Impact of Microscopically Positive (≤1 mm) Distal Margins on Disease Recurrence in Rectal Cancer Treated by Neoadjuvant Chemoradiotherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Study Design and Endpoints
2.3. Multidisciplinary Treatment of Rectal Cancer
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Included Patients
3.2. Type of Neoadjuvant Treatment
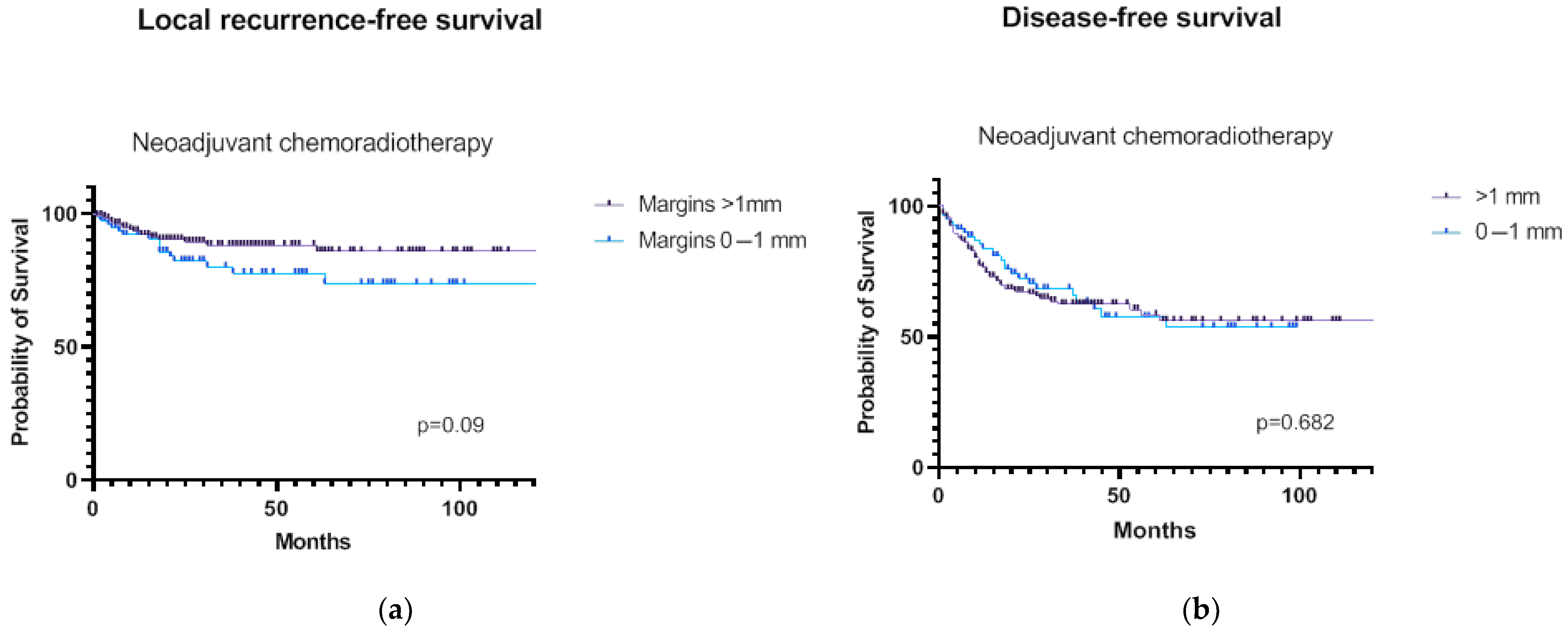
3.3. Survival Analyses
3.4. Predictors of LRRFS in Patients Treated by nCRT
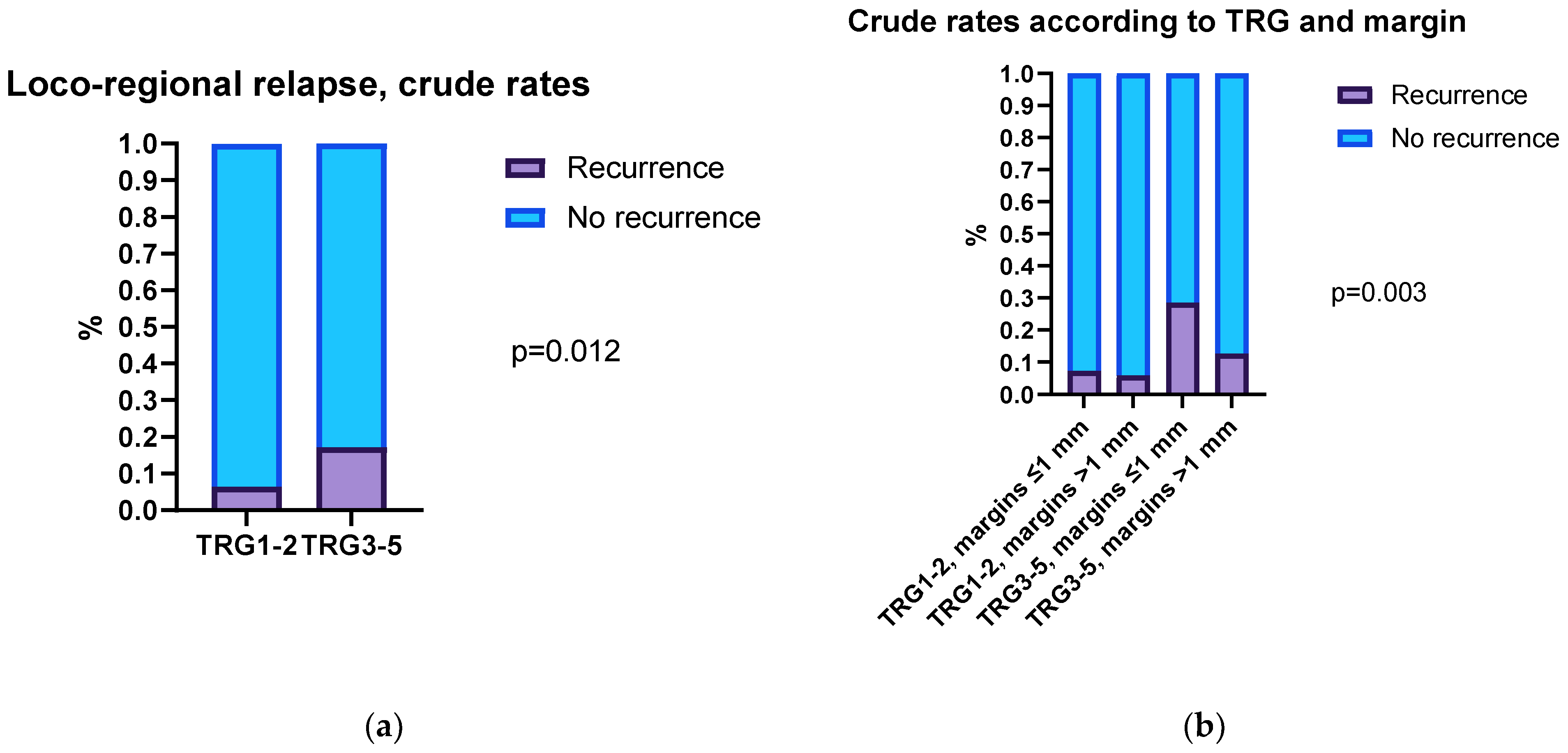
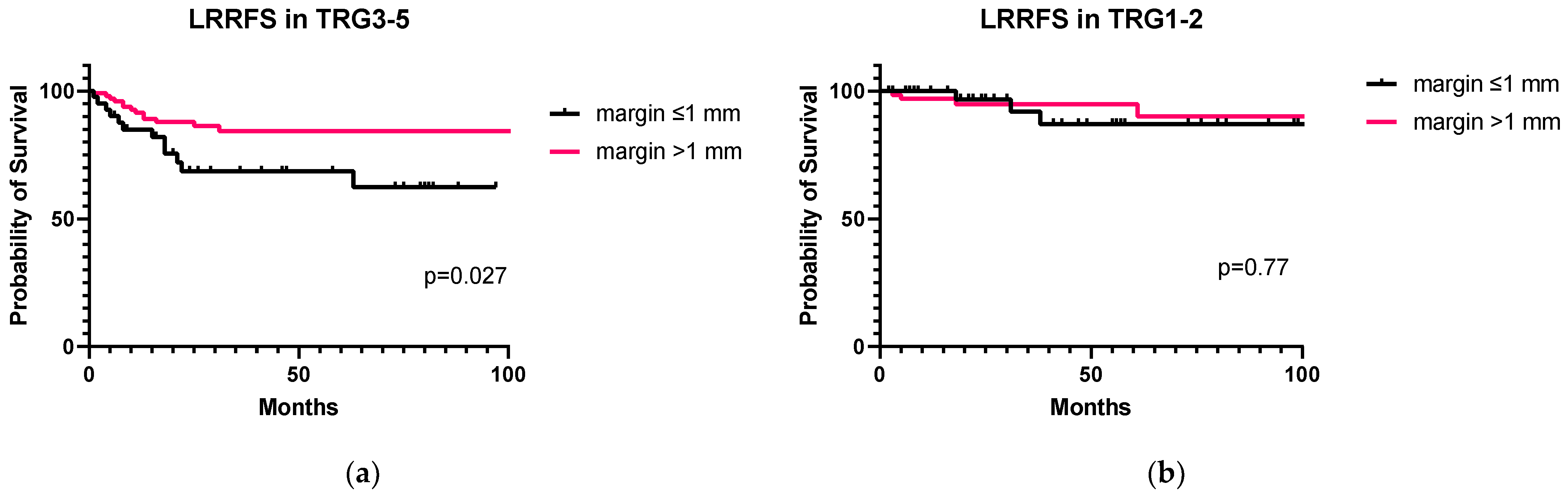
3.5. Correlation between Distal Margins, Tumor Regression Grade, and LRRFS in nCRT Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, H.; Wang, P.Y.; Wu, Y.C.; Liu, Y.C. Is a Distal Resection Margin of ≤1 cm Safe in Patients with Intermediate- to Low-Lying Rectal Cancer? A Systematic Review and Meta-Analysis. J. Gastrointest. Surg. 2022, 26, 1791–1803. [Google Scholar] [CrossRef] [PubMed]
- Park, I.J.; Kim, J.C. Adequate length of the distal resection margin in rectal cancer: From the oncological point of view. J. Gastrointest. Surg. 2010, 14, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Manegold, P.; Taukert, J.; Neeff, H.; Fichtner-Feigl, S.; Thomusch, O. The minimum distal resection margin in rectal cancer surgery and its impact on local recurrence—A retrospective cohort analysis. Int. J. Surg. 2019, 69, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Kwok, S.P.; Lau, W.Y.; Leung, K.L.; Liew, C.T.; Li, A.K. Prospective analysis of the distal margin of clearance in anterior resection for rectal carcinoma. Br. J. Surg. 1996, 83, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Kameda, K.; Furusawa, M.; Mori, M.; Sugimachi, K. Proposed distal margin for resection of rectal cancer. Jpn. J. Cancer Res. 1990, 81, 100–104. [Google Scholar] [CrossRef]
