Oligo-Metastatic Cancers: Putative Biomarkers, Emerging Challenges and New Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
2. The Origin of OMD: The Concept of “Metastatic Virulence”
3. OMD Clinical Contexts
- Genuine (or “de novo”) OMD: it is considered the “purest” phenotype of OMD, when the cancer has no prior history of polymetastatic disease. It is useful to distinguish between synchronous and metachronous OMD, which refer to the diagnosis being made within or after 6 months of the primary cancer diagnosis, respectively.
- Induced OMD: the polymetastatic cancer has become limited to a small number of metastatic sites (OMD) following systemic treatment.
- Repeat OMD: OMD that recurs after a previous diagnosis and treatment for OMD.
- Repeat and induced can be associated with different imaging dynamics (i.e., repeat oligo-recurrence vs. induced oligo-recurrence; both indicate new oligometastatic lesions from OMD or polymetastatic disease, respectively):
- Oligorecurrence: OMD that recurs after initial treatment during a treatment-free period.
- Oligoprogression: the OMD progresses during active systemic treatment.
- Oligopersistence: the OMD persists after initial treatment.
4. Epidemiology of OMD
5. Definitive Local Therapies in OMD
6. Biomarkers of OMD
| Author, Year | Tumor Type | Biomarker | Clinical Significance |
|---|---|---|---|
| Lussier, 2011 [88] | Mixed tumor histologies. | OligomiRNAs. | MicroRNAs expression patterns associated with OMD. |
| Turajlic, 2018 [89] | Clear-cell renal cell carcinoma. | 9p loss. Low intra-tumor heterogeneity of primary cancer. High genomic somatic copy-number alterations. | The patients with these characteristics develop poly-metastatic disease. |
| PBRM1 and SETD2 mutations in primary tumor. | These genetic features associate with oligo-metastases and attenuated progression. | ||
| Pitroda, 2018 [90] | Colorectal cancer. | “Canonical” and “immune” molecular subtypes in primary tumor. | They associate with long-term survival and OMD. |
| Ottaiano, 2020 [91] | Colorectal cancer. | KRAS regression from primary to metastatic lesions. ERBB2 p.Pro1170Ala. | They associate with lung-limited OMD. |
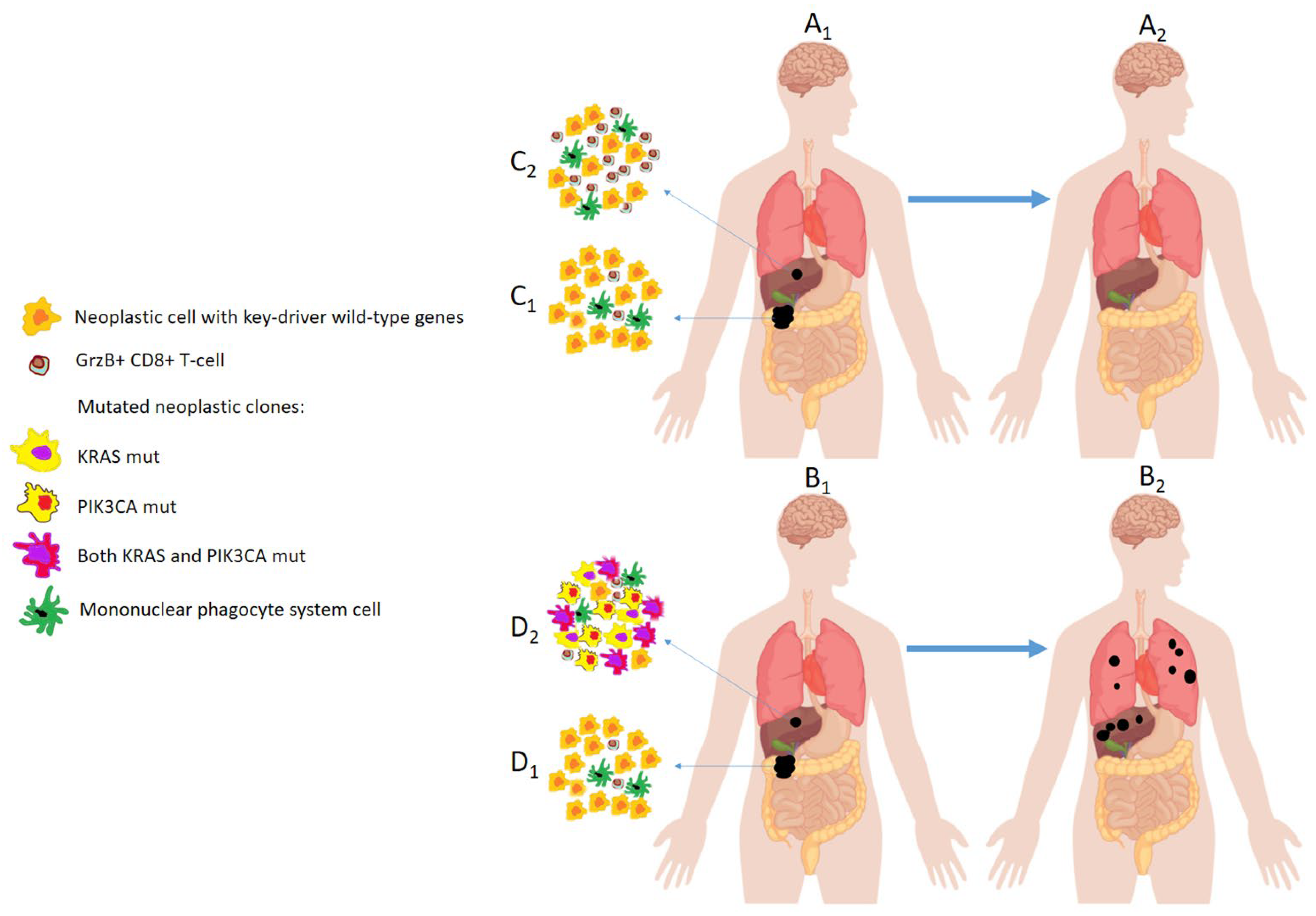
| Ottaiano, 2020 [92] | Colorectal cancer. | Loss of KRAS and SMAD4 alterations from primary to metastatic lesions. High granzyme-B+ T-cell infiltration into metastatic tumor. | The patients with these characteristics remain with liver-limited OMD for long time. |
| Gain in KRAS, PIK3CA and SMAD4 alterations. Scarce granzyme-B+ T-cells infiltration. | The patients with these characteristics develop poly-metastatic widely diffusive disease. | ||
| Ottaiano, 2022 [93] | Colorectal cancer. | KRAS regression from primary to metastatic lesions. HLA-C7 aplotype. | The patients with these characteristics remain oligometastatic for long time. |
| Ottaiano, 2022 [103] | Colorectal cancer. | Absence of TCF7L2 variants, low frequency of type 2 diabetes-associated genetic polymorphisms. | The patients with this characteristic have persistent OMD. |
