Role of Adjuvant Chemotherapy in Stage I Pure Ovarian Immature Teratoma: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources, Search Strategy, and Selection Criteria
- (1)
- Patients with stage IA G2-G3 and/or IB-IC POIT of any grade confirmed by pathology;
- (2)
- Randomized controlled trials (RCTs) or prospective, retrospective cohort studies that included POIT treated with surgery alone and surgery with adjuvant chemotherapy;
- (3)
- Studies that exactly reported outcomes (death or recurrence) of POIT based on intervention (surgery or surgery with adjuvant chemotherapy), corresponding stage (FIGO stage IA, IB, or IC), and/or WHO grade (G1, G2, or G3).
- (1)
- POIT of other stages/grades or topics, or studies that enrolled less than 10 cases of POIT that met the inclusion criteria;
- (2)
- MOGCTs of other pathology subtypes;
- (3)
- Patients reported in case reports, letters, personal opinions, conference abstracts, and non-English literature;
- (4)
- Studies with ambiguous clinical outcomes or unclear tumor stage/grade.
2.2. Data Extraction and Quality Assessment
2.3. Outcomes and Subgroup Setting
2.4. Statistical Analysis
3. Results
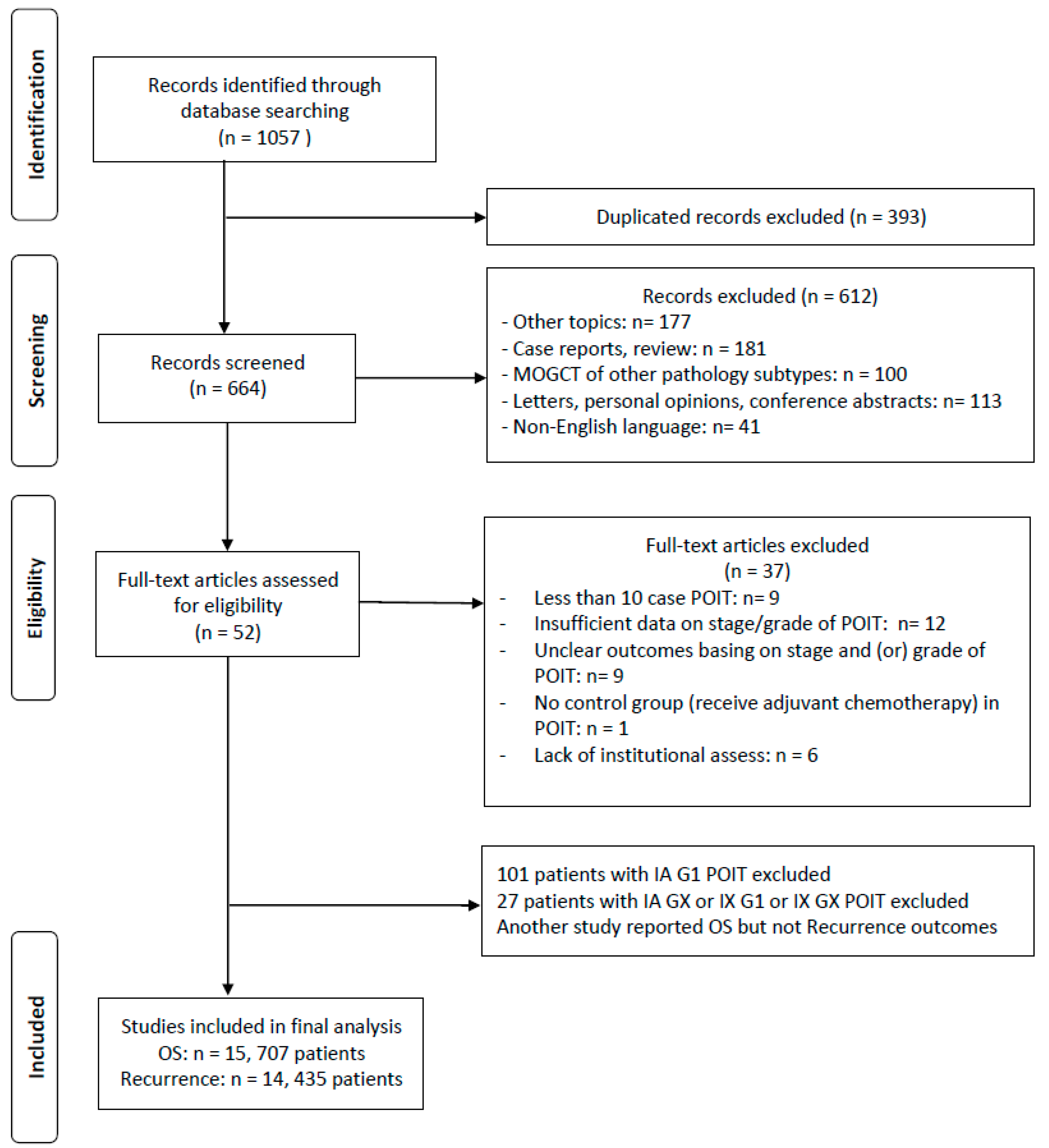
3.1. Systematic Review and Characteristics of the Included Studies
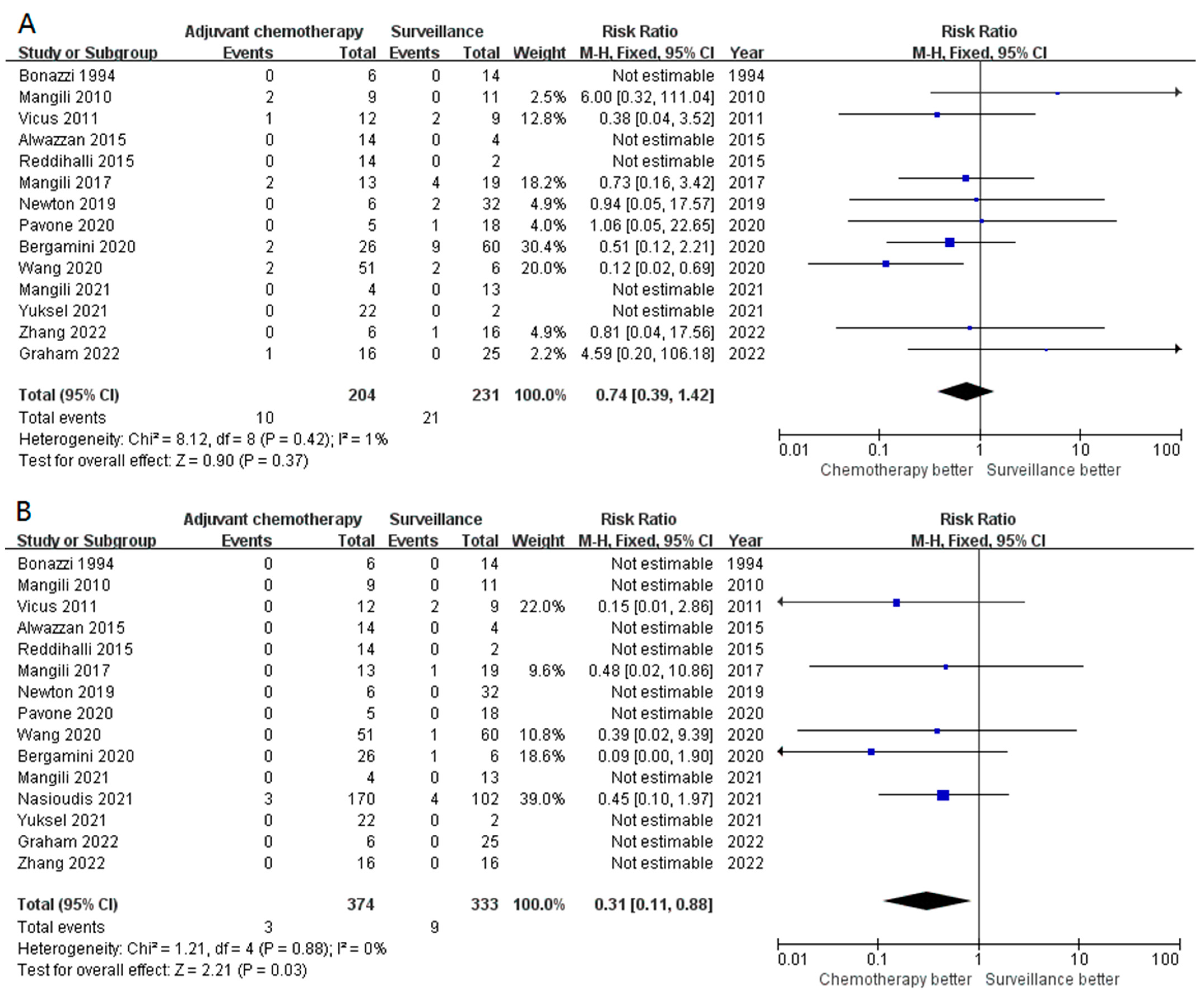
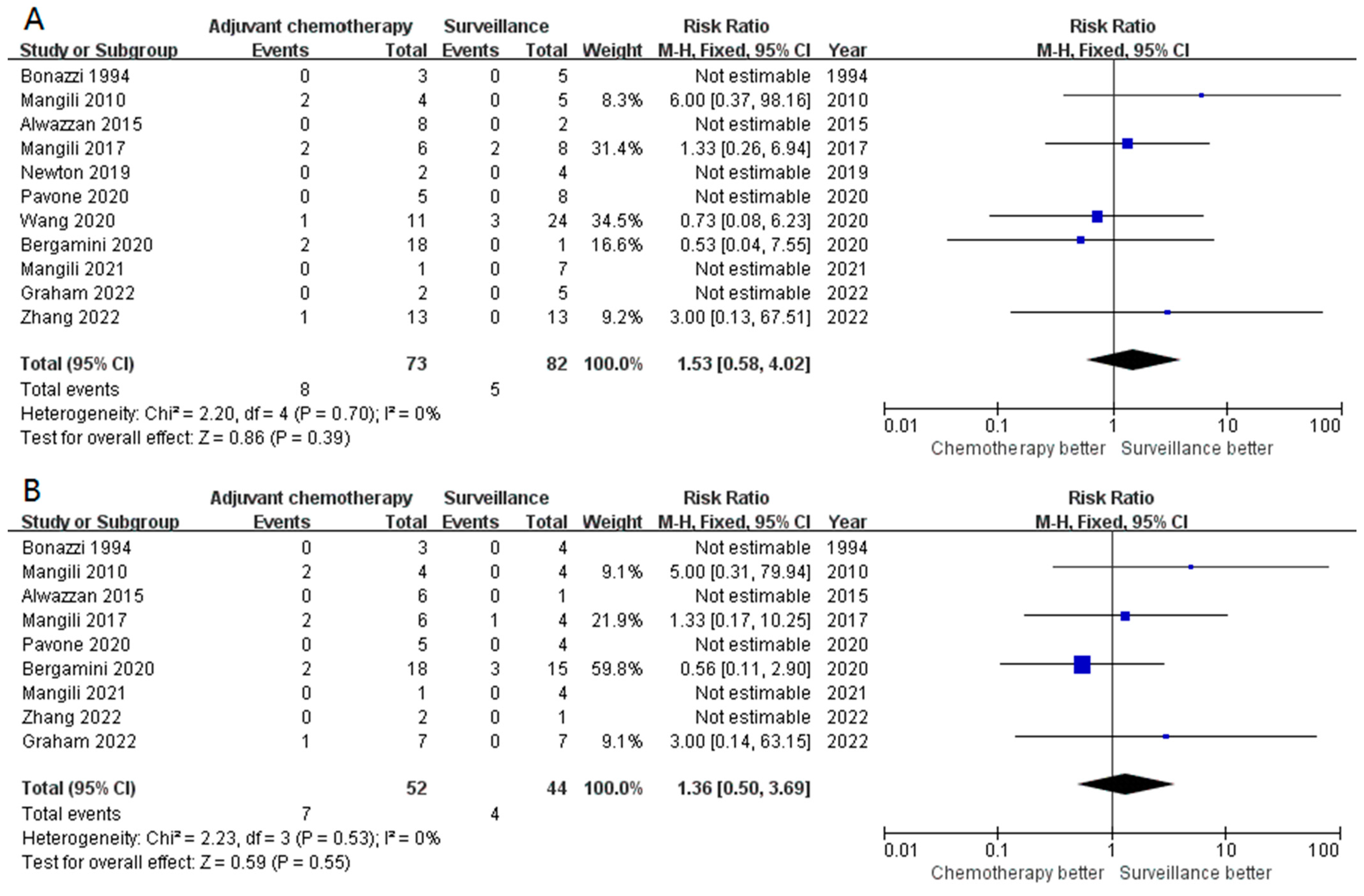
3.2. Primary Outcomes
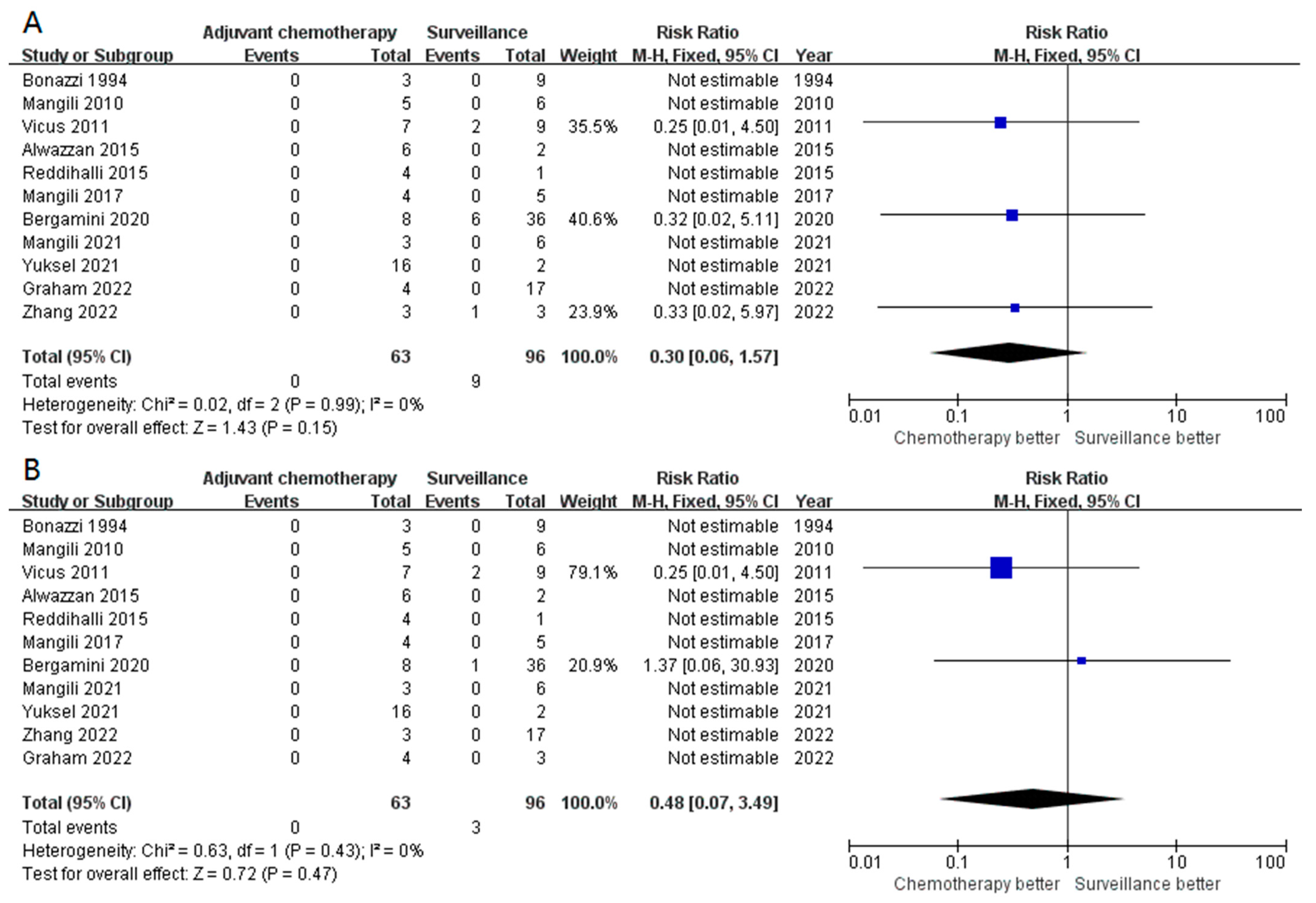
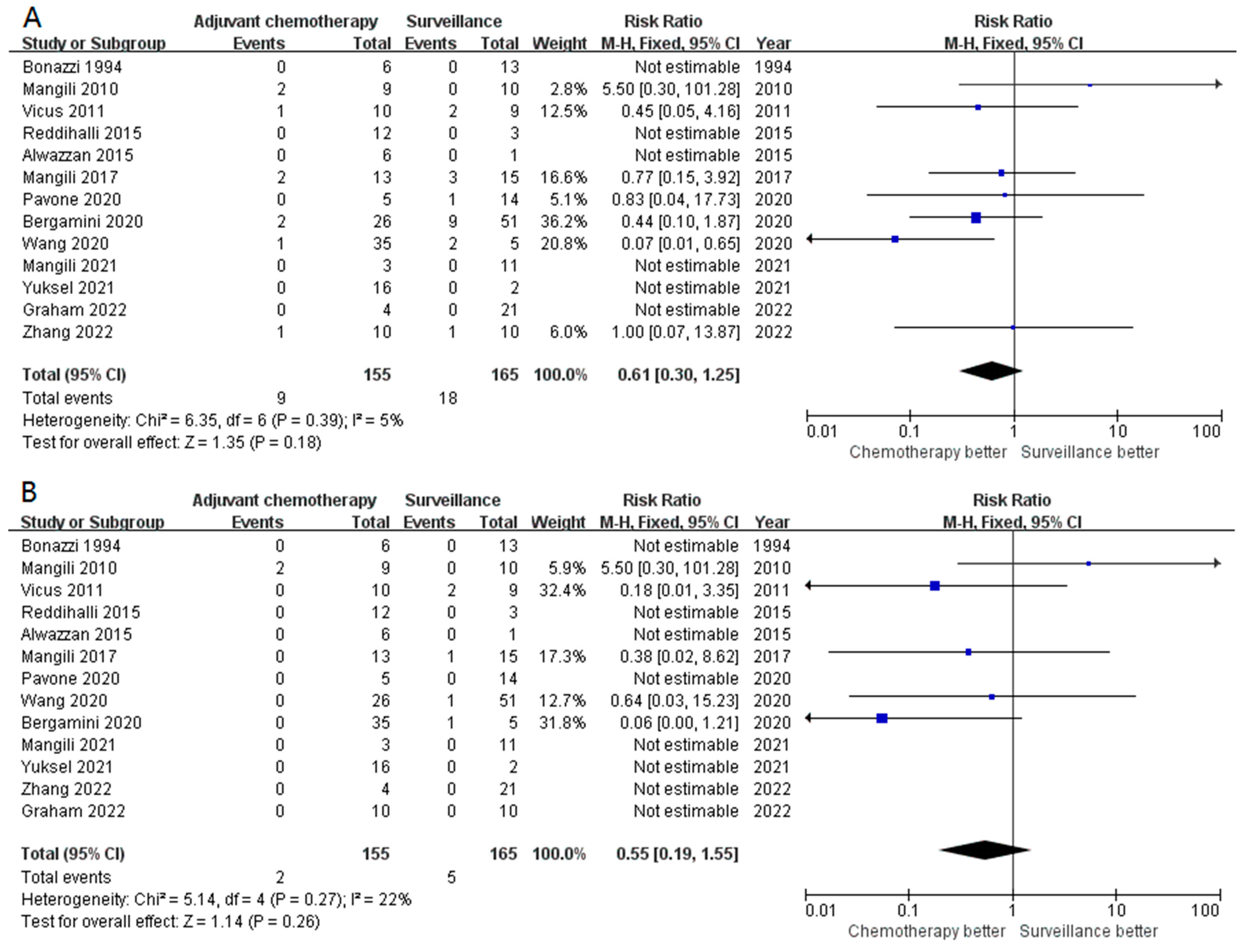
3.3. Secondary Outcomes (Subgroup Analysis)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Smith, H.O.; Berwick, M.; Verschraegen, C.F.; Wiggins, C.; Lansing, L.; Muller, C.Y.; Qualls, C.R. Incidence and survival rates for female malignant germ cell tumors. Obstet. Gynecol. 2006, 107, 1075–1085. [Google Scholar] [CrossRef]