- Mukkai Krishnamurty, D.; Wise, P.E. Importance of surgical margins in rectal cancer. J. Surg. Oncol. 2016, 113, 323–332. [Google Scholar] [CrossRef]
- Wilkinson, N. Management of Rectal Cancer. Surg. Clin. N. Am. 2020, 100, 615–628. [Google Scholar] [CrossRef]
- Nash, G.M.; Weiss, A.; Dasgupta, R.; Gonen, M.; Guillem, J.G.; Wong, W.D. Close distal margin and rectal cancer recurrence after sphincter-preserving rectal resection. Dis. Colon Rectum 2010, 53, 1365–1373. [Google Scholar] [CrossRef]
- Rutkowski, A.; Nowacki, M.P.; Chwalinski, M.; Oledzki, J.; Bednarczyk, M.; Liszka-Dalecki, P.; Gornicki, A.; Bujko, K. Acceptance of a 5-mm distal bowel resection margin for rectal cancer: Is it safe? Color. Dis. 2012, 14, 71–78. [Google Scholar] [CrossRef]
- Zeng, W.G.; Liu, M.J.; Zhou, Z.X.; Wang, Z.J. A Distal Resection Margin of ≤1 mm and Rectal Cancer Recurrence After Sphincter-Preserving Surgery: The Role of a Positive Distal Margin in Rectal Cancer Surgery. Dis. Colon Rectum 2017, 60, 1175–1183. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Quirke, P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J. Clin. Oncol. 2008, 26, 303–312. [Google Scholar] [CrossRef]
- Kasi, A.; Abbasi, S.; Handa, S.; Al-Rajabi, R.; Saeed, A.; Baranda, J.; Sun, W. Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2030097. [Google Scholar] [CrossRef]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef]
- Conroy, T.; Bosset, J.F.; Etienne, P.L.; Rio, E.; François, É.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef]
- Papaccio, F.; Roselló, S.; Huerta, M.; Gambardella, V.; Tarazona, N.; Fleitas, T.; Roda, D.; Cervantes, A. Neoadjuvant Chemotherapy in Locally Advanced Rectal Cancer. Cancers 2020, 12, 3611. [Google Scholar] [CrossRef]