7. Timings of DLTs and Systemic Treatments
8. Technological Limits for Studying OMD
9. Exploring the OMD from Cancer Biopsies: Pros and Cons
10. Does Chemotherapy Induces Genetic Remodeling in OMD?
11. Heterogeneity of Initial Tumor Burden in Clinical Trials
12. Identification of OMD
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohno, Y.; Ozawa, Y.; Koyama, H.; Yoshikawa, T.; Takenaka, D.; Nagata, H.; Ueda, T.; Ikeda, H.; Toyama, H. State of the Art MR Imaging for Lung Cancer TNM Stage Evaluation. Cancers 2023, 15, 950. [Google Scholar] [CrossRef] [PubMed]
- Cserni, G.; Chmielik, E.; Cserni, B.; Tot, T. The New TNM-Based Staging of Breast Cancer. Virchows Arch. 2018, 472, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.N. Colorectal Cancer: Preoperative Evaluation and Staging. Surg. Oncol. Clin. N. Am. 2022, 31, 127–141. [Google Scholar] [CrossRef]
- Santorsola, M.; Di Lauro, V.; Nasti, G.; Caraglia, M.; Capuozzo, M.; Perri, F.; Cascella, M.; Misso, G.; Ottaiano, A. Tumour Burden Reporting in Phase III Clinical Trials of Metastatic Lung, Breast, and Colorectal Cancers: A Systematic Review. Cancers 2022, 14, 3262. [Google Scholar] [CrossRef]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Ottaiano, A.; Santorsola, M.; Caraglia, M.; Circelli, L.; Gigantino, V.; Botti, G.; Nasti, G. Genetic Regressive Trajectories in Colorectal Cancer: A New Hallmark of Oligo-Metastatic Disease? Transl. Oncol. 2021, 14, 101131. [Google Scholar] [CrossRef]
- Katsuno, Y.; Lamouille, S.; Derynck, R. TGF-β Signaling and Epithelial-Mesenchymal Transition in Cancer Progression. Curr. Opin. Oncol. 2013, 25, 76–84. [Google Scholar] [CrossRef]
- Lynch, M.; Ackerman, M.S.; Gout, J.F.; Long, H.; Sung, W.; Thomas, W.K. Genetic Drift, Selection and the Evolution of the Mutation Rate. Nat. Rev. Genet. 2016, 17, 704–714. [Google Scholar] [CrossRef]
- Mohan, V.; Das, A.; Sagi, I. Emerging Roles of ECM Remodeling Processes in Cancer. Semin. Cancer Biol. 2020, 62, 192–200. [Google Scholar] [CrossRef]
- Jeong, J.H.; Ojha, U.; Lee, Y.M. Pathological angiogenesis and inflammation in tissues. Arch. Pharm. Res. 2021, 44, 1–15. [Google Scholar] [CrossRef]
- Li, H.; Zimmerman, S.E.; Weyemi, U. Genomic instability and metabolism in cancer. Int. Rev. Cell Mol. Biol. 2021, 364, 241–265. [Google Scholar] [CrossRef] [PubMed]
- Kaszak, I.; Witkowska-Piłaszewicz, O.; Niewiadomska, Z.; Dworecka-Kaszak, B.; Ngosa Toka, F.; Jurka, P. Role of Cadherins in Cancer-A Review. Int. J. Mol. Sci. 2020, 21, 7624. [Google Scholar] [CrossRef] [PubMed]
- Mejia, A.; Schulz, S.; Hyslop, T.; Weinberg, D.S.; Waldman, S.A. Molecular staging individualizing cancer management. J. Surg. Oncol. 2012, 105, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Niibe, Y.; Chang, J.Y.; Onishi, H.; Salama, J.; Hiraki, T.; Yamashita, H. Oligometastases/oligo-recurrence of lung cancer. J. Clin. Oncol. 2014, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Lievens, Y.; Guckenberger, M.; Gomez, D.; Hoyer, M.; Iyengar, P.; Kindts, I. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother. Oncol. 2020, 148, 157–166. [Google Scholar] [CrossRef]
- Withers, H.R.; Lee, S.P. Modeling growth kinetics and statistical distribution of oligometastases. Semin. Radiat. Oncol. 2016, 16, 111–119. [Google Scholar] [CrossRef]
- Niibe, Y.; Jingu, K.; Onishi, H. Oligometastases: History and future vision of breast cancer. Transl. Cancer Res. 2020, 9, 5028–5031. [Google Scholar] [CrossRef]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; Nandita, M.D.; Dingemans, A.M.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and classification of oligometastatic disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef]
- Ashworth, A.B.; Senan, S.; Palma, D.A.; Riquet, M.; Ahn, Y.C.; Ricardi, U.; Congedo, M.T.; Gomez, D.R.; Wright, G.M.; Melloni, G.; et al. An Individual Patient Data Meta-Analysis of Outcomes and Prognostic Factors after Treatment of Oligometastatic Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2014, 15, 346–355. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, Q.; Cai, F.; Li, H.; Zhou, Y. Local Therapy Treatment Conditions for Oligometastatic Non-Small Cell Lung Cancer. Front. Oncol. 2022, 12, 1028132. [Google Scholar] [CrossRef]
