Quality Indicators Compliance and Survival Outcomes in Breast Cancer according to Age in a Certified Center
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Collection
2.4. Statistical Analysis
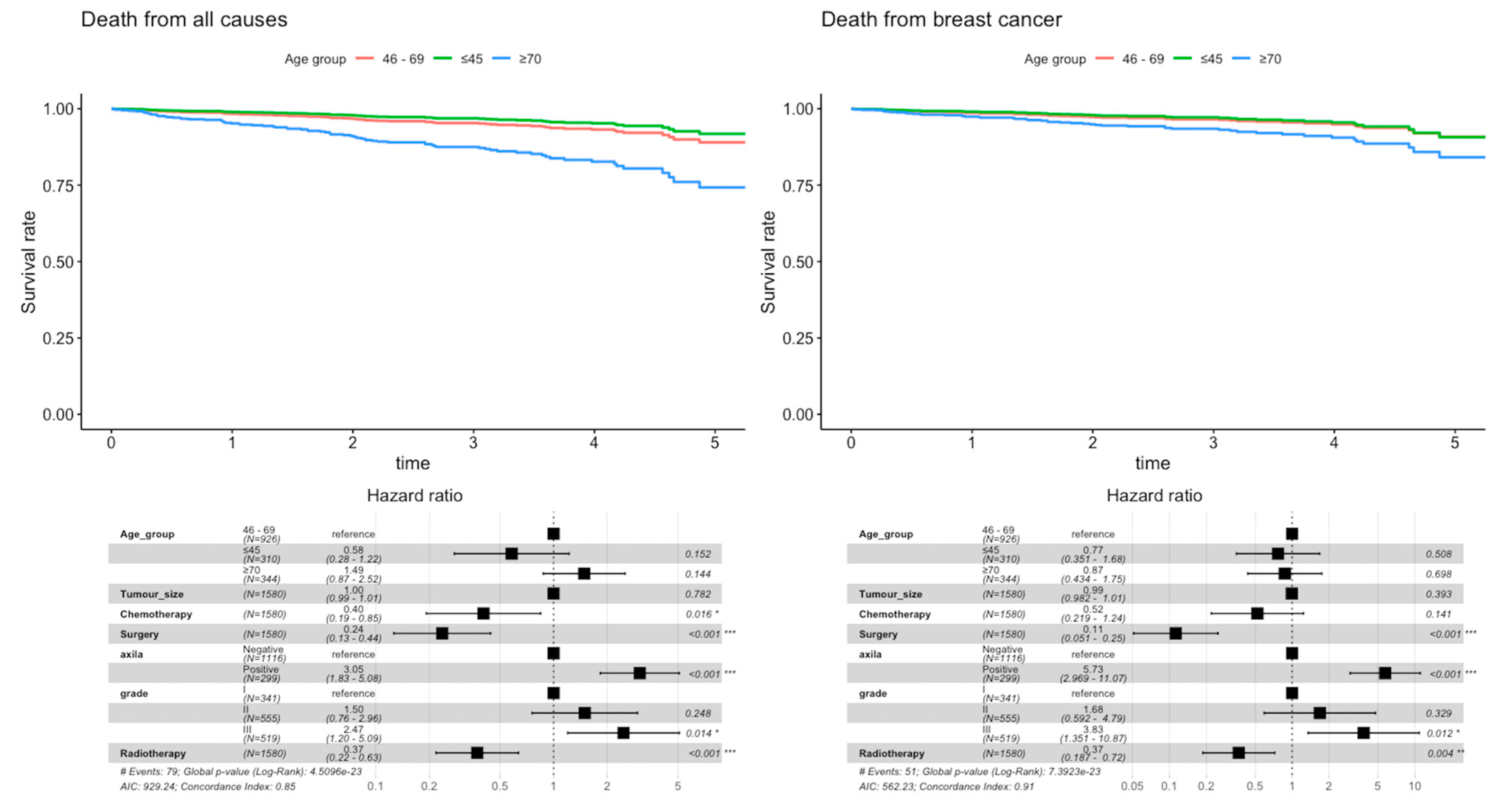
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blamey, R.W.; Hornmark-Stenstam, B.; Ball, G.; Blichert-Toft, M.; Cataliotti, L.; Fourquet, A.; Gee, J.; Holli, K.; Jakesz, R.; Kerin, M.; et al. ONCOPOOL—A European database for 16,944 cases of breast cancer. Eur. J. Cancer 2010, 46, 56–71. [Google Scholar] [CrossRef]
- De Angelis, R.; Sant, M.; Coleman, M.P.; Francisci, S.; Baili, P.; Pierannunzio, D.; Trama, A.; Visser, O.; Brenner, H.; Ardanaz, E.; et al. Cancer survival in Europe 1999–2007 by country and age: Results of EUROCARE-5—A population-based study. Lancet Oncol. 2014, 15, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Sant, M.; Lopez, M.D.; Agresti, R.; Pérez, M.J.S.; Holleczek, B.; Bielska-Lasota, M.; Dimitrova, N.; Innos, K.; Katalinic, A.; Langseth, H.; et al. Survival of women with cancers of breast and genital organs in Europe 1999–2007: Results of the EUROCARE-5 study. Eur. J. Cancer 2015, 51, 2191–2205. [Google Scholar] [CrossRef] [PubMed]
- Fietz, T.; Zahn, M.O.; Köhler, A.; Engel, E.; Frank, M.; Kruggel, L.; Jänicke, M.; Marschner, N.; TMK-Group. Routine treatment and outcome of breast cancer in younger versus elderly patients: Results from the SENORA project of the prospective German TMK cohort study. Breast Cancer Res. Treat. 2018, 167, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Plichta, J.K.; Thomas, S.M.; Vernon, R.; Fayanju, O.M.; Rosenberger, L.H.; Hyslop, T.; Hwang, E.S.; Greenup, R.A. Breast cancer tumor histopathology, stage at presentation, and treatment in the extremes of age. Breast Cancer Res. Treat. 2020, 180, 227–235. [Google Scholar] [CrossRef]
- Syed, B.M.; Green, A.R.; Rakha, E.A.; Morgan, D.A.; Ellis, I.O.; Cheung, K.L. Age-related biology of early-stage operable breast cancer and its impact on clinical outcome. Cancers 2021, 13, 1417. [Google Scholar] [CrossRef] [PubMed]
- Biganzoli, L.; Battisti, N.M.L.; Wildiers, H.; McCartney, A.; Colloca, G.; Kunkler, I.H.; Cardoso, M.J.; Cheung, K.L.; De Glas, N.A.; Trimboli, R.M.; et al. Updated recommendations regarding the management of older patients with breast cancer: A joint paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG). Lancet Oncol. 2021, 22, e327–e340. [Google Scholar] [CrossRef]
- Cornell, L.F.; Mclaughlin, S.A.; Pruthi, S.; Mussallem, D.M. Special considerations of breast cancer management in the elderly. Breast Cancer Manag. 2020, 9, BMT46. [Google Scholar] [CrossRef]
