; Stevens, A.; Monk, P.; Martin, D.; Diaz, D.A.; Wang, S.J. Radiotherapy in Oligometastatic, Oligorecurrent and Oligoprogressive Prostate Cancer: A Mini-Review. Front. Oncol. 2022, 12, 932637. [Google Scholar] [CrossRef] [PubMed]
- Scorsetti, M.; Alongi, F.; Filippi, A.R.; Pentimalli, S.; Navarria, P.; Clerici, E.; Castiglioni, S.; Tozzi, A.; Reggiori, G.; Mancosu, P.; et al. Long-term local control achieved after hypofractionated stereotactic body radiotherapy for adrenal gland metastases: A retrospective analysis of 34 patients. Acta Oncol. 2012, 51, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Alexandrescu, S.T.; Croitoru, A.E.; Grigorie, R.T.; Tomescu, D.R.; Droc, G.; Grasu, M.C.; Popescu, I. Aggressive surgical approach in patients with adrenal-only metastases from hepatocellular carcinoma enables higher survival rates than standard systemic therapy. Hepatobiliary Pancreat. Dis. Int. 2021, 20, 28–33. [Google Scholar] [CrossRef]
- Gunjur, A.; Duong, C.; Ball, D.; Siva, S. Surgical and ablative therapies for the management of adrenal ‘oligometastases’—A systematic review. Cancer Treat. Rev. 2014, 40, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Holy, R.; Piroth, M.; Pinkawa, M.; Eble, M.J. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther. Onkol. 2011, 187, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Rudra, S.; Malik, R.; Ranck, M.C.; Farrey, K.; Golden, D.W.; Hasselle, M.D.; Weichselbaum, R.R.; Salama, J.K. Stereotactic body radiation therapy for curative treatment of adrenal metastases. Technol. Cancer Res. Treat. 2013, 12, 217–224. [Google Scholar] [CrossRef]
- Chance, W.W.; Nguyen, Q.N.; Mehran, R.; Welsh, J.W.; Gomez, D.R.; Balter, P.; Komaki, R.; Liao, Z.; Chang, J.Y. Stereotactic ablative radiotherapy for adrenal gland metastases: Factors influencing outcomes, patterns of failure, and dosimetric thresholds for toxicity. Pract. Radiat. Oncol. 2017, 7, e195–e203. [Google Scholar] [CrossRef]
- Wysocka, B.; Kassam, Z.; Lockwood, G.; Brierley, J.; Dawson, L.A.; Buckley, C.A.; Jaffray, D.; Cummings, B.; Kim, J.; Wong, R.; et al. Interfraction and respiratory organ motion during conformal radiotherapy in gastric cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 53–59. [Google Scholar] [CrossRef]
- Knybel, L.; Cvek, J.; Otahal, B.; Jonszta, T.; Molenda, L.; Czerny, D.; Skacelikova, E.; Rybar, M.; Dvorak, P.; Feltl, D. The analysis of respiration-induced pancreatic tumor motion based on reference measurement. Radiat. Oncol. 2014, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Hu, Y.; Liu, J.; Cao, A.N.; Ye, L.X.; Zeng, Z.C. Respiratory motion of adrenal gland metastases: Analyses using four-dimensional computed tomography images. Phys. Med. 2017, 38, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Rai, H.; Haas, J.; Witten, M.; Blacksburg, S.; Schneider, J.G. A Retrospective Review of CyberKnife Stereotactic Body Radiotherapy for Adrenal Tumors (Primary and Metastatic): Winthrop University Hospital Experience. Front. Oncol. 2015, 5, 185. [Google Scholar] [CrossRef] [PubMed]
- Palacios, M.A.; Bohoudi, O.; Bruynzeel, A.M.E.; van Sorsen de Koste, J.R.; Cobussen, P.; Slotman, B.J.; Lagerwaard, F.J.; Senan, S. Role of Daily Plan Adaptation in MR-Guided Stereotactic Ablative Radiation Therapy for Adrenal Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 426–433. [Google Scholar] [CrossRef]
- Michalet, M.; Bettaieb, O.; Khalfi, S.; Ghorbel, A.; Valdenaire, S.; Debuire, P.; Ailleres, N.; Draghici, R.; De Meric De Bellefon, M.; Charissoux, M.; et al. Stereotactic MR-Guided Radiotherapy for Adrenal Gland Metastases: First Clinical Results. J. Clin. Med. 2022, 12, 291. [Google Scholar] [CrossRef]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Alva, A.; Baine, M.; Beckermann, K.; Carlo, M.I.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; et al. Kidney Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 71–90. [Google Scholar] [CrossRef]
- Siva, S.; Correa, R.J.M.; Warner, A.; Staehler, M.; Ellis, R.J.; Ponsky, L.; Kaplan, I.D.; Mahadevan, A.; Chu, W.; Gandhidasan, S.; et al. Stereotactic Ablative Radiotherapy for >/=T1b Primary Renal Cell Carcinoma: A Report from the International Radiosurgery Oncology Consortium for Kidney (IROCK). Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 941–949. [Google Scholar] [CrossRef]
- Siva, S.; Ali, M.; Correa, R.J.M.; Muacevic, A.; Ponsky, L.; Ellis, R.J.; Lo, S.S.; Onishi, H.; Swaminath, A.; McLaughlin, M.; et al. 5-year outcomes after stereotactic ablative body radiotherapy for primary renal cell carcinoma: An individual patient data meta-analysis from IROCK (the International Radiosurgery Consortium of the Kidney). Lancet Oncol. 2022, 23, 1508–1516. [Google Scholar] [CrossRef]
- Sonier, M.; Chu, W.; Lalani, N.; Erler, D.; Cheung, P.; Korol, R. Implementation of a volumetric modulated arc therapy treatment planning solution for kidney and adrenal stereotactic body radiation therapy. Med. Dosim. 2016, 41, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Prins, F.M.; Stemkens, B.; Kerkmeijer, L.G.W.; Barendrecht, M.M.; de Boer, H.J.; Vonken, E.P.A.; Lagendijk, J.J.W.; Tijssen, R.H.N. Intrafraction Motion Management of Renal Cell Carcinoma With Magnetic Resonance Imaging-Guided Stereotactic Body Radiation Therapy. Pract. Radiat. Oncol. 2019, 9, e55–e61. [Google Scholar] [CrossRef] [PubMed]
