The Use of San-Huang-Xie-Xin-Tang Reduces the Mortality Rate among Breast Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Design and Cohort
2.3. Study Outcome
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Breast Cancer Patients
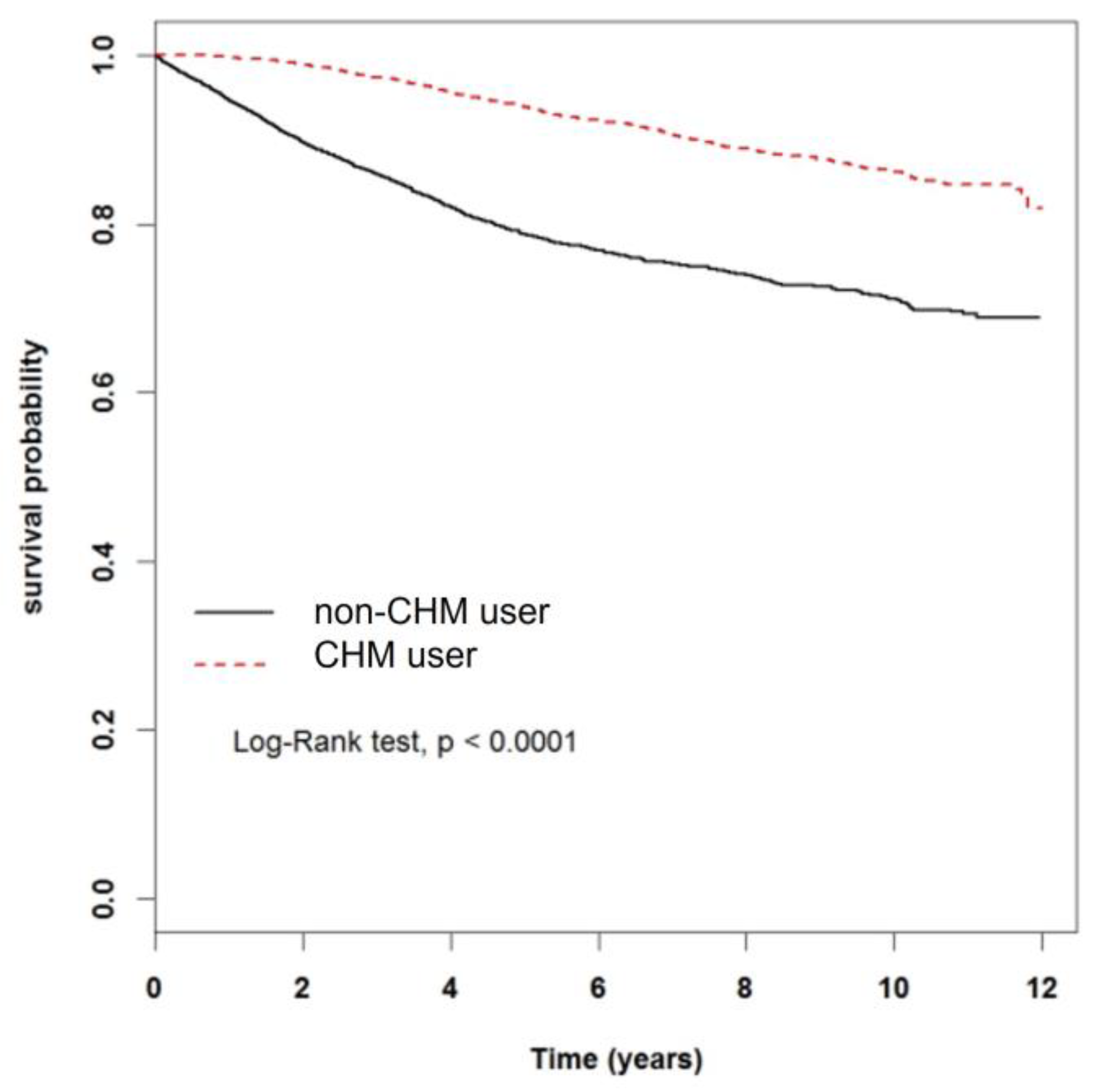
3.2. Effect of CHM on Mortality Rate of Breast Cancer Patients
3.3. The Cumulative Days and Annual Average Dose of CHM Use Influence the Breast Cancer Mortality Rate
3.4. The Impact of SHXXT and Its Constituents on the Breast Cancer Mortality Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Youlden, D.R.; Cramb, S.M.; Dunn, N.A.; Muller, J.M.; Pyke, C.M.; Baade, P.D. The descriptive epidemiology of female breast cancer: An international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012, 36, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Kamangar, F.; Dores, G.M.; Anderson, W.F. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006, 24, 2137–2150. [Google Scholar] [CrossRef] [PubMed]
- Banas, T.; Juszczyk, G.; Pitynski, K.; Nieweglowska, D.; Ludwin, A.; Czerw, A. Incidence and mortality rates in breast, corpus uteri, and ovarian cancers in poland (1980–2013): An analysis of population-based data in relation to socioeconomic changes. OncoTargets Ther. 2016, 9, 5521–5530. [Google Scholar]
- Liu, F.C.; Lin, H.T.; Kuo, C.F.; See, L.C.; Chiou, M.J.; Yu, H.P. Epidemiology and survival outcome of breast cancer in a nationwide study. Oncotarget 2017, 8, 16939–16950. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.C.; Wu, G.J.; Lu, Y.S.; Lin, C.H.; Hsiung, C.A. Associations between medical conditions and breast cancer risk in asians: A nationwide population-based study in taiwan. PLoS ONE 2015, 10, e0143410. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Magalhaes, M.C.; Jelovac, D.; Connolly, R.; Wolff, A.C. Treatment of her2-positive breast cancer. Breast 2014, 23, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, H.; Wu, W.; Yu, W.; Li, Y.; Bai, J.; Luo, B.; Li, S. Acupuncture for pain management in cancer: A systematic review and meta-analysis. Evid. Based Complement. Altern. Med. ECAM 2016, 2016, 1720239. [Google Scholar] [CrossRef] [PubMed]
- Chung, V.C.; Wu, X.; Lu, P.; Hui, E.P.; Zhang, Y.; Zhang, A.L.; Lau, A.Y.; Zhao, J.; Fan, M.; Ziea, E.T.; et al. Chinese herbal medicine for symptom management in cancer palliative care: Systematic review and meta-analysis. Medicine 2016, 95, e2793. [Google Scholar] [CrossRef] [PubMed]