- Tsutsui, T.; Yamaki, H.; Kumagai, T.; Omori, C.; Kobayashi, H.; Kakizaki, Y.; Miyashita, Y. Small Cell Lung Cancer with Thyroid Gland Oligometastasis: A Case Report. Thorac. Cancer 2021, 12, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Can, A.S.; Köksal, G. Thyroid Metastasis from Small Cell Lung Carcinoma: A Case Report and Review of the Literature. J. Med. Case Rep. 2015, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Kassar, S.; Samaha, R.; Aoun, R.; Khoury, M.; Kattan, J. The Concept of Oligometastatic Disease in Gastric Cancer: Reality or Fiction? Future Oncol. 2022, 18, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Deek, M.P.; Tran, P.T. Oligometastatic and Oligoprogression Disease and Local Therapies in Prostate Cancer. Cancer J. 2020, 26, 137–143. [Google Scholar] [CrossRef]
- Kimura, T.; Fujiwara, T.; Kameoka, T.; Adachi, Y.; Kariya, S. The Current Role of Stereotactic Body Radiation Therapy (SBRT) in Hepatocellular Carcinoma (HCC). Cancers 2022, 14, 4383. [Google Scholar] [CrossRef]
- Kimura, T.; Doi, Y.; Takahashi, S.; Kubo, K.; Imano, N.; Takeuchi, Y.; Takahashi, I.; Nishibuchi, I.; Murakami, Y.; Kenjo, M.; et al. An Overview of Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 271–279. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Sun, C.; Li, Z.; Fei, H.; Zhao, D. Does Surgical Resection Significantly Prolong the Long-Term Survival of Patients with Oligometastatic Pancreatic Ductal Adenocarcinoma? A Cross-Sectional Study Based on 18 Registries. J. Clin. Med. 2023, 12, 513. [Google Scholar] [CrossRef]
- Palluzzi, E.; Marchetti, C.; Cappuccio, S.; Avesani, G.; Macchia, G.; Gambacorta, M.A.; Cocciolillo, F.; Scambia, G.; Fagotti, A. Management of Oligometastatic Ovarian Cancer Recurrence During PARP Inhibitor Maintenance. Int. J. Gynecol. Cancer 2022, 32, 1164–1170. [Google Scholar] [CrossRef]
- Shen, J.; Tao, Y.; He, L.; Guan, H.; Zhen, H.; Liu, Z.; Zhang, F. Clinical Application of Radiotherapy in Patients with Oligometastatic Ovarian Cancer: A Sharp Tool to Prolong the Interval of Systemic Treatment. Discov. Oncol. 2022, 13, 82. [Google Scholar] [CrossRef]
- Duan, Y.; Qin, W.; Yang, L.; Zou, B.; Qie, W.; Song, R.; Xue, L.; Wang, L. Safety and Efficacy of Concurrent or Sequential Radiotherapy Plus (PD-1) Inhibitors in Oligometastatic Esophageal Cancer. Cancer Manag. Res. 2023, 15, 55–65. [Google Scholar] [CrossRef]
- Goetze, T.O.; Al-Batran, S.E. Perspectives on the Management of Oligometastatic Disease in Esophago-Gastric Cancer. Cancers 2022, 14, 5200. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Kalra, B.; Mulani, J.; Jain, J.; Gurram, L.; Mittal, P.; Alone, M.; Ghosh, J.; Rath, S.; Gulia, S.; et al. Salvage (Re)Radiation in Oligometastatic and Oligorecurrent Cervical Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Christ, S.M.; Heesen, P.; Muehlematter, U.J.; Pohl, K.; William Thiel, G.; Willmann, J.; Ahmadsei, M.; Kroese, T.E.; Mayinger, M.; Balermpas, P.; et al. Recognition of and Treatment Recommendations for Oligometastatic Disease in Multidisciplinary Tumor Boards. Clin. Transl. Radiat. Oncol. 2022, 38, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Galata, C.; Wimmer, E.; Kasper, B.; Wenz, F.; Reißfelder, C.; Jakob, J. Multidisciplinary Tumor Board Recommendations for Oligometastatic Malignancies: A Prospective Single-Center Analysis. Oncol Res Treat. 2019, 42, 87–94. [Google Scholar] [CrossRef]
- Aoki, S.; Yamashita, H.; Abe, O.; Nakagawa, K. Stereotactic Body Radiotherapy (SBRT) for Oligo-Metastatic Liver Metastases from Breast Cancer, as an Effective and Safe Alternative to Surgery: A Review. Transl. Cancer Res. 2020, 9, 5087–5095. [Google Scholar] [CrossRef]
- Niibe, Y.; Hayakawa, K. Oligometastases and Oligo-Recurrence: The New Era of Cancer Therapy. Jpn. J. Clin. Oncol. 2010, 40, 107–111. [Google Scholar] [CrossRef]
- Izzo, F.; Granata, V.; Grassi, R.; Fusco, R.; Palaia, R.; Delrio, P.; Carrafiello, G.; Azoulay, D.; Petrillo, A.; Curley, S.A. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist 2019, 24, e990–e1005. [Google Scholar] [CrossRef]
- Nieuwenhuizen, S.; Dijkstra, M.; Puijk, R.S.; Geboers, B.; Ruarus, A.H.; Schouten, E.A.; Nielsen, K.; de Vries, J.J.J.; Bruynzeel, A.M.E.; Scheffer, H.J.; et al. Microwave Ablation, Radiofrequency Ablation, Irreversible Electroporation, and Stereotactic Ablative Body Radiotherapy for Intermediate Size (3–5 cm) Unresectable Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Curr. Oncol. Rep. 2022, 24, 793–808. [Google Scholar] [CrossRef]
