New Horizons in Metastatic Colorectal Cancer: Prognostic Role of CD44 Expression
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Histological, Immunohistochemical, and Molecular Analysis
2.2. Statistical Analysis
3. Results
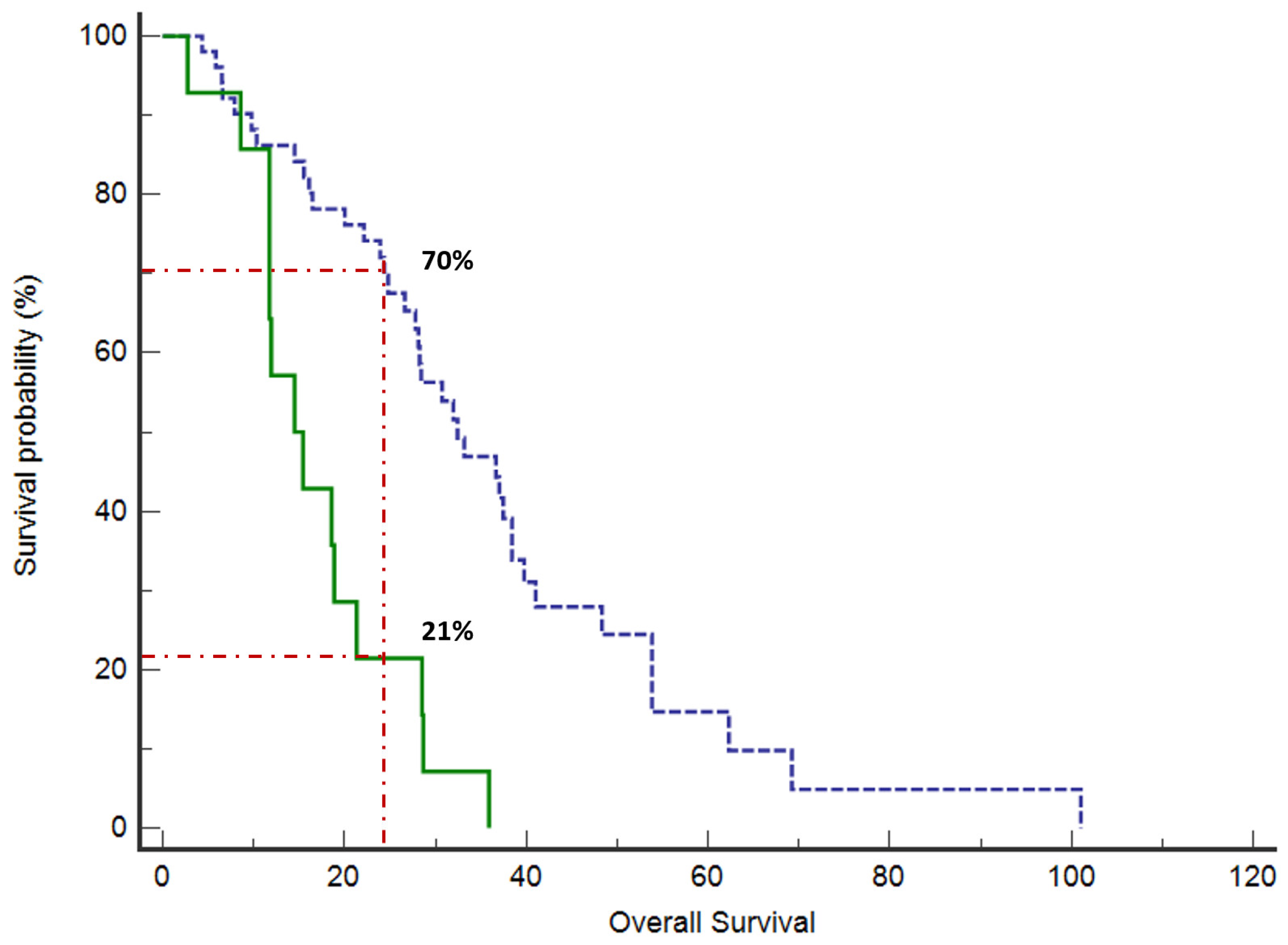
Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. The global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Ziranu, P.; Lai, E.; Schirripa, M.; Puzzoni, M.; Persano, M.; Pretta, A.; Munari, G.; Liscia, N.; Pusceddu, V.; Loupakis, F.; et al. The Role of p53 Expression in Patients with RAS/BRAF Wild-Type Metastatic Colorectal Cancer Receiving Irinotecan and Cetuximab as Later Line Treatment. Target. Oncol. 2021, 16, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.; Liscia, N.; Donisi, C.; Mariani, S.; Tolu, S.; Pretta, A.; Persano, M.; Pinna, G.; Balconi, F.; Pireddu, A.; et al. Molecular-Biology-Driven Treatment for Metastatic Colorectal Cancer. Cancers 2020, 12, 1214. [Google Scholar] [CrossRef] [PubMed]
- Puzzoni, M.; Ziranu, P.; Demurtas, L.; Lai, E.; Mariani, S.; Liscia, N.; Soro, P.; Pretta, A.; Impera, V.; Camera, S.; et al. Why precision medicine should be applied across the continuum of care for metastatic colorectal cancer patients. Future Oncol. 2020, 16, 4337–4339, Erratum in Future Oncol. 2020, 16, 219. [Google Scholar] [CrossRef]
- Giampieri, R.; Lupi, A.; Ziranu, P.; Bittoni, A.; Pretta, A.; Pecci, F.; Persano, M.; Giglio, E.; Copparoni, C.; Crocetti, S.; et al. Retrospective Comparative Analysis of KRAS G12C vs. Other KRAS Mutations in mCRC Patients Treated With First-Line Chemotherapy Doublet + Bevacizumab. Front. Oncol. 2021, 11, 736104. [Google Scholar] [CrossRef]
- Tamas, K.; Walenkamp, A.M.; de Vries, E.G.; van Vugt, M.A.; Beets-Tan, R.G.; van Etten, B.; de Groot, D.J.; Hospers, G.A. Rectal and colon cancer: Not just a different anatomic site. Cancer Treat. Rev. 2015, 41, 671–679. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Pollett, A.; Gallinger, S.; Dick, J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007, 445, 106–110. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R. Identification and expansion of human colon-cancer-initiating cells. Nature 2007, 445, 111–115. [Google Scholar] [CrossRef]
- Zhu, L.; Gibson, P.; Currle, D.S.; Tong, Y.; Richardson, R.J.; Bayazitov, I.T.; Poppleton, H.; Zakharenko, S.; Ellison, D.W.; Gilbertson, R.J. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 2009, 457, 603–607. [Google Scholar] [CrossRef]