- Roselló, S.; Papaccio, F.; Roda, D.; Tarazona, N.; Cervantes, A. The role of chemotherapy in localized and locally advanced rectal cancer: A systematic revision. Cancer Treat. Rev. 2018, 63, 156–171. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Fernández-Martínez, D.; Rodríguez-Infante, A.; Otero-Díez, J.L.; Baldonedo-Cernuda, R.F.; Mosteiro-Díaz, M.P.; García-Flórez, L.J. Is my life going to change?—A review of quality of life after rectal resection. J. Gastrointest. Oncol. 2020, 11, 91–101. [Google Scholar] [CrossRef]
- George, T.; Allegra, C.; Yothers, G. Neoadjuvant rectal (NAR) score: A new surrogate endpoint in rectal cancer clinical trials. Curr. Color. Cancer Rep. 2015, 11, 275–280. [Google Scholar] [CrossRef]
- Guedj, N.; Maggiori, L.; Poté, N.; Norkowski, E.; Cros, J.; Bedossa, P.; Panis, Y. Distal intramural and tumor spread in the mesorectum after neoadjuvant radiochemotherapy in rectal cancer: About 124 consecutive patients. Hum. Pathol. 2016, 52, 164–172. [Google Scholar] [CrossRef]
- Inoue, A.; Sheedy, S.P.; Wells, M.L.; Mileto, A.; Goenka, A.H.; Ehman, E.C.; Yalon, M.; Murthy, N.S.; Mathis, K.L.; Behm, K.T.; et al. Rectal cancer pelvic recurrence: Imaging patterns and key concepts to guide treatment planning. Abdom. Radiol. 2023, in press. [CrossRef] [PubMed]
- Shirouzu, K.; Isomoto, H.; Kakegawa, T. Distal spread of rectal cancer and optimal distal margin of resection for sphincter-preserving surgery. Cancer 1995, 76, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Özer, İ.; Zengin, N.İ.; Çaycı, H.M.; Yüksel, A.; Dalgıç, T.; Ulaş, M.; Bostancı, E.B.; Akoğlu, M. Distal spread and tumor regression patterns following preoperative chemoradiotherapy in rectal cancer patients. Turk. J. Med. Sci. 2021, 51, 2978–2985. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, O.; Nakanishi, M.; Murayama, Y.; Kuriu, Y.; Kokuba, Y.; Otsuji, E. Flattened tumor requires a more careful attention for residual distal cancer spread in locally advanced lower rectal carcinoma after chemoradiotherapy. Dig. Surg. 2015, 32, 159–165. [Google Scholar] [CrossRef]
- Grüter, A.A.J.; van Lieshout, A.S.; van Oostendorp, S.E.; Ket, J.C.F.; Tenhagen, M.; den Boer, F.C.; Hompes, R.; Tanis, P.J.; Tuynman, J.B. Required distal mesorectal resection margin in partial mesorectal excision: A systematic review on distal mesorectal spread. Tech. Coloproctol. 2023, 27, 11–21. [Google Scholar] [CrossRef]
- Keranmu, A.; Liu, H.N.; Wu, Y.C.; Liu, T.T.; Li, C.; Guo, T.A.; Liu, F.Q.; Zheng, H.T.; Xu, Y. A negative-doughnut distal resection margin less than 5 mm does not affect prognosis in rectal cancer. J. Surg. Oncol. 2018, 118, 536–543. [Google Scholar]
- Yoon, G.; Kim, S.M.; Kim, H.J.; Seo, A.N. Clinical influence of cancer stem cells on residual disease after preoperative chemoradiotherapy for rectal cancer. Tumor Biol. 2016, 37, 3571–3580. [Google Scholar] [CrossRef]
- Phang, P.T.; Kennecke, H.; McGahan, C.E.; Macfarlane, J.; McGregor, G.; Hay, J.H. Predictors of positive radial margin status in a population-based cohort of patients with rectal cancer. Curr. Oncol. 2008, 15, 98–103. [Google Scholar] [CrossRef]
- Sung, S.; Kim, S.H.; Lee, J.H.; Nam, T.K.; Jeong, S.; Jang, H.S.; Song, J.H.; Lee, J.W.; Bae, J.M.; Lee, J.H. Continuous Effect of Radial Resection Margin on Recurrence and Survival in Rectal Cancer Patients Who Receive Preoperative Chemoradiation and Curative Surgery: A Multicenter Retrospective Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 647–653. [Google Scholar] [CrossRef]