- Horie, T.; Kanemitsu, Y.; Takamizawa, Y.; Moritani, K.; Tsukamoto, S.; Shida, D. Prognostic Differences Between Oligometastatic and Polymetastatic Disease After Resection in Patients with Colorectal Cancer and Hepatic or Lung Metastases: Retrospective Analysis of a Large Cohort at a Single Institution. Surgery 2023, 173, 328–334. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ichiba, T.; Sakuyama, T.; Arakawa, Y.; Nagasaki, E.; Aiba, K.; Nogi, H.; Kawase, K.; Takeyama, H.; Toriumi, Y.; et al. Possible Clinical Cure of Metastatic Breast Cancer: Lessons from Our 30-Year Experience with Oligometastatic Breast Cancer Patients and Literature Review. Breast Cancer 2012, 19, 218–237. [Google Scholar] [CrossRef]
- Macagno, A.; de Nonneville, A.; Annede, P.; Piana, G.; Pougnet, I.; Daidj, N.; Moureau-Zabotto, L.; Darreon, J.; Padovani, L.; Bertucci, F.; et al. Repeated Multimodality Ablative Therapies for Oligorecurrent Pulmonary Metastatic Disease. Curr. Oncol. 2022, 29, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Patrone, R.; Izzo, F.; Palaia, R.; Granata, V.; Nasti, G.; Ottaiano, A.; Pasta, G.; Belli, A. Minimally Invasive Surgical Treatment of Intrahepatic Cholangiocarcinoma: A Systematic Review. World J. Gastrointest. Oncol. 2021, 13, 2203–2215. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jiang, J.; Xiao, D.; Zhou, Y.; Chen, Y.; Yang, H.; Wang, L.; Zeng, J.; He, B.; He, R.; et al. Robotic-Assisted Thoracic Surgery following Neoadjuvant Chemoimmunotherapy in Patients with Stage III Non-Small Cell Lung Cancer: A Real-World Prospective Cohort Study. Front. Oncol. 2022, 12, 969545. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, H.; Jingu, K.; Umezawa, R.; Yamamoto, T.; Ishikawa, Y.; Takahashi, N.; Katagiri, Y.; Kadoya, N. Stereotactic Radiotherapy for Oligometastases in Lymph Nodes-A Review. Technol. Cancer Res. Treat. 2018, 17. [Google Scholar] [CrossRef]
- Macbeth, F.; Abratt, R. Stereotactic Radiotherapy and Oligometastases. Lancet Oncol. 2021, 22, e132. [Google Scholar] [CrossRef]
- Lee, Y.; Auh, S.L.; Wang, Y.; Burnette, B.; Meng, Y.; Beckett, M.; Sharma, R.; Chin, R.; Tu, T.; Weichselbaum, R.R. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Blood 2009, 114, 589–595. [Google Scholar] [CrossRef]
- Lugade, A.A.; Sorensen, E.W.; Gerber, S.A.; Moran, J.P.; Frelinger, J.G.; Lord, E.M. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J. Immunol. 2008, 180, 3132–3139. [Google Scholar] [CrossRef]
- Hennel, R.; Brix, N.; Seidl, K.; Ernst, A.; Scheithauer, H.; Belka, C.; Lauber, K. Release of monocyte migration signals by breast cancer cell lines after ablative and fractionated γ-irradiation. Radiat. Oncol. 2014, 9, 85. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef]
- Golden, E.B.; Demaria, S.; Schiff, P.; Chachoua, A.; Formenti, S.C. An Abscopal Response to Radiation and Ipilimumab in a Patient with Metastatic Non–Small Cell Lung Cancer. Cancer Immunol. Res. 2013, 1, 365–372. [Google Scholar] [CrossRef]
- Gulley, J.; Arlen, P.M.; Bastian, A.; Morin, S.; Marte, J.; Beetham, P.; Tsang, K.-Y.; Yokokawa, J.; Hodge, J.W.; Ménard, C. Combining a Recombinant Cancer Vaccine with Standard Definitive Radiotherapy in Patients with Localized Prostate Cancer. Clin. Cancer Res. 2005, 11, 3353–3362. [Google Scholar] [CrossRef] [PubMed]
- Filatenkov, A.; Baker, J.; Mueller, A.M.; Kenkel, J.; Ahn, G.-O.; Dutt, S.; Zhang, N.; Kohrt, H.; Jensen, K.; Dejbakhsh-Jones, S. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin. Cancer Res. 2015, 21, 3727–3739. [Google Scholar] [CrossRef] [PubMed]
- Tinguely, P.; Dal, G.; Bottai, M.; Nilsson, H.; Freedman, J.; Engstrand, J. Microwave Ablation versus Resection for Colorectal Cancer Liver Metastases—A Propensity Score Analysis from a Population-Based Nationwide Registry. Eur. J. Surg. Oncol. 2020, 46, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Berber, E. Role of Thermal Ablation in the Management of Colorectal Liver Metastasis. Hepatobiliary Surg. Nutr. 2020, 9, 49–58. [Google Scholar] [CrossRef]
- Melnick, K.; Shin, D.; Dastmalchi, F.; Kabeer, Z.; Rahman, M.; Tran, D.; Ghiaseddin, A. Role of Laser Interstitial Thermal therapy in the Management of Primary and Metastatic Brain Tumors. Curr. Treat. Options Oncol. 2021, 22, 108. [Google Scholar] [CrossRef]
- Bargellini, I.; Bozzi, E.; Lorenzoni, G.; Boni, G.; Bianchi, F.; Traino, C.A.; Masi, G.; Cioni, R.; Crocetti, L. Role of Transhepatic Arterial Radioembolization in Metastatic Colorectal Cancer. Cardiovasc. Intervent. Radiol. 2022, 45, 1579–1589. [Google Scholar] [CrossRef]
- Shamimi-Noori, S.; Gonsalves, C.F.; Shaw, C.M. Metastatic Liver Disease: Indications for Locoregional Therapy and Supporting Data. Semin. Intervent. Radiol. 2017, 34, 145–166. [Google Scholar] [CrossRef]