- Todaro, M.; Gaggianesi, M.; Catalano, V.; Benfante, A.; Iovino, F.; Biffoni, M.; Apuzzo, T.; Sperduti, I.; Volpe, S.; Cocorullo, G.; et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell 2014, 14, 342–356. [Google Scholar] [CrossRef] [PubMed]
- de Sousa e Melo, F.; Kurtova, A.V.; Harnoss, J.M.; Kljavin, N.; Hoeck, J.D.; Hung, J.; Anderson, J.E.; Storm, E.E.; Modrusan, Z.; Koeppen, H.; et al. A distinct role for Lgr5(+) stem cells in primary and metastatic colon cancer. Nature 2017, 543, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Lee, H.W.; Hur, K.Y.; Kim, J.J.; Park, G.S.; Jang, S.H.; Song, Y.S.; Jang, K.S.; Paik, S.S. Cancer stem cell markers CD133 and CD24 correlate with invasiveness and differentiation in colorectal adenocarcinoma. World J. Gastroenterol. 2009, 15, 2258–2264. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Song, X.; Chen, Z.; Li, X.; Li, M.; Liu, H.; Li, J. CD133 expression and the prognosis of colorectal cancer: A systematic review and meta-analysis. PLoS ONE 2013, 8, e56380. [Google Scholar] [CrossRef] [PubMed]
- Jao, S.W.; Chen, S.F.; Lin, Y.S.; Chang, Y.C.; Lee, T.Y.; Wu, C.C.; Jin, J.S.; Nieh, S. Cytoplasmic CD133 expression is a reliable prognostic indicator of tumor regression after neoadjuvant concurrent chemoradiotherapy in patients with rectal cancer. Ann. Surg. Oncol. 2012, 19, 3432–3440. [Google Scholar] [CrossRef]
- Jing, F.; Kim, H.J.; Kim, C.H.; Kim, Y.J.; Lee, J.H.; Kim, H.R. Colon cancer stem cell markers CD44 and CD133 in patients with colorectal cancer and synchronous hepatic metastases. Int. J. Oncol. 2015, 46, 1582–1588. [Google Scholar] [CrossRef]
- Lin, E.H.; Hassan, M.; Li, Y.; Zhao, H.; Nooka, A.; Sorenson, E.; Xie, K.; Champlin, R.; Wu, X.; Li, D. Elevated circulating endothelial progenitor marker CD133 messenger RNA levels predict colon cancer recurrence. Cancer 2007, 110, 534–542. [Google Scholar] [CrossRef]
- Iinuma, H.; Watanabe, T.; Mimori, K.; Adachi, M.; Hayashi, N.; Tamura, J.; Matsuda, K.; Fukushima, R.; Okinaga, K.; Sasako, M.; et al. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes’ stage B and C colorectal cancer. J. Clin. Oncol. 2011, 29, 1547–1555. [Google Scholar] [CrossRef]
