Cellular Therapies in Chronic Lymphocytic Leukemia and Richter’s Transformation: Recent Developments in Chimeric Antigen Receptor T-Cells, Natural Killer Cells, and Allogeneic Stem Cell Transplant
Abstract
Simple Summary
Abstract
1. Introduction
2. What’s “New” in AlloHSCT for CLL and RT: Focus on Patients with Prior Novel-Agent Exposure
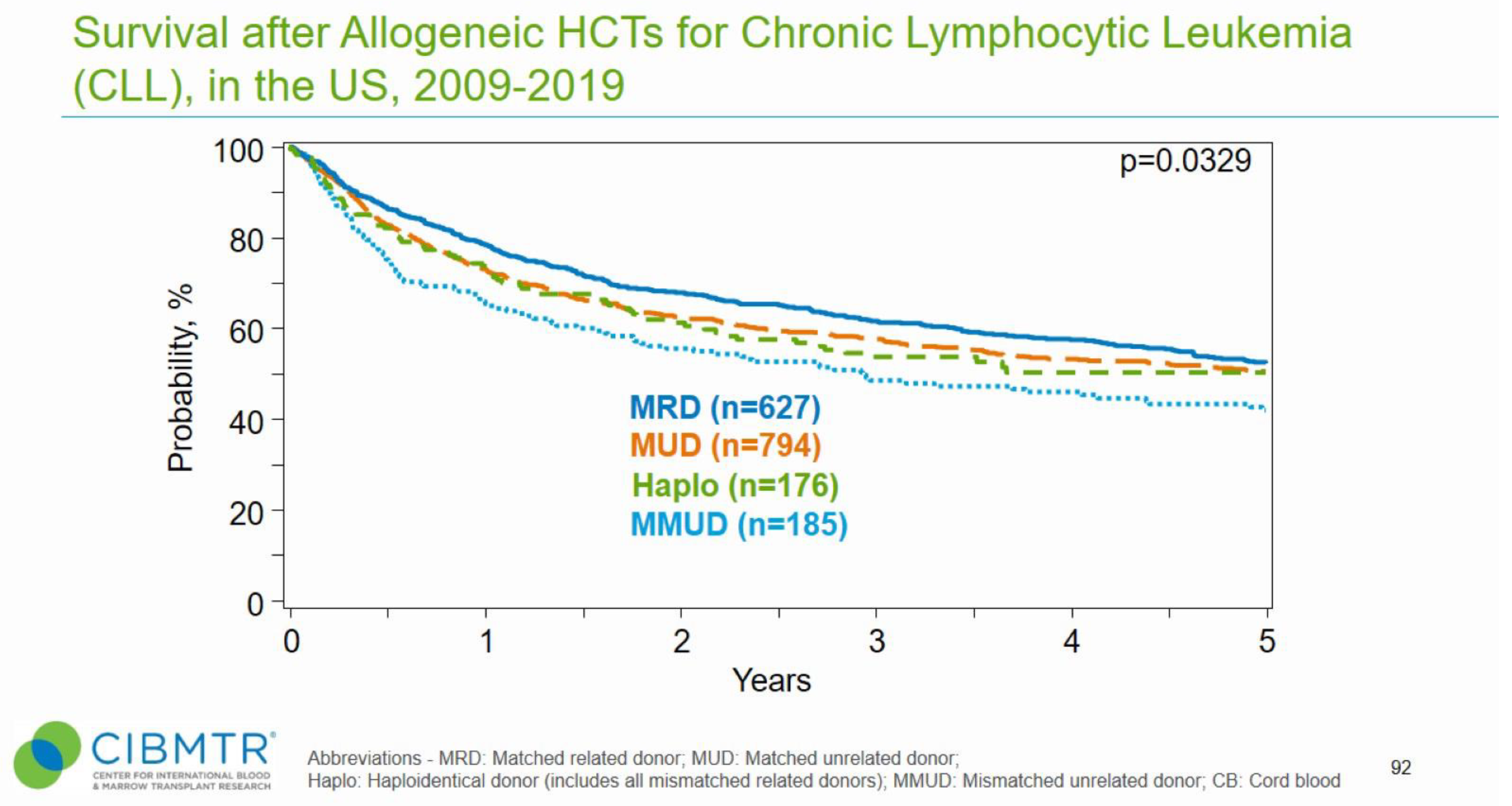
2.1. AlloHSCT in CLL
2.2. AlloHSCT for RT
2.3. AlloHSCT Limitations
3. CD19 CAR-T Therapies Approved in Other Indications
| CAR-T Product | Approved Indication(s) | Construct | Studied in CLL and/or RT |
|---|---|---|---|
| Lisocabtagene maraleucel (Breyanzi) | Adult patients with relapsed(rel)/refractory(ref) large B-cell lymphoma including diffuse large B-cell lymphoma (DLBCL) NOS, including DLBCL arising from indolent lymphoma, high-grade B-cell lymphoma, primary mediastinal lymphoma, and follicular lymphoma (FL) grade 3B who are refractory to firstline chemoimmunotherapy or relapsed within 12 months, who are rel/ref after firstline and are ineligible for HSCT due to comorbidities or age, or rel/ref after two or more lines of systemic therapy [38,39] | Anti-CD19 4-1-BB CD3z CD4:CD8 1:1 | CLL [36,37,40] and RT [36,41] |
| Tisagenlecleucel (Kymriah) | Adult patients with rel/ref DLBCL after two or more lines of systemic therapy, including DLBCL NOS, high grade B-cell lymphoma, and DLBCL arising from FL, adult patients with rel/ref FL after two or more lines of systemic therapy, and young patients (up to age 25) with refractory or in second or later relapse B-cell precursor acute lymphoblastic leukemia (ALL) [42,43,44] | Anti-CD19 4-1BB CD3z | CLL [7,45,46,47] |
| Brexucabtagene autoleucel (Tecartus) | Patients with rel/ref mantle cell lymphoma and adult patients with relapsed or refractory B-cell precursor ALL [48,49] | Anti-CD19 CD28 CD3z Manufacturing step added to remove CD19 tumor cells | CLL [50] |
| Axicabtagene ciloleucel (Yescarta) | Adult patients with large B-cell lymphoma that is refractory to firstline chemotherapy or that relapses within 12 months of firstline chemotherapy, adult patients with rel/ref large B-cell lymphoma after 2 or more lines of systemic therapy, including DLBCL NOS, primary media-stinal large B-cell lymphoma, and DLBCL arising from FL, and FL after 2 or more lines of systemic therapy [51,52,53] | Anti-CD19 CD28 CD3z | CLL [54] and RT [55] |
4. Emerging Strategies to Improve CAR-T Efficacy
4.1. Ibrutinib and Other Combinations
4.2. Novel CAR-T Targets and Constructs
4.3. Allogeneic CAR-T
4.4. Natural Killer CARs
4.5. CAR-T in Richter’s Transformation
5. Future Directions
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chavez, J.C.; Yassine, F.; Sandoval-Sus, J.; Kharfan-Dabaja, M.A. Anti-CD19 chimeric antigen receptor T-cell therapy in B-cell lymphomas: Current status and future directions. Int. J. Hematol. Oncol. 2021, 10, IJH33. [Google Scholar] [CrossRef]
