Subgroup Analysis of Overall Survival among Smoking and Non-Smoking Elderly Patients with HNSCC
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria and Data Acquisition
2.2. Diagnostic and Treatment Procedure
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
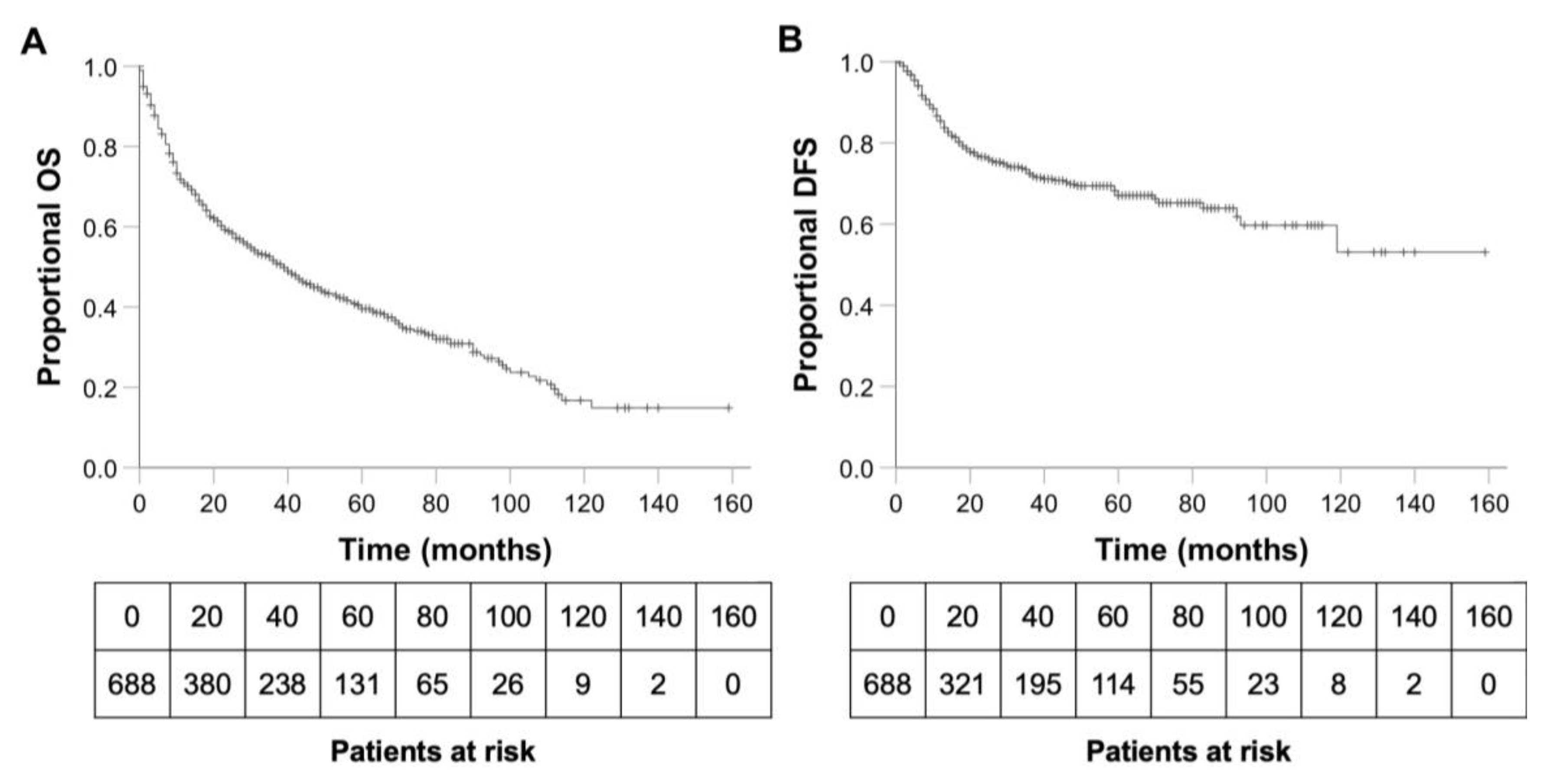
3.2. OS, DFS and Predictors of Survival
3.3. Predictors of a Negative Smoking Status in HNSCC Patients
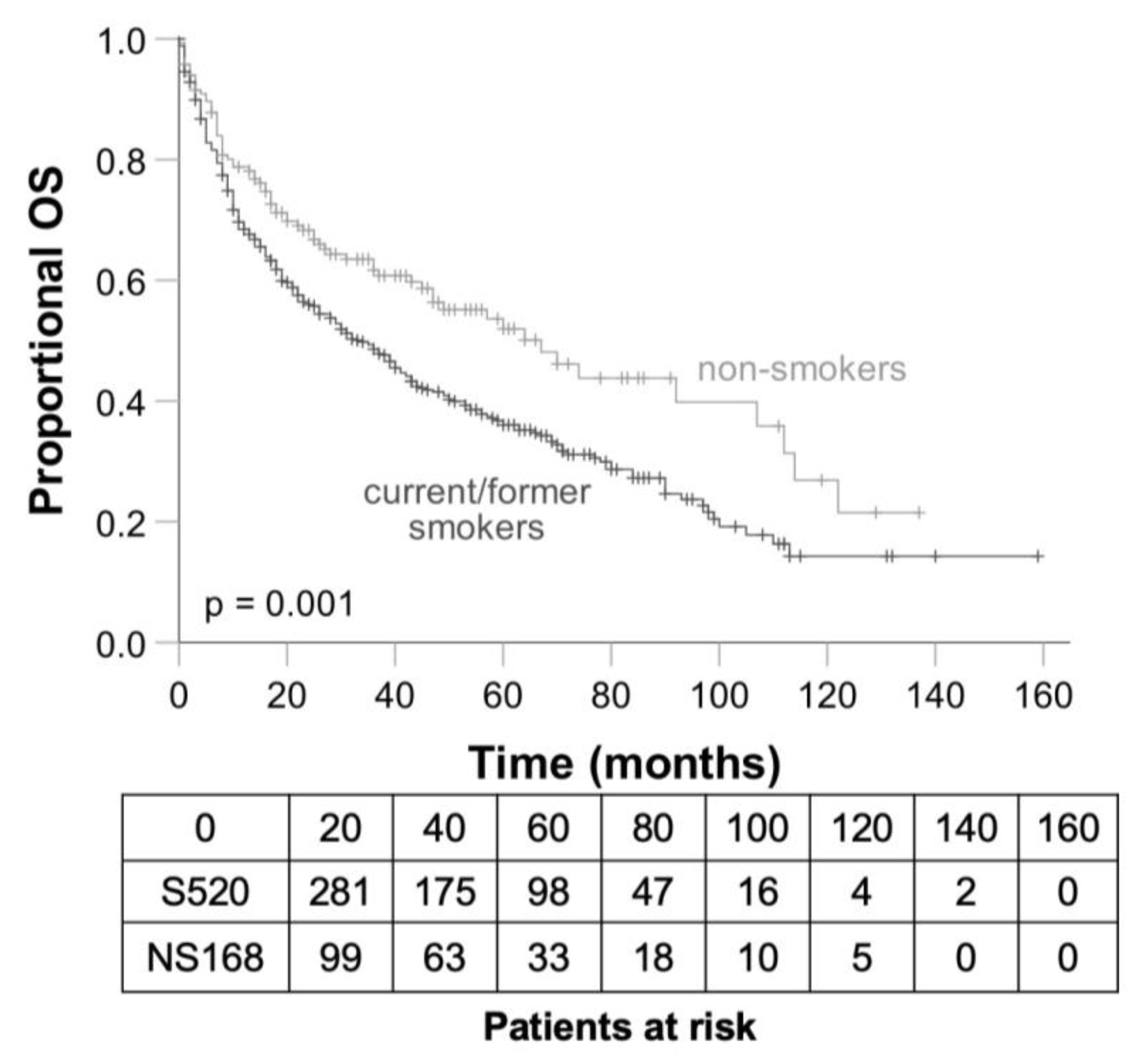
3.4. Differences between Smokers and Non-Smokers Concerning OS and DFS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, B.; Buttmann-Schweiger, N.; Dahm, S.; Fiebig, J.; Franke, M.; Gurung-Schönfeld, I.; Haberland, J.; Imhoff, M.; Kraywinkel, K.; Starker, A.; et al. Mundhöhle und Rachen (C00-C14). In Krebs in Deutschland für 2017/2018, 13th ed.; Gesellschaft der epidemiologischen Krebsregister in Deutschland, E.V., Ed.; Robert Koch-Institut: Berlin, Germny, 2021; pp. 32–35. [Google Scholar]
- The World Bank. Life Expectancy at Birth, Total (Years), European Union. Available online: https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=EU&name_desc=false (accessed on 31 March 2021).
- Smith, B.D.; Smith, G.L.; Hurria, A.; Hortobagyi, G.N.; Buchholz, T.A. Future of cancer incidence in the United States: Burdens upon an aging, changing nation. J. Clin. Oncol. 2009, 27, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Hurria, A.; Dale, W.; Mooney, M.; Rowland, J.H.; Ballman, K.V.; Cohen, H.J.; Muss, H.B.; Schilsky, R.L.; Ferrell, B.; Extermann, M.; et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J. Clin. Oncol. 2014, 32, 2587–2594. [Google Scholar] [CrossRef]
- Schmidt, B.L.; Dierks, E.J.; Homer, L.; Potter, B. Tobacco smoking history and presentation of oral squamous cell carcinoma. J. Oral. Maxillofac. Surg. 2004, 62, 1055–1058. [Google Scholar] [CrossRef]
- Morse, D.E.; Psoter, W.J.; Cleveland, D.; Cohen, D.; Mohit-Tabatabai, M.; Kosis, D.L.; Eisenberg, E. Smoking and drinking in relation to oral cancer and oral epithelial dysplasia. Cancer Causes Control 2007, 18, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M. 3. Cancers attributable to consumption of alcohol in the UK in 2010. Br. J. Cancer 2011, 105 (Suppl. S2), S14–S18. [Google Scholar] [CrossRef]