- Sprenger, T.; Conradi, L.C.; Beissbarth, T.; Ermert, H.; Homayounfar, K.; Middel, P.; Ruschoff, J.; Wolff, H.A.; Schuler, P.; Ghadimi, B.M.; et al. Enrichment of CD133-expressing cells in rectal cancers treated with preoperative radiochemotherapy is an independent marker for metastasis and survival. Cancer 2013, 119, 26–35. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Z.G.; Du, C.Z.; Wang, H.W.; Yan, L.; Gu, J. Cancer stem cell marker CD133+ tumour cells and clinical outcome in rectal cancer. Histopathology 2009, 55, 284–293. [Google Scholar] [CrossRef]
- Weichert, W.; Denkert, C.; Burkhardt, M.; Gansukh, T.; Bellach, J.; Altevogt, P.; Dietel, M.; Kristiansen, G. Cytoplasmic CD24 expression in colo-rectal cancer independently correlates with shortened patient survival. Clin. Cancer Res. 2005, 11, 6574–6581. [Google Scholar] [CrossRef] [PubMed]
- Aimola, V.; Fanni, D.; Gerosa, C.; Cerrone, G.; Ziranu, P.; Pretta, A.; Murru, R.; Piras, M.; Cau, F.; Zorcolo, L.; et al. Balance between the stem cell marker CD44 and CDX2 expression in colorectal cancer. Ann. Res. Oncol. 2022, 2, 160–166. [Google Scholar] [CrossRef]
- Du, L.; Wang, H.; He, L.; Zhang, J.; Ni, B.; Wang, X.; Jin, H.; Cahuzac, N.; Mehrpour, M.; Lu, Y.; et al. CD44 is of functional importance for colorectal cancer stem cells. Clin. Cancer Res. 2008, 14, 6751–6760. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.; Todaro, M.; de Sousa Mello, F.; Sprick, M.R.; Kemper, K.; Perez Alea, M.; Richel, D.J.; Stassi, G.; Medema, J.P. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc. Natl. Acad. Sci. USA 2008, 105, 13427–13432. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Franklin, D.M.; Leddy, H.A.; Robey, P.G.; Storms, R.W.; Gimble, J.M. Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell. Physiol. 2001, 189, 54–63. [Google Scholar] [CrossRef]
- Domev, H.; Amit, M.; Laevsky, I.; Dar, A.; Itskovitz-Eldor, J. Efficient engineering of vascularized ectopic bone from human embryonic stem cell-derived mesenchymal stem cells. Tissue Eng. Part A 2012, 18, 2290–2302. [Google Scholar] [CrossRef]
- Yin, T.; Wang, G.; He, S.; Liu, Q.; Sun, J.; Wang, Y. Human cancer cells with stem cell-like phenotype exhibit enhanced sensitivity to the cytotoxicity of IL-2 and IL-15 activated natural killer cells. Cell. Immunol. 2016, 300, 41–45. [Google Scholar] [CrossRef]
- Screaton, G.R.; Bell, M.V.; Bell, J.I.; Jackson, D.G. The identification of a new alternative exon with highly restricted tissue expression in transcripts encoding the mouse Pgp-1 (CD44) homing receptor. Comparison of all 10 variable exons between mouse, human, and rat. J. Biol. Chem. 1993, 268, 12235–12238. [Google Scholar] [CrossRef]
- Prochazka, L.; Tesarik, R.; Turanek, J. Regulation of alternative splicing of CD44 in cancer. Cell. Signal. 2014, 26, 2234–2239. [Google Scholar] [CrossRef]
- Banerjee, S.; Modi, S.; McGinn, O.; Zhao, X.; Dudeja, V.; Ramakrishnan, S.; Saluja, A.K. Impaired synthesis of stromal components in response to Minnelide improves vascular function, drug delivery, and survival in pancreatic cancer. Clin. Cancer Res. 2016, 22, 415–425. [Google Scholar] [CrossRef]
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: From adhesion molecules to siignaling regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Zoller, M. CD44: Can a cancer-initiating cell profit from an abundantly expressed molecule? Nat. Rev. Cancer 2011, 11, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Jung, K.-H.; Lee, J.H.; Moon, S.H.; Cho, Y.S.; Lee, K.-H. 89Zr anti-CD44 immuno-PET monitors CD44 expression on splenic myeloid cells and HT29 colon cancer cells. Sci. Rep. 2021, 11, 3876–3886. [Google Scholar] [CrossRef]
- Xu, H.; Niu, M.; Yuan, X.; Wu, K.; Liu, A. CD44 a tumor biomarker and therapeutic target. Exp. Hematol. Oncol. 2020, 9, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Yamano, S.; Gi, M.; Tago, Y.; Doi, K.; Okada, S.; Hirayama, Y.; Tachibana, H.; Ishii, N.; Fujioka, M.; Tatsumi, K.; et al. Role of deltaNp63(pos)CD44v(pos) cells in the development of N-nitroso-tris-chloroethylurea-induced peripheral-type mouse lung squamous cell carcinomas. Cancer Sci. 2016, 107, 123–132. [Google Scholar] [CrossRef]
- Orian-Rousseau, V.; Chen, L.; Sleeman, J.P.; Herrlich, P.; Ponta, H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 2002, 16, 3074–3086. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Ichikawa, Y.; Zheng, Y.W.; Oshima, T.; Miyata, H.; Nakazawa, K.; Guan, H.B.; Shiozawa, M.; Akaike, M.; Watanabe, K.; et al. Prognostic significance of CD44 variant 2 upregulation in colorectal cancer. Br. J. Cancer 2014, 111, 365–374. [Google Scholar] [CrossRef]
- Li, Z.; Chen, K.; Jiang, P.; Zhang, X.; Li, X.; Li, Z. CD44v/CD44s expression patterns are associated with the survival of pancreatic carcinoma patients. Diagn. Pathol. 2014, 9, 79. [Google Scholar] [CrossRef]