- Morgan, J.L.; George, J.; Holmes, G.; Martin, C.; Reed, M.W.R.; Ward, S.; Walters, S.J.; Cheung, K.L.; Audisio, R.A.; Wyld, L. Breast cancer surgery in older women: Outcomes of the Bridging Age Gap in breast cancer study. Br. J. Surg. 2020, 107, 1468–1479. [Google Scholar] [CrossRef]
- Wyld, L.; Reed, M.W.R.; Morgan, J.; Collins, K.; Ward, S.; Holmes, G.R.; Bradburn, M.; Walters, S.; Burton, M.; Herbert, E.; et al. Bridging the age gap in breast cancer. Impacts of omission of breast cancer surgery in older women with oestrogen receptor positive early breast cancer. A risk stratified analysis of survival outcomes and quality of life. Eur. J. Cancer 2021, 142, 48–62. [Google Scholar] [CrossRef]
- Hamelinck, V.C.; Bastiaannet, E.; Pieterse, A.H.; Merkus, J.W.; Jannink, I.; den Hoed, I.D.; van de Velde, C.J.; Liefers, G.J.; Stiggelbout, A.M. A prospective comparison of younger and older patients’ preferences for breast-conserving surgery versus mastectomy in early breast cancer. J. Geriatr. Oncol. 2018, 9, 170–173. [Google Scholar] [CrossRef]
- Esposito, E.; Sollazzo, V.; di Micco, R.; Cervotti, M.; Luglio, G.; Benassai, G.; Mozzillo, P.; Perrotta, S.; Desiato, V.; Amato, B.; et al. Can axillary node dissection be safely omitted in the elderly? A retrospective study on axillary management of early breast cancer in older women. Int. J. Surg. 2016, 33, S114–S118. [Google Scholar] [CrossRef]
- Dal Maso, L.; Panato, C.; Tavilla, A.; Guzzinati, S.; Serraino, D.; Mallone, S.; Botta, L.; Boussari, O.; Capocaccia, R.; Colonna, M.; et al. EUROCARE-5 Working Group. Cancer cure for 32 cancer types: Results from the EUROCARE-5 study. Int. J. Epidemiol. 2020, 49, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Del Turco, M.R.; Ponti, A.; Bick, U.; Biganzoli, L.; Cserni, G.; Cutuli, B.; Decker, T.; Dietel, M.; Gentilini, O.; Kuehn, T.; et al. Quality indicators in breast cancer care. EUSOMA position paper. Eur. J. Cancer 2010, 46, 2344–2356. [Google Scholar] [CrossRef]
- Biganzoli, L.; Marotti, L.; Hart, C.D.; Cataliotti, L.; Cutuli, B.; Kühn, T.; Mansel, R.E.; Ponti, A.; Poortmans, P.; Regitnig, P.; et al. Quality indicators in breast cancer care: An update from the EUSOMA working group. Eur. J. Cancer 2017, 86, 59–81. [Google Scholar] [CrossRef] [PubMed]