- Zane, K.E.; Cloyd, J.M.; Mumtaz, K.S.; Wadhwa, V.; Makary, M.S. Metastatic Disease to the Liver: Locoregional Therapy Strategies and Outcomes. World J. Clin. Oncol. 2021, 12, 725–745. [Google Scholar] [CrossRef]
- Bolton, J.S.; Vauthey, J.N.; Sauter, E.R. Colorectal Cancer: Surgical Management of Recurrent and Metastatic Disease. J. Natl. Med. Assoc. 1988, 80, 561–564. [Google Scholar]
- Nicosia, L.; Figlia, V.; Ricottone, N.; Cuccia, F.; Mazzola, R.; Giaj-Levra, N.; Ricchetti, F.; Rigo, M.; Jafari, F.; Maria Magrini, S.; et al. Stereotactic Body Radiotherapy (SBRT) and Concomitant Systemic Therapy in Oligoprogressive Breast Cancer Patients. Clin. Exp. Metastasis 2022, 39, 581–588. [Google Scholar] [CrossRef]
- Sponholz, S.; Koch, A.; Mese, M.; Becker, S.; Sebastian, M.; Fischer, S.; Trainer, S.; Schreiner, W. Lung Cancer Resection after Immunochemotherapy vs. Chemotherapy in Oligometastatic NSCLC. Thorac. Cardiovasc. Surg. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.C.; Zhang, J.X.; Wang, F.; Yu, J.; Chen, D. The Effect of Immunotherapy on Oligometastatic Non-Small Cell Lung Cancer Patients by Sites of Metastasis. Front. Immunol. 2022, 13, 1039157. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wu, H.; Zhang, Y.; Chen, Z.; Qi, Z.; Wang, M.; Cheng, P. Locoregional Therapy for Oligometastatic Cervical Cancer: A Single-Center Retrospective Study. Int. J. Gynecol. Cancer 2023, 33, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.N.; Yue, P.; Hao, Y.; Wu, S.Y.; Dong, B.Q.; Wu, Q.; Song, Z.B.; Chen, M. Definitive Local Therapy for Extracranial Single-Organ Oligorecurrent Non-Small-Cell Lung Cancer: A Single Institutional Retrospective Study. Medicine 2022, 101, e31918. [Google Scholar] [CrossRef]
- Virbel, G.; Cox, D.G.; Olland, A.; Falcoz, P.E.; Le Fevre, C.; Schott, R.; Antoni, D.; Noel, G. Outcome of Lung Oligometastatic Patients Treated with Stereotactic Body Irradiation. Front. Oncol. 2022, 12, 945189. [Google Scholar] [CrossRef]
- Ye, W.; Song, Z.; Lin, Z. Camrelizumab and Apatinib Combined with Radiotherapy Is Effective in Advanced Oligometastatic Non-Small-Cell Lung Cancer. Evid. Based Complement. Alternat. Med. 2022, 2022, 5067402. [Google Scholar] [CrossRef]
- Stefanovic, M.; Calvet, G.; Pérez-Montero, H.; Esteve, A.; Bujalance, M.V.; Navarro-Martín, A.; Fernández, M.D.A.; González, F.F.; Borras, S.M.; Borbalas, A.L.; et al. Stereotactic Body Radiation Therapy in the Treatment of Cancer Patients with Oligometastatic Disease: A Real World Study. Cancers 2023, 25, 199–206. [Google Scholar] [CrossRef]
- Lee, H.Y.; Kim, S.H.; Lee, H.Y.; Lee, Y.M.; Han, J.Y.; Cho, H.; Lee, H.K. Successful Treatment of Induced Oligometastasis and Repeated Oligoprogression of Advanced Lung Adenocarcinoma with Immunotherapy and Radiotherapy. Thorac. Cancer 2022, 13, 1998–2000. [Google Scholar] [CrossRef]
- Wang, X.S.; Bai, Y.F.; Verma, V.; Yu, R.L.; Tian, W.; Ao, R.; Deng, Y.; Xia, J.L.; Zhu, X.Q.; Liu, H.; et al. Randomized Trial of First-Line Tyrosine Kinase Inhibitor with or without Radiotherapy for Synchronous Oligometastatic EGFR-Mutated NSCLC. J. Natl. Cancer Inst. 2022. [Google Scholar] [CrossRef]
- Iyengar, P.; Wardak, Z.; Gerber, D.E.; Tumati, V.; Ahn, C.; Hughes, R.S.; Dowell, J.E.; Cheedella, N.; Nedzi, L.; Westover, K.D.; et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e173501. [Google Scholar] [CrossRef]
- Ruers, T.; Van Coevorden, F.; Punt, C.J.; Pierie, J.P.; Borel-Rinkes, I.; Ledermann, J.A.; Poston, G.; Bechstein, W.; Lentz, M.A.; Mauer, M.; et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J. Natl. Cancer Inst. 2017, 109, djx015. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.R.; Tang, C.; Zhang, J.; Blumenschein, G.R., Jr.; Hernandez, M.; Lee, J.J.; Ye, R.; Palma, D.A.; Louie, A.V.; Camidge, D.R.; et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J. Clin. Oncol. 2019, 37, 1558–1565. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.G.; Baretti, M.; Gerold, J.M.; Makohon-Moore, A.P.; Daud, A.; Iacobuzio-Donahue, C.A.; Azad, N.S.; Kinzler, K.W.; Nowak, M.A.; Vogelstein, B. An Analysis of Genetic Heterogeneity in Untreated Cancers. Nat. Rev. Cancer 2019, 19, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Punt, C.J.A.; Koopman, M.; Vermeulen, L. From Tumour Heterogeneity to Advances in Precision Treatment of Colorectal Cancer. Nat. Rev. Clin. Oncol. 2017, 14, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Jass, J.R. CRC a Multipathway Disease. Crit. Rev. Oncog. 2006, 12, 273–287. [Google Scholar] [CrossRef]