- Brown, R.L.; Reinke, L.M.; Damerow, M.S.; Perez, D.; Chodosh, L.A.; Yang, J.; Cheng, C. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J. Clin. Investig. 2011, 121, 1064–1074. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, C.; Chang, K.; Karnad, A.; Jagirdar, J.; Kumar, A.P.; Freeman, J.W. CD44 Expression Level and Isoform Contributes to Pancreatic Cancer Cell Plasticity, Invasiveness, and Response to Therapy. Clin. Cancer Res. 2016, 22, 5592–5604. [Google Scholar] [CrossRef]
- Wang, C.; Xie, J.; Guo, J.; Manning, H.C.; Gore, J.C.; Guo, N. Evaluation of CD44 and CD133 as cancer stem cell markers for colorectal cancer. Oncol. Rep. 2012, 28, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Madjd, Z.; Mehrjerdi, A.Z.; Sharifi, A.M.; Molanaei, S.; Shahzadi, S.Z.; Asadi-Lari, M. CD44+ cancer cells express higher levels of the anti-apoptotic protein Bcl-2 in breast tumors. Cancer Immun. 2009, 9, 4–10. [Google Scholar] [PubMed]
- El-Emshaty, H.; Hassan, D.; El-Hemaly, M.; Ismail, H. Clinical Association of CD44 Expression with Proliferative Activity and Apoptotic State in Egyptian Patients Suffering from Ulcerative Colitis and Colorectal Carcinoma. Asian Pac. J. Cancer Prev. 2021, 22, 3577–3583. [Google Scholar] [CrossRef]
- Lakshman, M.; Subramaniam, V.; Rubenthiran, U.; Jothy, S. CD44 promotes resistance to apoptosis in human colon cancer cells. Exp. Mol. Pathol. 2004, 77, 18–25. [Google Scholar] [CrossRef]
- Naor, D.; Wallach-Dayan, S.B.; Zahalka, M.A.; Sionov, R.V. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin. Cancer Biol. 2008, 18, 260–267. [Google Scholar] [CrossRef]
- Prasetyanti, P.R.; Medema, J.P. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol. Cancer 2017, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Vahidian, F.; Duijf, P.H.G.; Safarzadeh, E.; Derakhshani, A.; Baghbanzadeh, A.; Baradaran, B. Interactions between cancer stem cells, immune system and some environmental components: Friends or foes? Immunol. Lett. 2019, 208, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.C.; Vizoso, F.J.; Corte, M.D.; Gava, R.R.; Corte, M.G.; Suárez, J.P.; García-Muñíz, J.L.; García-Morán, M. CD44s expression in resectable colorectal cancers and surrounding mucosa. Cancer Investig. 2004, 22, 878–885. [Google Scholar] [CrossRef]