- Kiderlen, M.; Ponti, A.; Tomatis, M.; Boelens, P.G.; Bastiaannet, E.; Wilson, R.; eusomaDB Working Group. Variations in compliance to quality indicators by age for 41,871 breast cancer patients across Europe: A European Society of Breast Cancer Specialists database analysis. Eur. J. Cancer 2015, 51, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, P.A.; Tomatis, M.; Marotti, L.; Van Dam, P.A.; Tomatis, M.; Marotti, L.; Heil, J.; Mansel, R.E.; Del Turco, M.R.; eusomaDB Working Group. Time trends (2006-2015) of quality indicators in EUSOMA-certified breast centres. Eur. J. Cancer 2017, 85, 15–22. [Google Scholar] [CrossRef]
- Maes-Carballo, M.; Gómez-Fandiño, Y.; Reinoso-Hermida, A.; Estrada-López, C.R.; Martín-Díaz, M.; Khan, K.S.; Bueno-Cavanillas, A. Quality indicators for breast cancer care: A systematic review. Breast 2021, 59, 221–231. [Google Scholar] [CrossRef]
- When Cancer Grows Old. The Socioeconomic Burden of Cancer and Ageing. A Sanofi and KPMG Report. Feb 2022. Available online: https://kpmg.com/sg/en/home/insights/2022/02/when-cancer-grows-old-sanofi-report.html (accessed on 4 May 2022).
- Derks, M.G.M.; Bastiaannet, E.; Kiderlen, M.; Hilling, D.E.; Boelens, P.G.; Walsh, P.M.; EURECCA Breast Cancer Group. Variation in treatment and survival of older patients with non-metastatic breast cancer in five European countries: A population-based cohort study from the EURECCA Breast Cancer Group. Br. J. Cancer 2018, 119, 121–129. [Google Scholar] [CrossRef]
- Al-Rashdan, A.; Xu, Y.; Quan, M.L.; Cao, J.Q.; Cheung, W.; Bouchard-Fortier, A.; Barbera, L. Higher-risk breast cancer in women aged 80 and older: Exploring the effect of treatment on survival. Breast 2021, 59, 203–210. [Google Scholar] [CrossRef]
- Frebault, J.; Bergom, C.; Cortina, C.S.; Shukla, M.E.; Zhang, Y.; Huang, C.C.; Kong, A.L. Invasive breast cancer treatment patterns in women age 80 and over: A report from the National Cancer Database. Clin. Breast Cancer 2022, 22, 49–59. [Google Scholar] [CrossRef]
- Jobsen, J.J.; van der Palen, J.; Siemerink, E.; Struikmans, H. Limited impact of breast cancer and non-breast malignancies on survival in older patients with early-stage breast cancer: Results of a large, single-center, population-based study. Clin. Oncol. 2022, 34, 355–362. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 26 January 2023).
- DuMontier, C.; Loh, K.P.; Soto-Perez-de-Celis, E.; Dale, W. Decision making in older adults with cancer. J. Clin. Oncol. 2021, 39, 2164–2174. [Google Scholar] [CrossRef]
- Osório, F.; Barros, A.S.; Peleteiro, B.; Barradas, A.R.; Urbano, J.; Fougo, J.L.; Leite-Moreira, A. Frailty-independent undertreatment negative impact on survival in older patients with breast cancer. J. Breast Cancer 2021, 24, 542–553. [Google Scholar] [CrossRef]
- NCCN Guidelines: Older Adult Oncology 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf (accessed on 4 May 2022).