- Pu, C.Y.; Lan, V.M.; Lan, C.F.; Lang, H.C. The determinants of traditional chinese medicine and acupuncture utilization for cancer patients with simultaneous conventional treatment. Eur. J. Cancer Care 2008, 17, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.T.; Chang, T.T.; Muo, C.H.; Wu, M.Y.; Sun, M.F.; Yeh, C.C.; Yen, H.R. Use of complementary traditional chinese medicines by adult cancer patients in taiwan: A nationwide population-based study. Integr. Cancer Ther. 2018, 17, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.T.; Lai, J.N.; Lo, P.C.; Chen, C.N.; Lin, J.G. Prescription of chinese herbal products is associated with a decreased risk of invasive breast cancer. Medicine 2017, 96, e7918. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Chiu, J.H. Use of chinese medicine by women with breast cancer: A nationwide cross-sectional study in taiwan. Complement. Ther. Med. 2011, 19, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hu, Y.; Xiang, L.; Li, S.; Yuan, Y.; Chen, X.; Zhang, Y.; Huang, W.; Meng, X.; Wang, P. San-huang-xie-xin-tang constituents exert drug-drug interaction of mutual reinforcement at both pharmacodynamics and pharmacokinetic level: A review. Front. Pharmacol. 2016, 7, 448. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Tseng, C.K.; Wu, S.F.; Chang, F.R.; Chiu, C.C.; Wu, Y.C. San-huang-xie-xin-tang extract suppresses hepatitis c virus replication and virus-induced cyclooxygenase-2 expression. J. Viral Hepat. 2011, 18, e315–e324. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Hsieh, M.T. Two-year experience with “san-huang-hsieh-hsin-tang” in essential hypertension. Am. J. Chin. Med. 1986, 14, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Hsieh, M.T.; Tsai, H.Y.; Chang, H.H.; Wang, T.F.; Shibuya, T. Studies on the “san-huang-hsieh-hsin-tang” in the treatment of essential hypertension. Taiwan Yi Xue Hui Za Zhi 1984, 83, 340–346. [Google Scholar]
- Tsai, H.H.; Chen, I.J.; Lo, Y.C. Effects of san-huang-xie-xin-tang on u46619-induced increase in pulmonary arterial blood pressure. J. Ethnopharmacol. 2008, 117, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.C.; Tsai, P.L.; Huang, Y.B.; Shen, K.P.; Tsai, Y.H.; Wu, Y.C.; Lai, Y.H.; Chen, I.J. San-huang-xie-xin-tang reduces lipopolysaccharides-induced hypotension and inflammatory mediators. J. Ethnopharmacol. 2005, 96, 99–106. [Google Scholar] [CrossRef]
- Shih, Y.T.; Chen, I.J.; Wu, Y.C.; Lo, Y.C. San-huang-xie-xin-tang protects against activated microglia- and 6-ohda-induced toxicity in neuronal sh-sy5y cells. Evid. Based Complement. Altern. Med. 2011, 2011, 429384. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.W.; Ahn, T.S.; Hong, N.R.; Jeong, H.S.; Jung, M.H.; Ha, K.T.; Kim, B.J. Effects of traditional chinese herbal medicine san-huang-xie-xin-tang on gastrointestinal motility in mice. World J. Gastroenterol. 2015, 21, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.T.; Wu, D.C.; Liu, C.M.; Yang, Y.C.; Chen, I.J.; Lo, Y.C. San-huang-xie-xin-tang inhibits helicobacter pylori-induced inflammation in human gastric epithelial ags cells. J. Ethnopharmacol. 