- Keller, B.; Bruynzeel, A.M.E.; Tang, C.; Swaminath, A.; Kerkmeijer, L.; Chu, W. Adaptive Magnetic Resonance-Guided Stereotactic Body Radiotherapy: The Next Step in the Treatment of Renal Cell Carcinoma. Front. Oncol. 2021, 11, 634830. [Google Scholar] [CrossRef]
- Rudra, S.; Fischer-Valuck, B.; Pachynski, R.; Daly, M.; Green, O. Magnetic Resonance Image Guided Stereotactic Body Radiation Therapy to the Primary Renal Mass in Metastatic Renal Cell Carcinoma. Adv. Radiat. Oncol. 2019, 4, 566–570. [Google Scholar] [CrossRef]
- Tetar, S.U.; Bohoudi, O.; Senan, S.; Palacios, M.A.; Oei, S.S.; Wel, A.M.V.; Slotman, B.J.; Moorselaar, R.; Lagerwaard, F.J.; Bruynzeel, A.M.E. The Role of Daily Adaptive Stereotactic MR-Guided Radiotherapy for Renal Cell Cancer. Cancers 2020, 12, 2763. [Google Scholar] [CrossRef] [PubMed]
- Lalani, A.-K.A.; Swaminath, A.; Pond, G.R.; Morgan, S.C.; Azad, A.; Chu, W.; Winquist, E.; Kapoor, A.; Bonert, M.; Bramson, J.L.; et al. Phase II trial of cytoreductive stereotactic hypofractionated radiotherapy with combination ipilimumab/nivolumab for metastatic kidney cancer (CYTOSHRINK). J. Clin. Oncol. 2022, 40, TPS398. [Google Scholar] [CrossRef]
- Siva, S.; Chesson, B.; Bressel, M.; Pryor, D.; Higgs, B.; Reynolds, H.M.; Hardcastle, N.; Montgomery, R.; Vanneste, B.; Khoo, V.; et al. TROG 15.03 phase II clinical trial of Focal Ablative STereotactic Radiosurgery for Cancers of the Kidney—FASTRACK II. BMC Cancer 2018, 18, 1030. [Google Scholar] [CrossRef]
- Early Stage Breast Cancer. Consent Statement. 1990. Available online: https://consensus.nih.gov/1990/1990earlystagebreastcancer081html.htm (accessed on 12 March 2023).
- Acharya, S.; Hsieh, S.; Michalski, J.M.; Shinohara, E.T.; Perkins, S.M. Distance to Radiation Facility and Treatment Choice in Early-Stage Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Ki, Y.; Jeon, H.; Kim, D.W.; Jung, J.; Kim, S.S. Who are the optimal candidates for partial breast irradiation? Asia Pac. J. Clin. Oncol. 2021, 17, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Meattini, I.; Marrazzo, L.; Saieva, C.; Desideri, I.; Scotti, V.; Simontacchi, G.; Bonomo, P.; Greto, D.; Mangoni, M.; Scoccianti, S.; et al. Accelerated Partial-Breast Irradiation Compared With Whole-Breast Irradiation for Early Breast Cancer: Long-Term Results of the Randomized Phase III APBI-IMRT-Florence Trial. J. Clin. Oncol. 2020, 38, 4175–4183. [Google Scholar] [CrossRef] [PubMed]
- Galalae, R.; Hannoun-Levi, J.M. Accelerated partial breast irradiation by brachytherapy: Present evidence and future developments. Jpn. J. Clin. Oncol. 2020, 50, 743–752. [Google Scholar] [CrossRef]
- Livi, L.; Meattini, I.; Marrazzo, L.; Simontacchi, G.; Pallotta, S.; Saieva, C.; Paiar, F.; Scotti, V.; De Luca Cardillo, C.; Bastiani, P.; et al. Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur. J. Cancer 2015, 51, 451–463. [Google Scholar] [CrossRef]
- Whelan, T.J.; Julian, J.A.; Berrang, T.S.; Kim, D.H.; Germain, I.; Nichol, A.M.; Akra, M.; Lavertu, S.; Germain, F.; Fyles, A.; et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): A randomised controlled trial. Lancet 2019, 394, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, W.R.; Roach, M.C.; Thomas, M.A.; Ochoa, L.; Altman, M.B.; Hernandez-Aya, L.F.; Cyr, A.E.; Margenthaler, J.A.; Zoberi, I. Long-Term Outcomes with 3-Dimensional Conformal External Beam Accelerated Partial Breast Irradiation. Pract. Radiat. Oncol. 2020, 10, e128–e135. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Fischer-Valuck, B.W.; Mazur, T.R.; Curcuru, A.; Sona, K.; Kashani, R.; Green, O.; Ochoa, L.; Mutic, S.; Zoberi, I.; et al. Magnetic Resonance Image Guided Radiation Therapy for External Beam Accelerated Partial-Breast Irradiation: Evaluation of Delivered Dose and Intrafractional Cavity Motion. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Price, A.T.; Kennedy, W.R.; Henke, L.E.; Brown, S.R.; Green, O.L.; Thomas, M.A.; Ginn, J.; Zoberi, I. Implementing stereotactic accelerated partial breast irradiation using magnetic resonance guided radiation therapy. Radiother. Oncol. 2021, 164, 275–281. [Google Scholar] [CrossRef]
- Crivellari, D.; Sun, Z.; Coates, A.S.; Price, K.N.; Thurlimann, B.; Mouridsen, H.; Mauriac, L.; Forbes, J.F.; Paridaens, R.J.; Castiglione-Gertsch, M.; et al. Letrozole compared with tamoxifen for elderly patients with endocrine-responsive early breast cancer: The BIG 1-98 trial. J. Clin. Oncol. 2008, 26, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.; Srinivas, S.; Antonarakis, E.S.; Armstrong, A.J.; Bekelman, J.E.; Cheng, H.; D’Amico, A.V.; Davis, B.J.; Desai, N.; Dorff, T.; et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.R.; Basak, R.; Mohiuddin, J.J.; Chen, R.C. Use of stereotactic body radiotherapy for prostate cancer in the United States from 2004 through 2012. Cancer 2016, 122, 2234–2241. [Google Scholar] [CrossRef]