- Bastos, P.; Gomes, T.; Ribeiro, L. Catechol-O-Methyltransferase (COMT): An Update on Its Role in Cancer, Neurological and Cardiovascular Diseases. Rev. Physiol. Biochem. Pharmacol. 2017, 173, 41–62. [Google Scholar] [CrossRef]
- Balzan, S.; Lubrano, V. LOX-1 Receptor: A Potential Link in Atherosclerosis and Cancer. Life Sci. 2018, 198, 79–86. [Google Scholar] [CrossRef]
- Ouyang, L.; Zhang, K.; Chen, J.; Wang, J.; Huang, H. Roles of Platelet Derived Growth Factor in Vascular Calcification. J. Cell. Physiol. 2018, 233, 2804–2814. [Google Scholar] [CrossRef]
- Ottaiano, A.; De Divitiis, C.; Capozzi, M.; Avallone, A.; Pisano, C.; Pignata, S. Obesity and Cancer: Biological Links and Treatment Implications. Curr. Cancer Drug Targets 2018, 18, 231–238. [Google Scholar] [CrossRef]
- Nechama, M.; Kwon, J.; Wei, S.; Kyi, A.T.; Welner, R.S.; Ben-Dov, I.Z. The IL-33-PIN1-IRAK-M Axis is Critical for Type 2 Immunity in IL-33-Induced Allergic Airway Inflammation. Nat. Commun. 2018, 9, 1603. [Google Scholar] [CrossRef] [PubMed]
- Marenholz, I.; Esparza-Gordillo, J.; Rüschendorf, F.; Bauerfeind, A.; Strachan, D.P.; Spycher, B.D. Meta-Analysis Identifies Seven Susceptibility Loci Involved in the Atopic March. Nat. Commun. 2015, 6, 8804. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, R.; Bożek, A.; Jarząb, J. Association between Cancer and Allergies. Allergy Asthma Clin. Immunol. 2016, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.Y.; Lai, P.Y.; Hu, J.M.; Hsu, C.H.; Chen, Y.C.; Tian, Y.F. Association between Atopic Dermatitis and CRC Risk: A Nationwide Cohort Study. Medicine 2020, 99, E18530. [Google Scholar] [CrossRef] [PubMed]
- Marazzi, I.; Greenbaum, B.D.; Low, D.H.P.; Guccione, E. Chromatin Dependencies in Cancer and Inflammation. Nat. Rev. Mol. Cell Biol. 2018, 19, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Allavena, P.; Garlanda, C.; Borrello, M.G.; Sica, A.; Mantovani, A. Pathways Connecting Inflammation and Cancer. Curr. Opin. Genet. Dev. 2008, 18, 3–10. [Google Scholar] [CrossRef]
- Borrello, M.G.; Degl’Innocenti, D.; Pierotti, M.A. Inflammation and Cancer: The Oncogene-Driven Connection. Cancer Lett. 2018, 267, 262–270. [Google Scholar] [CrossRef]
- Lussier, Y.A.; Xing, H.R.; Salama, J.K.; Khodarev, N.N.; Huang, Y.; Zhang, Q.; Khan, S.A.; Yang, X.; Hasselle, M.D.; Darga, T.E.; et al. MicroRNA Expression Characterizes Oligometastasis(es). PLoS ONE 2011, 6, e28650. [Google Scholar] [CrossRef]
- Turajlic, S.; Xu, H.; Litchfield, K.; Rowan, A.; Horswell, S.; Chambers, T.; O’Brien, T.; Lopez, J.I.; Watkins, T.B.K.; Nicol, D.; et al. Tracking Cancer Evolution Reveals Constrained Routes to Metastases: TRACERx Renal. Cell 2018, 173, 581–594. [Google Scholar] [CrossRef]
- Pitroda, S.P.; Khodarev, N.N.; Huang, L.; Uppal, A.; Wightman, S.C.; Ganai, S.; Joseph, N.; Pitt, J.; Brown, M.; Forde, M.; et al. Integrated Molecular Subtyping Defines a Curable Oligometastatic State in Colorectal Liver Metastasis. Nat. Commun. 2018, 9, 1793. [Google Scholar] [CrossRef]
- Ottaiano, A.; Caraglia, M.; Di Mauro, A.; Botti, G.; Lombardi, A.; Galon, J.; Luce, A.; D’Amore, L.; Perri, F.; Santorsola, M.; et al. Evolution of Mutational Landscape and Tumor Immune-Microenvironment in Liver Oligo-Metastatic Colorectal Cancer. Cancers 2020, 12, 3073. [Google Scholar] [CrossRef] [PubMed]
- Ottaiano, A.; Circelli, L.; Lombardi, A.; Scala, S.; Martucci, N.; Galon, J.; Buonanno, M.; Scognamiglio, G.; Botti, G.; Hermitte, F.; et al. Genetic trajectory and immune microenvironment of lung-specific oligometastatic colorectal cancer. Cell Death Dis. 2020, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Ottaiano, A.; de Vera d’Aragona, R.P.; Trotta, A.M.; Santorsola, M.; Napolitano, M.; Scognamiglio, G.; Tatangelo, F.; Grieco, P.; Zappavigna, S.; Granata, V.; et al. Characterization of KRAS Mutational Regression in Oligometastatic Patients. Front Immunol. 2022, 13, 898561. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Robinson, P.N.; Wang, K. Phenolyzer: Phenotype-based prioritization of candidate genes for human diseases. Nat. Methods 2015, 12, 841–843. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Z.; Li, Y.; Peng, H.; Liu, J.; Zhang, J.; Xiao, X. The Role of CREBBP/EP300 and Its Therapeutic Implications in Hematological Malignancies. Cancers 2023, 15, 1219. [Google Scholar] [CrossRef]
- Stevens, A.P.; Spangler, B.; Wallner, S.; Kreutz, M.; Dettmer, K.; Oefner, P.J.; Bosserhoff, A.-K. Direct and Tumor Microenvironment Mediated Influences of 5′-Deoxy-5′-(Methylthio)Adenosine on Tumor Progression of Malignant Melanoma. Cancers 2009, 106, 210–219. [Google Scholar] [CrossRef]
- Zhong, Y.; Lu, K.; Zhu, S.; Li, W.; Sun, S. Characterization of methylthioadenosin phosphorylase (MTAP) expression in colorectal cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2082–2087. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Y.; Yang, F.; Liu, S.; Zhu, Z.; Lei, Z.; Guo, J. Functions of EpCAM in physiological processes and diseases (Review). Int. J. Mol. Med. 2018, 42, 1771–1785. [Google Scholar] [CrossRef]
- Barbero, S.; Mielgo, A.; Torres, V.; Teitz, T.; Shields, D.J.; Mikolon, D.; Bogyo, M.; Barilà, D.; Lahti, J.M.; Schlaepfer, D.; et al. Caspase-8 association with the focal adhesion complex promotes tumor cell migration and metastasis. Cancer Res. 2009, 69, 3755–3763. [Google Scholar] [CrossRef]
- Zhang, X.; Gureasko, J.; Shen, K.; Cole, P.A.; Kuriyan, J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 2006, 125, 1137–1149. [Google Scholar] [CrossRef]
- Wang, N.; Cao, Y.; Si, C.; Shao, P.; Su, G.; Wang, K.; Bao, J.; Yang, L. Emerging Role of ERBB2 in Targeted Therapy for Metastatic Colorectal Cancer: Signaling Pathways to Therapeutic Strategies. Cancers 2022, 14, 5160. [Google Scholar] [CrossRef] [PubMed]
- Minn, A.J.; Gupta, G.P.; Siegel, P.M.; Bos, P.D.; Shu, W.; Giri, D.D.; Viale, A.; Olshen, A.B.; Gerald, W.L.; Massagué, J. Genes that mediate breast cancer metastasis to lung. Nature 2005, 436, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Güttlein, L.N.; Benedetti, L.G.; Fresno, C.; Spallanzani, R.G.; Mansilla, S.F.; Rotondaro, C.; Raffo Iraolagoitia, X.L.; Salvatierra, E.; Bravo, A.I.; Fernández, E.A.; et al. Predictive Outcomes for HER2-enriched Cancer Using Growth and Metastasis Signatures Driven By SPARC. Mol. Cancer Res. 2017, 15, 304–316. [Google Scholar] [CrossRef]
- Ottaiano, A.; Circelli, L.; Santorsola, M.; Savarese, G.; Fontanella, D.; Gigantino, V.; Di Mauro, A.; Capuozzo, M.; Zappavigna, S.; Lombardi, A.; et al. Metastatic colorectal cancer and type 2 diabetes: Prognostic and genetic interactions. Mol. Oncol. 2022, 16, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Liu, L. The Wnt signaling pathway effector TCF7L2 and type 2 diabetes mellitus. Mol. Endocrinol. 2008, 22, 2383–2392. [Google Scholar] [CrossRef]
- Huang, F.; Wu, G.; Yang, K. Oligometastasis and oligo-recurrence: More than a mirage. Radiat. Oncol. 2014, 9, 230. [Google Scholar] [CrossRef]
- Von Bubnoff, A. Next-generation sequencing: The race is on. Cell 2008, 132, 721–723. [Google Scholar] [CrossRef]
- Metzker, M.L. Sequencing technologies—The next generation. Nat. Rev. Genet. 2010, 11, 31–46. [Google Scholar] [CrossRef]
- Pinto, C.; Biffoni, M.; Popoli, P.; Marchetti, A.; Marchetti, P.; Martini, N.; Normanno, N. Molecular tests and target therapies in oncology: Recommendations from the Italian workshop. Fut. Oncol. 2021, 17, 3529–3539. [Google Scholar] [CrossRef]
- Curtius, K.; Wright, N.A.; Graham, T.A. An evolutionary perspective on field cancerization. Nat. Rev. Cancer 2018, 18, 19–32. [Google Scholar] [CrossRef]
- Capuozzo, M.; Santorsola, M.; Bocchetti, M.; Perri, F.; Cascella, M.; Granata, V.; Celotto, V.; Gualillo, O.; Cossu, A.M.; Nasti, G.; et al. p53: From Fundamental Biology to Clinical Applications in Cancer. Biology 2022, 11, 1325. [Google Scholar] [CrossRef]
- Lin, B.; Hui, J.; Mao, H. Nanopore Technology and Its Applications in Gene Sequencing. Biosensors 2021, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, J.A.; Bohländer, P.; Dai, M.; Filius, M.; Howard, C.J.; van Kooten, X.F.; Ohayon, S.; Pomorski, A.; Schmid, S.; Aksimentiev, A.; et al. The Emerging Landscape of Single-Molecule Protein Sequencing Technologies. Nat. Methods 2021, 18, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Testa, U.; Pelosi, E.; Castelli, G. Molecular characterization of lung adenocarcinoma combining whole exome sequencing, copy number analysis and gene expression profiling. Expert. Rev. Mol. Diagn. 2022, 22, 77–100. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.M.; Weiskirchen, R.; Damm, F.; Strzelecka, P.M. Single-cell omics: Overview, analysis, and application in biomedical science. J. Cell. Biochem. 2021, 122, 1571–1578. [Google Scholar] [CrossRef]
- Mullegama, S.V.; Alberti, M.O.; Au, C.; Li, Y.; Toy, T.; Tomasian, V.; Xian, R.R. Nucleic Acid Extraction from Human Biological Samples. Methods Mol. Biol. 2019, 1897, 359–383. [Google Scholar] [CrossRef]