- Sengsayadeth, S.; Savani, B.N.; Oluwole, O.; Dholaria, B. Overview of approved CAR-T therapies, ongoing clinical trials, and its impact on clinical practice. eJHaem 2021, 3, 6–10. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Bahmanyar, M.; Vakil, M.K.; Al-Awsi, G.R.L.; Kouhpayeh, S.A.; Mansoori, H.; Mansoori, Y.; Salahi, A.; Nikfar, G.; Tavassoli, A.; Behmard, E.; et al. Opportunities and obstacles for the melanoma immunotherapy using T cell and chimeric antigen receptor T (CAR-T) applications: A literature review. Mol. Biol. Rep. 2022, 49, 10627–10633. [Google Scholar] [CrossRef] [PubMed]
- Guzman, G.; Reed, M.R.; Bielamowicz, K.; Koss, B.; Rodriguez, A. CAR-T Therapies in solid tumors: Opportunities and challenges. Curr. Oncol. Rep. 2023. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.L.; Levine, B.L.; Kalos, M.; Bagg, A.; June, C.H. Chimeric antigen receptor—Modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011, 365, 725–733. [Google Scholar] [CrossRef]
- Heyman, B.M.; Tzachanis, D.; Kipps, T.J. Recent advances in CAR T-cell therapy for patients with chronic lymphocytic leukemia. Cancers 2022, 14, 1715. [Google Scholar] [CrossRef]
- Aronson, J.H.; Skånland, S.S.; Roeker, L.E.; Thompson, M.C.; Mato, A.R. Approach to a patient with “double refractory” chronic lymphocytic leukemia: “Double, double toil and trouble” (Shakespeare). Am. J. Hematol. 2022, 97, S19–S25. [Google Scholar] [CrossRef]
- Lew, T.E.; Lin, V.S.; Cliff, E.R.S.; Blombery, P.; Thompson, E.R.; Handunnetti, S.M.; Westerman, D.A.; Kuss, B.J.; Tam, C.S.; Huang, D.C.S.; et al. Outcomes of patients with CLL sequentially resistant to both BCL2 and BTK inhibition. Blood Adv. 2021, 5, 4054–4058. [Google Scholar] [CrossRef]
- Mato, A.R.; Hess, L.M.; Chen, Y.; Abada, P.B.; Konig, H.; Pagel, J.M.; Walgren, R.A. Outcomes for patients with chronic lymphocytic leukemia (CLL) previously treated with both a covalent BTK and BCL2 inhibitor in the United States: A real-world database study. Clin. Lymphoma Myeloma Leuk. 2022, 23, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.C.; Roeker, L.E.; Coombs, C.C.; Jensen, J.L.; Kamdar, M.; Skarbnik, A.; Pagel, J.M.; Bailey, N.; Pu, J.J.; Jacobs, R.; et al. Addressing a new challenge in chronic lymphocytic leukemia: Outcomes of therapies after exposure to both a covalent bruton’s tyrosine kinase inhibitor and venetoclax. Blood 2021, 138, 2628. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, W. Richter transformation of chronic lymphocytic leukemia in the era of novel agents. Clin. Adv. Hematol. Oncol. 2020, 18, 348–357. [Google Scholar] [PubMed]
- Elnair, R.; Ellithi, M.; Kallam, A.; Shostrom, V.; Bociek, R.G. Outcomes of Richter’s transformation of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): An analysis of the SEER database. Ann. Hematol. 2021, 100, 2513–2519. [Google Scholar] [CrossRef] [PubMed]
- Auletta, J.J.; Kou, J.; Chen, M.; Shaw, B.E. Current use and outcome of hematopoietic stem cell transplantation. CIBMTR US summary slides. 2021. Available online: https://cibmtr.org/CIBMTR/Resources/Summary-Slides-Reports (accessed on 15 October 2022).
