Risk Factors of Complications from Central Bisectionectomy (H458) for Hepatocellular Carcinoma: A Multi-Institutional Single-Arm Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients, Research Collaborators, and Ethics
2.2. Study Design and Parameters
2.3. Statistical Analysis
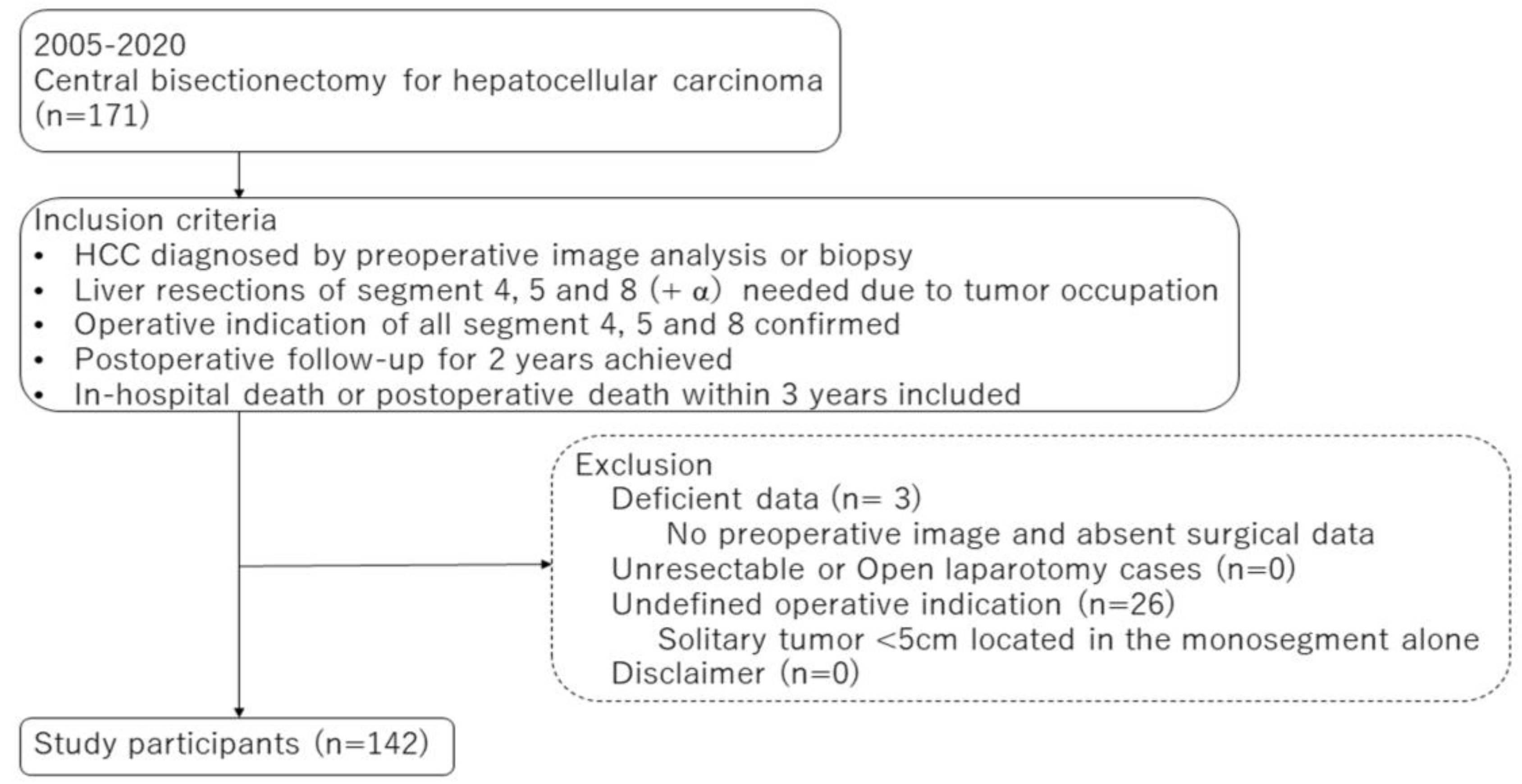
3. Results
3.1. Study Population and Selected Patient Characteristics
3.2. Relationship between Variables and Postoperative Morbidities and Mortality
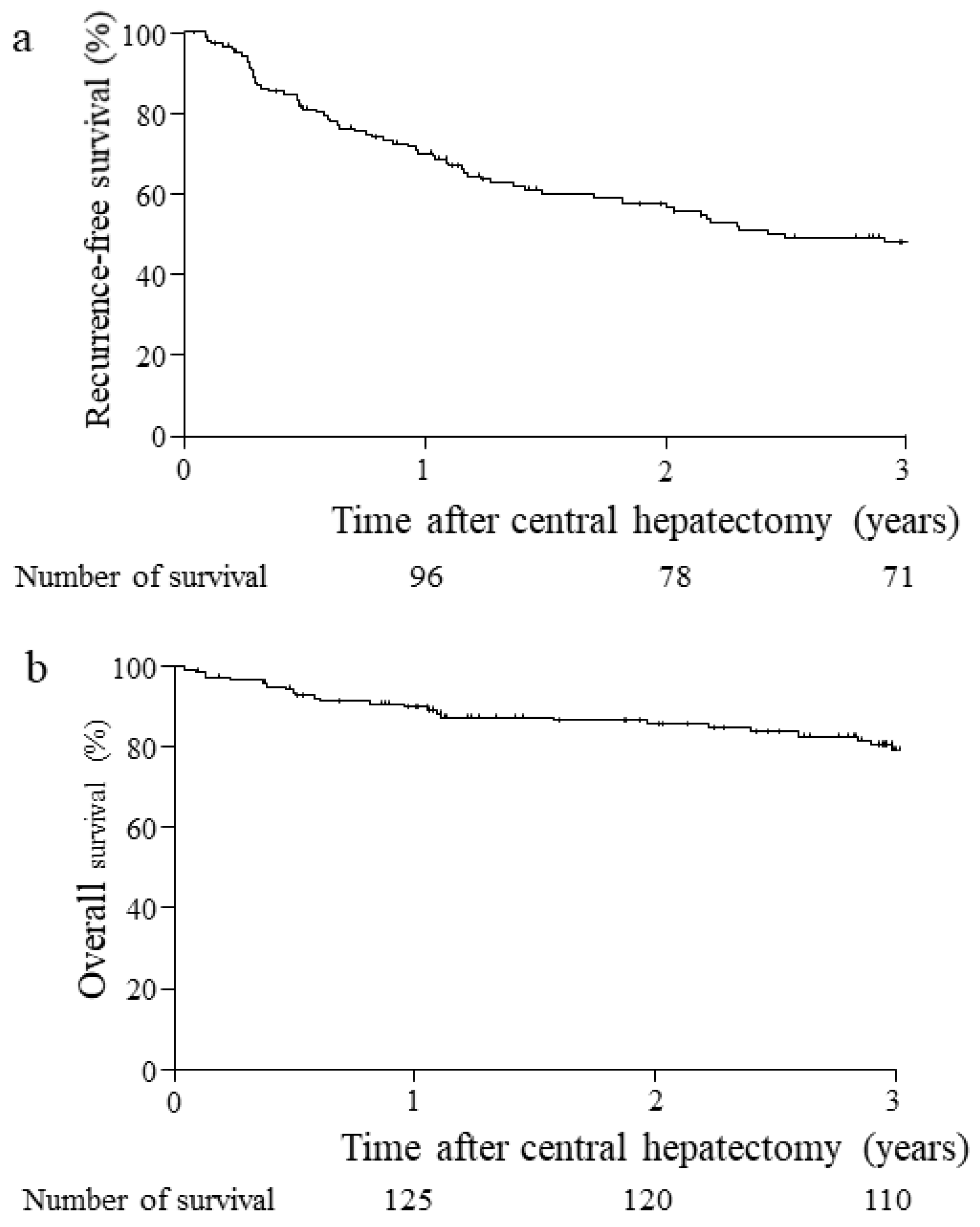
3.3. Postoperative Patient Outcomes for Three Years
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, J.W.; Chen, M.; Colombo, N.; Roberts, L.R.; Schwartz, M.; Chen, P.J.; Kudo, M.; Johnson, P.; Wagner, S.; Orsini, L.S.; et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The RIDGE Study. Liver Int. 2015, 35, 2155–2166. [Google Scholar] [PubMed]
- Yang, L.Y.; Chang, R.M.; Lau, W.Y.; Ou, D.P.; Wu, W.; Zeng, Z.J. Mesohepatectomy for centrally located large hepatocellular carcinoma: Indications, techniques, and outcomes. Surgery 2014, 156, 1177–1187. [Google Scholar] [PubMed]
- Orimo, T.; Kamiyama, T.; Kakisaka, T.; Shimada, S.; Nagatsu, A.; Asahi, Y.; Sakamoto, Y.; Kamachi, H.; Taketomi, A. Central Hepatectomy Versus Major Hepatectomy for Centrally Located Hepatocellular Carcinoma: A Propensity Score Matching Study. Ann. Surg. Oncol. 2021, 28, 6769–6779. [Google Scholar]
- Scudamore, C.H.; Buczkowski, A.K.; Shayan, H.; Ho, S.G.; Legiehn, G.M.; Chung, S.W.; Owen, D.A. Mesohepatectomy. Am. J. Surg. 2000, 179, 356–360. [Google Scholar]
- McBride, C.N.; Wallace, S. Cancer of the right lobe of the liver. Arch. Surg. 1972, 105, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Makuuchi, M.; Yamazaki, S.; Gunvén, P. Central bisegmentectomy of the liver: Experience in 16 patients. World J. Surg. 1989, 13, 786–790. [Google Scholar]
- Nagino, M.; DeMatteo, R.; Lang, H.; Cherqui, D.; Malago, M.; Kawakatsu, S.; DeOliveira, M.L.; Adam, R.; Aldrighetti, L.; Boudjema, K.; et al. Proposal of a New Comprehensive Notation for Hepatectomy: The “New World” Terminology. Ann. Surg. 2021, 274, 1–3. [Google Scholar]
- Yanaga, K. Central bisectionectomy (bisegmentectomy) of the liver (with video). J. Hepatobiliary Pancreat. Sci. 2012, 19, 44–47. [Google Scholar] [CrossRef]
- Llovet, J.M.; De Baere, T.; Kulik, L.; Haber, P.K.; Greten, T.F.; Meyer, T.; Lencioni, R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293–313. [Google Scholar] [PubMed]
- Hidaka, M.; Eguchi, S.; Okuda, K.; Beppu, T.; Shirabe, K.; Kondo, K.; Takami, Y.; Ohta, M.; Shiraishi, M.; Ueno, S.; et al. Impact of Anatomical Resection for Hepatocellular Carcinoma with Microportal Invasion (vp1): A Multi-institutional Study by the Kyushu Study Group of Liver Surgery. Ann. Surg. 2020, 271, 339–346. [Google Scholar]