- Wielenga, V.J.; van der Neut, R.; Offerhaus, G.J. Pals ST: CD44 glycoproteins in colorectal cancer expression function and prognostic value. Adv. Cancer Res. 2000, 77, 169–187. [Google Scholar]
- Bendardaf, R.; Elzagheid, A.; Lamlum, H.; Ristamäki, R.; Collan, Y.; Pyrhönen, S. E-cadherin CD44s and CD44v6 correlate with tumour differentiation in colorectal cancer. Oncol. Rep. 2005, 13, 831–835. [Google Scholar] [CrossRef]
- Liu, Y.J.; Yan, P.S.; Li, J.; Jia, J.F. Expression and significance of CD44s CD44v6 and nm23 mRNA in human cancer. World J. Gastroenterol. 2005, 11, 6601–6606. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.F.; Bronson, R.T.; Ilagan, J.; Cantor, H.; Schmits, R.; Mak, T.W. Absence of the CD44 gene prevents sarcoma metastasis. Cancer Res. 2002, 62, 2281–2286. [Google Scholar]
- Clara, J.A.; Monge, C.; Yang, Y.; Takebe, N. Targeting signalling pathways and the immune microenvironment of cancer stem cells—A clinical update. Nat. Rev. Clin. Oncol. 2020, 17, 204–232. [Google Scholar] [CrossRef] [PubMed]
- Ropponen, K.M.; Eskelinen, M.J.; Lipponen, P.K.; Alhava, E.; Kosma, V.M. Expression of CD44 and variant proteins in human colorectal cancer and its relevance for prognosis. Scand J. Gastroenterol. 1998, 33, 301–309. [Google Scholar] [PubMed]
- Ylagan, L.R.; Scholes, J.; Demopoulos, R. CD44 a marker of squamous differentiation in adenosquamous neoplasms. Arch. Pathol. Lab. Med. 2000, 124, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Dallas, M.R.; Liu, G.; Chen, W.; Thomas, S.N.; Wirtz, D.; Huso, D.L.; Konstantopoulos, K. Divergent roles of CD44 and carcinoembryonic antigen in colon cancer metastasis. FASEB J. 2012, 26, 2648–2656. [Google Scholar] [CrossRef]
- Carr, N.J.; Emory, T.S.; Sobin, L.H. Epithelial neoplasms of the appendix and colorectum: An analysis of cell proliferation, apoptosis and expression of p53, CD44 and bcl-2. Arch. Pathol. Lab. Med. 2002, 126, 837–841. [Google Scholar] [CrossRef]
- Zhao, L.H.; Lin, Q.L.; Wei, J.; Huai, Y.L.; Wang, K.J.; Yan, H.Y. CD44v6 expression in patients with stage II or stage III sporadic colorectal cancer is superior to CD44 expression for predicting progression. Int. J. Clin. Exp. Pathol. 2015, 8, 692–701. [Google Scholar]
- Holah, N.S.; Aiad, H.A.; Asaad, N.Y.; Elkhouly, E.A.; Lasheen, A.G. Evaluation of the role of CD44 as a cancer stem cell marker in colorectal carcinoma: Immunohistochemical study. Menoufia Med. J. 2017, 30, 174–183. [Google Scholar]