- Dreger, P. Is there a role for cellular therapy in chronic lymphocytic leukemia? Cancer J. 2021, 27, 297–305. [Google Scholar] [CrossRef]
- van Gelder, M.; de Wreede, L.C.; Bornhäuser, M.; Niederwieser, D.; Karas, M.; Anderson, N.S.; Gramatzki, M.; Dreger, P.; Michallet, M.; Petersen, E.; et al. Long-term survival of patients with CLL after allogeneic transplantation: A report from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2017, 52, 372–380. [Google Scholar] [CrossRef]
- Brown, J.R.; Kim, H.T.; Armand, P.; Cutler, C.; Fisher, D.C.; Ho, V.; Koreth, J.; Ritz, J.; Wu, C.; Antin, J.H.; et al. Long-term follow-up of reduced-intensity allogeneic stem cell transplantation for chronic lymphocytic leukemia: Prognostic model to predict outcome. Leukemia 2013, 27, 362–369. [Google Scholar] [CrossRef]
- Kharfan-Dabaja, M.A.; Pidala, J.; Kumar, A.; Terasawa, T.; Djulbegovic, B. Comparing efficacy of reduced-toxicity allogeneic hematopoietic cell transplantation with conventional chemo-(immuno) therapy in patients with relapsed or refractory CLL: A Markov decision analysis. Bone Marrow Transplant. 2012, 47, 1164–1170. [Google Scholar] [CrossRef]
- Delgado, J.; Pillai, S.; Phillips, N.; Brunet, S.; Pratt, G.; Briones, J.; Lovell, R.; Martino, R.; Ewing, J.; Sureda, A.; et al. Does reduced-intensity allogeneic transplantation confer a survival advantage to patients with poor prognosis chronic lymphocytic leukaemia? A case-control retrospective analysis. Ann. Oncol. 2009, 20, 2007–2012. [Google Scholar] [CrossRef]
- Paul, S.; Tsai, H.-L.; Lowery, P.; Fuchs, E.J.; Luznik, L.; Bolaños-Meade, J.; Swinnen, L.J.; Shanbhag, S.; Wagner-Johnston, N.; Varadhan, R.; et al. Allogeneic haploidentical blood or marrow transplantation with post-transplantation cyclophosphamide in chronic lymphocytic leukemia. Biol. Blood Marrow Transplant. 2020, 26, 502–508. [Google Scholar] [CrossRef]
- Hoffmann, A.; Dietrich, S.; Hain, S.; Rieger, M.; Hegenbart, U.; Sellner, L.; Ho, A.D.; Müller-Tidow, C.; Dreger, P. Allogeneic transplantation in high-risk chronic lymphocytic leukemia: A single-center, intent-to-treat analysis. Haematologica 2019, 104, e304–e306. [Google Scholar] [CrossRef]
- Mato, A.R.; Roeker, L.E.; Jacobs, R.; Hill, B.T.; Lamanna, N.; Brander, D.; Shadman, M.; Ujjani, C.S.; Yazdy, M.S.; Perini, G.F.; et al. Assessment of the efficacy of therapies following venetoclax discontinuation in CLL reveals BTK inhibition as an effective strategy. Clin. Cancer Res. 2020, 26, 3589–3596. [Google Scholar] [CrossRef]
- Jones, J.A.; Mato, A.R.; Wierda, W.G.; Davids, M.S.; Choi, M.; Cheson, B.D.; Furman, R.R.; Lamanna, N.; Barr, P.M.; Zhou, L.; et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: An interim analysis of a multicentre, open-label, phase 2 trial. Lancet Oncol. 2018, 19, 65–75. [Google Scholar] [CrossRef]
- Lin, V.S.; Lew, T.E.; Handunnetti, S.M.; Blombery, P.; Nguyen, T.; Westerman, D.A.; Kuss, B.J.; Tam, C.S.; Roberts, A.W.; Seymour, J.F.; et al. BTK inhibitor therapy is effective in patients with CLL resistant to venetoclax. Blood 2020, 135, 2266–2270. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Wang, X.V.; Hanson, C.A.; Paietta, E.M.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: Updated results of the E1912 trial. Blood 2022, 140, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Roeker, L.E.; Dreger, P.; Brown, J.R.; Lahoud, O.B.; Eyre, T.A.; Brander, D.M.; Skarbnik, A.; Coombs, C.C.; Kim, H.T.; Davids, M.; et al. Allogeneic stem cell transplantation for chronic lymphocytic leukemia in the era of novel agents. Blood Adv. 2020, 4, 3977–3989. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Milton, D.R.; Jabbour, E.J.; Gulbis, A.M.; Kadia, T.; Jain, N.; Ledesma, C.; Burger, J.; Ferrajoli, A.; Wierda, W.; et al. Clinical outcome of allogeneic stem cell transplantation in patients with B-cell lymphoid malignancies following treatment with targeted small molecule inhibitors. Leuk. Lymphoma 2022, 63, 885–893. [Google Scholar] [CrossRef]
- Kim, H.T.; Shaughnessy, C.J.; Rai, S.C.; Reynolds, C.; Ho, V.T.; Cutler, C.; Koreth, J.; Gooptu, M.; Romee, R.; Nikiforow, S.; et al. Allogeneic hematopoietic cell transplantation after prior targeted therapy for high-risk chronic lymphocytic leukemia. Blood Adv. 2020, 4, 4113–4123. [Google Scholar] [CrossRef]
- Lahoud, O.B.; Devlin, S.M.; Maloy, M.A.; Roeker, L.E.; Dahi, P.B.; Ponce, D.M.; Gyurkocza, B.; Koehne, G.; Young, J.W.; Castro-Malaspina, H.R.; et al. Reduced-intensity conditioning hematopoietic stem cell transplantation for chronic lymphocytic leukemia and Richter’s transformation. Blood Adv. 2021, 5, 2879–2889. [Google Scholar] [CrossRef] [PubMed]
- Farina, L.; Barretta, F.; Scarfò, L.; Bruno, B.; Patriarca, F.; Frustaci, A.M.; Coscia, M.; Salvetti, C.; Quaresmini, G.; Fanin, R.; et al. Refractory and 17p-deleted chronic lymphocytic leukemia: Improving survival with pathway inhibitors and allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 2020, 26, e256–e262. [Google Scholar] [CrossRef]