- Ikai, I.; Takayasu, K.; Omata, M.; Okita, K.; Nakanuma, Y.; Matsuyama, Y.; Makuuchi, M.; Kojiro, M.; Ichida, T.; Arii, S.; et al. A modified Japan Integrated Stage score for prognostic assessment in patients with hepatocellular carcinoma. J. Gastroenterol. 2006, 41, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Liver Cancer Study Group of Japan. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, 6th ed.; Kanehara & Co., Ltd.: Tokyo, Japan, 2015; pp. 8–51. [Google Scholar]
- Qiu, J.; Chen, S.; Du, C. The prognostic value of a classification system for centrally located liver tumors in the setting of hepatocellular carcinoma after mesohepatectomy. Surg. Oncol. 2016, 25, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Garden, O.J.; Padbury, R.; Brooke-Smith, M.; Crawford, M.; Adam, R.; Koch, M.; Makuuchi, M.; Dematteo, R.P.; Christophi, C.; et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149, 713–724. [Google Scholar] [CrossRef]
- Brooke-Smith, M.; Figueras, J.; Ullah, S.; Rees, M.; Vauthey, J.N.; Hugh, T.J.; Garden, O.J.; Fan, S.T.; Crawford, M.; Makuuchi, M.; et al. Prospective evaluation of the International Study Group for Liver Surgery definition of bile leak after a liver resection and the role of routine operative drainage: An international multicentre study. HPB 2015, 17, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, B.; He, W.; Wei, Y.G.; Du, Z.G.; Jiang, L. Mesohepatectomy versus extended hemihepatectomy for centrally located hepatocellular carcinoma. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Kubota, K.; Hasegawa, K.; Kubo, S.; Izumi, N.; Kokudo, N.; Sakamoto, M.; Shiina, S.; Takayama, T.; Nakashima, O.; et al. Significance of the surgical hepatic resection margin in patients with a single hepatocellular carcinoma. Br. J. Surg. 2020, 107, 113–120. [Google Scholar] [CrossRef]
- Strasberg, S.M.; Linehan, D.C.; Hawkins, W.G. Isolation of right main and right sectional portal pedicles for liver resection without hepatotomy or inflow occlusion. J. Am. Coll. Surg. 2008, 206, 390–396. [Google Scholar] [CrossRef]
- Fan, S.T. Precise hepatectomy guided by the middle hepatic vein. Hepatobiliary Pancreat. Dis. Int. 2007, 6, 430–434. [Google Scholar]
- Makuuchi, M.; Yamamoto, J.; Takayama, T.; Kosuge, T.; Gunvén, P.; Yamazaki, S.; Hasegawa, H. Extrahepatic division of the right hepatic vein in hepatectomy. Hepato-Gastroenterol. 1991, 38, 176–179. [Google Scholar]
- Nanashima, A.; Tobinaga, S.; Araki, M.; Nonaka, T.; Abo, T.; Hidaka, S.; Takeshita, H.; Sawai, T.; Nagayasu, T. Double liver hanging manoeuvre for central hepatectomy. HPB 2009, 11, 529–531. [Google Scholar] [CrossRef]
- Beppu, T.; Imai, K.; Okuda, K.; Eguchi, S.; Kitahara, K.; Taniai, N.; Ueno, S.; Shirabe, K.; Ohta, M.; Kondo, K.; et al. Anterior approach for right hepatectomy with hanging maneuver for hepatocellular carcinoma: A multi-institutional propensity score-matching study. J. Hepatobiliary Pancreat. Sci. 2017, 24, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Schadde, E.; Schnitzbauer, A.A.; Tschuor, C.; Raptis, D.A.; Bechstein, W.O.; Clavien, P.A. Systematic review and meta-analysis of feasibility, safety, and efficacy of a novel procedure: Associating liver partition and portal vein ligation for staged hepatectomy. Ann. Surg. Oncol. 2015, 22, 3109–3120. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, A.; Ramouz, A.; Golriz, M.; Khajeh, E.; Hackert, T.; Müller-Stich, B.; Strobel, O.; Hoffmann, K.; Büchler, M.W.; Liver Cancer Center Heidelberg (LCCH). Long-term outcomes of mesohepatectomy for centrally located liver tumors: Two-decade single-center experience. J. Am. Coll. Surg. 2022, 235, 257–266. [Google Scholar] [CrossRef]
- Vauthey, J.N.; Dixon, E.; Abdalla, E.K.; Helton, W.S.; Pawlik, T.M.; Taouli, B.; Brouquet, A.; Adams, R.B.; American Hepato-Pancreato-Biliary Association; Society of Surgical Oncology; et al. Pretreatment assessment of hepatocellular carcinoma: Expert consensus statement. HPB 2010, 12, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Yamazaki, S.; Maku-uchi, M.; Shimamura, Y. 108 hépatectomies pour tumeurs et quelques lesions bénignes. Chirrurgie 1080, 106, 346–349. [Google Scholar]
- Yamashita, Y.; Yamamoto, H.; Miyata, H.; Kakeji, Y.; Kitagawa, Y.; Yamaue, H.; Yamamoto, M.; Baba, H. Risk factors for bile leakage: Latest analysis of 10 102 hepatectomies for hepatocellular carcinoma from the Japanese national clinical database. J. Hepatobiliary Pancreat. Sci. 2021, 28, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, A.; Abo, T.; Hamasaki, K.; Wakata, K.; Kunizaki, M.; Tou, K.; Takeshita, H.; Hidaka, S.; Sawai, T.; Tsuchiya, T.; et al. Predictors of intraoperative blood loss in patients undergoing hepatectomy. Surg. Today 2013, 43, 485–493. [Google Scholar] [CrossRef]
- Shirabe, K.; Kajiyama, K.; Harimoto, N.; Tsujita, E.; Wakiyama, S.; Maehara, Y. Risk factors for massive bleeding during major hepatectomy. World J. Surg. 2010, 34, 1555–1562. [Google Scholar] [CrossRef]
- Orcutt, S.T.; Anaya, D.A. Liver Resection and Surgical Strategies for Management of Primary Liver Cancer. Cancer Control. 2018, 25, 1073274817744621. [Google Scholar] [CrossRef]
- Ye, Q.; Zeng, C.; Wang, Y.; Ming, Y.; Wan, Q.; Ye, S.; Xiong, Y.; Ling, L. Long-Term Outcomes of Ante-Situm Resection and Auto-Transplantation in Conventionally Unresectable Hepatocellular Carcinoma: A Single-Center Experience. Ann. Transplant. 2018, 23, 81–88. [Google Scholar] [CrossRef]
- Shindoh, J.; Kawamura, Y.; Kobayashi, Y.; Kobayashi, M.; Akuta, N.; Okubo, S.; Suzuki, Y.; Hashimoto, M. Prognostic Impact of Surgical Intervention After Lenvatinib Treatment for Advanced Hepatocellular Carcinoma. Ann. Surg. Oncol. 2021, 28, 7663–7672. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Ricci, A.D.; Federico, A.D.; Frega, G.; Palloni, A.; Tavolari, S.; Brandi, G. Predictive Biomarkers for Checkpoint Inhibitor-Based Immunotherapy in Hepatocellular Carcinoma: Where Do We Stand? Front. Oncol. 2021, 11, 803133. [Google Scholar] [CrossRef] [PubMed]