- ESMO Handbook of Cancer in the Senior Patient. (n.d.). Available online: https://oncologypro.esmo.org/education-library/esmo-handbooks/cancer-in-the-senior-patient (accessed on 4 May 2022).
- Ribnikar, D.; Ribeiro, J.M.; Pinto, D.; Sousa, B.; Pinto, A.C.; Gomes, E.; Cardoso, F. Breast cancer under age 40: A different approach. Curr. Treat. Options Oncol. 2015, 16, 16. [Google Scholar] [CrossRef]
- Paluch-Shimon, S.; Cardoso, F.; Partridge, A.H.; Abulkhair, O.; Azim Jr, H.A.; Bianchi-Micheli, G.; Pagani, O. ESO-ESMO 4th International consensus guidelines for breast cancer in young women (BCY4). Ann. Oncol. 2020, 31, 674–696. [Google Scholar] [CrossRef] [PubMed]
- Diab, S.G.; Elledge, R.M.; Clark, G.M. Tumor characteristics and clinical outcome of elderly women with breast cancer. J. Natl. Cancer Inst. 2000, 92, 550–556. [Google Scholar] [CrossRef]
- Bastiaannet, E.; Liefers, G.J.; de Craen, A.J.M.; Kuppen, P.J.K.; van de Water, W.; Portielje, J.E.A.; Westendorp, R.G.J. Breast cancer in elderly compared to younger patients in the Netherlands: Stage at diagnosis, treatment and survival in 127,805 unselected patients. Breast Cancer Res. Treat. 2010, 124, 801–807. [Google Scholar] [CrossRef] [PubMed]
- de Kruijf, E.M.; Bastiaannet, E.; Rubertá, F.; de Craen, A.J.; Kuppen, P.J.; Smit, V.T.; Liefers, G.J. Comparison of frequencies and prognostic effect of molecular subtypes between young and elderly breast cancer patients. Mol. Oncol. 2014, 8, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, E.O.; Deal, A.M.; Anders, C.K.; Prat, A.; Perou, C.M.; Carey, L.A.; Muss, H.B. Age-specific changes in intrinsic breast cancer subtypes: A focus on older women. Oncologist 2014, 19, 1076–1083. [Google Scholar] [CrossRef]
- Johansson, A.L.V.; Trewin, C.B.; Hjerkind, K.V.; Ellingjord-Dale, M.; Johannesen, T.B.; Ursin, G. Breast cancer-specific survival by clinical subtype after 7 years follow-up of young and elderly women in a nationwide cohort. Int. J. Cancer 2019, 144, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Lodi, M.; Scheer, L.; Reix, N.; Heitz, D.; Carin, A.J.; Thiébaut, N.; Mathelin, C. Breast cancer in elderly women and altered clinico-pathological characteristics: A systematic review. Breast Cancer Res. Treat. 2017, 166, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Pons-Tostivint, E.; Daubisse-Marliac, L.; Grosclaude, P.; Goddard, J.; Morel, C.; Dunet, C.; Group, T.E. Multidisciplinary team meeting and EUSOMA quality indicators in breast cancer care: A French regional multicenter study. Breast 2019, 46, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.I.; Salviat, F.; Laki, F.; Falcou, M.C.; Carton, M.; Poortmans, P.; Kirova, Y.M. Outcomes of postoperative radiation therapy for breast cancer in older women according to age and comorbidity status: An observational retrospective study in 752 patients. J. Geriatr. Oncol. 2018, 9, 600–605. [Google Scholar] [CrossRef]
- Hughes, K.S.; Schnaper, L.A.; Bellon, J.R.; Cirrincione, C.T.; Berry, D.A.; McCormick, B.; Wood, W.C. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: Long-term follow-up of CALGB 9343. J. Clin. Oncol. 2013, 31, 2382–2387. [Google Scholar] [CrossRef]
- Brain, E.; Caillet, P.; de Glas, N.; Biganzoli, L.; Cheng, K.; Dal Lago, L.; Wildiers, H. HER2-targeted treatment for older patients with breast cancer: An expert position paper from the International Society of Geriatric Oncology. J. Geriatr. Oncol. 2019, 10, 1003–1013. [Google Scholar] [CrossRef]

| Characteristic | N | ≤45 y, N = 310 1 | 46–69 y, N = 926 1 | ≥70 y, N = 344 1 | p-Value 2 |
|---|---|---|---|---|---|
| Sex | 1580 | 0.6 | |||
| Woman | 310 (100%) | 923 (99.7%) | 344 (100%) | ||
| Man | 0 (0%) | 3 (0.3%) | 0 (0%) | ||
| Body Mass Index (BMI) | 1580 | <0.001 | |||
| Not evaluated | 91 (29.4%) | 421 (45.5%) | 235 (68.3%) | ||
| <18.5–24.9 | 133 (42.9%) | 186 (20.0%) | 35 (10.2%) | ||
| 25.0–29.9 | 59 (19.0%) | 199 (21.5%) | 39 (11.3%) | ||
| ≥30.0 | 27 (8.7%) | 120 (13.0%) | 35 (10.2%) | ||
| Referral from screening programme | 1580 | 3 (1.0%) | 283 (30.6%) | 15 (4.4%) | <0.001 |
| Clinical examination/Suspicious of malignancy (yes) | 1580 | 258 (83.2%) | 614 (66.3%) | 274 (79.7%) | <0.001 |
| Side location of the lesion | 1580 | 0.5 | |||
| Left | 158 (51.0%) | 497 (53.7%) | 173 (50.3%) | ||
| Right | 152 (49.0%) | 429 (46.3%) | 171 (49.7%) | ||
| Disease extent by imaging or clinical examination | 1580 | <0.001 | |||
| Localized | 259 (83.5%) | 837 (90.4%) | 324 (94.2%) | ||
| Multicentric/Multifocal | 51 (16.5%) | 89 (9.6%) | 20 (5.8%) | ||
| Magnetic Resonance Imaging (RMI) (yes) | 1580 | 225 (72.6%) | 375 (40.5%) | 51 (14.8%) | <0.001 |
| Median Tumor size by imaging or physical examination [mm, (IQR)] | 1551 | 20 (15, 32) | 17 (11, 27) | 20 (12, 30) | <0.001 |
| Axillary staging (including only invasive tumors) | 1415 | 0.001 | |||
| Negative | 204 (71.1%) | 664 (81.0%) | 248 (79.2%) | ||
| Positive | 83 (28.9%) | 151 (19.0%) | 65 (20.8%) | ||
| TNM stage | 1580 | <0.001 | |||
| 0 | 22 (7.1%) | 107 (11.6%) | 28 (8.1%) | ||
| I | 125 (40.3%) | 483 (52.1%) | 154 (44.8%) | ||
| II | 143 (46.1%) | 288 (31.1%) | 136 (39.5%) | ||
| III | 17 (5.5%) | 15 (1.6%) | 14 (4.1%) | ||
| IV | 3 (1.0%) | 33 (3.6%) | 12 (3.5%) | ||
| Invasive histological type at biopsy (including invasive and microinvasive tumors) | 1423 | 0.2 | |||
| Ductal/No Special Type (NST) | 240 (83.3%) | 658 (80.3%) | 239 (75.6%) | ||
| Lobular | 23 (8.0%) | 82 (10.0%) | 35 (11.1%) | ||
| Other | 25 (8.7%) | 79 (9.7%) | 42 (13.3%) | ||
| Final pathology | 1580 | <0.001 | |||
| In situ | 22 (7.1%) | 107 (11.5%) | 28 (8.1%) | ||
| Invasive (including microinvasive) | 236 (76.1%) | 762 (82.3%) | 314 (91.3%) | ||
| Invasive at biopsy only with pathological complete response | 52 (16.8%) | 57 (6.2%) | 2 (0.6%) | ||
| Modified Bloom–Richardson Grade (including only invasive tumors) | 1415 | <0.001 | |||
| G1 | 41 (14.3%) | 209 (25.6%) | 91 (29.0%) | ||
| G2 | 93 (32.4%) | 320 (39.3%) | 142 (45.4%) | ||
| G3 | 153 (53.3%) | 286 (35.1%) | 80 (25.6%) | ||
| Lymphovascular invasion (including operated invasive tumors) | 1283 | <0.001 | |||
| No | 154 (65.8%) | 610 (81.0%) | 250 (84.5%) | ||