- Meguid, R.A.; Slidell, M.B.; Wolfgang, C.L.; Chang, D.C.; Ahuja, N. Is there a difference inn survival between right-versus left-sided colon cancers? Ann. Surg. Oncol. 2008, 15, 2388–2394. [Google Scholar] [CrossRef]
- Demurtas, L.; Puzzoni, M.; Giampieri, R.; Ziranu, P.; Pusceddu, V.; Mandolesi, A.; Cremolini, C.; Masi, G.; Gelsomino, F.; Antoniotti, C.; et al. The role of primary tumour sidedness, EGFR gene copy number and EGFR promoter methylation in RAS/BRAF wild-type colorectal cancer patients receiving irinotecan/cetuximab. Br. J. Cancer 2017, 117, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Schumann, J.; Stanko, K.; Schliesser, U.; Appelt, C.; Sawitzki, B. Differences in CD44 Surface Expression Levels and Function Discriminates IL-17 and IFN-γ Producing Helper T Cells. PLoS ONE 2015, 10, e0132479, Erratum in PLoS ONE 2015, 10, e0143986. [Google Scholar] [CrossRef]
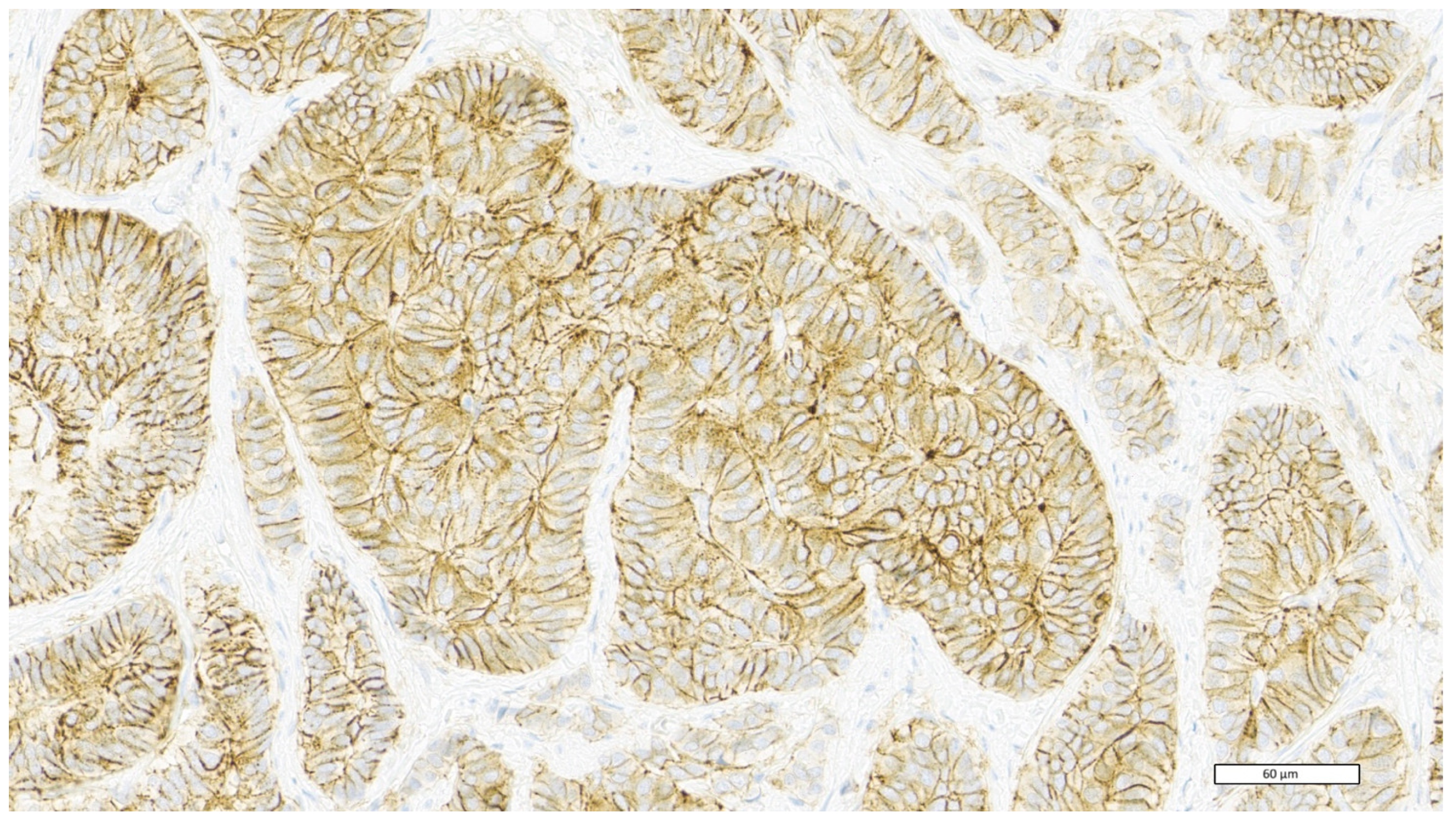
| CD44 Expression | Score |
|---|---|
| Negative or weak membrane staining in less than 10% of tumour cells | 0 |
| Weak membrane staining in at least 10% of tumour cells or moderate membrane staining in less than 10% of tumour cells | 1+ |
| Moderate membrane staining in at least 10% of tumour cells or intense membrane staining in less than 10% of tumour cells | 2+ |
| Intense membrane staining in at least 10% of tumour cells | 3+ |
| Low CD44 Expression | High CD44 Expression | |
|---|---|---|
Gender
| 28 (54.9%) 23 (45.1%) | 11 (78.6%) 3 (21.4%) |
Age
| 39 (76.5%) 12 (23.5%) | 8 (57.2%) 6 (42.8%) |
Stage at diagnosis
| 20 (39.2%) 31 (60.8%) | 1 (7.1%) 13 (92.9%) |
CDX-2
| 48 (94.1%) 3 (5.9%) | 11 (78.6%) 3 (21.4%) |
Site of primary tumour
| 38 (74.5%) 13 (25.5%) | 10 (71.4%) 4 (28.6%) |
Surgery of the primary tumour
| 46 (90.2%) 5 (9.8%) | 7 (50%) 7 (50%) |
Tumour Grade
| 41 (80.4%) 10 (19.6%) | 6 (42.8%) 8 (57.2%) |
Metastases sites
| 19 (37.3%) 32 (62.7%) | 3 (21.4%) 11 (78.6%) |
Liver Metastases
| 34 (66.7%) 17 (33.3%) | 12 (85.7%) 2 (14.3%) |
Peritoneal Metastases
| 16 (31.4%) 35 (68.6%) | 6 (42.9%) 8 (57.1%) |
K-RAS/N-RAS mutational status
| 27 (52.9%) 24 (47.1%) | 7 (50%) 7 (50%) |
B-RAF mutational status
| 46 (90.2%) 5 (9.8%) | 9 (64.3%) 5 (35.7%) |
First-line chemotherapy
| 6 (11.8%) 42 (82.3%) 3 (5.9%) | 2 (14.3%) 8 (57.1%) 4 (28.6%) |
First-line biological drug