- Viscardi, G.; Tralongo, A.C.; Massari, F.; Lambertini, M.; Mollica, V.; Rizzo, A.; Comito, F.; Liello, R.D.; Alfieri, S.; Imbimbo, M.; et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: A systematic review and meta-analysis. Eur. J. Cancer 2022, 177, 175–185. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual authors(s) and contributor(s), and not MDPI and/or editor(s). The MDPI and/or editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

| Variables (n = 142) | n, (%), Median Value [Minimum–Maximum] |
|---|---|
| Age (years) | 73 [36–87] * |
| Sex (Male/Female) | 118 (83)/24 (17) |
| ASA-PS (n = 137) | |
| 1/2/3 | 30 (22)/93 (68)/14 (10) |
| Background liver status | |
| Chronic liver injury, non/chronic hepatitis/cirrhosis | 39/103 (73) |
| non-BC (NAFLD 22, PBC 1, other 7) | 72 (51) |
| B | 21 (15) |
| C | 49 (34) # |
| Portal hypertension, yes | 4 (3) |
| Ascites, yes | 2 (1) (controllable, mild) |
| Splenomegaly, no/mild/moderate or more | 122 (85)/19 (14)/1 (1) |
| Preoperative liver functions | |
| Child–Pugh classification | A 138/B 4 (3) |
| Liver damage grade | A 121/B 14 (10) |
| ICGR15 (%) | 11.2 [1.3–25] |
| Total bilirubin (mg/dL) | 0.7 [0.3–2.6] |
| Albumin (g/dL) | 4.1 [2.3–4.9] |
| Prothrombin activity (%) | 95 [44–139] |
| Platelet counts (× 104/mm3) | 18.2 [9.8–77] |
| C-reactive protein (mg/dL) | 0.19 [0.02–10.1] |
| Alfa-feto protein (ng/mL) | 13.3 [1.1–383,541] |
| PIVKA-II (mAU/mL) | 323 [9–269,391] |
| Tumor location diagnosed before operation | |
| Main location, S8/S4/S5 | 25 (18)/107 (75)/10 (7) |
| S1 involved, yes | 9 (6) |
| Number of HCC | 1.2 [range 1–6] * |
| 1/2/3/4/5/6 | 103 (73)/24 (17)/8 (6)/2 (1)/2 (1)/3 (2) |
| Size | 6.0 [range 4–14] * |
| 0–4.9/5–9.9/over 10 cm | 1 (1)/10 (6)/160 (93) |
| Macrovascular involvement no/yes § | 130 (92)/12 (8) |
| Portal vein no/yes | 136 (96)/6 (4) |
| Hepatic venous no/yes | 134 (94)/8 (6) |
| Distance to major vessels | |
| Hilar Glisson, >10/10- >0/0 mm | 106 (75)/24 (17)/11 (8); Stenosis, 7 |
| MHV, >10/10- >0/0 mm | 96 (68)/13 (9)/32 (23) |
| RHV, >10/10- >0/0 mm | 115 (82)/12 (8)/14 (10) |
| IVC >10/10- >0/0 mm | 131 (93)/7 (5)/3 (2) |
| Right diaphragm involvement, yes | 26 (19) |
| Qiu’s classification system of centrally located HCC ¶ | |
| Type I/II/III/IV | 7 (5)/49 (35)/75 (53)/5 (3)/6 (4) |
| Preoperative cancer treatment | |
| TAE/chemotherapy/brachytherapy | 15 (11)/1 (1)/2 (2) |
| Surgical records | |
| Additional resection except H4581, yes | 8 (6) |
| S1 combined resection, yes | 8 (6) |
| Estimated resection volume (cm3) | 380 [28–1243] |
| Expose hilar plate Glisson sheath, yes | 93 (66) |
| root of MHV/entire RHV/front of IVC | 125 (87)/100 (71)/51 (36) |
| Hepatic inflow occlusion (Pringle), no/yes | 13/129 (91) |
| Time (min) | 115 [15–322] |
| Taping of main HV root, yes | 18 (13) |
| CVP before transection (mmHg) (n = 88) | 6.0 [1–11] |
| Operating time (min) | 475 [282–1807] |
| Intraoperative blood loss factors (iBL) | |