| Yes | 80 (34.2%) | 143 (19.0%) | 46 (15.5%) | ||
| Oestrogen receptor status (including only invasive tumors) | 1415 | 0.044 | |||
| Not performed | 1 (0.3%) | 0 (0%) | 0 (0%) | ||
| Negative | 63 (22.0%) | 135 (16.6%) | 48 (15.3%) | ||
| Positive | 223 (77.7%) | 680 (83.4%) | 265 (84.7%) | ||
| Progesterone receptor status (including only invasive tumors) | 1415 | 0.4 | |||
| Not performed | 0 (0%) | 6 (0.7%) | 4 (1.3%) | ||
| Negative | 89 (31.0%) | 258 (31.7%) | 92 (29.4%) | ||
| Positive | 198 (69.0%) | 551 (67.6%) | 217 (69.3%) | ||
| HER2 overexpression (including only invasive tumors) | 1415 | <0.001 | |||
| Not Performed | 0 (0%) | 0 (0%) | 18 (5.8%) | ||
| Negative | 226 (78.7%) | 685 (84.0%) | 263 (84.0%) | ||
| Positive | 61 (21.3%) | 130 (16.0%) | 32 (10.2%) | ||
| Proliferation activity index (Ki67) in invasive G2 tumors | 555 | <0.001 | |||
| Not performed | 57 (61.3%) | 203 (63.4%) | 122 (85.9%) | ||
| <5% | 4 (4.3%) | 21 (6.6%) | 4 (2.8%) | ||
| 5–30% | 23 (24.7%) | 82 (25.6%) | 13 (9.2%) | ||
| >30% | 9 (9.7%) | 14 (4.4%) | 3 (2.1%) | ||
| Molecular subtyping (including only invasive tumours) | 1415 | <0.001 | |||
| Luminal A-like | 182 (63.4%) | 585 (71.8%) | 224 (71.5%) | ||
| Luminal B-like | 43 (15.0%) | 94 (11.5%) | 26 (8.3%) | ||
| HER2 missing | 0 (0%) | 0 (0%) | 18 (5.8%) | ||
| HER2 positive | 20 (7.0%) | 43 (5.3%) | 11 (3.5%) | ||
| Triple Negative | 42 (14.6%) | 93 (11.4%) | 34 (10.9%) | ||
| BRCA1 + BRCA2 | 1580 | <0.001 | |||
| No genetic assessment | 123 (39.7%) | 784 (84.7%) | 334 (97.1%) | ||
| Negative | 173 (55.8%) | 129 (13.9%) | 8 (2.3%) | ||
| Positive | 14 (4.5%) | 13 (1.4%) | 2 (0.6%) | ||
| First treatment | 1580 | <0.001 | |||
| Surgery | 190 (61.3%) | 717 (77.4%) | 210 (61.0%) | ||
| Neoadjuvant chemotherapy | 116 (37.4%) | 169 (18.3%) | 16 (4.7%) | ||
| Primary endocrine therapy | 4 (1.3%) | 37 (4.0%) | 111 (32.3%) | ||
| Support treatment/Surveillance/ Patient refusal | 0 (0%) | 3 (0.3%) | 7 (2.0%) | ||
| Surgery | 1580 | <0.001 | |||
| Breast conservative surgery | 170 (54.9%) | 655 (70.8%) | 194 (56.4%) | ||
| Mastectomy | 138 (44.5%) | 243 (26.2%) | 90 (26.2%) | ||
| No surgery | 2 (0.6%) | 28 (3.0%) | 60 (17.4%) | ||
| Endocrine therapy (yes) | 1580 | 237 (76.5%) | 721 (77.9%) | 271 (78.8%) | 0.8 |
| Chemotherapy (yes) | 1415 | 96 (33.4 %) | 260 (31.9%) | 40 (12.8%) | <0.001 |
| Radiotherapy (yes) | 1580 | 211 (68.1%) | 687 (74.2%) | 164 (47.7%) | <0.001 |
| ≤45 Years | 46–69 Years | ≥70 Years | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Evidence | Mand/Recom | Min. Req. (%) | Target (%) | Cases/ Total | Result (%) | 95% I.C. | Cases/Total | Result (%) | 95% I.C. | Cases/Total | Result (%) | 95% I.C. | ||
| 1 | Cancers who underwent pre-operative physical examination, mammography/ultrasound of both breasts and axillae | III | Mandatory | >90 | >95 | 289/310 | 93.2 | (89.7; 95.7) | 895/926 | 96.7 | (95.2; 97.7) | 328/344 | 95.3 | (92.4; 97.2) |
| 3a | Invasive cancers who underwent axillary staging by ultrasound +/− FNA/CNB | III | Recommended | 85 | 95 | 287/287 | 100 | (98.4; 100) | 815/815 | 100 | (99.4; 100) | 313/313 | 100 | (98.5; 100) |