- Widmark, A.; Gunnlaugsson, A.; Beckman, L.; Thellenberg-Karlsson, C.; Hoyer, M.; Lagerlund, M.; Kindblom, J.; Ginman, C.; Johansson, B.; Bjornlinger, K.; et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 2019, 394, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Ferrer, M.; Suarez, J.F.; Multicentric Spanish Group of Clinically Localized Prostate Cancer. Comparison of quality of life after stereotactic body radiotherapy and surgery for early-stage prostate cancer. Radiat. Oncol. 2012, 7, 194. [Google Scholar] [CrossRef] [PubMed]
- Brand, D.H.; Tree, A.C.; Ostler, P.; van der Voet, H.; Loblaw, A.; Chu, W.; Ford, D.; Tolan, S.; Jain, S.; Martin, A.; et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): Acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019, 20, 1531–1543. [Google Scholar] [CrossRef]
- Nicosia, L.; Mazzola, R.; Rigo, M.; Figlia, V.; Giaj-Levra, N.; Napoli, G.; Ricchetti, F.; Corradini, S.; Ruggieri, R.; Alongi, F. Moderate versus extreme hypofractionated radiotherapy: A toxicity comparative analysis in low- and favorable intermediate-risk prostate cancer patients. J. Cancer Res. Clin. Oncol. 2019, 145, 2547–2554. [Google Scholar] [CrossRef]
- Kasivisvanathan, V.; Rannikko, A.S.; Borghi, M.; Panebianco, V.; Mynderse, L.A.; Vaarala, M.H.; Briganti, A.; Budaus, L.; Hellawell, G.; Hindley, R.G.; et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N. Engl. J. Med. 2018, 378, 1767–1777. [Google Scholar] [CrossRef]
- Sidaway, P. MRI improves diagnosis. Nat. Rev. Clin. Oncol. 2018, 15, 345. [Google Scholar] [CrossRef] [PubMed]
- Wibmer, A.G.; Vargas, H.A.; Hricak, H. Role of MRI in the diagnosis and management of prostate cancer. Future Oncol. 2015, 11, 2757–2766. [Google Scholar] [CrossRef]
- Teunissen, F.R.; Wortel, R.C.; Hes, J.; Willigenburg, T.; de Groot-van Breugel, E.N.; de Boer, J.C.; van Melick, H.H.; Verkooijen, H.M. Adaptive magnetic resonance-guided neurovascular-sparing radiotherapy for preservation of erectile function in prostate cancer patients. Phys. Imaging Radiat. Oncol. 2021, 20, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Kerkmeijer, L.G.; Groen, V.H.; Pos, F.J.; Haustermans, K.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.C.; den Boer, J.C.; Zijp, J.V.; Vulpen, M.V.; et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients with Localized Prostate Cancer: Results from the FLAME Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 787–796. [Google Scholar] [CrossRef]
- Tocco, B.R.; Kishan, A.U.; Ma, T.M.; Kerkmeijer, L.G.W.; Tree, A.C. MR-Guided Radiotherapy for Prostate Cancer. Front. Oncol. 2020, 10, 616291. [Google Scholar] [CrossRef]
- Cuccia, F.; Corradini, S.; Mazzola, R.; Spiazzi, L.; Rigo, M.; Bonu, M.L.; Ruggieri, R.; Buglione di Monale, E.B.M.; Magrini, S.M.; Alongi, F. MR-Guided Hypofractionated Radiotherapy: Current Emerging Data and Promising Perspectives for Localized Prostate Cancer. Cancers 2021, 13, 1791. [Google Scholar] [CrossRef]
- Fawaz, Z.S.; Yassa, M.; Nguyen, D.H.; Vavassis, P. Fiducial marker implantation in prostate radiation therapy: Complication rates and technique. Cancer Radiother. 2014, 18, 736–739. [Google Scholar] [CrossRef]
- Gill, S.; Li, J.; Thomas, J.; Bressel, M.; Thursky, K.; Styles, C.; Tai, K.H.; Duchesne, G.M.; Foroudi, F. Patient-reported complications from fiducial marker implantation for prostate image-guided radiotherapy. Br. J. Radiol. 2012, 85, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, A.; Mitchell, A.; Tree, A.; Barnes, H.; Bower, L.; Chick, J.; Goodwin, E.; Herbert, T.; Lawes, R.; McNair, H.; et al. Daily adaptive radiotherapy for patients with prostate cancer using a high field MR-linac: Initial clinical experiences and assessment of delivered doses compared to a C-arm linac. Clin. Transl. Radiat. Oncol. 2020, 23, 35–42. [Google Scholar] [CrossRef]
- Tetar, S.U.; Bruynzeel, A.M.E.; Oei, S.S.; Senan, S.; Fraikin, T.; Slotman, B.J.; Moorselaar, R.; Lagerwaard, F.J. Magnetic Resonance-guided Stereotactic Radiotherapy for Localized Prostate Cancer: Final Results on Patient-reported Outcomes of a Prospective Phase 2 Study. Eur. Urol. Oncol. 2021, 4, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Alongi, F.; Rigo, M.; Figlia, V.; Cuccia, F.; Giaj-Levra, N.; Nicosia, L.; Ricchetti, F.; Sicignano, G.; De Simone, A.; Naccarato, S.; et al. 1.5 T MR-guided and daily adapted SBRT for prostate cancer: Feasibility, preliminary clinical tolerability, quality of life and patient-reported outcomes during treatment. Radiat. Oncol. 2020, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Bruynzeel, A.M.E.; Tetar, S.U.; Oei, S.S.; Senan, S.; Haasbeek, C.J.A.; Spoelstra, F.O.B.; Piet, A.H.M.; Meijnen, P.; Bakker van der Jagt, M.A.B.; Fraikin, T.; et al. A Prospective Single-Arm Phase 2 Study of Stereotactic Magnetic Resonance Guided Adaptive Radiation Therapy for Prostate Cancer: Early Toxicity Results. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.M.; Ballas, L.K.; Wilhalme, H.; Sachdeva, A.; Chong, N.; Sharma, S.; Yang, T.; Basehart, V.; Reiter, R.E.; Saigal, C.; et al. Quality-of-Life Outcomes and Toxicity Profile among Patients with Localized Prostate Cancer after Radical Prostatectomy Treated with Stereotactic Body Radiation: The SCIMITAR Multicenter Phase 2 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 142–152. [Google Scholar] [CrossRef]
- Kishan, A.U.; Ma, T.M.; Lamb, J.M.; Casado, M.; Wilhalme, H.; Low, D.A.; Sheng, K.; Sharma, S.; Nickols, N.G.; Pham, J.; et al. Magnetic Resonance Imaging-Guided vs Computed Tomography-Guided Stereotactic Body Radiotherapy for Prostate Cancer: The MIRAGE Randomized Clinical Trial. JAMA Oncol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Redmond, K.J.; Robertson, S.; Lo, S.S.; Soltys, S.G.; Ryu, S.; McNutt, T.; Chao, S.T.; Yamada, Y.; Ghia, A.; Chang, E.L.; et al. Consensus Contouring Guidelines for Postoperative Stereotactic Body Radiation Therapy for Metastatic Solid Tumor Malignancies to the Spine. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Redler, G.; Stevens, T.; Cammin, J.; Malin, M.; Green, O.; Mutic, S.; Pitroda, S.; Aydogan, B. Dosimetric Feasibility of Utilizing the ViewRay Magnetic Resonance Guided Linac System for Image-guided Spine Stereotactic Body Radiation Therapy. Cureus 2019, 11, e6364. [Google Scholar] [CrossRef]
- Stradiotti, P.; Curti, A.; Castellazzi, G.; Zerbi, A. Metal-related artifacts in instrumented spine. Techniques for reducing artifacts in CT and MRI: State of the art. Eur. Spine J. 2009, 18 (Suppl. 1), 102–108. [Google Scholar] [CrossRef] [PubMed]
- Paulson, E.S.; Erickson, B.; Schultz, C.; Allen Li, X. Comprehensive MRI simulation methodology using a dedicated MRI scanner in radiation oncology for external beam radiation treatment planning. Med. Phys. 2015, 42, 28–39. [Google Scholar] [CrossRef]
- Spieler, B.; Samuels, S.E.; Llorente, R.; Yechieli, R.; Ford, J.C.; Mellon, E.A. Advantages of Radiation Therapy Simulation with 0.35 Tesla Magnetic Resonance Imaging for Stereotactic Ablation of Spinal Metastases. Pract. Radiat. Oncol. 2020, 10, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Weichselbaum, R.R.; Hellman, S. Oligometastases revisited. Nat. Rev. Clin. Oncol. 2011, 8, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R., Jr.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R.; et al. Local Consolidative Therapy vs. Maintenance Therapy or Observation for Patients with Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef]
- Chmura, S.; Winter, K.A.; Robinson, C.; Pisansky, T.M.; Borges, V.; Al-Hallaq, H.; Matuszak, M.; Park, S.S.; Yi, S.; Hasan, Y.; et al. Evaluation of Safety of Stereotactic Body Radiotherapy for the Treatment of Patients With Multiple Metastases: Findings from the NRG-BR001 Phase 1 Trial. JAMA Oncol. 2021, 7, 845–852. [Google Scholar] [CrossRef]
- Derynda, B.R.; Liveringhouse, C.L.; Bryant, J.M.; Rosenberg, S.A. MR-Guided Radiation Therapy for Oligometastatic Malignancies. Appl. Rad. Oncol. 2021, 10, 25–32. [Google Scholar]
- Tyran, M.; Cao, M.; Raldow, A.C.; Dang, A.; Lamb, J.; Low, D.A.; Steinberg, M.L.; Lee, P. Stereotactic Magnetic Resonance-guided Online Adaptive Radiotherapy for Oligometastatic Breast Cancer: A Case Report. Cureus 2018, 10, e2368. [Google Scholar] [CrossRef]
- Haque, W.; Crane, C.H.; Krishnan, S.; Delclos, M.E.; Javle, M.; Garrett, C.R.; Wolff, R.A.; Das, P. Reirradiation to the abdomen for gastrointestinal malignancies. Radiat. Oncol. 2009, 4, 55. [Google Scholar] [CrossRef]
- Valentini, V.; Morganti, A.G.; Gambacorta, M.A.; Mohiuddin, M.; Doglietto, G.B.; Coco, C.; De Paoli, A.; Rossi, C.; Di Russo, A.; Valvo, F.; et al. Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: A multicentric phase II study. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1129–1139. [Google Scholar] [CrossRef]
- Hunt, A.; Das, P.; Minsky, B.D.; Koay, E.J.; Krishnan, S.; Herman, J.M.; Taniguchi, C.; Koong, A.; Smith, G.L.; Holliday, E.B. Hyperfractionated abdominal reirradiation for gastrointestinal malignancies. Radiat. Oncol. 2018, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Koom, W.S.; Choi, Y.; Shim, S.J.; Cha, J.; Seong, J.; Kim, N.K.; Nam, K.C.; Keum, K.C. Reirradiation to the pelvis for recurrent rectal cancer. J. Surg. Oncol. 2012, 105, 637–642. [Google Scholar] [CrossRef]
- Tao, R.; Tsai, C.J.; Jensen, G.; Eng, C.; Kopetz, S.; Overman, M.J.; Skibber, J.M.; Rodriguez-Bigas, M.; Chang, G.J.; You, Y.N.; et al. Hyperfractionated accelerated reirradiation for rectal cancer: An analysis of outcomes and toxicity. Radiother. Oncol. 2017, 122, 146–151. [Google Scholar] [CrossRef]
- Chuong, M.D.; Bryant, J.M.; Herrera, R.; McCulloch, J.; Contreras, J.; Kotecha, R.; Romaguera, T.; Alvarez, D.; Hall, M.D.; Rubens, M.; et al. Dose-Escalated Magnetic Resonance Image-Guided Abdominopelvic Reirradiation With Continuous Intrafraction Visualization, Soft Tissue Tracking, and Automatic Beam Gating. Adv. Radiat. Oncol. 2022, 7, 100840. [Google Scholar] [CrossRef] [PubMed]
- Cuccia, F.; Rigo, M.; Figlia, V.; Giaj-Levra, N.; Mazzola, R.; Nicosia, L.; Ricchetti, F.; Trapani, G.; De Simone, A.; Gurrera, D.; et al. 1.5T MR-Guided Daily Adaptive Stereotactic Body Radiotherapy for Prostate Re-Irradiation: A Preliminary Report of Toxicity and Clinical Outcomes. Front. Oncol. 2022, 12, 858740. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Weygand, J.; Hwang, K.P.; Mohamed, A.S.; Ding, Y.; Fuller, C.D.; Lai, S.Y.; Frank, S.J.; Zhou, J. Magnetic Resonance Imaging of Glucose Uptake and Metabolism in Patients with Head and Neck Cancer. Sci. Rep. 2016, 6, 30618. [Google Scholar] [CrossRef]
- Salzillo, T.C.; Mawoneke, V.; Weygand, J.; Shetty, A.; Gumin, J.; Zacharias, N.M.; Gammon, S.T.; Piwnica-Worms, D.; Fuller, G.N.; Logothetis, C.J.; et al. Measuring the Metabolic Evolution of Glioblastoma throughout Tumor Development, Regression, and Recurrence with Hyperpolarized Magnetic Resonance. Cells 2021, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Perez, M.R.; Lee, J.; Kang, Y.; Pratt, M.; Salzillo, T.C.; Weygand, J.; Zacharias, N.M.; Gammon, S.T.; Koay, E.J.; et al. Combining Hyperpolarized Real-Time Metabolic Imaging and NMR Spectroscopy to Identify Metabolic Biomarkers in Pancreatic Cancer. J. Proteome Res. 2019, 18, 2826–2834. [Google Scholar] [CrossRef]
- Maziero, D.; Straza, M.W.; Ford, J.C.; Bovi, J.A.; Diwanji, T.; Stoyanova, R.; Paulson, E.S.; Mellon, E.A. MR-Guided Radiotherapy for Brain and Spine Tumors. Front. Oncol. 2021, 11, 626100. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Grenier, P.; Cabanis, E.; Laval-Jeantet, M. MR imaging of intravoxel incoherent motions: Application to diffusion and perfusion in neurologic disorders. Radiology 1986, 161, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, T.; Korogi, Y.; Kochi, M.; Ikushima, I.; Shigematu, Y.; Hirai, T.; Okuda, T.; Liang, L.; Ge, Y.; Komohara, Y.; et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J. Magn. Reson. Imaging 1999, 9, 53–60. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Malkin, M.G.; Rand, S.D.; Connelly, J.M.; Quinsey, C.; LaViolette, P.S.; Bedekar, D.P.; Schmainda, K.M. Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J. Magn. Reson. Imaging 2010, 31, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Hein, P.A.; Eskey, C.J.; Dunn, J.F.; Hug, E.B. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: Tumor recurrence versus radiation injury. AJNR Am. J. Neuroradiol. 2004, 25, 201–209. [Google Scholar]
- Decker, G.; Murtz, P.; Gieseke, J.; Traber, F.; Block, W.; Sprinkart, A.M.; Leitzen, C.; Buchstab, T.; Lutter, C.; Schuller, H.; et al. Intensity-modulated radiotherapy of the prostate: Dynamic ADC monitoring by DWI at 3.0 T. Radiother. Oncol. 2014, 113, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Bains, L.J.; Zweifel, M.; Thoeny, H.C. Therapy response with diffusion MRI: An update. Cancer Imaging 2012, 12, 395–402. [Google Scholar] [CrossRef] [PubMed]
- McGarry, S.D.; Hurrell, S.L.; Kaczmarowski, A.L.; Cochran, E.J.; Connelly, J.; Rand, S.D.; Schmainda, K.M.; LaViolette, P.S. Magnetic Resonance Imaging-Based Radiomic Profiles Predict Patient Prognosis in Newly Diagnosed Glioblastoma before Therapy. Tomography 2016, 2, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Kim, H.S.; Jo, Y.; Yoo, R.E.; Choi, S.H.; Nam, S.J.; Kim, J.H. Radiomics prognostication model in glioblastoma using diffusion- and perfusion-weighted MRI. Sci. Rep. 2020, 10, 4250. [Google Scholar] [CrossRef] [PubMed]
- Kooreman, E.S.; van Houdt, P.J.; Nowee, M.E.; van Pelt, V.W.J.; Tijssen, R.H.N.; Paulson, E.S.; Gurney-Champion, O.J.; Wang, J.; Koetsveld, F.; van Buuren, L.D.; et al. Feasibility and accuracy of quantitative imaging on a 1.5 T MR-linear accelerator. Radiother. Oncol. 2019, 133, 156–162. [Google Scholar] [CrossRef]
- Thorwarth, D.; Ege, M.; Nachbar, M.; Monnich, D.; Gani, C.; Zips, D.; Boeke, S. Quantitative magnetic resonance imaging on hybrid magnetic resonance linear accelerators: Perspective on technical and clinical validation. Phys. Imaging Radiat. Oncol. 2020, 16, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Habrich, J.; Boeke, S.; Nachbar, M.; Nikolaou, K.; Schick, F.; Gani, C.; Zips, D.; Thorwarth, D. Repeatability of diffusion-weighted magnetic resonance imaging in head and neck cancer at a 1.5 T MR-Linac. Radiother. Oncol. 2022, 174, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kooreman, E.S.; van Houdt, P.J.; Keesman, R.; Pos, F.J.; van Pelt, V.W.J.; Nowee, M.E.; Wetscherek, A.; Tijssen, R.H.N.; Philippens, M.E.P.; Thorwarth, D.; et al. ADC measurements on the Unity MR-linac—A recommendation on behalf of the Elekta Unity MR-linac consortium. Radiother. Oncol. 2020, 153, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cao, M.; Sheng, K.; Gao, Y.; Chen, A.; Kamrava, M.; Lee, P.; Agazaryan, N.; Lamb, J.; Thomas, D.; et al. Longitudinal diffusion MRI for treatment response assessment: Preliminary experience using an MRI-guided tri-cobalt 60 radiotherapy system. Med. Phys. 2016, 43, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Shaverdian, N.; Yang, Y.; Hu, P.; Hart, S.; Sheng, K.; Lamb, J.; Cao, M.; Agazaryan, N.; Thomas, D.; Steinberg, M.; et al. Feasibility evaluation of diffusion-weighted imaging using an integrated MRI-radiotherapy system for response assessment to neoadjuvant therapy in rectal cancer. Br. J. Radiol. 2017, 90, 20160739. [Google Scholar] [CrossRef]
- Kalbasi, A.; Kamrava, M.; Chu, F.I.; Telesca, D.; Van Dams, R.; Yang, Y.; Ruan, D.; Nelson, S.D.; Dry, S.M.; Hernandez, J.; et al. A Phase II Trial of 5-Day Neoadjuvant Radiotherapy for Patients with High-Risk Primary Soft Tissue Sarcoma. Clin. Cancer Res. 2020, 26, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ghodrati, V.; Kalbasi, A.; Fu, J.; Ruan, D.; Cao, M.; Wang, C.; Eilber, F.C.; Bernthal, N.; Bukata, S.; et al. Prediction of soft tissue sarcoma response to radiotherapy using longitudinal diffusion MRI and a deep neural network with generative adversarial network-based data augmentation. Med. Phys. 2021, 48, 3262–3372. [Google Scholar] [CrossRef]
- Lewis, B.; Guta, A.; Mackey, S.; Gach, H.M.; Mutic, S.; Green, O.; Kim, T. Evaluation of diffusion-weighted MRI and geometric distortion on a 0.35T MR-LINAC at multiple gantry angles. J. Appl. Clin. Med. Phys. 2021, 22, 118–125. [Google Scholar] [CrossRef]
- Weygand, J.; Armstrong, T.; Bryant, J.M.; Andreozzi, J.; Oraiqat, I.M.; Liveringhouse, C.L.; Latifi, K.; Yamoah, K.; Costello, J.R.; Frakes, J.M.; et al. Accurate, repeatable, and geometrically precise diffusion-weighted imaging on a 0.35 T MRI-guided linear accelerator. In Proceedings of the Annual European Society for Radiotherapy and Oncology (ESTRO) Meeting, Vienna, Austria, 24–28 March 2023. [Google Scholar]
- Oderinde, O.M.; Shirvani, S.M.; Olcott, P.D.; Kuduvalli, G.; Mazin, S.; Larkin, D. The technical design and concept of a PET/CT linac for biology-guided radiotherapy. Clin. Transl. Radiat. Oncol. 2021, 29, 106–112. [Google Scholar] [CrossRef]
- Warburg, O. The Metabolism of Carcinoma Cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Sullivan, L.B.; Gui, D.Y.; Vander Heiden, M.G. Altered metabolite levels in cancer: Implications for tumour biology and cancer therapy. Nat. Rev. Cancer 2016, 16, 680–693. [Google Scholar] [CrossRef]
- Phelps, M.E.; Hoffman, E.J.; Mullani, N.A.; Ter-Pogossian, M.M. Application of annihilation coincidence detection to transaxial reconstruction tomography. J. Nucl. Med. 1975, 16, 210–224. [Google Scholar]
- Ter-Pogossian, M.M.; Phelps, M.E.; Hoffman, E.J.; Mullani, N.A. A positron-emission transaxial tomograph for nuclear imaging (PETT). Radiology 1975, 114, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Phelps, M.E.; Huang, S.C.; Hoffman, E.J.; Selin, C.; Sokoloff, L.; Kuhl, D.E. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: Validation of method. Ann. Neurol. 1979, 6, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Posse, S.; Otazo, R.; Dager, S.R.; Alger, J. MR spectroscopic imaging: Principles and recent advances. J. Magn. Reson. Imaging 2013, 37, 1301–1325. [Google Scholar] [CrossRef]
- Van Zijl, P.C.; Yadav, N.N. Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? Magn. Reson. Med. 2011, 65, 927–948. [Google Scholar] [CrossRef]
- Wu, B.; Warnock, G.; Zaiss, M.; Lin, C.; Chen, M.; Zhou, Z.; Mu, L.; Nanz, D.; Tuura, R.; Delso, G. An overview of CEST MRI for non-MR physicists. EJNMMI Phys. 2016, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Ardenkjaer-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef]
- Salzillo, T.C.; Hu, J.; Nguyen, L.; Whiting, N.; Lee, J.; Weygand, J.; Dutta, P.; Pudakalakatti, S.; Millward, N.Z.; Gammon, S.T.; et al. Interrogating Metabolism in Brain Cancer. Magn. Reson. Imaging Clin. N. Am. 2016, 24, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Bogner, W.; Gruber, S.; Trattnig, S.; Chmelik, M. High-resolution mapping of human brain metabolites by free induction decay (1)H MRSI at 7 T. NMR Biomed. 2012, 25, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Hangel, G.; Cadrien, C.; Lazen, P.; Furtner, J.; Lipka, A.; Heckova, E.; Hingerl, L.; Motyka, S.; Gruber, S.; Strasser, B.; et al. High-resolution metabolic imaging of high-grade gliomas using 7T-CRT-FID-MRSI. Neuroimage Clin. 2020, 28, 102433. [Google Scholar] [CrossRef]
- De Feyter, H.M.; Behar, K.L.; Corbin, Z.A.; Fulbright, R.K.; Brown, P.B.; McIntyre, S.; Nixon, T.W.; Rothman, D.L.; de Graaf, R.A. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci. Adv. 2018, 4, eaat7314. [Google Scholar] [CrossRef]
- Korzowski, A.; Weinfurtner, N.; Mueller, S.; Breitling, J.; Goerke, S.; Schlemmer, H.P.; Ladd, M.E.; Paech, D.; Bachert, P. Volumetric mapping of intra- and extracellular pH in the human brain using (31) P MRSI at 7T. Magn. Reson. Med. 2020, 84, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Bogner, W.; Otazo, R.; Henning, A. Accelerated MR spectroscopic imaging-a review of current and emerging techniques. NMR Biomed. 2021, 34, e4314. [Google Scholar] [CrossRef] [PubMed]
- Henning, A.; Fuchs, A.; Murdoch, J.B.; Boesiger, P. Slice-selective FID acquisition, localized by outer volume suppression (FIDLOVS) for (1)H-MRSI of the human brain at 7 T with minimal signal loss. NMR Biomed. 2009, 22, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Hovener, J.B.; Schwaderlapp, N.; Lickert, T.; Duckett, S.B.; Mewis, R.E.; Highton, L.A.; Kenny, S.M.; Green, G.G.; Leibfritz, D.; Korvink, J.G.; et al. A hyperpolarized equilibrium for magnetic resonance. Nat. Commun. 2013, 4, 2946. [Google Scholar] [CrossRef]