| Intraoperative blood loss (mL) | 852 [42–23,336] |
| Red cell transfusion, no/yes | 88/54 (38) |
| Injury of preserved main vessels, yes | 15 (11) |
| Histology | |
| Diagnosis (HCC, combined type HCC) | 136/6 (4) |
| Occupied location | |
| Main location, S8/S4/S5 | 25 (18)/107 (75)/10 (7) |
| S1 involved, yes | 9 (6) |
| Histological tumor findings | |
| F (fibrotic grade) ‡ 0/1/2/3/4 (n = 98) | 15 (11)/50 (37)/28 (21)/25 (18)/18 (13) |
| Normal liver/chronic hepatitis/cirrhosis | 1 (11)5/109 (76)/18 (13) |
| A (necro-inflammatory grade) ‡ 0/1/2/3 | 15 (15)/56 (57)/24 (25)/3 (3) |
| Surgical margin (mm) | 4.0 [0–26] |
| 0/0.1–5/>5 mm | 29/58/55 |
| Postoperative outcomes | |
| Complications | |
| Liver failure (PHLF), no/A/B/C ** | 85 (60)/39/12/6 |
| Bile leakage (PHBL), no/A/B/C ** | 87 (61)/28/26/1 |
| Prolonged ascites >7 days, yes | 18 (13) |
| Post-hepatectomy hemorrhage (PHH) | |
| Radiological or surgical intervention, yes | nil |
| Organ space SSI, yes | 21 (15) |
| Others | 32 |
| Mortality, yes | 4 (3) |
| Within 30 days, yes | 1 (0.5) |
| Hospital stay (days) | 26 [8–176] |
| Prognosis within 3 years | |
| 1st cancer recurrence, yes | 71 (51); liver 60, extra-liver 10, lung 1 |
| Relapse-free survival period (days) | 1362 ± 139 (mean ± standard deviation) |
| rate (1-, 2-, 3-year) | 70%, 56%, 48% |
| Prognosis | |
| Cancer-free survival | 109 |
| Dead, in-hospital/HCC/other disease | 4/25/4 |
| Overall survival period (days) | 4838 ± 216 (mean ± standard deviation) |
| rate (1-, 2-, 3-year) | 90%, 86%, 81% |
| Variables | PHLF | PHBL | Total Morbidity # | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| None | A | B | C | None | A | B | C | No | Yes | |
| Demographics | ||||||||||
| ASA-PS | ||||||||||
| 1 | 0 | 5 | 0 | 1 | 5 | 0 | 1 | 0 | 6 | 5 |
| 2 | 3 | 16 | 3 | 2 | 5 | 15 | 4 | 0 | 15 | 9 |
| 3 | 68 | 16 | 8 | 1 | 65 | 12 | 15 | 1 | 56 | 37 |
| 4 | 9 | 2 | 1 | 2 ** | 8 | 1 | 5 | 0 ** | 5 | 9 |
| Liver functions | ||||||||||
| Liver damage grade | ||||||||||
| A | 78 | 30 | 9 | 4 | 73 | 25 | 22 | 1 | 74 | 8 |
| B | 6 | 4 | 3 | 1 | 8 | 3 | 3 | 0 | 8 | 6 |
| ICGR15 (%) | 12.2 (6.6) | 12.6 (6.6) | 24.5 (16.9) | 9.7 (3.8) | 13.6 (8.3) | 14.9 (10.6) | 11.1 (6.8) | 4.1 | 14.0 (8.2) | 12.5 (9.2) |
| Bilirubin (mg/dL) | 0.80 (0.3) | 0.83 (0.4) | 0.84 (0.4) | 0.77 (0.3) | 0.80 (0.3) | 0.81 (0.3) | 0.85 (0.5) | 0.6 | 0.79 (0.3) | 0.83 (0.4) |
| Albumin (g/dL) | 4.0 (0.4) | 4.2 (0.4) | 3.9 (0.5) | 3.8 (0.9) | 4.0 (0.4) | 4.3 (0.3) | 3.9 (0.6) * | 3.7 | 4.1 (0.4) | 3.9 (0.5) ** |
| PT (%) | 95 (17) | 94 (11) | 87 (13) | 90 (10) | 94 (15) | 93 (10) | 94 (19) | 88 | 94 (15) | 94 (15) |
| Platelet (/mm3) | 19.6 (7.2) | 20.4 (7.5) | 24.3 (17.3) | 20.9 (7.3) | 20.1 (9.6) | 20.9 (6.7) | 19.8 (6.7) | 29.2 | 20.7 (9.9) | 19.7 (6.2) |
| CRP (mg/dL) | 0.57 (1.5) | 0.39 (0.4) | 0.27 (0.3) | 2.23 (2.8) * | 0.57 (1.5) | 0.35 (0.4) | 0.8 (1.5) | 0.55 | 0.50 (1.4) | 0.65 (1.3) |
| Imaging of tumor | ||||||||||
| Location (main) | ||||||||||