| 3b | Cancers (invasive or in situ) with a pre-operative confirmed diagnosis (B5 or C5) | III | Mandatory | 85 | 90 | 310/310 | 100 | (98.4; 100) | 926/926 | 100 | (99.5; 100) | 344/344 | 100 | (98.6; 100) |
| 4a | Invasive cancers with histological type, grading, ER/HER2, pN, margins, vascular invasion & size recorded | II | Mandatory | >95 | >98 | 243/288 | 84.4 | (79.5; 88.3) | 748/819 | 91.3 | (89.1; 93.1) | 245/316 | 77.5 | (72.4; 81.9) |
| 4b | Non-invasive cancers with histological pattern, grading, size, margins & ER recorded | II | Mandatory | >95 | >98 | 17/22 | 77.3 | (54.2; 91.3) | 65/107 | 60.7 | (50.8; 69.9) | 12/28 | 42.9 | (25.0; 62.6) |
| 5 | Waiting time ≤ 6 weeks between the date of first diagnostic examination (mammogram/ultrasound) and surgery/other treatment | IV | Recommended | 80 | 90 | 194/310 | 62.6 | (56.9; 67.9) | 476/926 | 51.4 | (48.1; 54.7) | 202/344 | 58.7 | (51.3; 62.0) |
| 6a | Cancers examined preoperatively by MRI (excluding PST) | IV | Recommended | 10 | NA | 102/164 | 62.2 | (54.3; 69.5) | 193/638 | 30.3 | (26.7; 34.0) | 38/294 | 12.9 | (9.4; 17.4) |
| 6b | Cancers treated with PST undergoing MRI | IV | Recommended | 60 | 90 | 107/123 | 87.0 | (79.4; 92.2) | 155/177 | 87.6 | (81.6; 91.9) | 10/19 | 52.6 | (29.5; 74.8) |
| 7 | Cancers refered for genetic counselling | IV | Recommended | 10 | NA | 187/310 | 60.3 | (54.6; 65.8) | 142/926 | 15.3 | (13.1; 17.9) | 10/344 | 2.9 | (1.5; 5.5) |
| 8 | Cancers discussed pre and postoperatively by a MDT | III | Mandatory | 90 | 99 | 310/310 | 100 | (98.5; 100) | 926/926 | 100 | (99.5; 100) | 344/344 | 100 | (98.6; 100) |
| 9a | Invasive cancers receiving just 1 operation (excl. reconstruction) | II | Mandatory | 80 | 90 | 276/288 | 95.8 | (92.6; 97.7) | 784/819 | 95.7 | (94.0; 97.0) | 307/316 | 97.2 | (94.5; 98.6) |
| 9b | DCIS receiving just 1 operation (excl. reconstruction) | II | Mandatory | 70 | 90 | 20/22 | 90.9 | (69.4; 98.4) | 87/99 | 87.9 | (79.4; 93.3) | 25/28 | 89.3 | (70.6; 97.2) |
| 9c | Immediate reconstruction after mastectomy | III | Recommended | 40 | 40 | 54/138 | 39.1 | (31.0; 47.8) | 79/243 | 32.5 | (26.7; 38.8) | 4/90 | 4.4 | (1.4; 11.6) |
| 10a | M0 invasive cancers receiving postoperative RT after BCT | I | Mandatory | 90 | 95 | 145/154 | 94.2 | (88.9; 97.1) | 542/565 | 95.9 | (93.9; 97.3) | 129/160 | 80.6 | (73.5; 86.4) |
| 10b | Cancers ≥ pN2a+ receiving post-mastectomy RT | I | Mandatory | 90 | 95 | 6/7 | 85.7 | (42.0; 99.2) | 11/18 | 61.1 | (36.1; 81.7) | 1/4 | 25.0 | (1.3; 78.1) |
| 10c | Cancers pN1 receiving post-mastectomy RT | I | Mandatory | 70 | 85 | 18/35 | 51.4 | (34.3; 68.3) | 37/60 | 61.7 | (48.2; 73.6) | 6/19 | 31.6 | (13.6; 56.5) |
| 11a | Invasive cancers cN0 who underwent SLNB only (excluding PST) | I | Mandatory | 90 | 95 | 146/147 | 99.3 | (95.7; 100) | 560/563 | 99.5 | (98.3; 99.9) | 73/82 | 89.0 | (79.7; 94.5) |
| 11b | No more than 5 nodes excised in invasive cancers who underwent SLNB | I | Recommended | 90 | 95 | 184/188 | 97.9 | (94.3; 99.3) | 573/590 | 97.1 | (95.3; 98.3) | 170/175 | 97.1 | (93.1; 98.9) |
| 11c | Invasive cancers ≤ 3cm (incl. DCIS component) who underwent BCT (BRCA patients excluded) | I | Mandatory | 70 | 85 | 80/105 | 76.2 | (66.7; 83.7) | 412/473 | 87.1 | (83.7; 89.9) | 119/146 | 81.5 | (74.1; 87.3) |
| 11d | Non-invasive cancers ≤ 2cm treated with BCT | II | Mandatory | 80 | 90 | 3/6 | 50.0 | (18.8; 81.2) | 46/48 | 95.8 | (84.6; 99.3) | 14/17 | 82.4 | (55.8; 95.3) |
| 11e | DCIS who do not undergo axillary clearance | II | Mandatory | 97 | 99 | 20/20 | 100 | (80.0; 100) | 102/103 | 99.0 | (93.9; 99.9) | 23/24 | 95.8 | (76.9; 99.8) |
| 12 | Endocrine sensitive invasive cancers receiving HT | I | Mandatory | 85 | 90 | 217/223 | 97.3 | (94.0; 98.9) | 662/680 | 97.4 | (95.8; 98.4) | 259/263 | 98.5 | (95.9; 99.5) |
| 13a | Invasive cancers ER negative (T > 1cm or N+) receiving adjuvant CT | I | Mandatory | 85 | 95 | 59/60 | 98.3 | (89.9; 99.9) | 117/121 | 96.7 | (91.2;98.9) | 27/44 | 61.4 | (45.5; 75.3) |
| 13b | Invasive cancers HER2 positive (T > 1cm or N+) treated with adjuvant CT who received adjuvant trastuzumab | I | Mandatory | 85 | 95 | 9/9 | 100 | (62.9; 100) | 45/47 | 95.7 | (84.3; 99.3) | 10/10 | 100 | (65.5; 100) |
| 13c | Invasive cancers HER2 positive treated with neo-adjuvant CT who received neo-adjuvant trastuzumab | I | Mandatory | 90 | 95 | 29/29 | 100 | (85.4; 100) | 44/44 | 100 | (90.0; 100) | 2/2 | 100 | (19.8; 100) |
| 13d | Inflammatory breast cancer who received neo-adjuvant CT | II | Mandatory | 90 | 95 | 3/3 | 100 | (31.0; 100) | 6/7 | 85.7 | (42.0; 99.2) | 1/1 | 100 | (5.5; 100) |
 Diagnostic Qis;
Diagnostic Qis;  Loco-regional treatment Qis;
Loco-regional treatment Qis;  Systemic treatment Qis;
Systemic treatment Qis;  Mandatory requirements Qis;
Mandatory requirements Qis;  Recommended requirements Qis;
Recommended requirements Qis;  Minimum standard Qis;
Minimum standard Qis;  Desirable target Qis;
Desirable target Qis;  QIs noncompliance.
QIs noncompliance.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osório, F.; Barros, A.S.; Peleteiro, B.; Amendoeira, I.; Fougo, J.L. Quality Indicators Compliance and Survival Outcomes in Breast Cancer according to Age in a Certified Center. Cancers 2023, 15, 1446. https://doi.org/10.3390/cancers15051446
Osório F, Barros AS, Peleteiro B, Amendoeira I, Fougo JL. Quality Indicators Compliance and Survival Outcomes in Breast Cancer according to Age in a Certified Center. Cancers. 2023; 15(5):1446. https://doi.org/10.3390/cancers15051446
Chicago/Turabian StyleOsório, Fernando, António S. Barros, Bárbara Peleteiro, Isabel Amendoeira, and José Luís Fougo. 2023. "Quality Indicators Compliance and Survival Outcomes in Breast Cancer according to Age in a Certified Center" Cancers 15, no. 5: 1446. https://doi.org/10.3390/cancers15051446
APA StyleOsório, F., Barros, A. S., Peleteiro, B., Amendoeira, I., & Fougo, J. L. (2023). Quality Indicators Compliance and Survival Outcomes in Breast Cancer according to Age in a Certified Center. Cancers, 15(5), 1446. https://doi.org/10.3390/cancers15051446