2007, 112, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.C.; Lin, Y.L.; Yu, K.L.; Lai, Y.H.; Wu, Y.C.; Ann, L.M.; Chen, I.J. San-huang-xie-xin-tang attenuates inflammatory responses in lipopolysaccharide-exposed rat lungs. J. Ethnopharmacol. 2005, 101, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Liou, S.F.; Ke, H.J.; Hsu, J.H.; Liang, J.C.; Lin, H.H.; Chen, I.J.; Yeh, J.L. San-huang-xie-xin-tang prevents rat hearts from ischemia/reperfusion-induced apoptosis through enos and mapk pathways. Evid. Based Complement. Altern. Med. 2011, 2011, 915051. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Hou, Y.C.; Lee Chao, P.D.; Shia, C.S.; Hsu, I.C.; Fang, S.H. Potential ex vivo immunomodulatory effects of san-huang-xie-xin-tang and its component herbs on mice and humans. J. Ethnopharmacol. 2010, 127, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.Y.; Wu, S.L.; Hsiang, C.Y.; Li, C.C.; Lai, T.Y.; Lo, H.Y.; Shen, W.S.; Lee, C.H.; Chen, J.C.; Wu, H.C.; et al. Relationship between san-huang-xie-xin-tang and its herbal components on the gene expression profiles in hepg2 cells. Am. J. Chin. Med. 2008, 36, 783–797. [Google Scholar] [CrossRef]
- Shia, C.S.; Hou, Y.C.; Juang, S.H.; Tsai, S.Y.; Hsieh, P.H.; Ho, L.C.; Chao, P.D. Metabolism and pharmacokinetics of san-huang-xie-xin-tang, a polyphenol-rich chinese medicine formula, in rats and ex-vivo antioxidant activity. Evid. Based Complement. Altern. Med. ECAM 2011, 2011, 721293. [Google Scholar] [CrossRef]
- Lo, Y.C.; Shih, Y.T.; Tseng, Y.T.; Hsu, H.T. Neuroprotective effects of san-huang-xie-xin-tang in the mpp(+)/mptp models of parkinson’s disease in vitro and in vivo. Evid. Based Complement. Altern. Med. ECAM 2012, 2012, 501032. [Google Scholar] [CrossRef] [PubMed]
- Li, X.K.; Motwani, M.; Tong, W.; Bornmann, W.; Schwartz, G.K. Huanglian, a chinese herbal extract, inhibits cell growth by suppressing the expression of cyclin b1 and inhibiting cdc2 kinase activity in human cancer cells. Mol. Pharmacol. 2000, 58, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.X.; Liu, J.; Wang, J.; He, C.; Li, F.P. The extract of huanglian, a medicinal herb, induces cell growth arrest and apoptosis by upregulation of interferon-beta and tnf-alpha in human breast cancer cells. Carcinogenesis 2005, 26, 1934–1939. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.C.; Lu, Y.C.; Cheng, C.S.; Chen, Y.W.; Lyu, P.C.; Lin, C.W.; Timms, J.F.; Chan, H.L. Proteomic and redox-proteomic analysis of berberine-induced cytotoxicity in breast cancer cells. J. Proteom. 2012, 75, 3158–3176. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Luo, J.; Brooks, C.L.; Nikolaev, A.Y.; Li, M. Dynamics of the p53 acetylation pathway. Novartis Found. Symp. 2004, 259, 197–205; discussion 195–223. [Google Scholar] [PubMed]