- Park, M.; Lim, J.; Lee, J.A.; Park, H.J.; Park, B.K.; Lim, M.C.; Park, S.Y.; Won, Y.J. Incidence and outcomes of malignant ovarian germ cell tumors in Korea, 1999–2017. Gynecol. Oncol. 2021, 163, 79–84. [Google Scholar] [CrossRef]
- Prat, J. FIGO’s staging classification for cancer of the ovary, fallopian tube, and peritoneum: Abridged republication. J. Gynecol. Oncol. 2015, 26, 87–89. [Google Scholar] [CrossRef]
- Robboy, S.J.; Scully, R.E. Ovarian teratoma with glial implants on the peritoneum. An analysis of 12 cases. Hum. Pathol. 1970, 1, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Morice, P.; Lorusso, D.; Prat, J.; Oaknin, A.; Pautier, P.; Colombo, N. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv1–iv18. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.M.; Cristea, M.; DeRosa, M.; Eisenhauer, E.L.; et al. Ovarian Cancer, Version 2.2020. J. Natl. Compr. Cancer Netw. 2021, 19, 191. [Google Scholar] [CrossRef]
- Pashankar, F.; Hale, J.P.; Dang, H.; Krailo, M.; Brady, W.E.; Rodriguez-Galindo, C.; Nicholson, J.C.; Murray, M.J.; Bilmire, D.F.; Stoneham, S.; et al. Is adjuvant chemotherapy indicated in ovarian immature teratomas? A combined data analysis from the Malignant Germ Cell Tumor International Collaborative. Cancer 2016, 122, 230–237. [Google Scholar] [CrossRef]
- Billmire, D.F.; Cullen, J.W.; Rescorla, F.J.; Davis, M.; Schlatter, M.G.; Olson, T.A.; Malogolowkin, M.H.; Pashankar, F.; Villaluna, D.; Krailo, M.; et al. Surveillance after initial surgery for pediatric and adolescent girls with stage I ovarian germ cell tumors: Report from the Children’s Oncology Group. J. Clin. Oncol. 