- Kim, H.T.; Baker, P.O.; Parry, E.; Davids, M.; Alyea, E.P.; Ho, V.T.; Cutler, C.; Koreth, J.; Gooptu, M.; Romee, R.; et al. Allogeneic hematopoietic cell transplantation outcomes in patients with Richter’s transformation. Haematologica 2021, 106, 3219–3222. [Google Scholar] [CrossRef]
- Aulakh, S.; Reljic, T.; Yassine, F.; Ayala, E.; Chavez, J.C.; Chanan-Khan, A.; Pinilla-Ibarz, J.; Kumar, A.; Kharfan-Dabaja, M.A. Allogeneic hematopoietic cell transplantation is an effective treatment for patients with Richter syndrome: A systematic review and meta-analysis. Hematol. Oncol. Stem Cell Ther. 2021, 14, 33–40. [Google Scholar] [CrossRef]
- Herrera, A.F.; Ahn, K.W.; Litovich, C.; Chen, Y.; Assal, A.; Bashir, Q.; Bayer, R.-L.; Coleman, M.; DeFilipp, Z.; Farhadfar, N.; et al. Autologous and allogeneic hematopoietic cell transplantation for diffuse large B-cell lymphoma-type Richter syndrome. Blood Adv. 2021, 5, 3528–3539. [Google Scholar] [CrossRef]
- Rossi, D.; Spina, V.; Deambrogi, C.; Rasi, S.; Laurenti, L.; Stamatopoulos, K.; Arcaini, L.; Lucioni, M.; Rocque, G.B.; Xu-Monette, Z.Y.; et al. The genetics of Richter syndrome reveals disease heterogeneity and predicts survival after transformation. Blood 2011, 117, 3391–3401. [Google Scholar] [CrossRef] [PubMed]
- Turtle, C.J.; Hay, K.A.; Hanafi, L.-A.; Li, D.; Cherian, S.; Chen, X.; Wood, B.; Lozanski, A.; Byrd, J.C.; Heimfeld, S.; et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J. Clin. Oncol. 2017, 35, 3010–3020. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, T.; Soumerai, J.D.; Dorritie, K.A.; Stephens, D.M.; Riedell, P.A.; Arnason, J.; Kipps, T.J.; Gillenwater, H.H.; Gong, L.; Yang, L.; et al. Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL. Blood 2022, 139, 1794–1806. [Google Scholar] [CrossRef]
- Kamdar, M.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F.; et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): Results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 2022, 399, 2294–2308. [Google Scholar] [CrossRef]
- Sehgal, A.; Hoda, D.; Riedell, P.A.; Ghosh, N.; Hamadani, M.; Hildebrandt, G.C.; Godwin, J.E.; Reagan, P.M.; Wagner-Johnston, N.; Essell, J.; et al. Lisocabtagene maraleucel as second-line therapy in adults with relapsed or refractory large B-cell lymphoma who were not intended for haematopoietic stem cell transplantation (PILOT): An open-label, phase 2 study. Lancet Oncol. 2022, 23, 1066–1077. [Google Scholar] [CrossRef]
- Wierda, W.G.; Dorritie, K.A.; Munoz, J.; Stephens, D.M.; Solomon, S.R.; Gillenwater, H.H.; Gong, L.; Yang, L.; Ogasawara, K.; Thorpe, J.; et al. Transcend CLL 004: Phase 1 cohort of lisocabtagene maraleucel (liso-cel) in combination with ibrutinib for patients with relapsed/refractory (R/R) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Blood 2020, 136, 39–40. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Bishop, M.R.; Dickinson, M.; Purtill, D.; Barba, P.; Santoro, A.; Hamad, N.; Kato, K.; Sureda, A.; Greil, R.; Thieblemont, C.; et al. Second-line tisagenlecleucel or standard care in aggressive b-cell lymphoma. N. Engl. J. Med. 2022, 386, 629–639. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in children and young adults with b-cell lymphoblastic leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in adult relapsed or refractory diffuse large b-cell lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.L.; Hwang, W.-T.; Frey, N.V.; Lacey, S.F.; Shaw, P.A.; Loren, A.W.; Bagg, A.; Marcucci, K.T.; Shen, A.; Gonzalez, V.; et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015, 7, 303ra139. [Google Scholar] [CrossRef]
- Frey, N.V.; Gill, S.; Hexner, E.O.; Schuster, S.; Nasta, S.; Loren, A.; Svoboda, J.; Stadtmauer, E.; Landsburg, D.J.; Mato, A.; et al. Long-term outcomes from a randomized dose optimization study of chimeric antigen receptor modified T cells in relapsed chronic lymphocytic leukemia. J. Clin. Oncol. 2020, 38, 2862–2871. [Google Scholar] [CrossRef]
- Porter, D.L.; Frey, N.V.; Melenhorst, J.J.; Hwang, W.-T.; Lacey, S.F.; Shaw, P.; Chew, A.; Marcucci, K.; Gill, S.; Loren, A.W.; et al. Randomized, phase II dose optimization study of chimeric antigen receptor (CAR) modified T cells directed against CD19 in patients (pts) with relapsed, refractory (R/R) CLL. J. Clin. Oncol. 2016, 34, 3009. [Google Scholar] [CrossRef]
- Shah, B.D.; Ghobadi, A.; Oluwole, O.O.; Logan, A.C.; Boissel, N.; Cassaday, R.D.; Leguay, T.; Bishop, M.R.; Topp, M.S.; Tzachanis, D.; et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: Phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet 2021, 398, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. Three-year follow-up of KTE-X19 in patients with relapsed/refractory mantle cell lymphoma, including high-risk subgroups, in the ZUMA-2 study. J. Clin. Oncol. 2023, 41, 555–567. [Google Scholar] [CrossRef]