- Cai, R.L.; Yan-Neale, Y.; Cueto, M.A.; Xu, H.; Cohen, D. Hdac1, a histone deacetylase, forms a complex with hus1 and rad9, two g2/m checkpoint rad proteins. J. Biol. Chem. 2000, 275, 27909–27916. [Google Scholar] [CrossRef]
- McClung, J.K.; Jupe, E.R.; Liu, X.T.; Dell’Orco, R.T. Prohibitin: Potential role in senescence, development, and tumor suppression. Exp. Gerontol. 1995, 30, 99–124. [Google Scholar] [CrossRef]
- Klumpp, S.; Krieglstein, J. Serine/threonine protein phosphatases in apoptosis. Curr. Opin. Pharmacol. 2002, 2, 458–462. [Google Scholar] [CrossRef]
- Garcia, A.; Cayla, X.; Guergnon, J.; Dessauge, F.; Hospital, V.; Rebollo, M.P.; Fleischer, A.; Rebollo, A. Serine/threonine protein phosphatases pp1 and pp2a are key players in apoptosis. Biochimie 2003, 85, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.W.; Xin, L.Y.; Xu, X.; Ji, X.X.; Fan, L.H. Epithelial-to-mesenchymal transition markers to predict response of berberine in suppressing lung cancer invasion and metastasis. J. Transl. Med. 2014, 12, 22. [Google Scholar] [CrossRef]
- Li, J.; Qiu, D.M.; Chen, S.H.; Cao, S.P.; Xia, X.L. Suppression of human breast cancer cell metastasis by coptisine in vitro. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 5747–5751. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.P.; Hsieh, Y.C.; Shia, C.S.; Hsu, P.W.; Chen, J.Y.; Hou, Y.C.; Hsieh, Y.W. Increased systemic exposure of methotrexate by a polyphenol-rich herb via modulation on efflux transporters multidrug resistance-associated protein 2 and breast cancer resistance protein. J. Pharm. Sci. 2016, 105, 343–349. [Google Scholar] [CrossRef]
- Wei, L.; Zhou, Y.; Dai, Q.; Qiao, C.; Zhao, L.; Hui, H.; Lu, N.; Guo, Q.L. Oroxylin a induces dissociation of hexokinase ii from the mitochondria and inhibits glycolysis by sirt3-mediated deacetylation of cyclophilin d in breast carcinoma. Cell Death Dis. 2013, 4, e601. [Google Scholar] [CrossRef]
- Zhang, J.; Anastasiadis, P.Z.; Liu, Y.; Thompson, E.A.; Fields, A.P. Protein kinase c (pkc) betaii induces cell invasion through a ras/mek-, pkc iota/rac 1-dependent signaling pathway. J. Biol. Chem. 2004, 279, 22118–22123. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Lu, N.; Ling, Y.; Chen, Y.; Hui, H.; Lu, Z.; Song, X.; Li, Z.; You, Q.; Guo, Q. Inhibitory effects of wogonin on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Toxicology 2011, 282, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.O. Effects of wogonin, wogonoside, and 3,5,7,2’,6’-pentahydroxyflavone on chemical mediator production in peritoneal exduate cells and immunoglobulin e of rat mesenteric lymph node lymphocytes. J. Ethnopharmacol. 