2014, 32, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.C.; Olson, T.A.; Cullen, J.W.; Billmire, D.F.; Marina, N.; Rescorla, F.; Davis, M.M.; London, W.B.; Lauer, S.J.; Giller, R.H.; et al. Treatment of children and adolescents with stage II testicular and stages I and II ovarian malignant germ cell tumors: A Pediatric Intergroup Study—Pediatric Oncology Group 9048 and Children’s Cancer Group 8891. J. Clin. Oncol. 2004, 22, 3563–3569. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhu, S.; Jia, C.; Cao, D.; Wu, M.; Shen, K.; Yang, J.; Xiang, Y. Role of staging surgery and adjuvant chemotherapy in adult patients with apparent stage I pure immature ovarian teratoma after fertility-sparing surgery. Int. J. Gynecol. Cancer 2020, 30, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, A.; Sarwar, N.; Ferrandina, G.; Scarfone, G.; Short, D.; Aguiar, X.; Camnasio, C.; Kaur, B.; Savage, P.M.; Cormio, G.; et al. Can we replace adjuvant chemotherapy with surveillance for stage IA-C immature ovarian teratomas of any grade? An international multicenter analysis. Eur. J. Cancer 2020, 137, 136–143. [Google Scholar] [CrossRef]
- Mangili, G.; Giorda, G.; Ferrandina, G.; Cormio, G.; Cassani, C.; Savarese, A.; Danese, S.; Carnelli, M.; Vasta, F.M.; Perrone, A.M.; et al. Surveillance alone in stage I malignant ovarian germ cell tumors: A MITO (Multicenter Italian Trials in Ovarian cancer) prospective observational study. Int. J. Gynecol. Cancer 2021, 31, 1242–1247. [Google Scholar] [CrossRef]