- Flinn, I.; Marris, M.; Wierda, W.G.; Coutre, S.; Pagel, J.M.; Byrd, J.C.; Goyal, L.; Goodman, K.; Zheng, Y.; Milletti, F.; et al. ZUMA-8: A phase 1/2 multicenter study evaluating KTE-X19 in patients (pts) with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL). J. Clin. Oncol. 2019, 37, TPS7566. [Google Scholar] [CrossRef]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.-A.; Kersten, M.-J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene ciloleucel as second-line therapy for large b-cell lymphoma. N. Engl. J. Med. 2022, 386, 640–654. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene ciloleucel CAR t-cell therapy in refractory large b-cell lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Jacobson, C.A.; Chavez, J.C.; Sehgal, A.R.; William, B.M.; Munoz, J.; Salles, G.; Munshi, P.N.; Casulo, C.; Maloney, D.G.; de Vos, S.; et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): A single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022, 23, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Kochenderfer, J.N.; Dudley, M.E.; Kassim, S.H.; Somerville, R.P.T.; Carpenter, R.O.; Stetler-Stevenson, M.; Yang, J.C.; Phan, G.Q.; Hughes, M.S.; Sherry, R.M.; et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2015, 33, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Kittai, A.S.; Bond, D.A.; William, B.; Saad, A.; Penza, S.; Efebera, Y.; Larkin, K.; Wall, S.A.; Choe, H.K.; Bhatnagar, B.; et al. Clinical activity of axicabtagene ciloleucel in adult patients with Richter syndrome. Blood Adv. 2020, 4, 4648–4652. [Google Scholar] [CrossRef] [PubMed]
- Melenhorst, J.J.; Chen, G.M.; Wang, M.; Porter, D.L.; Chen, C.; Collins, M.A.; Gao, P.; Bandyopadhyay, S.; Sun, H.; Zhao, Z.; et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 2022, 602, 503–509. [Google Scholar] [CrossRef]
- Cappell, K.M.; Sherry, R.M.; Yang, J.C.; Goff, S.L.; Vanasse, D.A.; McIntyre, L.; Rosenberg, S.A.; Kochenderfer, J.N. Long-term follow-up of anti-CD19 chimeric antigen receptor T-cell therapy. J. Clin. Oncol. 2020, 38, 3805–3815. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Kenderian, S.S.; Flinn, I.W.; Hill, B.T.; Maris, M.; Ghia, P.; Byrne, M.; Bartlett, N.L.; Pagel, J.M.; Zheng, Y.; et al. ZUMA-8: A phase 1 study of KTE-X19, an anti-cd19 chimeric antigen receptor (CAR) T-cell therapy, in patients with relapsed/refractory chronic lymphocytic leukemia. Blood 2022, 140, 7454–7456. [Google Scholar] [CrossRef]
- Fraietta, J.A.; Beckwith, K.A.; Patel, P.R.; Ruella, M.; Zheng, Z.; Barrett, D.M.; Lacey, S.F.; Melenhorst, J.J.; McGettigan, S.E.; Cook, D.R.; et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood 2016, 127, 1117–1127. [Google Scholar] [CrossRef]
- Long, M.; Beckwith, K.; Do, P.; Mundy, B.L.; Gordon, A.; Lehman, A.M.; Maddocks, K.J.; Cheney, C.; Jones, J.A.; Flynn, J.M.; et al. Ibrutinib treatment improves T cell number and function in CLL patients. J. Clin. Investig. 2017, 127, 3052–3064. [Google Scholar] [CrossRef]
- Ruella, M.; Kenderian, S.S.; Shestova, O.; Klichinsky, M.; Melenhorst, J.J.; Wasik, M.A.; Lacey, S.F.; June, C.H.; Gill, S. Kinase inhibitor ibrutinib to prevent cytokine-release syndrome after anti-CD19 chimeric antigen receptor T cells for B-cell neoplasms. Leukemia 2017, 31, 246–248. [Google Scholar] [CrossRef]
- Gill, S.; Vides, V.; Frey, N.V.; Hexner, E.O.; Metzger, S.; O’Brien, M.; Hwang, W.-T.; Brogdon, J.L.; Davis, M.M.; Fraietta, J.A.; et al. Anti-CD19 CAR T cells in combination with ibrutinib for the treatment of chronic lymphocytic leukemia. Blood Adv. 2022, 6, 5774–5785. [Google Scholar] [CrossRef]
- Gauthier, J.; Hirayama, A.V.; Purushe, J.; Hay, K.A.; Lymp, J.; Li, D.H.; Yeung, C.C.S.; Sheih, A.; Pender, B.S.; Hawkins, R.M.; et al. Feasibility and efficacy of CD19-targeted CAR T cells with concurrent ibrutinib for CLL after ibrutinib failure. Blood 2020, 135, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Scrivener, S.; Goddard, R.; Kaminski, E.; Prentice, A. Abnormal T-cell function in B-cell chronic lymphocytic leukaemia. Leuk. Lymphoma 2003, 44, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Riches, J.C.; Davies, J.K.; McClanahan, F.; Fatah, R.; Iqbal, S.; Agrawal, S.; Ramsay, A.G.; Gribben, J.G. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood 2013, 121, 1612–1621. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.A.; Alanio, C.; Svoboda, J.; Nasta, S.D.; Landsburg, D.J.; Lacey, S.F.; Ruella, M.; Bhattacharyya, S.; Wherry, E.J.; Schuster, S.J. Pembrolizumab for B-cell lymphomas relapsing after or refractory to CD19-directed CAR T-cell therapy. Blood 2022, 139, 1026–1038. [Google Scholar] [CrossRef]
- Siddiqi, T.; Abramson, J.S.; Lee, H.J.; Schuster, S.; Hasskarl, J.; Montheard, S.; Dell Aringa, J.; Thompson, E.; Ananthakrishnan, R.; Lunning, M. Safety of lisocabtagene maraleucel given with durvalumab in patients with relapsed/refractory aggressive b-cell non hodgkin lymphoma: First results from the platform study. Hematol. Oncol. 2019, 37, 171–172. [Google Scholar] [CrossRef]