- Belli, F.; Sorrentino, L.; Gallino, G.; Gronchi, A.; Scaramuzza, D.; Valvo, F.; Cattaneo, L.; Cosimelli, M. A proposal of an updated classification for pelvic relapses of rectal cancer to guide surgical decision-making. J. Surg. Oncol. 2020, 122, 350–359. [Google Scholar] [CrossRef]
- Lim, J.W.; Chew, M.H.; Lim, K.H.; Tang, C.L. Close distal margins do not increase rectal cancer recurrence after sphincter-saving surgery without neoadjuvant therapy. Int. J. Color. Dis. 2012, 27, 1285–1294. [Google Scholar] [CrossRef]
- Shimada, Y.; Takii, Y.; Maruyama, S.; Ohta, T. Intramural and mesorectal distal spread detected by whole-mount sections in the determination of optimal distal resection margin in patients undergoing surgery for rectosigmoid or rectal cancer without preoperative therapy. Dis. Colon Rectum 2011, 54, 1510–1520. [Google Scholar] [CrossRef]
- Leo, E.; Belli, F.; Miceli, R.; Mariani, L.; Gallino, G.; Battaglia, L.; Vannelli, A.; Andreola, S. Distal clearance margin of 1 cm or less: A safe distance in lower rectum cancer surgery. Int. J. Color. Dis. 2009, 24, 317–322. [Google Scholar] [CrossRef]
- Moore, H.G.; Riedel, E.; Minsky, B.D.; Saltz, L.; Paty, P.; Wong, D.; Cohen, A.M.; Guillem, J.G. Adequacy of 1-cm distal margin after restorative rectal cancer resection with sharp mesorectal excision and preoperative combined-modality therapy. Ann. Surg. Oncol. 2003, 10, 80–85. [Google Scholar] [CrossRef]
- Fan, W.H.; Xiao, J.; An, X.; Jiang, W.; Li, L.R.; Gao, Y.H.; Chen, G.; Kong, L.H.; Lin, J.Z.; Wang, J.P.; et al. Patterns of recurrence in patients achieving pathologic complete response after neoadjuvant chemoradiotherapy for rectal cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 1461–1467. [Google Scholar] [CrossRef]
- NCCN Rectal Cancer Guidelines. Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf (accessed on 13 March 2023).