- Parkin, D.M. 2. Tobacco-attributable cancer burden in the UK in 2010. Br. J. Cancer 2011, 105 (Suppl. S2), S6–S13. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, A.; Breik, O.; Koo, K.; Iseli, T.; Nastri, A.; Fua, T.; Rischin, D.; McCullough, M.; Wiesenfeld, D. Non-smoking, non-drinking elderly females, a 5year follow-up of a clinically distinct cohort of oral squamous cell carcinoma patients. Oral. Oncol. 2018, 86, 113–120. [Google Scholar] [CrossRef]
- Andersen, A.O.; Jensen, J.S.; Jakobsen, K.K.; Stampe, H.; Nielsen, K.J.; Wessel, I.; Christensen, A.; Andersen, E.; Friborg, J.; Gronhoj, C.; et al. The impact of tobacco smoking on survival of patients with oral squamous cell carcinoma: A population-based retrospective study. Acta Oncol. 2022, 61, 449–458. [Google Scholar] [CrossRef]
- Dahlstrom, K.R.; Little, J.A.; Zafereo, M.E.; Lung, M.; Wei, Q.; Sturgis, E.M. Squamous cell carcinoma of the head and neck in never smoker-never drinkers: A descriptive epidemiologic study. Head Neck 2008, 30, 75–84. [Google Scholar] [CrossRef]
- Pytynia, K.B.; Grant, J.R.; Etzel, C.J.; Roberts, D.B.; Wei, Q.; Sturgis, E.M. Matched-pair analysis of survival of never smokers and ever smokers with squamous cell carcinoma of the head and neck. J. Clin. Oncol. 2004, 22, 3981–3988. [Google Scholar] [CrossRef] [PubMed]
- Fortin, A.; Wang, C.S.; Vigneault, E. Influence of smoking and alcohol drinking behaviors on treatment outcomes of patients with squamous cell carcinomas of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Fazel, A.; Quabius, E.S.; Gonzales-Donate, M.; Laudien, M.; Herzog, A.; Kress, K.; Schleicher, T.; Fabian, A.; Huber, K.; Hoffmann, M. Alteration of smoking habit at time of first diagnosis influences survival of patients with HNSCC. Mol. Clin. Oncol. 2020, 13, 50. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Mehanna, H.; Beech, T.; Nicholson, T.; El-Hariry, I.; McConkey, C.; Paleri, V.; Roberts, S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck 2013, 35, 747–755. [Google Scholar] [CrossRef]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-associated oropharyngeal cancer: Epidemiology, molecular biology and clinical management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef]
- Mahal, B.A.; Catalano, P.J.; Haddad, R.I.; Hanna, G.J.; Kass, J.I.; Schoenfeld, J.D.; Tishler, R.B.; Margalit, D.N. Incidence and Demographic Burden of HPV-Associated Oropharyngeal Head and Neck Cancers in the United States. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1660–1667. [Google Scholar] [CrossRef]
- Mendez, D.; Warner, K.E. Adult cigarette smoking prevalence: Declining as expected (not as desired). Am. J. Public Health 2004, 94, 251–252. [Google Scholar] [CrossRef]
- Farshadpour, F.; Hordijk, G.J.; Koole, R.; Slootweg, P.J. Non-smoking and non-drinking patients with head and neck squamous cell carcinoma: A distinct population. Oral. Dis. 2007, 13, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Oh, L.J.; Asher, R.; Veness, M.; Smee, R.; Goldstein, D.; Gopalakrishna Iyer, N.; Balasubramanian, D.; Low, T.H.; Palme, C.E.; Gupta, R.; et al. Effect of age and gender in non-smokers with oral squamous cell carcinoma: Multi-institutional study. Oral. Oncol. 2021, 116, 105210. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.; Barrowman, R.; McCullough, M.; Iseli, T.; Wiesenfeld, D. Non-smoking non-drinking elderly females: A clinically distinct subgroup of oral squamous cell carcinoma patients. Int. J. Oral. Maxillofac. Surg. 2013, 42, 929–933. [Google Scholar] [CrossRef]
- Statistisches Bundesamt. Gesundheitszustand Und -Relevantes Verhalten; Rauchgewohnheiten Nach Altersgruppen. Available online: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Gesundheit/Gesundheitszustand-Relevantes-Verhalten/Tabellen/rauchverhalten-insgesamt.html#fussnote-2-95630 (accessed on 30 October 2022).
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; John Wiley & Sons: Chichester, UK, 2017. [Google Scholar]
- Boeker, R.; Stromberger, C.; Heiland, M.; Beck-Broichsitter, B.; Hofmann, V.M.; Neumann, K.; Ochsenreither, S.; Olze, H.; Dommerich, S.; Piwonski, I.; et al. Carcinoma of Unknown Primary and the 8th Edition TNM Classification for Head and Neck Cancer. Laryngoscope 2021, 131, E2534–E2542. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Guidelines, Head and Neck Cancers. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1437 (accessed on 1 January 2022).
- Karnofsky, D.A.; Abelmann, W.H.; Craver, L.F.; Burchenal, J.H. The use of the nitrogen mustards in the palliative treatment of carcinoma. Cancer 1948, 1, 634–656. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.A.; Altman, D.G. Basic statistical reporting for articles published in biomedical journals: The “Statistical Analyses and Methods in the Published Literature” or the SAMPL Guidelines. Int. J. Nurs. Stud. 2015, 52, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Graessle, R.; Stromberger, C.; Heiland, M.; Doll, C.; Hofmann, V.M.; Klinghammer, K.; Tinhofer, I.; Olze, H.; Beck, M.; Arens, P.; et al. Predictors for Adherence to Treatment Strategies in Elderly HNSCC Patients. Cancers 2022, 14, 423. [Google Scholar] [CrossRef]
- Stromberger, C.; Yedikat, B.; Coordes, A.; Tinhofer, I.; Kalinauskaite, G.; Budach, V.; Zschaeck, S.; Raguse, J.D.; Kofla, G.; Heiland, M.; et al. Prognostic Factors Predict Oncological Outcome in Older Patients With Head and Neck Cancer Undergoing Chemoradiation Treatment. Front. Oncol. 2020, 10, 566318. [Google Scholar] [CrossRef]
- Sanabria, A.; Carvalho, A.L.; Vartanian, J.G.; Magrin, J.; Ikeda, M.K.; Kowalski, L.P. Comorbidity is a prognostic factor in elderly patients with head and neck cancer. Ann. Surg. Oncol. 2007, 14, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Beynon, R.A.; Lang, S.; Schimansky, S.; Penfold, C.M.; Waylen, A.; Thomas, S.J.; Pawlita, M.; Waterboer, T.; Martin, R.M.; May, M.; et al. Tobacco smoking and alcohol drinking at diagnosis of head and neck cancer and all-cause mortality: Results from head and neck 5000, a prospective observational cohort of people with head and neck cancer. Int. J. Cancer 2018, 143, 1114–1127. [Google Scholar] [CrossRef]
- Duffy, S.A.; Ronis, D.L.; McLean, S.; Fowler, K.E.; Gruber, S.B.; Wolf, G.T.; Terrell, J.E. Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. J. Clin. Oncol. 2009, 27, 1969–1975. [Google Scholar] [CrossRef]
- Sharp, L.; McDevitt, J.; Carsin, A.E.; Brown, C.; Comber, H. Smoking at diagnosis is an independent prognostic factor for cancer-specific survival in head and neck cancer: Findings from a large, population-based study. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2579–2590. [Google Scholar] [CrossRef]
- Dubin, S.; Griffin, D. Lung Cancer in Non-Smokers. Mo. Med. 2020, 117, 375–379. [Google Scholar] [PubMed]
- Alsharairi, N.A. The Effects of Dietary Supplements on Asthma and Lung Cancer Risk in Smokers and Non-Smokers: A Review of the Literature. Nutrients 2019, 11, 725. [Google Scholar] [CrossRef] [PubMed]
- Idris, S.; Baqays, A.; Isaac, A.; Chau, J.K.M.; Calhoun, K.H.; Seikaly, H. The effect of second hand smoke in patients with squamous cell carcinoma of the head and neck. J. Otolaryngol. Head Neck Surg. 2019, 48, 33. [Google Scholar] [CrossRef] [PubMed]
- Dequanter, D.; Shahla, M.; Paulus, P.; Boutremans, E.; Lothaire, P. Should older head and neck patients be treated differently? Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 165–168. [Google Scholar] [CrossRef]
- Srinivasalu, V.K.; Subramaniam, N.; Balasubramanian, D.; Kumar, N.; Philip, A.; Susan, A.; Pushpaja, K.U.; Nair, A.R.; Thankappan, K.; Jose, W.; et al. Concurrent chemoradiotherapy for head and neck cancers in older patients: Outcomes and their determinants. Indian J. Cancer 2019, 56, 261–266. [Google Scholar] [CrossRef]
- Okuyama, K.; Yanamoto, S.; Michi, Y.; Shibata, E.; Tsuchiya, M.; Yokokawa, M.; Naruse, T.; Tomioka, H.; Kuroshima, T.; Shimamoto, H.; et al. Multicenter retrospective analysis of clinicopathological features and prognosis of oral tongue squamous cell carcinoma in adolescent and young adult patients. Medicine 2021, 100, e27560. [Google Scholar] [CrossRef]
- Rummer, A.; Schulz, R.J. Geriatrie: Vermeidung des Drehtüreffekts. Dtsch. Ärzteblatt 2012, 109, A746–A748. [Google Scholar]
- Arbeitsgruppe der Bundesarbeitsgemeinschaft der Klinisch-Geriatrischen Einrichtungen e.V. (BAG). Abgrenzungskriterien der Geriatrie. —Version V1.3. Available online: http://www.geriatrie-drg.de/public/docs/Abgrenzungskriterien_Geriatrie_V13_16-03-04.pdf (accessed on 5 November 2022).
- Piccirillo, J.F. Importance of comorbidity in head and neck cancer. Laryngoscope 2000, 110, 593–602. [Google Scholar] [CrossRef]
- Singh, B.; Bhaya, M.; Stern, J.; Roland, J.T.; Zimbler, M.; Rosenfeld, R.M.; Har-El, G.; Lucente, F.E. Validation of the Charlson comorbidity index in patients with head and neck cancer: A multi-institutional study. Laryngoscope 1997, 107, 1469–1475. [Google Scholar] [CrossRef]
- Saiyed, F.K.; Guo, T.; Johnson, F.; Myers, J.N. Characterizing distant metastases and survival in oropharyngeal squamous cell carcinoma. Head Neck 2021, 43, 2101–2109. [Google Scholar] [CrossRef]
- Roth, G.M.; Shick, R.M. Effect of smoking on the cardiovascular system of man. Circulation 1958, 17, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Hammond, E.C.; Horn, D. Smoking and death rates--report on forty-four months of follow-up of 187,783 men. By E. Cuyler Hammond and Daniel Horn, 1958. CA Cancer J. Clin. 1988, 38, 28–58. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Z.; Zheng, L. Association of smoking with coronary artery disease and myocardial infarction: A Mendelian randomization study. Eur. J. Prev. Cardiol. 2021, 28, e11–e12. [Google Scholar] [CrossRef] [PubMed]
- Wendt, M.; Hammarstedt-Nordenvall, L.; Zupancic, M.; Friesland, S.; Landin, D.; Munck-Wikland, E.; Dalianis, T.; Nasman, A.; Marklund, L. Long-Term Survival and Recurrence in Oropharyngeal Squamous Cell Carcinoma in Relation to Subsites, HPV, and p16-Status. Cancers 2021, 13, 2553. [Google Scholar] [CrossRef]
- Marklund, L.; Holzhauser, S.; de Flon, C.; Zupancic, M.; Landin, D.; Kolev, A.; Haeggblom, L.; Munck-Wikland, E.; Hammarstedt-Nordenvall, L.; Dalianis, T.; et al. Survival of patients with oropharyngeal squamous cell carcinomas (OPSCC) in relation to TNM 8—Risk of incorrect downstaging of HPV-mediated non-tonsillar, non-base of tongue carcinomas. Eur. J. Cancer 2020, 139, 192–200. [Google Scholar] [CrossRef]
- Antonsson, A.; Neale, R.E.; Boros, S.; Lampe, G.; Coman, W.B.; Pryor, D.I.; Porceddu, S.V.; Whiteman, D.C. Human papillomavirus status and p16(INK4A) expression in patients with mucosal squamous cell carcinoma of the head and neck in Queensland, Australia. Cancer Epidemiol. 2015, 39, 174–181. [Google Scholar] [CrossRef]
- Dronkers, E.A.; Mes, S.W.; Wieringa, M.H.; van der Schroeff, M.P.; Baatenburg de Jong, R.J. Noncompliance to guidelines in head and neck cancer treatment; associated factors for both patient and physician. BMC Cancer 2015, 15, 515. [Google Scholar] [CrossRef]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total | Current/Former Smokers | Non-Smokers | p Value |
|---|---|---|---|---|
| n = 688 | n = 520 | n = 168 | ||
| Sex—no. (%) | <0.001 | |||
| Male | 493 (71.7) | 390 (75.0) | 103 (61.3) | |
| Female | 195 (28.3) | 130 (25.0) | 65 (38.7) | |
| Age at initial diagnosis of HNSCC, years | <0.001 | |||
| Median (range) | 74 (26) | 74 (26) | 76 (22) | |
| Pack years | <0.001 | |||
| Median (range) | 49 (197) | 49 (197) | 0 (0) | |
| Alcohol abuse—no. (%) | <0.001 | |||
| No ethanol consumption | 317 (63.0) | 209 (55.3) | 108 (86.4) | |
| Ethanol consumption | 186 (37.0) | 169 (44.7) | 17 (13.6) | |
| Additional cancer diagnoses—no. (%) | 0.012 | |||
| Other cancers | 244 (35.5) | 198 (38.1) | 46 (27.4) | |
| None | 444 (64.5) | 322 (61.9) | 122 (72.6) | |
| Number of additional cancer diagnoses—no. (%) | 0.041 | |||
| 0 | 444 (64.5) | 322 (61.9) | 122 (72.6) | |
| 1 | 189 (27.5) | 154 (29.6) | 35 (20.8) | |
| ≥2 | 55 (8.0) | 44 (8.5) | 11 (6.5) | |
| Charlson comorbidity index—no. (%) | 0.301 | |||
| ≤5 | 444 (64.5) | 330 (63.5) | 114 (67.9) | |
| ≥6 | 244 (35.5) | 190 (36.5) | 54 (32.1) | |
| Karnofsky performance status—no. (%) | 0.023 | |||
| ≤70% | 370 (54.0) | 292 (56.5) | 78 (46.4) | |
| ≥80% | 315 (46.0) | 225 (43.5) | 90 (53.6) | |
| Death due to cancer—no. (%) | <0.001 | |||
| Survived | 293 (47.4) | 199 (43.1) | 94 (60.3) | |
| Non-cancer-associated | 69 (11.2) | 57 (12.3) | 12 (7.7) | |
| Cancer-associated | 256 (41.4) | 206 (44.6) | 50 (32.1) | |
| Recurrence—no. (%) | 0.406 | |||
| Positive | 152 (22.1) | 111 (21.3) | 41 (24.4) | |
| HNSCC characteristics | ||||
| Tumour site—no. (%) | 0.066 1 | |||
| Oropharynx | 202 (29.4) | 155 (29.8) | 47 (28.0) | |
| Oral cavity | 240 (34.9) | 173 (33.3) | 67 (39.9) | |
| Larynx | 154 (22.4) | 116 (22.3) | 38 (22.6) | |
| Hypopharynx | 62 (9.0) | 56 (10.8) | 6 (3.6) | |
| Nasal/paranasal sinuses | 24 (3.5) | 16 (3.1) | 8 (4.8) | |
| Nasopharynx | 6 (0.9) | 4 (0.8) | 2 (1.2) | |
| P16 in oropharynx carcinoma—no. (%) | 0.001 | |||
| Positive | 69 (51.1) | 46 (43.8) | 23 (76.7) | |
| Grading—no. (%) | 0.387 | |||
| G1 | 59 (9.4) | 42 (8.8) | 17 (11.1) | |
| G2 | 412 (65.3) | 319 (66.7) | 93 (60.8) | |
| G3 | 160 (25.4) | 117 (24.5) | 43 (28.1) | |
| T classification—no. (%) | 0.121 | |||
| T1–2 | 341 (49.6) | 249 (47.9) | 92 (54.8) | |
| T3–4 | 347 (50.4) | 271 (52.1) | 76 (45.2) | |
| N classification—no. (%) | 0.121 | |||
| Positive | 347 (50.4) | 271 (52.1) | 76 (45.2) | |
| M classification—no. (%) | 0.887 | |||
| Positive | 30 (4.4) | 23 (4.4) | 7 (4.2) | |
| UICC stage (8th edition)—no. (%) | 0.119 | |||
| 0–II | 272 (39.5) | 197 (37.9) | 75 (44.6) | |
| III–IV | 416 (60.5) | 323 (62.1) | 93 (55.4) | |
| Intention of therapy—no. (%) | 0.332 | |||
| Curative | 528 (77.1) | 392 (75.8) | 136 (81.0) | |
| Palliative | 111 (16.2) | 87 (16.8) | 24 (14.3) | |
| Curative, discontinued | 46 (6.7) | 38 (7.4) | 8 (4.8) | |
| Treatment received—no. (%) | 0.046 1 | |||
| Palliative/BSC | 49 (7.2) | 41 (8.0) | 8 (4.8) | |
| Pall. R(C)T | 46 (6.8) | 36 (7.0) | 10 (6.1) | |
| Surgery | 258 (38.1) | 179 (35.0) | 79 (47.9) | |
| Surgery + adj. R(C)T | 101 (14.9) | 78 (15.2) | 23 (13.9) | |
| Def. R(C)T | 212 (31.3) | 171 (33.4) | 41 (24.8) | |
| Pall. CT | 11 (1.6) | 7 (1.4) | 4 (2.4) | |
| Treatment recommendation—no. (%) | 0.002 1 | |||
| Palliative/BSC | 16 (2.5) | 12 (2.5) | 4 (2.5) | |
| Pall. R(C)T | 51 (7.9) | 42 (8.6) | 9 (5.6) | |
| Surgery | 216 (33.3) | 143 (29.2) | 73 (45.6) | |
| Surgery + adj. R(C)T | 133 (20.5) | 104 (21.3) | 29 (18.1) | |
| Def. R(C)T | 225 (34.7) | 184 (37.6) | 41 (25.6) | |
| Pall. CT | 8 (1.2) | 4 (0.8) | 4 (2.5) | |
| Adherence—no. (%) | 0.038 | |||
| Non-adherent | 89 (13.9) | 75 (15.5) | 14 (8.9) | |
| Adherent | 551 (86.1) | 408 (84.5) | 143 (91.1) |
| UICC Stage | Total | |||
|---|---|---|---|---|
| I–II | III–IV | |||
| Death due to cancer | Survived | 158 (53.9%) | 135 (46.1%) | 293 (100.0%) |
| Non-cancer-associated | 34 (49.3%) | 35 (50.7%) | 69 (100.0%) | |
| Cancer associated | 59 (23.0%) | 197 (77.0%) | 256 (100.0%) | |
| Total | 251 (40.6%) | 367 (59.4%) | 618 (100.0%) | |
| Univariate Analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Total | Current/Former Smokers | Non-Smokers | |||||||
| n = 688 | Mean OS (Months/% 1) | p Value | n = 520 | Mean OS (Months/% 1) | p Value | n = 168 | Mean OS (Months/% 1) | p Value | ||
| Sex | 0.515 | 0.868 | 0.519 | |||||||
| Male | 493 | 57/39.2 | 390 | 55/36.7 | 103 | 63/50.3 | ||||
| Female | 195 | 58/40.4 | 130 | 48/34.1 | 65 | 75/54.6 | ||||
| Age at initial diagnosis of HNSCC | 0.073 | <0.001 | 0.094 | |||||||
| 70–74 years | 356 | 63/41.4 | 302 | 59/37.8 | 54 | 83/63.2 | ||||
| 75–79 years | 225 | 53/40.3 | 159 | 47/38.0 | 66 | 62/45.8 | ||||
| 80–84 years | 71 | 39/33.8 | 45 | 32/21.7 | 26 | 51/57.8 | ||||
| 85–89 years | 25 | 46/31.7 | 12 | 42/27.3 | 13 | 46/38.5 | ||||
| Older than 90 years | 11 | 19/27.7 | 2 | 4/0.0 | 9 | 23/34.6 | ||||
| Tobacco exposure | 0.001 | |||||||||
| Non-smokers | 168 | 69/52.0 | ||||||||
| Current/former smokers | 520 | 54/36.0 | ||||||||
| Alcohol abuse | <0.001 | <0.001 | 0.004 | |||||||
| No ethanol consumption | 317 | 64/49.3 | 209 | 57/46.0 | 108 | 77/56.5 | ||||
| Ethanol consumption | 186 | 41/25.0 | 169 | 41/24.8 | 17 | 32/26.5 | ||||
| Additional cancer diagnoses | 0.430 | 0.280 | 0.186 | |||||||
| Other cancers | 244 | 53/35.5 | 198 | 48/31.0 | 46 | 81/63.9 | ||||
| No | 444 | 60/42.2 | 322 | 57/39.9 | 122 | 64/48.0 | ||||
| Karnofsky performance status | <0.001 | <0.001 | <0.001 | |||||||
| ≤70% | 370 | 38/23.0 | 292 | 36/21.4 | 78 | 46/30.2 | ||||
| ≥80% | 315 | 79/58.4 | 225 | 77/54.7 | 90 | 86/68.1 | ||||
| Charlson comorbidity index | <0.001 | <0.001 | 0.014 | |||||||
| ≤5 | 444 | 68/47.1 | 330 | 65/43.5 | 114 | 75/58.3 | ||||
| ≥6 | 244 | 39/25.9 | 190 | 33/23.0 | 54 | 52/38.7 | ||||
| Site of primary tumour | <0.001 | 0.004 | <0.001 | |||||||
| Oropharynx | 202 | 59/40.4 | 155 | 51/33.8 | 47 | 85/64.1 | ||||
| Oral cavity | 240 | 51/37.7 | 173 | 48/35.1 | 67 | 58/46.9 | ||||
| Larynx | 154 | 69/47.2 | 116 | 67/45.0 | 38 | 77/55.0 | ||||
| Hypopharynx | 62 | 29/22.4 | 56 | 29/21.8 | 6 | 32/25.0 | ||||
| Paranasal sinus | 24 | 55/50.3 | 16 | 51/52.4 | 8 | 62/46.9 | ||||
| Nasopharynx | 6 | 19/16.7 | 4 | 27/25.0 | 2 | 5/0.0 | ||||
| P16 in Oropharynx-Carcinoma | 0.022 | 0.283 | 0.209 | |||||||
| Positive | 69 | 72/49.2 | 46 | 52/36.1 | 23 | 106/72.4 | ||||
| Negative | 66 | 42/33.8 | 59 | 41/31.4 | 7 | 51/64.3 | ||||
| Grading | 0.107 | 0.074 | 0.605 | |||||||
| G1 | 59 | 61/45.9 | 42 | 55/41.4 | 17 | 77/58.6 | ||||
| G2 | 412 | 61/41.1 | 319 | 58/38.5 | 93 | 68/51.7 | ||||
| G3 | 160 | 50/34.1 | 117 | 45/28.5 | 43 | 66/50.7 | ||||
| T classification | <0.001 | <0.001 | <0.001 | |||||||
| T1–2 | 341 | 77/52.1 | 249 | 71/48.7 | 92 | 88/63.2 | ||||
| T3–4 | 347 | 38/27.1 | 271 | 34/24.1 | 76 | 49/38.4 | ||||
| N classification | <0.001 | <0.001 | 0.063 | |||||||
| Positive | 347 | 47/30.2 | 271 | 42/26.0 | 76 | 62/45.9 | ||||
| Negative | 341 | 65/48.9 | 249 | 62/46.3 | 92 | 73/57.1 | ||||
| M classification | <0.001 | <0.001 | <0.001 | |||||||
| Positive | 30 | 18/7.9 | 23 | 16/4.6 | 7 | 32/28.6 | ||||
| Negative | 658 | 61/41.0 | 497 | 57/37.5 | 161 | 71/53.0 | ||||
| UICC stage (8th edition) | <0.001 | <0.001 | <0.001 | |||||||
| 0–II | 272 | 77/56.7 | 197 | 71/53.1 | 75 | 98/67.0 | ||||
| III–IV | 416 | 42/28.4 | 323 | 40/25.2 | 93 | 50/40.2 | ||||
| Treatment received | <0.001 | <0.001 | <0.001 | |||||||
| Palliative/BSC | 49 | 10/2.8 | 41 | 10/3.8 | 8 | 9/0.0 | ||||
| Pall. R(C)T | 46 | 12/12.2 | 36 | 9/3.9 | 10 | 22/48.0 | ||||
| Surgery | 258 | 78/59.6 | 179 | 74/55.5 | 79 | 86/72.1 | ||||
| Surgery + adj. R(C)T | 101 | 62/37.7 | 78 | 60/34.9 | 23 | 74/50.2 | ||||
| Def. R(C)T | 212 | 46/33.1 | 171 | 43/32.0 | 41 | 55/37.3 | ||||
| Pall. CT | 11 | 9/18.2 | 7 | 9/0.0 | 4 | 7/25.0 | ||||
| Treatment recommendation | <0.001 | <0.001 | <0.001 | |||||||
| Palliative/BSC | 16 | 8/0.0 | 12 | 7/0.0 | 4 | 13/0.0 | ||||
| Pall. R(C)T | 51 | 12/10.9 | 42 | 9/3.5 | 9 | 29/66.7 | ||||
| Surgery | 216 | 82/63.1 | 143 | 78/59.4 | 73 | 89/72.9 | ||||
| Surgery + adj. R(C)T | 133 | 62/37.7 | 104 | 60/36.2 | 29 | 68/45.8 | ||||
| Def. R(C)T | 225 | 42/30.1 | 184 | 39/28.9 | 41 | 50/35.1 | ||||
| Pall. CT | 8 | 15/0.0 | 4 | 12/0.0 | 4 | 17/50.0 | ||||
| Adherence to treatment recommendation | <0.001 | <0.001 | <0.001 | |||||||
| Non-adherent | 89 | 26/16.9 | 75 | 27/17.3 | 14 | 15/21.4 | ||||
| Adherent | 551 | 66/44.2 | 408 | 62/40.3 | 143 | 76/56.6 | ||||
| Recurrence | 0.801 | 0.669 | 0.803 | |||||||
| Positive | 152 | 53/34.4 | 111 | 49/34.2 | 41 | 61/31.4 | ||||
| Negative | 536 | 59/41.2 | 409 | 55/36.8 | 127 | 70/57.0 | ||||
| Implementation of therapy | <0.001 | <0.001 | <0.001 | |||||||
| Discontinued | 44 | 13/7.6 | 41 | 14/8.1 | 3 | 5/0.0 | ||||
| Rejected | 47 | 37/26.1 | 36 | 39/27.7 | 11 | 17/27.3 | ||||
| Carried out | 585 | 64/43.1 | 434 | 60/39.4 | 151 | 73/55.1 | ||||
| Intention of therapy | <0.001 | <0.001 | <0.001 | |||||||
| Curative | 528 | 69/47.0 | 392 | 65/43.4 | 136 | 77/58.6 | ||||
| Palliative | 111 | 14/6.1 | 87 | 11/3.0 | 24 | 16/22.1 | ||||
| Curative, discontinued | 46 | 44/31.2 | 38 | 45/32.3 | 8 | 22/37.5 | ||||
| Multivariate Analysis | |||||
|---|---|---|---|---|---|
| Variable | n = 688 | HR | 95% CI | p Value | |
| Age at initial diagnosis of HNSCC | 1.303 | 1.055–1.609 | 0.014 | ||
| ≤75 | 410 | ||||
| ≥76 | 278 | ||||
| Tobacco exposure | 1.475 | 1.134–1.919 | 0.004 | ||
| Non-smokers | 168 | ||||
| Current/former smokers | 520 | ||||
| Charlson comorbidity index | 1.471 | 1.194–1.813 | <0.001 | ||
| ≤5 | 444 | ||||
| ≥6 | 244 | ||||
| Karnofsky performance status | 0.504 | 0.405–0.627 | <0.001 | ||
| ≤70% | 370 | ||||
| ≥80% | 315 | ||||
| UICC stage (8th edition) | 2.177 | 1.745–2.716 | <0.001 | ||
| 0–II | 272 | ||||
| III–IV | 416 | ||||
| Current/Former Smokers | |||||
| Variable | n = 520 | HR | 95% CI | p Value | |
| Age at initial diagnosis of HNSCC | 1.404 | 1.116–1.766 | 0.004 | ||
| ≤75 | 339 | ||||
| ≥76 | 181 | ||||
| Charlson comorbidity index | 1.508 | 1.198–1.898 | <0.001 | ||
| ≤5 | 330 | ||||
| ≥6 | 190 | ||||
| Karnofsky performance status | 0.514 | 0.403–0.655 | <0.001 | ||
| ≤70% | 292 | ||||
| ≥80% | 225 | ||||
| UICC stage (8th edition) | 2.031 | 1.593–2.589 | <0.001 | ||
| 0–II | 197 | ||||
| III–IV | 323 | ||||
| Non-Smokers | |||||
| Variable | n = 168 | HR | 95% CI | p Value | |
| Age at initial diagnosis of HNSCC | 0.886 | 0.531–1.478 | 0.643 | ||
| ≤75 | 71 | ||||
| ≥76 | 97 | ||||
| Charlson comorbidity index | 1.341 | 0.805–2.232 | 0.260 | ||
| ≤5 | 114 | ||||
| ≥6 | 54 | ||||
| Karnofsky performance status | 0.443 | 0.260–0.754 | 0.003 | ||
| ≤70% | 78 | ||||
| ≥80% | 90 | ||||
| UICC stage (8th edition) | 2.974 | 1.729–5.117 | <0.001 | ||
| 0–II | 75 | ||||
| III–IV | 93 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graessle, R.; Stromberger, C.; Beck, M.; Heiland, M.; Hofmann, V.M.; Olze, H.; Dommerich, S.; Gauger, U.; Piwonski, I.; Coordes, A. Subgroup Analysis of Overall Survival among Smoking and Non-Smoking Elderly Patients with HNSCC. Cancers 2023, 15, 1842. https://doi.org/10.3390/cancers15061842
Graessle R, Stromberger C, Beck M, Heiland M, Hofmann VM, Olze H, Dommerich S, Gauger U, Piwonski I, Coordes A. Subgroup Analysis of Overall Survival among Smoking and Non-Smoking Elderly Patients with HNSCC. Cancers. 2023; 15(6):1842. https://doi.org/10.3390/cancers15061842
Chicago/Turabian StyleGraessle, Raphaela, Carmen Stromberger, Marcus Beck, Max Heiland, Veit M. Hofmann, Heidi Olze, Steffen Dommerich, Ulrich Gauger, Iris Piwonski, and Annekatrin Coordes. 2023. "Subgroup Analysis of Overall Survival among Smoking and Non-Smoking Elderly Patients with HNSCC" Cancers 15, no. 6: 1842. https://doi.org/10.3390/cancers15061842
APA StyleGraessle, R., Stromberger, C., Beck, M., Heiland, M., Hofmann, V. M., Olze, H., Dommerich, S., Gauger, U., Piwonski, I., & Coordes, A. (2023). Subgroup Analysis of Overall Survival among Smoking and Non-Smoking Elderly Patients with HNSCC. Cancers, 15(6), 1842. https://doi.org/10.3390/cancers15061842