- Jäger, U.; Worel, N.; McGuirk, J.; Riedell, P.A.; Fleury, I.; Borchmann, P.; Du, Y.; Abdelhady, A.M.; Han, X.; Martinez-Prieto, M.; et al. Safety and efficacy of tisagenlecleucel (tisa-cel) plus pembrolizumab (pembro) in patients (pts) with relapsed/refractory diffuse large B-cell lymphoma (r/r DLBCL): Updated analysis of the phase 1b PORTIA study. J. Clin. Oncol. 2021, 39, e19537. [Google Scholar] [CrossRef]
- Jacobson, C.A.; Locke, F.L.; Miklos, D.B.; Herrera, A.F.; Westin, J.R.; Lee, J.; Rossi, J.M.; Zheng, L.; Avanzi, M.P.; Roberts, Z.J.; et al. End of phase 1 results from Zuma-6: Axicabtagene ciloleucel (Axi-Cel) in combination with atezolizumab for the treatment of patients with refractory diffuse large B cell lymphoma. Blood 2018, 132, 4192. [Google Scholar] [CrossRef]
- Ruella, M.; Barrett, D.M.; Kenderian, S.S.; Shestova, O.; Hofmann, T.J.; Perazzelli, J.; Klichinsky, M.; Aikawa, V.; Nazimuddin, F.; Kozlowski, M.; et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J. Clin. Investig. 2016, 126, 3814–3826. [Google Scholar] [CrossRef]
- Shadman, M.; Yeung, C.C.; Redman, M.; Lee, S.Y.; Lee, D.H.; Ra, S.; Ujjani, C.S.; Dezube, B.J.; Poh, C.; Warren, E.H.; et al. High efficacy and low toxicity of MB-106, a third generation CD20 targeted CAR-T for treatment of relapsed/refractory B-NHL and CLL. Transplant. Cell. Ther. 2022, 28, S182–S183. [Google Scholar] [CrossRef]
- Qin, H.; Dong, Z.; Wang, X.; Cheng, W.A.; Wen, F.; Xue, W.; Sun, H.; Walter, M.; Wei, G.; Smith, D.L.; et al. CAR T cells targeting BAFF-R can overcome CD19 antigen loss in B cell malignancies. Sci. Transl. Med. 2019, 11, eaaw9414. [Google Scholar] [CrossRef] [PubMed]
- Scarfò, I.; Ormhøj, M.; Frigault, M.J.; Castano, A.P.; Lorrey, S.; Bouffard, A.A.; van Scoyk, A.; Rodig, S.J.; Shay, A.J.; Aster, J.C.; et al. Anti-CD37 chimeric antigen receptor T cells are active against B- and T-cell lymphomas. Blood 2018, 132, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Ramakrishna, S.; Nguyen, S.; Fountaine, T.J.; Ponduri, A.; Stetler-Stevenson, M.; Yuan, C.M.; Haso, W.; Shern, J.F.; Shah, N.N.; et al. Preclinical development of bivalent chimeric antigen receptors targeting both CD19 and CD22. Mol. Ther. Oncolytics 2018, 11, 127–137. [Google Scholar] [CrossRef]
- Spiegel, J.Y.; Patel, S.; Muffly, L.; Hossain, N.M.; Oak, J.; Baird, J.H.; Frank, M.J.; Shiraz, P.; Sahaf, B.; Craig, J.; et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: A phase 1 trial. Nat. Med. 2021, 27, 1419–1431. [Google Scholar] [CrossRef]
- Shah, N.N.; Johnson, B.D.; Schneider, D.; Zhu, F.; Szabo, A.; Keever-Taylor, C.A.; Krueger, W.; Worden, A.A.; Kadan, M.J.; Yim, S.; et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: A phase 1 dose escalation and expansion trial. Nat. Med. 2020, 26, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Zhang, Y.; Liu, Y.; Ji, X.; Zhang, W.-Y.; Guo, Y.; Han, X.; Ti, D.; Dai, H.; Wang, C.; et al. Optimized tandem CD19/CD20 CAR-engineered T cells in refractory/relapsed B cell lymphoma. Blood 2020, 136, 1632–1644. [Google Scholar] [CrossRef]
- Ramos, C.A.; Savoldo, B.; Torrano, V.; Ballard, B.; Zhang, H.; Dakhova, O.; Liu, E.; Carrum, G.; Kamble, R.T.; Gee, A.P.; et al. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J. Clin. Investig. 2016, 126, 2588–2596. [Google Scholar] [CrossRef]
- Ranganathan, R.; Shou, P.; Ahn, S.; Sun, C.; West, J.; Savoldo, B.; Dotti, G. CAR T cells targeting human immunoglobulin light chains eradicate mature B-cell malignancies while sparing a subset of normal B cells. Clin. Cancer Res. 2021, 27, 5951–5960. [Google Scholar] [CrossRef]
- Cui, B.; Ghia, E.M.; Chen, L.; Rassenti, L.Z.; DeBoever, C.; Widhopf, G.F.; Yu, J.; Neuberg, D.S.; Wierda, W.G.; Rai, K.R.; et al. High-level ROR1 associates with accelerated disease progression in chronic lymphocytic leukemia. Blood 2016, 128, 2931–2940. [Google Scholar] [CrossRef]
- Hudecek, M.; Lupo-Stanghellini, M.-T.; Kosasih, P.L.; Sommermeyer, D.; Jensen, M.C.; Rader, C.; Riddell, S.R. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin. Cancer Res. 2013, 19, 3153–3164. [Google Scholar] [CrossRef]
- Faitschuk, E.; Hombach, A.A.; Frenzel, L.P.; Wendtner, C.-M.; Abken, H. Chimeric antigen receptor T cells targeting Fc μ receptor selectively eliminate CLL cells while sparing healthy B cells. Blood 2016, 128, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Condomines, M.; van der Stegen, S.J.C.; Perna, F.; Kloss, C.C.; Gunset, G.; Plotkin, J.; Sadelain, M. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 2015, 28, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Palomba, M.L.; Batlevi, C.L.; Riviere, I.; Wang, X.; Senechal, B.; Furman, R.R.; Bernal, Y.; Hall, B.M.; Pineda, R.J.; et al. A phase I first-in-human clinical trial of CD19-targeted 19-28z/4-1BBL “armored” CAR T cells in patients with relapsed or refractory NHL and CLL including richter’s transformation. Blood 2018, 132, 224. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Fang, C.; Zhang, N.; Wang, Y.; Chen, R.; Li, Y.; Tu, S. Donor-derived and off-the-shelf allogeneic anti-CD19 CAR T-cell therapy for R/R ALL and NHL: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2022, 179, 103807. [Google Scholar] [CrossRef]