- da Cunha Santos, G.; Saieg, M.A. Preanalytic specimen triage: Smears, cell blocks, cytospin preparations, transport media, and cytobanking. Cancer Cytopathol. 2017, 125, 455–464. [Google Scholar] [CrossRef]
- Angelova, M.; Mlecnik, B.; Vasaturo, A.; Bindea, G.; Fredriksen, T.; Lafontaine, L.; Buttard, B.; Morgand, E.; Bruni, D.; Jouret-Mourin, A.; et al. Evolution of Metastases in Space and Time under Immune Selection. Cell 2018, 175, 751–765. [Google Scholar] [CrossRef]
- Germani, M.M.; Borelli, B.; Boraschi, P.; Antoniotti, C.; Ugolini, C.; Urbani, L.; Morelli, L.; Fontanini, G.; Masi, G.; Cremolini, C.; et al. The management of colorectal liver metastases amenable of surgical resection: How to shape treatment strategies according to clinical, radiological, pathological and molecular features. Cancer Treat. Rev. 2022, 106, 102382. [Google Scholar] [CrossRef]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): A randomised controlled trial. Lancet 2008, 371, 1007–1016. [Google Scholar] [CrossRef]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): Long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013, 14, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Groelly, F.J.; Fawkes, M.; Dagg, R.A.; Blackford, A.N.; Tarsounas, M. Targeting DNA damage response pathways in cancer. Nat. Rev. Cancer 2023, 23, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Ottaiano, A.; Scotti, V.; De Divitiis, C.; Capozzi, M.; Romano, C.; Cassata, A.; Casaretti, R.; Silvestro, L.; Nappi, A.; Vicario, V.; et al. Integration of Stereotactic Radiotherapy in the Treatment of Metastatic Colorectal Cancer Patients: A Real Practice Study with Long-Term Outcome and Prognostic Factors. Oncotarget 2018, 9, 35251–35265. [Google Scholar] [CrossRef]
- Kim, S.I.; Cassella, C.R.; Byrne, K.T. Tumor Burden and Immunotherapy: Impact on Immune Infiltration and Therapeutic Outcomes. Front. Immunol. 2021, 11, 629722. [Google Scholar] [CrossRef] [PubMed]
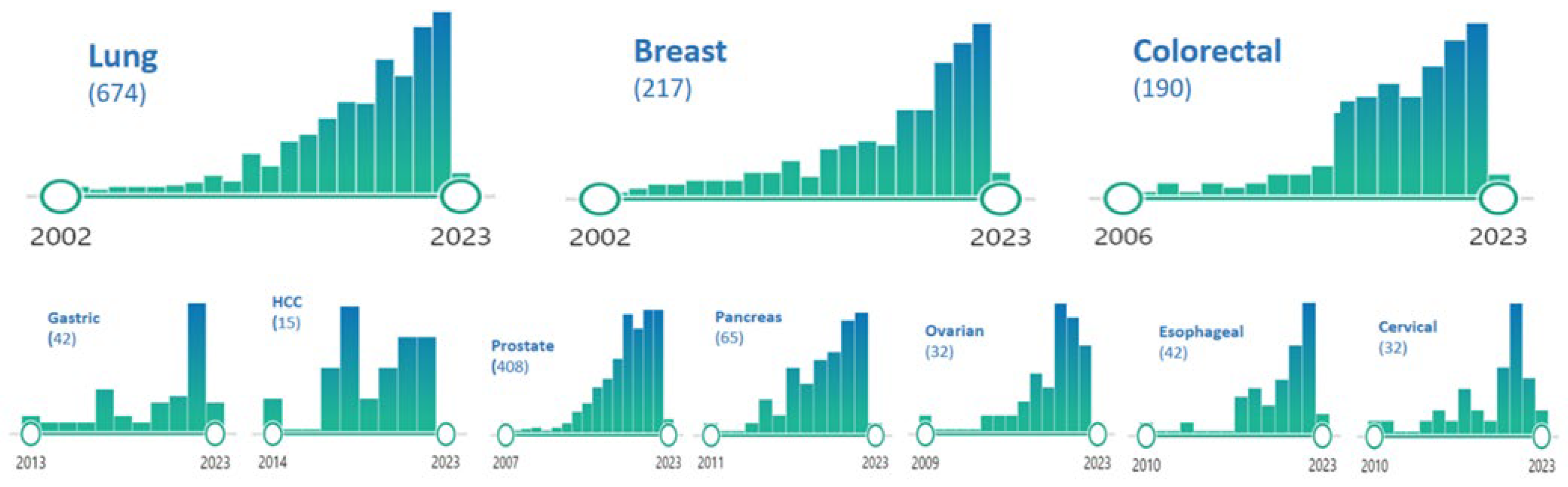
| Cancer | Incidence of Oligo-Metastatic Disease (% on Metastatic Presentation) |
|---|---|
| Lung (NSCLC) | 5 |
| Lung (SCLC) | Undefined (extremely rare) |
| Breast | 5–20 |
| Colorectal | 10–15 |
| Stomach | 5 |
| HCC | 10–40 |
| Prostate | 10–30 |
| Pancreatic | 5 |
| Ovarian | 5–15 |
| Esophageal | 5 |
| Cervical | 5–15 |
| Type of Cancer | Targetable Mutations | Maximum No. of Lesions | Arms | No. of Patients | mPFS (Months) | p | mOS (Months) | p |
|---|---|---|---|---|---|---|---|---|
| NSCLC | Not permitted | 6 | SRT + CT | 14 | 9.7 | NR | ||
| CT | 15 | 3.5 | 0.01 | 17.0 | NR | |||
| CRC | Permitted | 10 liver met | RFA + resection + CT | 51 | 16.8 | 45.6 | ||
| CT | 57 | 9.9 | 0.005 | 40.5 | 0.01 | |||
| NSCLC | Permitted | 3 | SRT or surgery | 25 | 14.2 | 41.2 | ||
| Observation or CT | 24 | 4.4 | 0.022 | 17.0 | 0.034 | |||
| Breast, CRC, NSCLC, prostate, others | Permitted | 5 | SRT + SC | 66 | 12.9 | 41.0 | ||
| SC | 33 | 6.0 | 0.0012 | 28.0 | 0.09 | |||
| NSCLC | Only EGFR-mutated cancers | 5 | SRT + TKI | 68 | 20.2 | 25.5 | ||
| TKI | 65 | 12.5 | <0.001 | 17.4 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ottaiano, A.; Santorsola, M.; Circelli, L.; Trotta, A.M.; Izzo, F.; Perri, F.; Cascella, M.; Sabbatino, F.; Granata, V.; Correra, M.; et al. Oligo-Metastatic Cancers: Putative Biomarkers, Emerging Challenges and New Perspectives. Cancers 2023, 15, 1827. https://doi.org/10.3390/cancers15061827
Ottaiano A, Santorsola M, Circelli L, Trotta AM, Izzo F, Perri F, Cascella M, Sabbatino F, Granata V, Correra M, et al. Oligo-Metastatic Cancers: Putative Biomarkers, Emerging Challenges and New Perspectives. Cancers. 2023; 15(6):1827. https://doi.org/10.3390/cancers15061827
Chicago/Turabian StyleOttaiano, Alessandro, Mariachiara Santorsola, Luisa Circelli, Anna Maria Trotta, Francesco Izzo, Francesco Perri, Marco Cascella, Francesco Sabbatino, Vincenza Granata, Marco Correra, and et al. 2023. "Oligo-Metastatic Cancers: Putative Biomarkers, Emerging Challenges and New Perspectives" Cancers 15, no. 6: 1827. https://doi.org/10.3390/cancers15061827
APA StyleOttaiano, A., Santorsola, M., Circelli, L., Trotta, A. M., Izzo, F., Perri, F., Cascella, M., Sabbatino, F., Granata, V., Correra, M., Tarotto, L., Stilo, S., Fiore, F., Martucci, N., Rocca, A. L., Picone, C., Muto, P., Borzillo, V., Belli, A., ... Caraglia, M. (2023). Oligo-Metastatic Cancers: Putative Biomarkers, Emerging Challenges and New Perspectives. Cancers, 15(6), 1827. https://doi.org/10.3390/cancers15061827