- Newton, C.; Murali, K.; Ahmad, A.; Hockings, H.; Graham, R.; Liberale, V.; Sarker, S.J.; Ledermann, J.; Berney, D.M.; Shamash, J.; et al. A multicentre retrospective cohort study of ovarian germ cell tumours: Evidence for chemotherapy de-escalation and alignment of paediatric and adult practice. Eur. J. Cancer 2019, 113, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Pavone, R.; Dijoud, F.; Galmiche, L.; Ro, V.; Hameury, F.; Sarnacki, S.; Orbach, D.; Briandet, C.; Pasquet, M.; Bertrand, A.; et al. Pure pediatric ovarian immature teratomas: The French experience. Pediatr. Blood Cancer 2020, 67, e28186. [Google Scholar] [CrossRef]
- Graham, R.; MacDonald, N.D.; Lockley, M.; Miller, R.; Butler, J.; Murali, K.; Sarker, S.J.; Banerjee, S.; Stoneham, S.; Shamash, J.; et al. Surgical management and outcomes for stage 1 malignant ovarian germ cell tumours: A UK multicentre retrospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 271, 138–144. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Bonazzi, C.; Peccatori, F.; Colombo, N.; Lucchini, V.; Cantù, M.G.; Mangioni, C. Pure ovarian immature teratoma, a unique and curable disease: 10 years’ experience of 32 prospectively treated patients. Obstet. Gynecol. 1994, 84, 598–604. [Google Scholar] [PubMed]
- Mangili, G.; Scarfone, G.; Gadducci, A.; Sigismondi, C.; Ferrandina, G.; Scibilia, G.; Viganò, R.; Tateo, S.; Villa, A.; Lorusso, D. Is adjuvant chemotherapy indicated in stage I pure immature ovarian teratoma (IT)? A multicentre Italian trial in ovarian cancer (MITO-9). Gynecol. Oncol. 2010, 119, 48–52. [Google Scholar] [CrossRef]
- Vicus, D.; Beiner, M.E.; Clarke, B.; Klachook, S.; Le, L.W.; Laframboise, S.; Mackay, H. Ovarian immature teratoma: Treatment and outcome in a single institutional cohort. Gynecol. Oncol. 2011, 123, 50–53. [Google Scholar] [CrossRef]
- Alwazzan, A.B.; Popowich, S.; Dean, E.; Robinson, C.; Lotocki, R.; Altman, A.D. Pure Immature Teratoma of the Ovary in Adults: Thirty-Year Experience of a Single Tertiary Care Center. Int. J. Gynecol. Cancer 2015, 25, 1616–1622. [Google Scholar] [CrossRef]
- Reddihalli, P.V.; Subbian, A.; Umadevi, K.; Rathod, P.S.; Krishnappa, S.; Nanaiah, S.P.; Bafna, U.D. Immature teratoma of ovary--outcome following primary and secondary surgery: Study of a single institution cohort. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 192, 17–21. [Google Scholar] [CrossRef]
- Mangili, G.; Sigismondi, C.; Lorusso, D.; Cormio, G.; Candiani, M.; Scarfone, G.; Mascilini, F.; Gadducci, A.; Mosconi, A.M.; Scollo, P.; et al. The role of staging and adjuvant chemotherapy in stage I malignant ovarian germ cell tumors (MOGTs): The MITO-9 study. Ann. Oncol. 2017, 28, 333–338. [Google Scholar] [CrossRef]
- Nasioudis, D.; Frey, M.K.; Chapman-Davis, E.; Caputo, T.A.; Holcomb, K.M. Surveillance Only for High-risk FIGO Stage IA/IB Malignant Ovarian Germ Cell Tumors: Results From a National Cancer Database. Am. J. Clin. Oncol. 2021, 44, 195–199. [Google Scholar] [CrossRef]
- Yuksel, D.; Ayhan, S.; Korkmaz, V.; Cakir, C.; Kilic, C.; Akgor, U.; Ozgul, N.; Kilic, F.; Ersak, B.; Esen, S.; et al. Retrospective Analysis of Pure Ovarian Immature Teratoma in Patients Aged 15–39 Years: A Turkish Multicenter Study. J. Adolesc. Young Adult Oncol. 2021, 10, 697–702. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Wang, J.; Zhang, Y.; Yang, J. Early stage ovarian immature teratoma, surveillance or chemotherapy after surgery? A propensity score matched analysis. Gynecol. Oncol. Rep. 2022, 40, 100976. [Google Scholar] [CrossRef] [PubMed]
- Skalleberg, J.; Solheim, O.; Fosså, S.D.; Småstuen, M.C.; Osnes, T.; Gundersen, P.O.M.; Bunne, M. Long-term ototoxicity in women after cisplatin treatment for ovarian germ cell cancer. Gynecol. Oncol. 2017, 145, 148–153. [Google Scholar] [CrossRef]
- Solheim, O.; Skalleberg, J.; Warncke, T.; Orstavik, K.; Trope, C.; Fossa, S.D. Long-term neurotoxicity and Raynaud’s phenomenon in patients treated with cisplatin-based chemotherapy for malignant ovarian germ cell tumor. Acta Obstet. Gynecol. Scand. 2019, 98, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Razak, A.R.A.; Li, L.; Bryant, A.; Diaz-Padilla, I. Chemotherapy for malignant germ cell ovarian cancer in adult patients with early stage, advanced and recurrent disease. Cochrane Database Syst. Rev. 2011, 2011, CD007584. [Google Scholar] [CrossRef]
- Jorge, S.; Jones, N.L.; Chen, L.; Hou, J.Y.; Tergas, A.I.; Burke, W.M.; Ananth, C.V.; Neugut, A.I.; Herhshman, D.L.; Wright, J.D. Characteristics, treatment and outcomes of women with immature ovarian teratoma, 1998-2012. Gynecol. Oncol. 2016, 142, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennn, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
| Author and Year Published | Design | Participant Disease | N (Stage I POIT) | Inclusion | Median Follow-Up * | Recurrence (Stage I POIT, Surveillance vs. Chemotherapy) | Death (Stage I POIT, Surveillance vs. Chemotherapy) |
|---|---|---|---|---|---|---|---|
| Bonazzi, 1994 | RS | POIT | 26 | Pathology-confirmed diagnosis of POIT between 1982–1991, any stage | 47 months | No event | No event |
| Mangili, 2010 | RS | MOGCTs | 28 | MOGCT diagnosis between 1982–2008 with complete clinical data and outcomes, any stage | 61 months | Two recurrences in the chemotherapy group | No event |