2003, 84, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Guo, Q.; You, Q.; Zhang, K.; Yang, Y.; Yu, J.; Liu, W.; Zhao, L.; Gu, H.; Hu, Y.; et al. Involvement of bax/bcl-2 in wogonin-induced apoptosis of human hepatoma cell line smmc-7721. Anti Cancer Drugs 2006, 17, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Tai, M.C.; Tsang, S.Y.; Chang, L.Y.; Xue, H. Therapeutic potential of wogonin: A naturally occurring flavonoid. CNS Drug Rev. 2005, 11, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Gao, Y.; Ling, Y.; Chen, Y.; Yang, Y.; Gu, H.Y.; Qi, Q.; Liu, W.; Wang, X.T.; You, Q.D.; et al. Wogonin suppresses tumor growth in vivo and vegf-induced angiogenesis through inhibiting tyrosine phosphorylation of vegfr2. Life Sci. 2008, 82, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Zingue, S.; Nde, C.B.M.; Michel, T.; Ndinteh, D.T.; Tchatchou, J.; Adamou, M.; Fernandez, X.; Fohouo, F.T.; Clyne, C.; Njamen, D. Ethanol-extracted cameroonian propolis exerts estrogenic effects and alleviates hot flushes in ovariectomized wistar rats. BMC Complement. Altern. Med. 2017, 17, 65. [Google Scholar] [CrossRef]
- Oumarou, M.R.; Zingue, S.; Bakam, B.Y.; Ateba, S.B.; Foyet, S.H.; Mbakop, F.T.T.; Njamen, D. Lannea acida a. Rich. (anacardiaceae) ethanol extract exhibits estrogenic effects and prevents bone loss in an ovariectomized rat model of osteoporosis. Evid. Based Complement. Altern. Med. ECAM 2017, 2017, 7829059. [Google Scholar] [CrossRef]
- Leung, E.; Kim, J.E.; Askarian-Amiri, M.; Finlay, G.J.; Baguley, B.C. Evidence for the existence of triple-negative variants in the mcf-7 breast cancer cell population. BioMed Res. Int. 2014, 2014, 836769. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.; Wang, Y.; Collins, L.C.; Kaplan, J.; Li, H.; Gelman, R.; Comander, A.H.; Gallagher, B.; Fetten, K.; Krag, K.; et al. Estrogen receptor positive breast cancers in brca1 mutation carriers: Clinical risk factors and pathologic features. Breast Cancer Res. BCR 2010, 12, R12. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.G.; Kang, S.C.; Kim, K.C.; Choung, E.S.; Zee, O.P. Screening of estrogenic and antiestrogenic activities from medicinal plants. Environ. Toxicol. Pharmacol. 2008, 25, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Park, S.; Choi, S.; Kim, S.H.; Kang, K.S. In vitro estrogenic and breast cancer inhibitory activities of chemical constituents isolated from rheum undulatum L. Molecules 2018, 23, 1215. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Z.; Li, S.; Ye, X.; Li, X.; He, K. Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia 2014, 92, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.W.; Chen, T.L.; Shih, Y.R.; Tsai, C.L.; Chang, C.C.; Liang, H.H.; Tseng, S.H.; Chien, S.C.; Wang, C.C. Adjunctive traditional chinese medicine therapy improves survival in patients with advanced breast cancer: A population-based study. