Tumour Necrosis Factor Alpha (TNF-α) and Oral Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction:
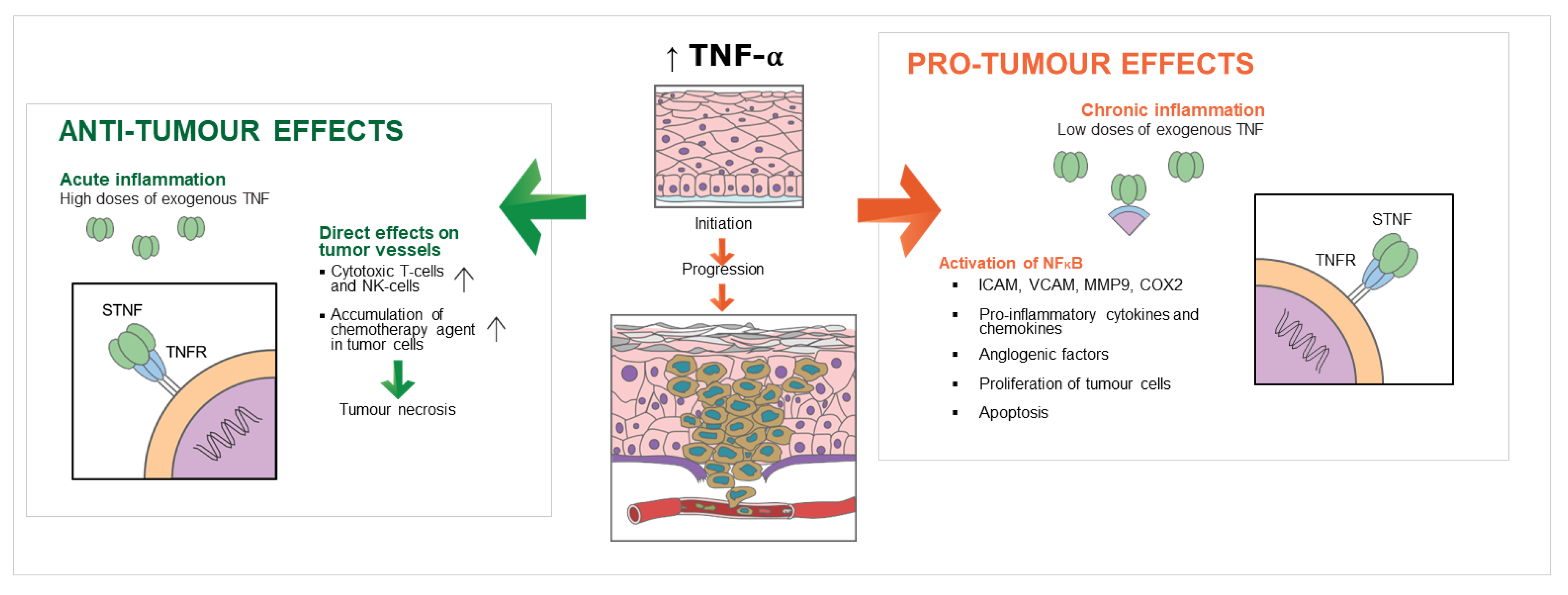
2. TNF-α, Cancer and SCC
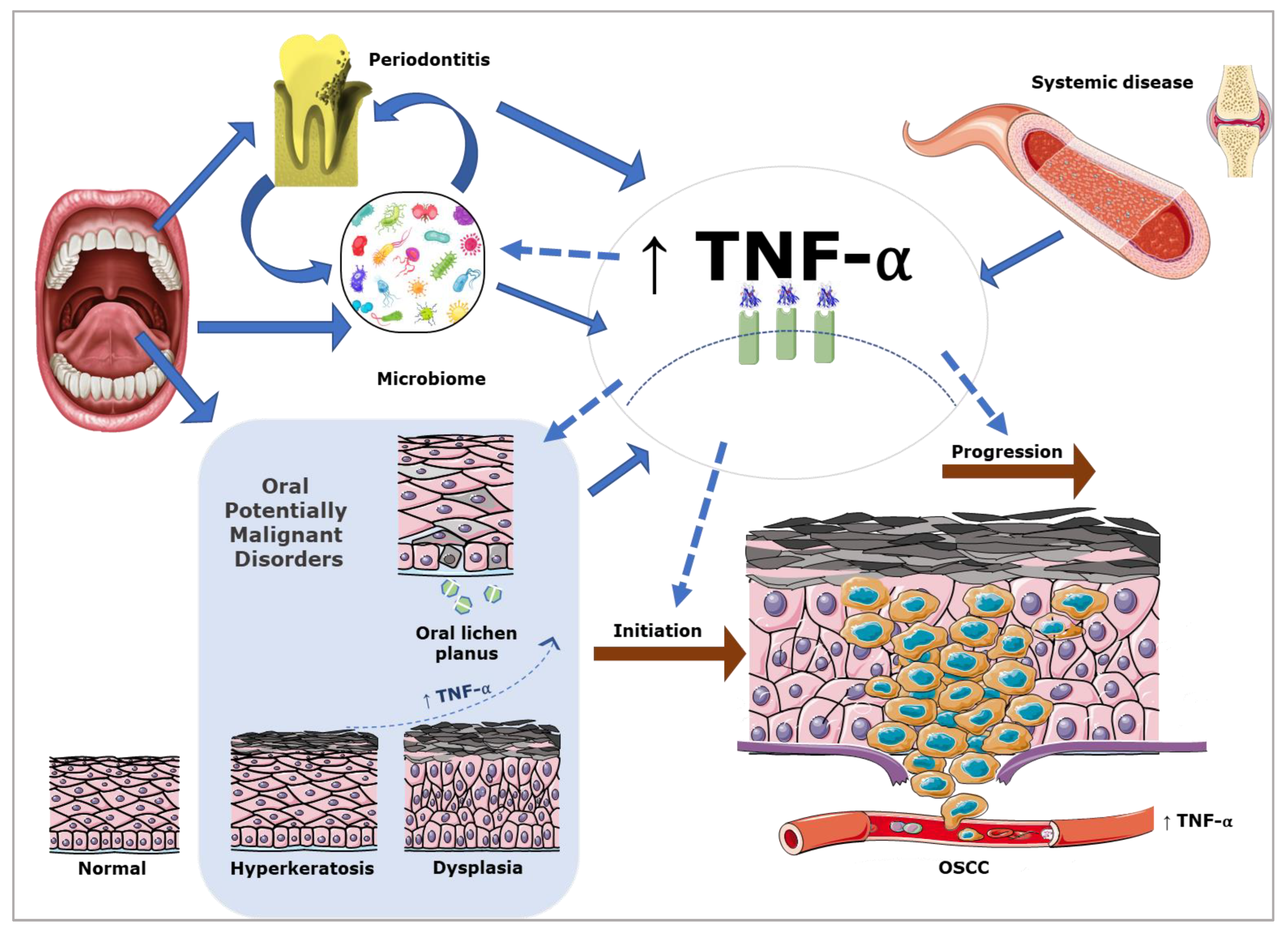
3. The Role of TNF-α in the Aetiology of OSCC
4. The Role of TNF-α in the Promotion of OSCC
5. OSCC Prognosis and TNF-α
6. TNF-α as a Biomarker in OSCC
| Outcome | Sample Type | Sensitivity/Specificity of Test | Levels Reported |
|---|---|---|---|
| Diagnosis and levels | |||
| Normal | Saliva | Sensitivity 39%, Specificity 100% for diagnosis of OSCC [67] | 4.5 ± 2.5 pg/mL [71] 38 ± 3.23 pg/mL [75] 3.0 ± 1.9 pg/mL [38] 4.1 ± 2.1 pg/mL [74] 8.6 ± 7.27 pg/mL [67] |
| Blood (Serum or Plasma) | Sensitivity 39%, Specificity 100% for diagnosis of OSCC [67] | 3.9 ± 2 pg/mL [71] 12.7 ± 4.89 pg/mL [77] 10.10 ± 6.08 pg/mL [67] | |
| Premalignant lesion | Saliva | Not described | 136.8 ± 59.6pg/mL [71] 30 ± 3.01 pg/mL [75] 10.5 ± 7.4 pg/mL [38] |
| Blood (Serum or Plasma) | Not described | 180.1 ± 52.4pg/mL [71] | |
| Premalignant disease | Saliva | Sensitivity 97%, Specificity 83% for diagnosis of OSCC [71] | 126.8 ± 59.2 pg/mL [71] |
| Blood (Serum or Plasma) | Sensitivity 72%, Specificity 75% for diagnosis of OSCC [71] | 166.5 ± 49.4 pg/mL [71] | |
| OSCC | Saliva | 311.9 ± 95.3 pg/mL [71] 34 ± 21.58 pg/mL [75] 28.9 ± 14.6 pg/mL [38] 35.2 ± 51.8 pg/mL [74] 27.75 ± 30.94 pg/mL [67] | |
| Blood (Serum or Plasma) | 225.1 ± 99.9 pg/mL [71] 45.8 ± 37.01 pg/mL [77] 11.65 ± 7.32 pg/mL [67] | ||
7. The Oral Microbiome and TNF-α in OSCC
8. TNF-α in Oral Potentially Malignant Disorders
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- AIHW. 2021. Available online: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/summary (accessed on 25 March 2022).
- Shield, K.D.; Ferlay, J.; Jemal, A.; Sankaranarayanan, R.; Chaturvedi, A.K.; Bray, F.; Soerjomataram, I. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J. Clin. 2017, 67, 51–64. [Google Scholar] [CrossRef]
- DeCoro, M.; Wilder-Smith, P. Potential of optical coherence tomography for early diagnosis of oral malignancies. Expert Rev Anticancer Ther. 2010, 10, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Niklander, S.E. Inflammatory Mediators in Oral Cancer: Pathogenic Mechanisms and Diagnostic Potential. Front. Oral Health 2021, 2, 642238. [Google Scholar] [CrossRef]
- Rao, S.K.; Pavicevic, Z.; Du, Z.; Kim, J.-G.; Fan, M.; Jiao, Y.; Rosebush, M.; Samant, S.; Gu, W.; Pfeffer, L.M.; et al. Pro-inflammatory genes as biomarkers and therapeutic targets in oral squamous cell carcinoma. J. Biol. Chem. 2010, 285, 32512–32521. [Google Scholar] [CrossRef] [PubMed]
- Glogauer, J.E.; Sun, C.X.; Bradley, G.; Magalhaes, M.A. Neutrophils Increase Oral Squamous Cell Carcinoma Invasion through an Invadopodia-Dependent Pathway. Cancer Immunol. Res. 2015, 3, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Goertzen, C.; Mahdi, H.; Laliberte, C.; Meirson, T.; Eymael, D.; Gil-Henn, H.; Magalhaes, M. Oral inflammation promotes oral squamous cell carcinoma invasion. Oncotarget 2018, 9, 29047–29063. [Google Scholar] [CrossRef]
- Epstein Shochet, G.; Brook, E.; Israeli-Shani, L.; Edelstein, E.; Shitrit, D. Fibroblast paracrine TNF-α signaling elevates integrin A5 expression in idiopathic pulmonary fibrosis (IPF). Respir. Res. 2017, 18, 122. [Google Scholar] [CrossRef]
- van Horssen, R.; Ten Hagen, T.L.; Eggermont, A.M. TNF-alpha in cancer treatment: Molecular insights, antitumor effects, and clinical utility. Oncologist 2006, 11, 397–408. [Google Scholar] [CrossRef]
- Lucas, R.; Hadizamani, Y.; Enkhbaatar, P.; Csanyi, G.; Caldwell, R.W.; Hundsberger, H.; Sridhar, S.; Lever, A.A.; Hudel, M.; Ash, D.; et al. Dichotomous Role of Tumor Necrosis Factor in Pulmonary Barrier Function and Alveolar Fluid Clearance. Front. Physiol. 2021, 12, 793251. [Google Scholar] [CrossRef]
- Wajant, H.; Beilhack, A. Targeting Regulatory T Cells by Addressing Tumor Necrosis Factor and Its Receptors in Allogeneic Hematopoietic Cell Transplantation and Cancer. Front. Immunol. 2019, 10, 2040. [Google Scholar] [CrossRef]
- Horiuchi, T.; Mitoma, H.; Harashima, S.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-alpha: Structure, function and interaction with anti-TNF agents. Rheumatology 2010, 49, 1215–1228. [Google Scholar] [CrossRef]
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the Control of the Life and Death Balance of Macrophages. Front. Cell Dev. Biol. 2019, 7, 91. [Google Scholar] [CrossRef]
- Sheng, Y.; Li, F.; Qin, Z. TNF Receptor 2 Makes Tumor Necrosis Factor a Friend of Tumors. Front. Immunol. 2018, 9, 1170. [Google Scholar] [CrossRef] [PubMed]
- Mercogliano, M.F.; Bruni, S.; Mauro, F.; Elizalde, P.V.; Schillaci, R. Harnessing Tumor Necrosis Factor Alpha to Achieve Effective Cancer Immunotherapy. Cancers 2021, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Cruceriu, D.; Baldasici, O.; Balacescu, O.; Berindan-Neagoe, I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: Molecular insights and therapeutic approaches. Cell Oncol. 2020, 43, 1–18. [Google Scholar] [CrossRef]
- Zhao, C.; Lu, X.; Bu, X.; Zhang, N.; Wang, W. Involvement of tumor necrosis factor-alpha in the upregulation of CXCR4 expression in gastric cancer induced by Helicobacter pylori. BMC Cancer 2010, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Egberts, J.H.; Cloosters, V.; Noack, A.; Schniewind, B.; Thon, L.; Klose, S.; Kettler, B.; von Forstner, C.; Kneitz, C.; Tepel, J.; et al. Anti-tumor necrosis factor therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2008, 68, 1443–1450. [Google Scholar] [CrossRef]
- Gupta, M.; Babic, A.; Beck, A.H.; Terry, K. TNF-α expression, risk factors, and inflammatory exposures in ovarian cancer: Evidence for an inflammatory pathway of ovarian carcinogenesis? Hum. Pathol. 2016, 54, 82–91. [Google Scholar] [CrossRef]
- Morgado, M.; Sutton, M.N.; Simmons, M.; Warren, C.R.; Lu, Z.; Constantinou, P.E.; Liu, J.; Francis, L.L.; Conlan, R.S.; Bast, R.C., Jr.; et al. Tumor necrosis factor-α and interferon-γ stimulate MUC16 (CA125) expression in breast, endometrial and ovarian cancers through NFκB. Oncotarget 2016, 7, 14871–14884. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Sethi, G.; Shanmugam, M.K.; Ramachandran, L.; Kumar, A.P.; Tergaonkar, V. Multifaceted link between cancer and inflammation. Biosci. Rep. 2012, 32, 1–15. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Ren, H.; Ma, J.; Sun, X.; Li, N.; Liu, C.; Huang, K.; Xu, M.; Ming, L. Molecular correlates and prognostic value of tmTNF-α expression in colorectal cancer of 5-Fluorouracil-Based Adjuvant Therapy. Cancer Biol. Ther. 2016, 17, 684–692. [Google Scholar] [CrossRef]
- Tang, D.; Tao, D.; Fang, Y.; Deng, C.; Xu, Q.; Zhou, J. TNF-Alpha Promotes Invasion and Metastasis via NF-Kappa B Pathway in Oral Squamous Cell Carcinoma. Med. Sci. Monit. Basic Res. 2017, 23, 141–149. [Google Scholar] [CrossRef]
- Roberts, R.A.; Kimber, I. Cytokines in non-genotoxic hepatocarcinogenesis. Carcinogenesis 1999, 20, 1397–1401. [Google Scholar] [CrossRef]
- Qu, Y.; Zhao, G.; Li, H. Forward and Reverse Signaling Mediated by Transmembrane Tumor Necrosis Factor-Alpha and TNF Receptor 2: Potential Roles in an Immunosuppressive Tumor Microenvironment. Front. Immunol. 2017, 8, 1675. [Google Scholar] [CrossRef]
- Kriegler, M.; Perez, C.; DeFay, K.; Albert, I.; Lu, S.D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: Ramifications for the complex physiology of TNF. Cell 1988, 53, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.; Albert, I.; DeFay, K.; Zachariades, N.; Gooding, L.; Kriegler, M. A nonsecretable cell surface mutant of tumor necrosis factor (TNF) kills by cell-to-cell contact. Cell 1990, 63, 251–258. [Google Scholar] [CrossRef]
- Burow, M.E.; Weldon, C.B.; Tang, Y.; Navar, G.L.; Krajewski, S.; Reed, J.C.; Hammond, T.G.; Clejan, S.; Beckman, B.S. Differences in susceptibility to tumor necrosis factor alpha-induced apoptosis among MCF-7 breast cancer cell variants. Cancer Res. 1998, 58, 4940–4946. [Google Scholar] [PubMed]
- Mei, Z.; Huang, J.; Qiao, B.; Lam, A.K. Immune checkpoint pathways in immunotherapy for head and neck squamous cell carcinoma. Int. J. Oral Sci. 2020, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef]
- Zandberg, D.P.; Strome, S.E. The role of the PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 2014, 50, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liu, X.; Song, J.H.; Cheng, Y.; Liu, Y.; Jia, Y.; Meltzer, S.J.; Wang, Z. TNFAIP8 overexpression: A potential predictor of lymphatic metastatic recurrence in pN0 esophageal squamous cell carcinoma after Ivor Lewis esophagectomy. Tumour Biol. 2016, 37, 10923–10934. [Google Scholar] [CrossRef]
- Arnott, C.H.; Scott, K.A.; Moore, R.J.; Robinson, S.C.; Thompson, R.G.; Balkwill, F.R. Expression of both TNF-alpha receptor subtypes is essential for optimal skin tumour development. Oncogene 2004, 23, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Gubernatorova, E.O.; Polinova, A.I.; Petropavlovskiy, M.M.; Namakanova, O.A.; Medvedovskaya, A.D.; Zvartsev, R.V.; Telegin, G.B.; Drutskaya, M.S.; Nedospasov, S.A. Dual Role of TNF and LTα in Carcinogenesis as Implicated by Studies in Mice. Cancers 2021, 13, 1775. [Google Scholar] [CrossRef]
- Serefoglou, Z.; Yapijakis, C.; Nkenke, E.; Vairaktaris, E. Genetic association of cytokine DNA polymorphisms with head and neck cancer. Oral Oncol. 2008, 44, 1093–1099. [Google Scholar] [CrossRef]
- Juretić, M.; Cerović, R.; Belušić-Gobić, M.; Brekalo Pršo, I.; Kqiku, L.; Špalj, S.; Pezelj-Ribarić, S. Salivary levels of TNF-α and IL-6 in patients with oral premalignant and malignant lesions. Folia Biol. 2013, 59, 99–102. [Google Scholar]
- Rhodus, N.L.; Ho, V.; Miller, C.S.; Myers, S.; Ondrey, F. NF-kappaB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect. Prev. 2005, 29, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, G.; Nandan, S.R.K.; Kulkarni, P.G. Salivary Tumour Necrosis Factor-α as a Biomarker in Oral Leukoplakia and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2019, 20, 2087–2093. [Google Scholar] [CrossRef]
- Baldwin, A.S. Regulation of cell death and autophagy by IKK and NF-κB: Critical mechanisms in immune function and cancer. Immunol. Rev. 2012, 246, 327–345. [Google Scholar] [CrossRef]
- Chu, Z.L.; McKinsey, T.A.; Liu, L.; Gentry, J.J.; Malim, M.H.; Ballard, D.W. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc. Natl. Acad. Sci. USA 1997, 94, 10057–10062. [Google Scholar] [CrossRef]
- Barberà, M.J.; Puig, I.; Domínguez, D.; Julien-Grille, S.; Guaita-Esteruelas, S.; Peiró, S.; Baulida, J.; Francí, C.; Dedhar, S.; Larue, L.; et al. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene 2004, 23, 7345–7354. [Google Scholar] [CrossRef]
- Chua, H.L.; Bhat-Nakshatri, P.; Clare, S.E.; Morimiya, A.; Badve, S.; Nakshatri, H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: Potential involvement of ZEB-1 and ZEB-2. Oncogene 2007, 26, 711–724. [Google Scholar] [CrossRef]
- Pham, C.G.; Bubici, C.; Zazzeroni, F.; Knabb, J.R.; Papa, S.; Kuntzen, C.; Franzoso, G. Upregulation of Twist-1 by NF-kappaB blocks cytotoxicity induced by chemotherapeutic drugs. Mol. Cell. Biol. 2007, 27, 3920–3935. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Allison, D.F.; Baranova, N.N.; Wamsley, J.J.; Katz, A.J.; Bekiranov, S.; Jones, D.R.; Mayo, M.W. NF-κB regulates mesenchymal transition for the induction of non-small cell lung cancer initiating cells. PLoS ONE 2013, 8, e68597. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.W.; Karakasheva, T.A.; Hicks, P.D.; Bass, A.J.; Rustgi, A.K. The tumor microenvironment in esophageal cancer. Oncogene 2016, 35, 5337–5349. [Google Scholar] [CrossRef]
- Semenza, G.L.; Ruvolo, P.P. Introduction to tumor microenvironment regulation of cancer cell survival, metastasis, inflammation, and immune surveillance. Biochim. Biophys. Acta 2016, 1863, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.Y.; Tang, S.J.; Chuang, M.J.; Cha, T.L.; Li, J.Y.; Sun, G.H.; Sun, K.H. TNF-α induces epithelial-mesenchymal transition of renal cell carcinoma cells via a GSK3β-dependent mechanism. Mol. Cancer Res. 2012, 10, 1109–1119. [Google Scholar] [CrossRef]
- Sun, Z.; Meng, Y.; Liu, G.; Jiang, Y.; Meng, Q.; Hu, S. Effect of interleukin-1β and tumor necrosis factor α gene silencing on mouse gastric cancer cell proliferation and migration. Oncol. Lett. 2016, 11, 2559–2565. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Qian, Y.; Dai, X.; Yang, L.; Chen, J.; Guo, S.; Hisamitsu, T. Antimetastatic effects of Celastrus orbiculatus on human gastric adenocarcinoma by inhibiting epithelial-mesenchymal transition and NF-κB/snail signaling pathway. Integr. Cancer Ther. 2015, 14, 271–281. [Google Scholar] [CrossRef]
- Furuta, H.; Osawa, K.; Shin, M.; Ishikawa, A.; Matsuo, K.; Khan, M.; Aoki, K.; Ohya, K.; Okamoto, M.; Tominaga, K.; et al. Selective inhibition of NF-κB suppresses bone invasion by oral squamous cell carcinoma in vivo. Int. J. Cancer 2012, 131, E625–E635. [Google Scholar] [CrossRef]
- Alves, A.; Diel, L.; Ramos, G.; Pinto, A.; Bernardi, L.; Yates, J., 3rd; Lamers, M. Tumor microenvironment and Oral Squamous Cell Carcinoma: A crosstalk between the inflammatory state and tumor cell migration. Oral Oncol. 2021, 112, 105038. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.P.; Gao, Z.L.; Zhou, M.L.; He, M.Y.; Xu, X.H.; Tao, D.T.; Yang, C.C.; Liu, L.K. Snail interacts with Id2 in the regulation of TNF-α-induced cancer cell invasion and migration in OSCC. Am. J. Cancer Res. 2015, 5, 1680–1691. [Google Scholar]
- Brinkman, B.M.; Wong, D.T. Disease mechanism and biomarkers of oral squamous cell carcinoma. Curr. Opin. Oncol. 2006, 18, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Almadori, G.; Bussu, F.; Paludetti, G. Should there be more molecular staging of head and neck cancer to improve the choice of treatments and thereby improve survival? Curr. Opin. Otolaryngol. Head Neck Surg. 2008, 16, 117–126. [Google Scholar] [CrossRef]
- Bradley, P.J.; MacLennan, K.; Brakenhoff, R.H.; Leemans, C.R. Status of primary tumour surgical margins in squamous head and neck cancer: Prognostic implications. Curr. Opin. Otolaryngol. Head Neck Surg 2007, 15, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, E.L.; Matrisian, L.M. Matrix metalloproteases in head and neck cancer. Head Neck 2006, 28, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef]
- Yamamoto, S.; Higuchi, Y.; Yoshiyama, K.; Shimizu, E.; Kataoka, M.; Hijiya, N.; Matsuura, K. ADAM family proteins in the immune system. Immunol. Today 1999, 20, 278–284. [Google Scholar] [CrossRef]
- Black, R.A. Tumor necrosis factor-alpha converting enzyme. Int. J. Biochem. Cell Biol. 2002, 34, 1–5. [Google Scholar] [CrossRef]
- Ge, L.; Baskic, D.; Basse, P.; Vujanovic, L.; Unlu, S.; Yoneyama, T.; Vujanovic, A.; Han, J.; Bankovic, D.; Szczepanski, M.J.; et al. Sheddase activity of tumor necrosis factor-alpha converting enzyme is increased and prognostically valuable in head and neck cancer. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2913–2922. [Google Scholar] [CrossRef]
- Santana, I.T.S.; Dos Santos, J.N.A.; de Almeida, V.L.; Ferreira, W.N.S.; Santos, E.M.; de Almeida Freitas, R.; Pinto, C.C.K.; de Carvalho Barreto, I.D.; de Matos, F.R. Association of PON1, TNF-α and TGF-β gene polymorphisms with prognosis in oral and oropharyngeal squamous cell carcinoma. Acta Odontol. Scand. 2021, 79, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ren, Y.; Dai, Z.J.; Wu, C.J.; Ji, Y.H.; Xu, J. IL-6, IL-8 and TNF-α levels correlate with disease stage in breast cancer patients. Adv. Clin. Exp. Med. 2017, 26, 421–426. [Google Scholar] [CrossRef]
- Xiao, Z.; Nie, K.; Han, T.; Cheng, L.; Zhang, Z.; Peng, W.; Shi, D. Development and Validation of a TNF Family-Based Signature for Predicting Prognosis, Tumor Immune Characteristics, and Immunotherapy Response in Colorectal Cancer Patients. J. Immunol. Res. 2021, 2021, 6439975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, P.; Zhang, C.; Luo, Y.; Zhang, G.; Zeng, Q.; Wang, L.; Yang, Z.; Sun, N.; He, J. Tumor Necrosis Factor Family Member Profile Predicts Prognosis and Adjuvant Chemotherapy Benefit for Patients With Small-Cell Lung Cancer. Front. Immunol. 2021, 12, 745769. [Google Scholar] [CrossRef] [PubMed]
- Caporossi, L.; Santoro, A.; Papaleo, B. Saliva as an analytical matrix: State of the art and application for biomonitoring. Biomarkers 2010, 15, 475–487. [Google Scholar] [CrossRef]
- Lee, L.T.; Wong, Y.K.; Hsiao, H.Y.; Wang, Y.W.; Chan, M.Y.; Chang, K.W. Evaluation of saliva and plasma cytokine biomarkers in patients with oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2018, 47, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Dikova, V.; Jantus-Lewintre, E.; Bagan, J. Potential Non-Invasive Biomarkers for Early Diagnosis of Oral Squamous Cell Carcinoma. J. Clin. Med. 2021, 10, 1658. [Google Scholar] [CrossRef]
- Aziz, S.; Ahmed, S.S.; Ali, A.; Khan, F.A.; Zulfiqar, G.; Iqbal, J.; Khan, A.A.; Shoaib, M. Salivary Immunosuppressive Cytokines IL-10 and IL-13 Are Significantly Elevated in Oral Squamous Cell Carcinoma Patients. Cancer Investig. 2015, 33, 318–328. [Google Scholar] [CrossRef]
- Dineshkumar, T.; Ashwini, B.K.; Rameshkumar, A.; Rajashree, P.; Ramya, R.; Rajkumar, K. Salivary and Serum Interleukin-6 Levels in Oral Premalignant Disorders and Squamous Cell Carcinoma: Diagnostic Value and Clinicopathologic Correlations. Asian Pac. J. Cancer Prev. 2016, 17, 4899–4906. [Google Scholar] [CrossRef]
- Krishnan, R.; Thayalan, D.K.; Padmanaban, R.; Ramadas, R.; Annasamy, R.K.; Anandan, N. Association of serum and salivary tumor necrosis factor-α with histological grading in oral cancer and its role in differentiating premalignant and malignant oral disease. Asian Pac. J. Cancer Prev. 2014, 15, 7141–7148. [Google Scholar] [CrossRef]
- Brinkmann, O.; Zhang, L.; Giannobile, W.V.; Wong, D.T. Salivary biomarkers for periodontal disease diagnostics. Expert Opin. Med. Diagn. 2011, 5, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Brailo, V.; Vucićević-Boras, V.; Cekić-Arambasin, A.; Alajbeg, I.Z.; Milenović, A.; Lukac, J. The significance of salivary interleukin 6 and tumor necrosis factor alpha in patients with oral leukoplakia. Oral Oncol. 2006, 42, 370–373. [Google Scholar] [CrossRef] [PubMed]
- SahebJamee, M.; Eslami, M.; AtarbashiMoghadam, F.; Sarafnejad, A. Salivary concentration of TNFalpha, IL1 alpha, IL6, and IL8 in oral squamous cell carcinoma. Med. Oral Patol. Oral Cir. Bucal. 2008, 13, E292–E295. [Google Scholar] [PubMed]
- Brailo, V.; Vucicevic-Boras, V.; Lukac, J.; Biocina-Lukenda, D.; Zilic-Alajbeg, I.; Milenovic, A.; Balija, M. Salivary and serum interleukin 1 beta, interleukin 6 and tumor necrosis factor alpha in patients with leukoplakia and oral cancer. Med. Oral Patol. Oral Cir. Bucal. 2012, 17, e10–e15. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Pezzi, M.E.; Cassi, D.; Pertinhez, T.A.; Spisni, A.; Meleti, M. Salivary Cytokines as Biomarkers for Oral Squamous Cell Carcinoma: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 6795. [Google Scholar] [CrossRef]
- Jablonska, E.; Piotrowski, L.; Grabowska, Z. Serum Levels of IL-1b, IL-6, TNF-a, sTNF-RI and CRP in Patients with Oral Cavity Cancer. Pathol. Oncol. Res. 1997, 3, 126–129. [Google Scholar] [CrossRef]
- Schiegnitz, E.; Kämmerer, P.W.; Schön, H.; Blatt, S.; Berres, M.; Sagheb, K.; Al-Nawas, B. Proinflammatory cytokines as serum biomarker in oral carcinoma-A prospective multi-biomarker approach. J. Oral Pathol. Med. 2018, 47, 268–274. [Google Scholar] [CrossRef]
- Skrinjar, I.; Brailo, V.; Vidovic-Juras, D.; Vucicevic-Boras, V.; Milenovic, A. Evaluation of pretreatment serum interleukin-6 and tumour necrosis factor alpha as a potential biomarker for recurrence in patients with oral squamous cell carcinoma. Med. Oral Patol. Oral Cir. Bucal. 2015, 20, e402–e407. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Banerjee, A.; Saikia, N.; Phookan, J.; Baruah, M.N.; Baruah, S. Negative regulation of natural killer cell in tumor tissue and peripheral blood of oral squamous cell carcinoma. Cytokine 2015, 76, 123–130. [Google Scholar] [CrossRef]
- Babiuch, K.; Kuśnierz-Cabala, B.; Kęsek, B.; Okoń, K.; Darczuk, D.; Chomyszyn-Gajewska, M. Evaluation of Proinflammatory, NF-kappaB Dependent Cytokines: IL-1α, IL-6, IL-8, and TNF-α in Tissue Specimens and Saliva of Patients with Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders. J. Clin. Med. 2020, 9, 867. [Google Scholar] [CrossRef]
- Chen, T.; Yu, W.H.; Izard, J.; Baranova, O.V.; Lakshmanan, A.; Dewhirst, F.E. The Human Oral Microbiome Database: A web accessible resource for investigating oral microbe taxonomic and genomic information. Database J. Biol. Databases Curation 2010, 2010, baq013. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of Oral Microbiome Signatures in Diagnosis and Prognosis of Oral Cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; Godoy-Vitorino, F.; Jedlicka, A.; Rodríguez-Hilario, A.; González, H.; Bondy, J.; Lawson, F.; Folawiyo, O.; Michailidi, C.; Dziedzic, A.; et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget 2016, 7, 51320–51334. [Google Scholar] [CrossRef]
- Perera, M.; Al-Hebshi, N.N.; Perera, I.; Ipe, D.; Ulett, G.C.; Speicher, D.J.; Chen, T.; Johnson, N.W. A dysbiotic mycobiome dominated by Candida albicans is identified within oral squamous-cell carcinomas. J. Oral Microbiol. 2017, 9, 1385369. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Yeh, Y.-M.; Yu, H.-Y.; Chin, C.-Y.; Hsu, C.-W.; Liu, H.; Huang, P.-J.; Hu, S.-N.; Liao, C.-T.; Chang, K.-P.; et al. Oral Microbiota Community Dynamics Associated With Oral Squamous Cell Carcinoma Staging. Front. Microbiol. 2018, 9, 862. [Google Scholar] [CrossRef]
- Sami, A.; Elimairi, I.; Stanton, C.; Ross, R.P.; Ryan, C.A. The Role of the Microbiome in Oral Squamous Cell Carcinoma with Insight into the Microbiome-Treatment Axis. Int. J. Mol. Sci. 2020, 21, 8061. [Google Scholar] [CrossRef] [PubMed]
- Szlosarek, P.; Charles, K.A.; Balkwill, F.R. Tumour necrosis factor-alpha as a tumour promoter. Eur. J. Cancer 2006, 42, 745–750. [Google Scholar] [CrossRef]
- Ha, N.H.; Woo, B.H.; Kim, D.J.; Ha, E.S.; Choi, J.I.; Kim, S.J.; Park, B.S.; Lee, J.H.; Park, H.R. Prolonged and repetitive exposure to Porphyromonas gingivalis increases aggressiveness of oral cancer cells by promoting acquisition of cancer stem cell properties. Tumour Biol. 2015, 36, 9947–9960. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, A.A.; Shelton, R.M.; Landini, G.; Cooper, P.R.; Milward, M.R. Periodontal pathogens promote epithelial-mesenchymal transition in oral squamous carcinoma cells in vitro. Cell Adhes. Migr. 2018, 12, 127–137. [Google Scholar] [CrossRef]
- Binder Gallimidi, A.; Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.M.; Nussbaum, G.; Elkin, M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 2015, 6, 22613–22623. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, F.; Iwamoto, Y.; Mineshiba, J.; Shimizu, A.; Soga, Y.; Murayama, Y. Periodontal disease and diabetes mellitus: The role of tumor necrosis factor-alpha in a 2-way relationship. J. Periodontol. 2003, 74, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, F.; Saccucci, M.; Di Carlo, G.; Lucchetti, R.; Pilloni, A.; Pranno, N.; Luzzi, V.; Valesini, G.; Polimeni, A. Periodontitis and Rheumatoid Arthritis: The Same Inflammatory Mediators? Mediat. Inflamm 2019, 2019, 6034546. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Wang, Q.; Chen, Q. The cytokine network involved in the host immune response to periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef]
- Yao, Y.; Shen, X.; Zhou, M.; Tang, B. Periodontal Pathogens Promote Oral Squamous Cell Carcinoma by Regulating ATR and NLRP3 Inflammasome. Front. Oncol. 2021, 11, 722797. [Google Scholar] [CrossRef]
- Vadovics, M.; Ho, J.; Igaz, N.; Alföldi, R.; Rakk, D.; Veres, É.; Szücs, B.; Horváth, M.; Tóth, R.; Szücs, A.; et al. Candida albicans Enhances the Progression of Oral Squamous Cell Carcinoma In Vitro and In Vivo. Mbio 2022, 13, e03144-21. [Google Scholar] [CrossRef]
- Alnuaimi, A.D.; Wiesenfeld, D.; O’Brien-Simpson, N.M.; Reynolds, E.C.; McCullough, M.J. Oral Candida colonization in oral cancer patients and its relationship with traditional risk factors of oral cancer: A matched case-control study. Oral Oncol. 2015, 51, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Orozco, A.S.; Zhou, X.; Filler, S.G. Mechanisms of the proinflammatory response of endothelial cells to Candida albicans infection. Infect. Immun. 2000, 68, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Tanimoto, T.; Taniai, M.; Taniguchi, M.; Ariyasu, T.; Arai, S.; Ohta, T.; Fukuda, S. Regulation of Candida albicans morphogenesis by tumor necrosis factor-alpha and potential for treatment of oral candidiasis. In Vivo 2007, 21, 25–32. [Google Scholar]
- Chadwick, J.W.; Macdonald, R.; Ali, A.A.; Glogauer, M.; Magalhaes, M.A. TNFα Signaling Is Increased in Progressing Oral Potentially Malignant Disorders and Regulates Malignant Transformation in an Oral Carcinogenesis Model. Front. Oncol. 2021, 11, 741013. [Google Scholar] [CrossRef]
- Kaur, J.; Jacobs, R. Proinflammatory cytokine levels in oral lichen planus, oral leukoplakia, and oral submucous fibrosis. J. Korean Assoc. Oral Maxillofac. Surg. 2015, 41, 171–175. [Google Scholar] [CrossRef]
- Hsu, H.J.; Yang, Y.H.; Shieh, T.Y.; Chen, C.H.; Kao, Y.H.; Yang, C.F.; Ko, E.C. Role of cytokine gene (interferon-γ, transforming growth factor-β1, tumor necrosis factor-α, interleukin-6, and interleukin-10) polymorphisms in the risk of oral precancerous lesions in Taiwanese. Kaohsiung J. Med. Sci. 2014, 30, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Sugermann, P.B.; Savage, N.W.; Seymour, G.J.; Walsh, L.J. Is there a role for tumor necrosis factor-alpha (TNF-alpha) in oral lichen planus? J. Oral Pathol. Med. 1996, 25, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Agha-Hosseini, F.; Moosavi, M.-S.; Sheykhbahaei, N. Association of Oral Lichen Planus and Its Treatment on Tumor Necrosis Factor-Alpha: A Review Literature and Meta-analysis. Middle East J. Rehabil. Health Stud. 2021, 8, e109577. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; Manzano-Moreno, F.J.; Ruiz, C.; Illescas-Montes, R. Salivary Biomarkers and Their Application in the Diagnosis and Monitoring of the Most Common Oral Pathologies. Int. J. Mol. Sci. 2020, 21, 5173. [Google Scholar] [CrossRef]
- Braybrooke, J.P.; Slade, A.; Deplanque, G.; Harrop, R.; Madhusudan, S.; Forster, M.D.; Gibson, R.; Makris, A.; Talbot, D.C.; Steiner, J.; et al. Phase I study of MetXia-P450 gene therapy and oral cyclophosphamide for patients with advanced breast cancer or melanoma. Clin. Cancer Res. 2005, 11, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
| TNF-α Inhibitor | Mechanism of Action and Clinical Translation |
|---|---|
| ADALIMUMAB | Monoclonal antibody with TNF-α as a target. No clinical studies at present. |
| ETANERCEPT | Fusion protein produced by recombinant DNA. Reduces the efficacy of TNF and works as a TNF antagonist. No clinical studies at present. |
| GOLIMUMAB | Monoclonal antibody with TNF-α as a target. The TNF-α antagonist golimumab has been assessed in an experimental metastatic murine model in vivo, specifically using OSCC cells depleted of interferon induced protein with tetratricopeptide repeats 2 (IFIT2), a protein known to promote cell death via apoptosis. TNF-α antagonists reduced angiogenesis, tumour growth, and metastasis [106]. |
| CERTOLIZUMAB | Pegylated monoclonal antibody directed against TNF-α. No clinical studies at present. |
| INFLIXIMAB | Chrimeric monoclonal antibody directed against TNF-α. No clinical studies at present. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brierly, G.; Celentano, A.; Breik, O.; Moslemivayeghan, E.; Patini, R.; McCullough, M.; Yap, T. Tumour Necrosis Factor Alpha (TNF-α) and Oral Squamous Cell Carcinoma. Cancers 2023, 15, 1841. https://doi.org/10.3390/cancers15061841
Brierly G, Celentano A, Breik O, Moslemivayeghan E, Patini R, McCullough M, Yap T. Tumour Necrosis Factor Alpha (TNF-α) and Oral Squamous Cell Carcinoma. Cancers. 2023; 15(6):1841. https://doi.org/10.3390/cancers15061841
Chicago/Turabian StyleBrierly, Gary, Antonio Celentano, Omar Breik, Elham Moslemivayeghan, Romeo Patini, Michael McCullough, and Tami Yap. 2023. "Tumour Necrosis Factor Alpha (TNF-α) and Oral Squamous Cell Carcinoma" Cancers 15, no. 6: 1841. https://doi.org/10.3390/cancers15061841
APA StyleBrierly, G., Celentano, A., Breik, O., Moslemivayeghan, E., Patini, R., McCullough, M., & Yap, T. (2023). Tumour Necrosis Factor Alpha (TNF-α) and Oral Squamous Cell Carcinoma. Cancers, 15(6), 1841. https://doi.org/10.3390/cancers15061841