- Nelson, S.J.; Kurhanewicz, J.; Vigneron, D.B.; Larson, P.E.; Harzstark, A.L.; Ferrone, M.; van Criekinge, M.; Chang, J.W.; Bok, R.; Park, I.; et al. Metabolic imaging of patients with prostate cancer using hyperpolarized[1-(1)(3)C]pyruvate. Sci. Transl. Med. 2013, 5, 198ra108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; van Zijl, P.C. Chemical exchange saturation transfer imaging and spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2006, 48, 109–136. [Google Scholar] [CrossRef]
- Meissner, J.E.; Korzowski, A.; Regnery, S.; Goerke, S.; Breitling, J.; Floca, R.O.; Debus, J.; Schlemmer, H.P.; Ladd, M.E.; Bachert, P.; et al. Early response assessment of glioma patients to definitive chemoradiotherapy using chemical exchange saturation transfer imaging at 7 T. J. Magn. Reson. Imaging 2019, 50, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Regnery, S.; Adeberg, S.; Dreher, C.; Oberhollenzer, J.; Meissner, J.E.; Goerke, S.; Windschuh, J.; Deike-Hofmann, K.; Bickelhaupt, S.; Zaiss, M.; et al. Chemical exchange saturation transfer MRI serves as predictor of early progression in glioblastoma patients. Oncotarget 2018, 9, 28772–28783. [Google Scholar] [CrossRef]
- Cusumano, D.; Boldrini, L.; Dhont, J.; Fiorino, C.; Green, O.; Gungor, G.; Jornet, N.; Kluter, S.; Landry, G.; Mattiucci, G.C.; et al. Artificial Intelligence in magnetic Resonance guided Radiotherapy: Medical and physical considerations on state of art and future perspectives. Phys. Med. 2021, 85, 175–191. [Google Scholar] [CrossRef]
- Bryant, J.M.; Saghand, P.G.; Latifi, K.; Frakes, J.; Hoffe, S.A.; Moros, E.; Mittauer, K.E.; Kotecha, R.; El Naqa, I.; Rosenberg, S.A. A novel multi-task hybrid deep neural network (DNN) predicts tumor progression during MRgRT. In Proceedings of the Annual European Society for Radiotherapy and Oncology (ESTRO) Meeting, Vienna, Austria, 24 March–28 March 2023. [Google Scholar]
- Botman, R.; Tetar, S.U.; Palacios, M.A.; Slotman, B.J.; Lagerwaard, F.J.; Bruynzeel, A.M.E. The clinical introduction of MR-guided radiation therapy from a RTT perspective. Clin. Transl. Radiat. Oncol. 2019, 18, 140–145. [Google Scholar] [CrossRef]
- Mittauer, K.; Paliwal, B.; Hill, P.; Bayouth, J.E.; Geurts, M.W.; Baschnagel, A.M.; Bradley, K.A.; Harari, P.M.; Rosenberg, S.; Brower, J.V.; et al. A New Era of Image Guidance with Magnetic Resonance-guided Radiation Therapy for Abdominal and Thoracic Malignancies. Cureus 2018, 10, e2422. [Google Scholar] [CrossRef] [PubMed]
- Kueng, R.; Guyer, G.; Volken, W.; Frei, D.; Stabel, F.; Stampanoni, M.F.M.; Manser, P.; Fix, M.K. Development of an extended Macro Monte Carlo method for efficient and accurate dose calculation in magnetic fields. Med. Phys. 2020, 47, 6519–6530. [Google Scholar] [CrossRef] [PubMed]
| Study Title | Sponsor | Site | Condition/Disease | Estimated Enrollment | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|
| A Master Protocol of Stereotactic Magnetic Resonance Guided Adaptive Radiation Therapy (SMART) | Dana–Farber Cancer Institute | All/Multiple sites | N/A | 1000 | NCT04115254 |
| The MR-Linac Technical Feasibility Protocol (UMBRELLA-II) | The Netherlands Cancer Institute | All/Multiple sites | N/A | 140 | NCT04351204 |
| The Multiple Outcome Evaluation of Radiation Therapy Using the MR-Linac Study (MOMENTUM) | UMC Utrecht | All/Multiple sites | N/A | 6000 | NCT04075305 |
| Magnetic Resonance Guided Radiation Therapy (CONFIRM) | Dana–Farber Cancer Institute | All/Multiple sites | Gastric Cancer, Invasive Breast Cancer, in Situ Breast Cancer, Mantle Cell Lymphoma, Larynx Cancer, Bladder Cancer | 70 | NCT04368702 |
| Immune Checkpoint Inhibitor and MR-guided SBRT for Limited Progressive Metastatic Carcinoma | Baptist Health South Florida | All/Multiple sites | Metastatic tumors | 52 | NCT04376502 |
| Stereotactic MRI-guided Adaptive Radiation Therapy (SMART) in One Fraction (SMART-ONE) | Baptist Health South Florida | All/Multiple sites | Oligometastatic cancer, up to 10 sites of disease | 30 | NCT04939246 |
| Real-Time MRI-Guided 3-Fraction Accelerated Partial Breast Irradiation in Early Breast Cancer (MAPBI) | University of Wisconsin, Madison | Breast | Breast Cancer, DCIS | 30 | NCT03936478 |
| MR-Linac Guided Adaptive FSRT for Brain Metastases From Non-small Cell Lung Cancer | Sun Yat-Sen University | Central Nervous System | Brain Metastases from Non-Small Cell Lung Cancer | 55 | NCT04946019 |
| Pilot Study of Same-session MR-only Simulation and Treatment With Stereotactic MRI-guided Adaptive Radiotherapy (SMART) for Oligometastases of the Spine | Washington University School of Medicine | Central Nervous System | Oligometastases of the Spine | 10 | NCT03878485 |
| Locally Advanced Pancreatic Cancer Treated With ABLAtivE Stereotactic MRI-guided Adaptive Radiation Therapy (LAP-ABLATE) | ViewRay Inc. | Gastrointestinal | Pancreatic Cancer | 267 | NCT05585554 |