| S8 | 13 | 11 | 1 | 0 | 15 | 10 | 0 | 0 | 20 | 5 |
| S4 | 64 | 27 | 11 | 5 | 65 | 17 | 24 | 1 | 56 | 51 * |
| S5 | 8 | 1 | 0 | 1 | 7 | 1 | 2 | 0 | 6 | 4 |
| S1 involvement (H4581), yes | 5 | 1 | 2 | 2 * | 8 | 1 | 1 | 0 | 3 | 7 |
| Number of tumors | ||||||||||
| 1 | 61 | 30 | 9 | 3 | 62 | 22 | 18 | 1 | 57 | 46 |
| 2 | 15 | 5 | 1 | 3 | 14 | 5 | 5 | 0 | 16 | 8 |
| 3≤ | 9 | 4 | 2 | 0 | 11 | 1 | 3 | 0 | 9 | 6 |
| Tumor size (mm) | ||||||||||
| 0–5 | 37 | 6 | 4 | 1 | 37 | 5 | 5 | 1 | 29 | 19 |
| 5–10 | 38 | 24 | 3 | 2 | 37 | 15 | 15 | 0 | 39 | 28 |
| 10< | 10 | 9 | 5 | 3 ** | 13 | 8 | 6 | 0 | 14 | 13 |
| Vascular invasion | ||||||||||
| 0 | 73 | 23 | 11 | 3 | 76 | 15 | 19 | 0 | 61 | 49 |
| 1 | 4 | 14 | 1 | 1 | 5 | 11 | 3 | 1 | 13 | 7 |
| 2 and 3 | 7 | 1 | 0 | 2 ** | 6 | 1 | 3 | 0 ** | 8 | 4 |
| Portal vein | ||||||||||
| 0 | 78 | 27 | 12 | 3 | 80 | 19 | 21 | 0 | 68 | 52 |
| 1 | 5 | 10 | 0 | 1 | 6 | 7 | 2 | 1 | 11 | 5 |
| 2 and 3 | 2 | 2 | 0 | 2 ** | 1 | 2 | 3 | 0 ** | 3 | 3 |
| Hepatic vein | ||||||||||
| 0 | 76 | 31 | 11 | 5 | 80 | 22 | 20 | 1 | 70 | 53 |
| 1 | 2 | 8 | 1 | 0 | 2 | 6 | 3 | 0 | 7 | 4 |
| 2 and 3 | 7 | 0 | 0 | 1 | 5 | 0 | 3 | 0 | 5 | 3 |
| Glisson pedicle stenosis | ||||||||||
| no | 81 | 37 | 11 | 5 | 84 | 27 | 23 | 0 | 79 | 55 |
| yes | 4 | 2 | 1 | 1 | 3 | 1 | 3 | 1 ** | 3 | 5 |
| Compression of LMHV root | ||||||||||
| no | 83 | 32 | 8 | 6 | 81 | 24 | 23 | 1 | 74 | 55 |
| yes | 2 | 7 | 4 | 0 ** | 6 | 4 | 3 | 0 | 8 | 5 |
| RHV root | ||||||||||
| no | 80 | 36 | 10 | 3 | 77 | 27 | 24 | 1 | 76 | 53 |
| yes | 5 | 3 | 2 | 3 ** | 10 | 1 | 2 | 0 | 6 | 7 |
| IVC | ||||||||||
| no | 84 | 38 | 10 | 5 | 84 | 26 | 26 | 1 | 79 | 58 |
| yes | 1 | 1 | 2 | 1* | 3 | 2 | 0 | 0 | 3 | 2 |
| Diaphragm | ||||||||||
| no | 75 | 29 | 9 | 3 | 74 | 21 | 20 | 1 | 68 | 48 |
| yes | 10 | 10 | 3 | 3* | 13 | 7 | 6 | 0 | 14 | 12 |
| Qiu’s CLLTs type | ||||||||||
| I | 4 | 2 | 1 | 0 | 3 | 1 | 2 | 1* | 4 | 3 |
| II | 25 | 18 | 4 | 2 | 29 | 12 | 8 | 0 | 30 | 19 |
| III | 55 | 17 | 5 | 2 | 52 | 13 | 14 | 0 | 47 | 32 |
| IV | 1 | 2 | 2 | 2* | 3 | 2 | 2 | 0 | 1 | 6 |
| Treatment related factors | ||||||||||
| Resected volume (cm3) | 355 (253) | 505 (223) | 563 (395) | 723 (485) | 62.9 (32.3) | 70.9 (36.3) | 73.9 (28.3) | 709 | 419 (293) | 420 (275) |
| Segment 1 partial resection | ||||||||||
| no | 81 | 37 | 11 | 5 | 83 | 27 | 23 | 1 | 79 | 55 |
| yes | 4 | 2 | 1 | 1 | 4 | 1 | 3 | 0 | 3 | 5 |
| Occlusion time (min) | 110 (57) | 87 (66) | 124 (91) | 116 (84) | 374 (289) | 546 (275) | 440 (239) | 99 (62) | 114 (67) | |
| Operating time (min) | ||||||||||
| <480 | 45 | 22 | 5 | 2 | 49 | 14 | 11 | 0 | 42 | 32 |
| ≥480 | 40 | 17 | 7 | 4 | 38 | 14 | 15 | 1 | 40 | 28 |
| Blood loss (mL) | ||||||||||
| <1500 | 68 | 25 | 5 | 1 | 66 | 18 | 15 | 0 | 61 | 38 |
| ≥1500 | 17 | 14 | 7 | 5 ** | 21 | 10 | 11 | 1 | 21 | 22 |
| Histological factors | ||||||||||
| Fibrotic grade | ||||||||||
| 0 | 7 | 8 | 0 | 0 | 6 | 5 | 4 | 0 | 8 | 7 |
| 1 | 31 | 12 | 6 | 1 | 33 | 11 | 5 | 1 | 31 | 19 |