| ≤1 mm (n = 83) | >1 mm (n = 172) | p Value | |
|---|---|---|---|
| Gender | 0.247 | ||
| Male | 55 (66.3%) | 101 (58.7%) | |
| Female | 28 (33.7%) | 71 (41.3%) | |
| ASA Score | 0.544 | ||
| ASA I–II | 65 (87.8%) | 124 (90.5%) | |
| ASA III | 9 (12.2%) | 13 (9.5%) | |
| Data missing | 9 | 35 | |
| Age | 59.9 ± 12.3 | 60.1 ± 11.1 | 0.897 |
| CEA | 6.3 ± 33.4 | 5.8 ± 9.8 | 0.856 |
| CA 19.9 | 12.6 ± 11.4 | 15.2 ± 15.5 | 0.175 |
| cT category | 0.886 | ||
| cT1 | 3 (3.6%) | 3 (1.7%) | |
| cT2 | 6 (7.2%) | 11 (6.4%) | |
| cT3a–b | 35 (42.2%) | 76 (44.2%) | |
| cT3c–d | 30 (36.2%) | 66 (38.4%) | |
| cT4 | 9 (10.8%) | 16 (9.3%) | |
| cN category | 0.022 | ||
| cN0 | 21 (25.3%) | 21 (12.2%) | |
| cN1 | 43 (51.8%) | 113 (65.7%) | |
| cN2 | 19 (22.9%) | 38 (22.1%) | |
| Distance from anal verge (cm) | 3.9 ± 2.3 | 6.1 ± 2.7 | <0.0001 |
| Type of neoadjuvant treatment | 0.51 | ||
| Long-course nCRT | 68 (81.9%) | 131 (76.2%) | |
| Short-course radiotherapy | 7 (8.5%) | 25 (14.5%) | |
| Total neoadjuvant therapy | 3 (3.6%) | 4 (2.3%) | |
| nCRT + Immunotherapy | 5 (6.0%) | 12 (7.0%) | |
| ypT category | 0.226 | ||
| ypT0 | 14 (16.9%) | 27 (15.7%) | |
| ypT1–2 | 33 (39.8%) | 51 (29.7%) | |
| ypT3 | 30 (36.1%) | 85 (49.4%) | |
| ypT4 | 6 (7.2%) | 9 (5.2%) | |
| ypN stage | 0.266 | ||
| ypN0 | 58 (69.9%) | 108 (62.8%) | |
| ypN+ | 25 (30.1%) | 64 (37.2%) | |
| Harvested lymph nodes | 14.6 ± 6.0 | 15.1 ± 7.7 | 0.604 |
| TRG (Mandard) | 0.502 | ||
| TRG1 | 14 (16.9%) | 27 (15.7%) | |
| TRG2 | 27 (32.5%) | 42 (24.4%) | |
| TRG3 | 26 (31.3%) | 59 (34.3%) | |
| TRG4 | 15 (18.1%) | 37 (21.5%) | |
| TRG5 | 1 (1.2%) | 7 (4.1%) | |
| pCR | 0.54 | ||
| Yes | 14 (16.9%) | 24 (14.0%) | |
| No | 69 (83.1%) | 148 (86.0%) | |
| NAR score | 0.205 | ||
| NAR < 8 | 16 (19.3%) | 30 (17.4%) | |
| NAR 8–16 | 44 (53.0%) | 75 (43.6%) | |
| NAR > 16 | 23 (27.7%) | 67 (39.0%) | |
| Anastomotic leak | 0.297 | ||
| Yes | 13 (15.7%) | 19 (11.0%) | |
| No | 70 (84.3%) | 153 (89.0%) | |
| Adiuvant chemotherapy | 0.109 | ||
| Yes | 44 (53.0%) | 95 (63.8%) | |
| No | 39 (47.0%) | 54 (36.2%) | |
| Data missing | 8 | 23 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p Value | HR | 95%CI | p Value | |
| Age | 0.98 | 0.96–1.01 | 0.267 | |||
| Gender | 1.01 | 0.50–2.10 | 0.971 | |||
| CEA | 1.00 | 0.99–1.01 | 0.165 | |||
| Distance from the anal verge | 0.95 | 0.84–1.06 | 0.377 | |||
| Short course vs. long course | 2.27 | 0.82–5.42 | 0.083 | |||