| Sequential Treatment With GEMBRAX and Then FOLFIRINOX Followed by Stereotactic MRI-guided Radiotherapy in Patients With Locally Advanced Pancreatic Cancer (GABRINOX-ART) | Institut du Cancer de Montpellier—Val d’Aurelle | Gastrointestinal | Pancreatic Cancer | 103 | NCT04570943 |
| MR-Guided Adaptive SBRT of Primary Tumor for Pain Control in Metastatic PDAC (MASPAC) | Ludwig-Maximilians—University of Munich | Gastrointestinal | Pancreatic Cancer | 92 | NCT05114213 |
| Stereotactic Radiotherapy vs. Best Supportive Care in Unfit Pancreatic Cancer Patients (PANCOSAR) | Amsterdam UMC | Gastrointestinal | Pancreatic Cancer | 98 | NCT05265663 |
| Precision Radiotherapy Using MR-linac for Pancreatic Neuroendocrine Tumours in MEN1 Patients (PRIME) | J.M. de Laat | Gastrointestinal | Pancreatic Neuroendocrine Tumors | 20 | NCT05037461 |
| MR-guided Pre-operative RT in Gastric Cancer | Washington University School of Medicine | Gastrointestinal | Gastric cancer | 36 | NCT04162665 |
| Magnetic Resonance-guided Adaptive Stereotactic Body Radiotherapy for Hepatic Metastases (MAESTRO) | University Hospital Heidelberg | Gastrointestinal | Liver Metastases | 90 | NCT05027711 |
| OAR-Based, Dose Escalated SBRT With Real-time Adaptive MRI Guidance for Liver Metastases | University of Wisconsin, Madison | Gastrointestinal | Liver Metastases | 48 | NCT04020276 |
| Adaptative MR-Guided Stereotactic Body Radiotherapy of Liver Tumors (RASTAF) | Centre Georges Francois Leclerc | Gastrointestinal | Liver Metastases | 46 | NCT04242342 |
| Radiotherapy With Iron Oxide Nanoparticles (SPION) on MR-Linac for Primary & Metastatic Hepatic Cancers | Allegheny Singer Research Institute | Gastrointestinal | Liver tumors | 25 | NCT04682847 |
| Stereotactic MRI-guided Radiation Therapy for Localized Prostate Cancer (SMILE) | University Hospital Heidelberg | Genitourinary | Prostate Cancer | 68 | NCT04845503 |
| Randomized Trial of Five or Two MRI-Guided Adaptive Radiotherapy Treatments for Prostate Cancer (FORT) | Weill Medical College of Cornell University | Genitourinary | Prostate Cancer | 136 | NCT04984343 |
| MR-linac Guided Ultra-hypofractionated RT for Prostate Cancer | Chinese Academy of Medical Sciences | Genitourinary | Prostate Cancer | 50 | NCT05183074 |
| Randomized Phase-II Trial of Salvage Radiotherapy for Prostate Cancer In 4 Weeks vs. 2 Weeks | Weill Medical College of Cornell University | Genitourinary | Prostate Cancer | 134 | NCT04422132 |
| MR-Linac for Head and Neck SBRT | Sunnybrook Health Sciences Centre | Head and Neck | Head and Neck Cancer | 30 | NCT04809792 |
| Nano-SMART: Nanoparticles with MR Guided SBRT in Centrally Located Lung Tumors and Pancreatic Cancer | Dana–Farber Cancer Institute | Thorax | Non-small Cell Lung Cancer, Pancreatic Cancer | 100 | NCT04789486 |
| Magnetic Resonance Guided Adaptive Stereotactic Body Radiotherapy for Lung Tumors in Ultra-central Location (MAGELLAN) | University Hospital Heidelberg | Thorax | Non-small Cell Lung Cancer, Metastatic tumors | 38 | NCT04925583 |
| Study of LUNG Stereotactic Adaptive Ablative Radiotherapy (LUNG STAAR) | Baptist Health South Florida | Thorax | Non-small Cell Lung Cancer | 60 | NCT04917224 |
| A Multicenter Phase-II Study of Stereotactic Radiotherapy for Centrally Located Lung Tumors (STRICT-LUNG STUDY) and Ultra-centrally Located Lung Tumors (STAR-LUNG STUDY) | Rigshospitalet, Denmark | Thorax | Primary Lung Cancer, Metastatic tumors | 138 | NCT05354596 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bryant, J.M.; Weygand, J.; Keit, E.; Cruz-Chamorro, R.; Sandoval, M.L.; Oraiqat, I.M.; Andreozzi, J.; Redler, G.; Latifi, K.; Feygelman, V.; et al. Stereotactic Magnetic Resonance-Guided Adaptive and Non-Adaptive Radiotherapy on Combination MR-Linear Accelerators: Current Practice and Future Directions. Cancers 2023, 15, 2081. https://doi.org/10.3390/cancers15072081
Bryant JM, Weygand J, Keit E, Cruz-Chamorro R, Sandoval ML, Oraiqat IM, Andreozzi J, Redler G, Latifi K, Feygelman V, et al. Stereotactic Magnetic Resonance-Guided Adaptive and Non-Adaptive Radiotherapy on Combination MR-Linear Accelerators: Current Practice and Future Directions. Cancers. 2023; 15(7):2081. https://doi.org/10.3390/cancers15072081
Chicago/Turabian StyleBryant, John Michael, Joseph Weygand, Emily Keit, Ruben Cruz-Chamorro, Maria L. Sandoval, Ibrahim M. Oraiqat, Jacqueline Andreozzi, Gage Redler, Kujtim Latifi, Vladimir Feygelman, and et al. 2023. "Stereotactic Magnetic Resonance-Guided Adaptive and Non-Adaptive Radiotherapy on Combination MR-Linear Accelerators: Current Practice and Future Directions" Cancers 15, no. 7: 2081. https://doi.org/10.3390/cancers15072081
APA StyleBryant, J. M., Weygand, J., Keit, E., Cruz-Chamorro, R., Sandoval, M. L., Oraiqat, I. M., Andreozzi, J., Redler, G., Latifi, K., Feygelman, V., & Rosenberg, S. A. (2023). Stereotactic Magnetic Resonance-Guided Adaptive and Non-Adaptive Radiotherapy on Combination MR-Linear Accelerators: Current Practice and Future Directions. Cancers, 15(7), 2081. https://doi.org/10.3390/cancers15072081