| 2 | 18 | 5 | 3 | 2 | 17 | 2 | 9 | 0 | 16 | 12 |
| ≥3 | 25 | 12 | 3 | 3 | 28 | 8 | 7 | 0 | 22 | 21 |
| Necroinflammatory grade (n = 98) | ||||||||||
| 0 | 9 | 6 | 0 | 0 | 9 | 3 | 3 | 0 | 8 | 7 |
| 1 | 32 | 15 | 5 | 4 | 35 | 12 | 9 | 0 | 36 | 20 |
| ≥2 | 16 | 5 | 4 | 2 | 19 | 4 | 4 | 0 | 14 | 13 |
| Background liver status | ||||||||||
| normal | 7 | 8 | 0 | 0 | 6 | 5 | 4 | 0 | 8 | 7 |
| chronic hepatitis | 64 | 28 | 11 | 6 | 67 | 21 | 20 | 1 | 64 | 45 |
| cirrhosis | 14 | 3 | 1 | 0 | 14 | 2 | 2 | 0 | 10 | 8 |
| Postoperative outcomes | ||||||||||
| Hospital stay (days) | 30 (24) | 35 (29) | 39 (22) | 66 (56) † | 27 (14) | 27 (30) | 64 (37) ‡ | 74 | 23 (10) | 49 (36) ** |
| Complications | ||||||||||
| Organ SSI | ||||||||||
| no | 76 | 31 | 11 | 3 | 85 | 27 | 9 | 0 | - | - |
| yes | 9 | 8 | 1 | 3 * | 2 | 1 | 17 | 1 ** | ||
| Prolonged ascites | ||||||||||
| no | 79 | 35 | 7 | 3 | 78 | 25 | 21 | 0 | - | - |
| yes | 6 | 4 | 5 | 3 ** | 9 | 3 | 5 | 1 ** | ||
| Mortality | ||||||||||
| no | 85 | 39 | 12 | 2 | 86 | 28 | 23 | 1 | - | - |
| yes | 0 | 0 | 0 | 4 ** | 1 | 0 | 3 | 0 | ||
| Variables | Significance | RR | 95% Confidence Interval | |
|---|---|---|---|---|
| (p Value) | Lower Limit | Upper Limit | ||
| PHLF | ||||
| CRP ≥ 0.2 mg/dL | 0.379 | 1.43 | 0.644 | 3.171 |
| Tumor size ≥ 10 cm | 0.556 | 1.473 | 0.405 | 5.356 |
| Segment 1 resection, yes | 0.254 | 2.347 | 0.541 | 10.18 |
| Vascular involvement of tumor, yes | 0.475 | 0.629 | 0.177 | 2.242 |
| Compression of middle & left HV confluence, yes | 0.05 | 6.818 | 0.995 | 46.93 |
| Compression of RHV, yes | 0.928 | 1.073 | 0.236 | 4.886 |
| Compression of IVC, yes | 0.472 | 0.338 | 0.018 | 6.502 |
| iBL ≥ 1500 mL | 0.025 | 2.791 | 1.14 | 6.826 |
| PHBL | ||||
| Albumin < 4 g/dL | 0.009 | 2.994 | 1.31 | 6.849 |
| Tumor size ≥ 10 cm | 0.255 | 1.809 | 0.651 | 5.028 |
| Glissonean pedicle stenosis, yes | 0.128 | 6.976 | 0.572 | 85.05 |
| Segment 1 resection, yes | 0.092 | 2.662 | 0.852 | 8.319 |
| Inflow hepatic occlusion ≥ 120 min | 0.914 | 1.045 | 0.472 | 2.314 |
| iBL ≥ 1500 mL | 0.099 | 2.072 | 0.871 | 4.93 |
| Total severe morbidities # | ||||
| Step 1 | ||||
| Total bilirubin ≥ 0.8 mg/dL | 0.776 | 1.208 | 0.329 | 4.432 |
| Albumin < 4 g/dL | 0.291 | 0.498 | 0.136 | 1.816 |
| CRP ≥ 0.2 mg/dL | 0.159 | 2.795 | 0.669 | 11.674 |
| Segment 1 resection, yes | 0.057 | 10.2 | 0.933 | 111.43 |
| Tumor size ≥ 6 cm | 0.071 | 3.562 | 0.897 | 14.14 |
| Vascular involvement of tumor, yes | 0.799 | 1.345 | 0.138 | 13.15 |
| Glissonean pedicle stenosis, yes | 0.205 | 7.149 | 0.341 | 150.04 |
| Compression of middle & left HV confluence, yes | 0.02 | 33.59 | 1.76 | 642.92 |
| Compression of RHV root, yes | 0.255 | 3.602 | 0.397 | 32.7 |
| Compression of IVC, yes | 0.037 | 0.008 | 0 | 0.755 |
| Inflow hepatic occlusion ≥ 120 min | 0.972 | 1.023 | 0.292 | 3.589 |
| Operation time ≥ 480 min | 0.164 | 0.367 | 0.089 | 1.506 |
| iBL ≥ 750 mL | 0.132 | 2.968 | 0.72 | 12.23 |
| Red cell blood transfusion, yes | 0.847 | 0.853 | 0.169 | 4.299 |
| Step 6 * | ||||
| Segment 1 resection, yes | 0.037 | 5.67 | 1.11 | 29.056 |
| Tumor size ≥ 6 cm | 0.013 | 3.749 | 1.33 | 10.589 |
| Glissonean pedicle stenosis, yes | 0.053 | 6.505 | 0.976 | 43.331 |
| Compression of middle & left HV confluence, yes | 0.032 | 8.948 | 1.21 | 66.002 |
| Compression of IVC, yes | 0.049 | 19.61 | 1.01 | 333.33 |
| iBL ≥ 1500 mL | ||||
|---|---|---|---|---|
| Significance | RR | 95% Confidence Interval | ||
| Variables | (p Value) | Lower Limit | Upper Limit | |
| Step (1) | ||||
| Albumin <4 g/dL | 0.18 | 5.139 | 1.328 | 19.895 |
| PT < 90% | 0.559 | 0.691 | 0.2 | 2.392 |
| CRP ≥ 0.2 mg/dL | 0.028 | 4.901 | 1.188 | 20.213 |
| Estimated liver volume ≥ 500 cm3 * | 0.065 | 4.745 | 0.907 | 24.824 |
| Tumor size ≥ 6.2 cm | 0.043 | 6.25 | 1.058 | 37.037 |
| ≥10 cm | 0.04 | 10.47 | 1.114 | 98.36 |
| Compression of RHV root, yes | 0.942 | 1.137 | 0.036 | 36.258 |
| Diaphragm compression by HCC, yes | 0.054 | 0.105 | 0.011 | 1.042 |
| Operating time > 480 min, yes | 0.169 | 3.414 | 0.594 | 19.618 |
| Resection of segment 1, yes | 0.051 | 9.598 | 0.988 | 93.254 |
| Step (11) ** | ||||
| Albumin < 4 g/dL | 0.017 | 3.795 | 1.267 | 11.374 |
| CRP ≥ 0.2 mg/dL | 0.03 | 3.74 | 1.137 | 12.304 |
| Tumor size ≥ 6.2 cm | 0.025 | 6.25 | 1.309 | 29.412 |
| ≥10 cm | 0.002 | 7.178 | 2.358 | 37.346 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanashima, A.; Eguchi, S.; Hisaka, T.; Kawasaki, Y.; Yamashita, Y.-i.; Ide, T.; Kuroki, T.; Yoshizumi, T.; Kitahara, K.; Endo, Y.; et al. Risk Factors of Complications from Central Bisectionectomy (H458) for Hepatocellular Carcinoma: A Multi-Institutional Single-Arm Analysis. Cancers 2023, 15, 1740. https://doi.org/10.3390/cancers15061740
Nanashima A, Eguchi S, Hisaka T, Kawasaki Y, Yamashita Y-i, Ide T, Kuroki T, Yoshizumi T, Kitahara K, Endo Y, et al. Risk Factors of Complications from Central Bisectionectomy (H458) for Hepatocellular Carcinoma: A Multi-Institutional Single-Arm Analysis. Cancers. 2023; 15(6):1740. https://doi.org/10.3390/cancers15061740
Chicago/Turabian StyleNanashima, Atsushi, Susumu Eguchi, Toru Hisaka, Yota Kawasaki, Yo-ichi Yamashita, Takao Ide, Tamotsu Kuroki, Tomoharu Yoshizumi, Kenji Kitahara, Yuichi Endo, and et al. 2023. "Risk Factors of Complications from Central Bisectionectomy (H458) for Hepatocellular Carcinoma: A Multi-Institutional Single-Arm Analysis" Cancers 15, no. 6: 1740. https://doi.org/10.3390/cancers15061740
APA StyleNanashima, A., Eguchi, S., Hisaka, T., Kawasaki, Y., Yamashita, Y.-i., Ide, T., Kuroki, T., Yoshizumi, T., Kitahara, K., Endo, Y., Utsunomiya, T., Kajiwara, M., Sakoda, M., Okamoto, K., Nagano, H., Takami, Y., & Beppu, T. (2023). Risk Factors of Complications from Central Bisectionectomy (H458) for Hepatocellular Carcinoma: A Multi-Institutional Single-Arm Analysis. Cancers, 15(6), 1740. https://doi.org/10.3390/cancers15061740