- Benjamin, R.; Jain, N.; Maus, M.V.; Boissel, N.; Graham, C.; Jozwik, A.; Yallop, D.; Konopleva, M.; Frigault, M.J.; Teshima, T.; et al. UCART19, a first-in-class allogeneic anti-CD19 chimeric antigen receptor T-cell therapy for adults with relapsed or refractory B-cell acute lymphoblastic leukaemia (CALM): A phase 1, dose-escalation trial. Lancet Haematol. 2022, 9, e833–e843. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, Y.; Yan, C.; Wang, Y.; Zhao, X.; Chen, Y.; Han, W.; Xu, L.; Zhang, X.; Liu, K.; et al. Donor-derived CD19-targeted T cell infusion eliminates B cell acute lymphoblastic leukemia minimal residual disease with no response to donor lymphocytes after allogeneic hematopoietic stem cell transplantation. Engineering 2019, 5, 150–155. [Google Scholar] [CrossRef]
- Rossig, C.; Pule, M.; Altvater, B.; Saiagh, S.; Wright, G.; Ghorashian, S.; Clifton-Hadley, L.; Champion, K.; Sattar, Z.; Popova, B.; et al. Vaccination to improve the persistence of CD19CAR gene-modified T cells in relapsed pediatric acute lymphoblastic leukemia. Leukemia 2017, 31, 1087–1095. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.-Q.; Zhang, R.-L.; Liu, F.; Wang, Y.; Yan, Z.-L.; Song, Y.-P.; Yang, T.; Li, P.; Wang, Z.; et al. Donor-derived CD19 CAR-T cell therapy of relapse of CD19-positive B-ALL post allotransplant. Leukemia 2021, 35, 1563–1570. [Google Scholar] [CrossRef]
- Magnani, C.F.; Gaipa, G.; Lussana, F.; Belotti, D.; Gritti, G.; Napolitano, S.; Matera, G.; Cabiati, B.; Buracchi, C.; Borleri, G.; et al. Sleeping beauty–engineered CAR T cells achieve antileukemic activity without severe toxicities. J. Clin. Investig. 2020, 130, 6021–6033. [Google Scholar] [CrossRef]
- Brudno, J.N.; Somerville, R.P.; Shi, V.; Rose, J.J.; Halverson, D.C.; Fowler, D.H.; Gea-Banacloche, J.C.; Pavletic, S.Z.; Hickstein, D.D.; Lu, T.L.; et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J. Clin. Oncol. 2016, 34, 1112–1121. [Google Scholar] [CrossRef]
- Benjamin, R.; Graham, C.; Yallop, D.; Jozwik, A.; Mirci-Danicar, O.C.; Lucchini, G.; Pinner, D.; Jain, N.; Kantarjian, H.; Boissel, N.; et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: Results of two phase 1 studies. Lancet 2020, 396, 1885–1894. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Hamadani, M.; Miklos, D.B.; Holmes, H.; Hinkle, J.; Kennedy-Wilde, J.; Maller, O.; Weinstein, M.; Galimi, F.; Lai, R.; et al. A phase 1 study of ADI-001: Anti-CD20 CAR-engineered allogeneic gamma delta (γδ) T cells in adults with B-cell malignancies. J. Clin. Oncol. 2022, 40, 7509. [Google Scholar] [CrossRef]
- Nastoupil, L.J.; O’Brien, S.; Holmes, H.E.; Dsouza, L.; Hart, D.; Matsuda, E.; Satterfield-Ledbetter, T.; Skoble, J.; Garner, E.; Bryan, M.; et al. P1455: First-in-human trial of CB-010, a crispr-edited allogeneic anti-CD19 car -T cell therapy with a PD-1 knock out, in patients with relapsed or refractory B cell non-hodgkin lymphoma (antler study). Hemasphere 2022, 6, 1337–1338. [Google Scholar] [CrossRef]
- Mehta, A.; Farooq, U.; Chen, A.; Mcguirk, J.; Ly, T.; Wong, L.; Cooley, S.; Valamehr, B.; Elstrom, R.; Chu, Y.W.; et al. Interim phase I clinical data of FT819-101, a study of the first-ever, off-the-shelf, iPSC-derived TCR-less CD19 CAR T-cell therapy for patients with relapsed/refractory B-cell malignancies. Blood 2022, 140 (Suppl. 1), 4577–4578. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Nath, R.; Munoz, J.; Tees, M.; Miklos, D.B.; Frank, M.J.; Malik, S.A.; Stevens, D.; Shin, C.R.; Balakumaran, A.; et al. ALPHA study: ALLO-501 produced deep and durable responses in patients with relapsed/refractory non-hodgkin’s lymphoma comparable to autologous CAR T. Blood 2021, 138, 3878. [Google Scholar] [CrossRef]
- Lekakis, L.J.; Locke, F.L.; Tees, M.; Neelapu, S.S.; Malik, S.A.; Hamadani, M.; Frank, M.J.; Popplewell, L.L.; Abramson, J.S.; de Vos, S.; et al. ALPHA2 study: ALLO-501A allogeneic CAR T in LBCL, updated results continue to show encouraging safety and efficacy with consolidation dosing. Blood 2021, 138, 649. [Google Scholar] [CrossRef]
- Shah, B.D.; Jacobson, C.A.; Solomon, S.; Jain, N.; Vainorius, M.; Heery, C.R.; He, F.C.; Reshef, R.; Herrera, A.F.; Akard, L.P.; et al. Preliminary safety and efficacy of PBCAR0191, an allogeneic, off-the-shelf CD19-targeting CAR-T product, in relapsed/refractory (r/r) CD19+ NHL. J. Clin. Oncol. 2021, 39, 7516. [Google Scholar] [CrossRef]
- Shah, B.D.; Jacobson, C.; Solomon, S.R.; Jain, N.; Johnson, M.C.; Vainorius, M.; Yu, L.; Heery, C.R.; List, A.F.; He, F.; et al. Allogeneic CAR-T PBCAR0191 with intensified lymphodepletion is highly active in patients with relapsed/refractory B-cell malignancies. Blood 2021, 138, 302. [Google Scholar] [CrossRef]