| 32 (62.8%) 7 (13.7%) 12 (23.5%) | 9 (64.3%) 2 (14.3%) 3 (21.4%) |
Second-line chemotherapy
| 3 (5.9%) 32 (62.7%) 16 (31.4%) | 1 (7.1%) 7 (50%) 6 (42.9%) |
Second-line biological drug
| 22 (43.1%) - 29 (56.9%) | 5 (35.7%) 1 (7.1%) 8 (57.2%) |
| Response | Low CD44 Expression | High CD44 Expression | p-Value |
|---|---|---|---|
| ORR first line, n (%) ORR second line, n (%) | 21 (41.2%) 4 (12.1%) | 6 (42.9%) - | p = 0.9 p = 0.3 |
| DCR first line, n (%) DCR second line, n (%) | 43 (87.8%) 26 (78.8) | 10 (71.4%) 4 (50%) | p = 0.14 p = 0.1 |
| Median PFS-1, mo (range) | 11.6 mo (7 to 93.5) | 9.5 mo (3.9 to 19.4) | p = 0.17 |
| Median PFS-2, mo (range) | 5.6 mo (3.5 to 26) | 2.9 (2.1 to 9.6) | p = 0.12 |
| Variable | OS—p-Value |
|---|---|
| Age (≥70 y) | 0.0166 |
| CDX2 negative | 0.09 |
| Stage IV at diagnosis | 0.0241 |
| Right-sided colon cancer | 0.8 |
| Inoperable colon cancer | 0.0008 |
| High-grade tumour | 0.0084 |
| Metastasis sites (single vs. multiple sites) | 0.27 |
| Liver Metastases | 0.16 |
| Peritoneal Metastases | 0.4 |
| B-RAF mutational status | 0.0111 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziranu, P.; Aimola, V.; Pretta, A.; Dubois, M.; Murru, R.; Liscia, N.; Cau, F.; Persano, M.; Deias, G.; Palmas, E.; et al. New Horizons in Metastatic Colorectal Cancer: Prognostic Role of CD44 Expression. Cancers 2023, 15, 1212. https://doi.org/10.3390/cancers15041212
Ziranu P, Aimola V, Pretta A, Dubois M, Murru R, Liscia N, Cau F, Persano M, Deias G, Palmas E, et al. New Horizons in Metastatic Colorectal Cancer: Prognostic Role of CD44 Expression. Cancers. 2023; 15(4):1212. https://doi.org/10.3390/cancers15041212
Chicago/Turabian StyleZiranu, Pina, Valentina Aimola, Andrea Pretta, Marco Dubois, Raffaele Murru, Nicole Liscia, Flaviana Cau, Mara Persano, Giulia Deias, Enrico Palmas, and et al. 2023. "New Horizons in Metastatic Colorectal Cancer: Prognostic Role of CD44 Expression" Cancers 15, no. 4: 1212. https://doi.org/10.3390/cancers15041212
APA StyleZiranu, P., Aimola, V., Pretta, A., Dubois, M., Murru, R., Liscia, N., Cau, F., Persano, M., Deias, G., Palmas, E., Loi, F., Migliari, M., Pusceddu, V., Puzzoni, M., Lai, E., Cascinu, S., Faa, G., & Scartozzi, M. (2023). New Horizons in Metastatic Colorectal Cancer: Prognostic Role of CD44 Expression. Cancers, 15(4), 1212. https://doi.org/10.3390/cancers15041212