| Laparoscopy vs. laparotomy | 0.27 | 0.015–1.29 | 0.204 | |||
| Anastomotic leakage | 1.32 | 0.45–3.16 | 0.569 | |||
| ypT category | ||||||
| ypT0 | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| ypT1 | 3.41 | 0.41–28.41 | 0.221 | 2.48 | 0.29–21.24 | 0.372 |
| ypT2 | 1.84 | 0.39–12.84 | 0.468 | 1.06 | 0.17–8.44 | 0.949 |
| ypT3 | 2.95 | 0.82–18.77 | 0.153 | 1.50 | 0.26–11.9 | 0.664 |
| ypT4 | 20.08 | 5.13–132.4 | 0.0001 | 9.25 | 1.31–83.92 | 0.003 |
| ypN stage | ||||||
| ypN0 | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| ypN1 | 2.07 | 0.98–4.29 | 0.782 | 1.08 | 0.44–2.52 | 0.869 |
| ypN2 | 1.19 | 0.28–3.58 | 0.052 | 0.77 | 0.17–2.59 | 0.702 |
| Mandard TRG | ||||||
| TRG1–2 | (ref) | (ref) | (ref) | (ref) | (ref) | (ref) |
| TRG3–5 | 2.90 | 1.32–7.28 | 0.013 | 2.30 | 0.83–7.91 | 0.143 |
| Distal margin ≤ 1 mm | 1.81 | 0.89–3.63 | 0.094 | 1.87 | 0.89–3.87 | 0.092 |
| MSI-H/dMMR | 2.59 | 0.40–9.49 | 0.213 | |||
| Adjuvant chemotherapy | 0.87 | 0.42–1.89 | 0.708 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorrentino, L.; Sileo, A.; Daveri, E.; Battaglia, L.; Guaglio, M.; Centonze, G.; Sabella, G.; Patti, F.; Villa, S.; Milione, M.; et al. Impact of Microscopically Positive (≤1 mm) Distal Margins on Disease Recurrence in Rectal Cancer Treated by Neoadjuvant Chemoradiotherapy. Cancers 2023, 15, 1828. https://doi.org/10.3390/cancers15061828
Sorrentino L, Sileo A, Daveri E, Battaglia L, Guaglio M, Centonze G, Sabella G, Patti F, Villa S, Milione M, et al. Impact of Microscopically Positive (≤1 mm) Distal Margins on Disease Recurrence in Rectal Cancer Treated by Neoadjuvant Chemoradiotherapy. Cancers. 2023; 15(6):1828. https://doi.org/10.3390/cancers15061828
Chicago/Turabian StyleSorrentino, Luca, Annaclara Sileo, Elena Daveri, Luigi Battaglia, Marcello Guaglio, Giovanni Centonze, Giovanna Sabella, Filippo Patti, Sergio Villa, Massimo Milione, and et al. 2023. "Impact of Microscopically Positive (≤1 mm) Distal Margins on Disease Recurrence in Rectal Cancer Treated by Neoadjuvant Chemoradiotherapy" Cancers 15, no. 6: 1828. https://doi.org/10.3390/cancers15061828
APA StyleSorrentino, L., Sileo, A., Daveri, E., Battaglia, L., Guaglio, M., Centonze, G., Sabella, G., Patti, F., Villa, S., Milione, M., Belli, F., & Cosimelli, M. (2023). Impact of Microscopically Positive (≤1 mm) Distal Margins on Disease Recurrence in Rectal Cancer Treated by Neoadjuvant Chemoradiotherapy. Cancers, 15(6), 1828. https://doi.org/10.3390/cancers15061828