- Herrera, L.; Santos, S.; Vesga, M.A.; Carrascosa, T.; Garcia-Ruiz, J.C.; Pérez-Martínez, A.; Juan, M.; Eguizabal, C. The race of CAR therapies: CAR-NK cells for fighting B-cell hematological cancers. Cancers 2021, 13, 5418. [Google Scholar] [CrossRef]
- Sabbah, M.; Jondreville, L.; Lacan, C.; Norol, F.; Vieillard, V.; Roos-Weil, D.; Nguyen, S. CAR-NK Cells: A chimeric hope or a promising therapy? Cancers 2022, 14, 3839. [Google Scholar] [CrossRef]
- Mizia-Malarz, A.; Sobol-Milejska, G. NK Cells as possible prognostic factor in childhood acute lymphoblastic leukemia. Dis. Markers 2019, 2019, 3596983. [Google Scholar] [CrossRef]
- Liu, E.; Tong, Y.; Dotti, G.; Shaim, H.; Savoldo, B.; Mukherjee, M.; Orange, J.; Wan, X.; Lu, X.; Reynolds, A.; et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2018, 32, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Bahmanyar, M.; Vakil, M.K.; Al-Awsi, G.R.L.; Kouhpayeh, S.A.; Mansoori, Y.; Mansoori, B.; Moravej, A.; Mazarzaei, A.; Ghasemian, A. Anticancer traits of chimeric antigen receptors (CARs)-natural killer (NK) cells as novel approaches for melanoma treatment. BMC Cancer 2022, 22, 1220. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Patel, S.; Patel, M.; Musni, K.; Anderson, M.; Cooley, S.; Valamehr, B.; Chu, W. 380 Preliminary results of an ongoing phase I trial of FT500, a first-in-class, off-the-shelf, induced pluripotent stem cell (iPSC) derived natural killer (NK) cell therapy in advanced solid tumors. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Cichocki, F.; Bjordahl, R.; Gaidarova, S.; Mahmood, S.; Abujarour, R.; Wang, H.; Tuininga, K.; Felices, M.; Davis, Z.B.; Bendzick, L.; et al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti–PD-1 therapy. Sci. Transl. Med. 2020, 12, eaaz5618. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Kerbauy, L.N.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Li, Y.; Basar, R.; Wang, G.; Liu, E.; Moyes, J.S.; Li, L.; Kerbauy, L.N.; Uprety, N.; Fathi, M.; Rezvan, A.; et al. KIR-based inhibitory CARs overcome CAR-NK cell trogocytosis-mediated fratricide and tumor escape. Nat. Med. 2022, 28, 2133–2144. [Google Scholar] [CrossRef]
- Cruz, C.R.Y.; Micklethwaite, K.; Savoldo, B.; Ramos, C.; Lam, S.; Ku, S.; Diouf, O.; Liu, E.; Barrett, A.J.; Ito, S.; et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: A phase 1 study. Blood 2013, 122, 2965–2973. [Google Scholar] [CrossRef]
- Mato, A.R.; Shah, N.N.; Jurczak, W.; Cheah, C.Y.; Pagel, J.M.; Woyach, J.A.; Fakhri, B.; Eyre, T.A.; Lamanna, N.; Patel, M.R.; et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): A phase 1/2 study. Lancet 2021, 397, 892–901. [Google Scholar] [CrossRef]
- Woyach, J.; Flinn, I.W.; Awan, F.T.; Eradat, H.; Brander, D.; Tees, M.; Parikh, S.A.; Phillips, T.; Wang, W.; Reddy, N.M.; et al. P682: Nemtabrutinib (mk-1026), a non-covalent inhibitor of wild-type and c481s mutated bruton tyrosine kinase for b-cell malignancies: Efficacy and safety of the phase 2 dose-expansion bellwave-001 study. HemaSphere 2022, 6, 578–579. [Google Scholar] [CrossRef]
- Hampel, P.J.; Rabe, K.G.; Call, T.G.; Ding, W.; Leis, J.F.; Kenderian, S.S.; Muchtar, E.; Wang, Y.; Koehler, A.B.; Parrondo, R.; et al. Combined ibrutinib and venetoclax for treatment of patients with ibrutinib-resistant or double-refractory chronic lymphocytic leukaemia. Br. J. Haematol. 2022, 199, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Hyak, J.M.; Huang, Y.; Rogers, K.A.; Bhat, S.A.; Grever, M.R.; Byrd, J.C.; Kittai, A.S.; Jones, D.; Miller, C.R.; Woyach, J.A. Combined BCL2 and BTK inhibition in CLL demonstrates efficacy after monotherapy with both classes. Blood Adv. 2022, 6, 5124–5127. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coombs, C.C.; Easaw, S.; Grover, N.S.; O’Brien, S.M. Cellular Therapies in Chronic Lymphocytic Leukemia and Richter’s Transformation: Recent Developments in Chimeric Antigen Receptor T-Cells, Natural Killer Cells, and Allogeneic Stem Cell Transplant. Cancers 2023, 15, 1838. https://doi.org/10.3390/cancers15061838
Coombs CC, Easaw S, Grover NS, O’Brien SM. Cellular Therapies in Chronic Lymphocytic Leukemia and Richter’s Transformation: Recent Developments in Chimeric Antigen Receptor T-Cells, Natural Killer Cells, and Allogeneic Stem Cell Transplant. Cancers. 2023; 15(6):1838. https://doi.org/10.3390/cancers15061838
Chicago/Turabian StyleCoombs, Catherine C., Saumya Easaw, Natalie S. Grover, and Susan M. O’Brien. 2023. "Cellular Therapies in Chronic Lymphocytic Leukemia and Richter’s Transformation: Recent Developments in Chimeric Antigen Receptor T-Cells, Natural Killer Cells, and Allogeneic Stem Cell Transplant" Cancers 15, no. 6: 1838. https://doi.org/10.3390/cancers15061838
APA StyleCoombs, C. C., Easaw, S., Grover, N. S., & O’Brien, S. M. (2023). Cellular Therapies in Chronic Lymphocytic Leukemia and Richter’s Transformation: Recent Developments in Chimeric Antigen Receptor T-Cells, Natural Killer Cells, and Allogeneic Stem Cell Transplant. Cancers, 15(6), 1838. https://doi.org/10.3390/cancers15061838