| Vicus, 2011 | RS | POIT | 32 | POIT of any stage histologically diagnosed between 1970–2005 | 4.8 years | Two recurrences vs. one recurrence | Two deaths in the chemotherapy group |
| Alwazzan, 2015 | RS | POIT | 22 | POIT of any stage/grade diagnosed between 1983 and 2013 | 60 months | No event | No event |
| Reddihalli, 2015 | RS | POIT | 16 | POIT of any stage/grade diagnosed between 1999 and 2011 had the exact follow-up data and clinical outcomes | 39 months | No event | No event |
| Mangili, 2017 | RS | MOGCTs | 49 | Stage I MOGCTs diagnosed between 1982 and 2014 that had clear outcomes | 59 months | Four cases vs. two cases | One death in surgery group |
| Newton, 2019 | RS | MOGCTs | 38 | MOGCTs diagnosed between 2005 and 2016 of any stage/grade who had clear outcomes | 56.6 months | Two recurrences in surgery group | No event |
| Pavone, 2020 | RS | POIT | 35 | Pediatric (no more than 18 years old) POIT of any stage/grade | 39.5 months | One relapse in surgery group (exclude IX G1) | No event |
| Wang, 2020 | RS | POIT | 75 | Stage I POIT aged over 18 years who underwent fertility-sparing surgery between 1986 and 2018 | 80.2 months | Two recurrences in each group (5-year RFS of 91.7% vs. 96.0%) | One death in surgery group |
| Bergamini, 2020 | RS | POIT | 108 | Post-puberal Stage I POIT diagnosed between 1985 and 2018 that had clear follow-up data | 64.3 months | Nine cases vs. two cases | One death in surgery group |
| Mangili, 2021 | PS | MOGCTs | 23 | Post-pubertal stage I MOGCT patients diagnosed between 2013 and 2019 | 46.2 months | No recurrence | No deaths |
| Nasioudis, 2021 | RS | MOGCTs | 272 | IA/IB grade 2–3 POIT, yolk sac, or mixed MOGCTs diagnosed between 2004 and 2014 with at least 1 month of follow-up | 63.8/61.7 months | NA | 95.0% vs. 97.3% (5-year OS rate, p = 0.22) |
| Yuksel, 2021 | RS | POIT | 40 | POIT patients aged between 15 and 39 years diagnosed between 1993 and 2019 | 60 months | No event | No event |
| Graham, 2022 | RS | MOGCTs | 39 | Histological diagnosis of stage I MOGCTs between 2005 and 2016 that had clear outcomes | 4.4 years | No event | No event |
| Zhang, 2022 | RS | POIT | 32 | Histologically confirmed POIT of stage I (except IA G1) before 2016 | 24 months | One recurrence in each group | No event |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wang, Y.; Zhang, X.; Zhang, T.; Yin, M.; Yang, J. Role of Adjuvant Chemotherapy in Stage I Pure Ovarian Immature Teratoma: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 1741. https://doi.org/10.3390/cancers15061741
Li S, Wang Y, Zhang X, Zhang T, Yin M, Yang J. Role of Adjuvant Chemotherapy in Stage I Pure Ovarian Immature Teratoma: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(6):1741. https://doi.org/10.3390/cancers15061741
Chicago/Turabian StyleLi, Sijian, Yuelin Wang, Xinyue Zhang, Tianyu Zhang, Min Yin, and Jiaxin Yang. 2023. "Role of Adjuvant Chemotherapy in Stage I Pure Ovarian Immature Teratoma: A Systematic Review and Meta-Analysis" Cancers 15, no. 6: 1741. https://doi.org/10.3390/cancers15061741
APA StyleLi, S., Wang, Y., Zhang, X., Zhang, T., Yin, M., & Yang, J. (2023). Role of Adjuvant Chemotherapy in Stage I Pure Ovarian Immature Teratoma: A Systematic Review and Meta-Analysis. Cancers, 15(6), 1741. https://doi.org/10.3390/cancers15061741






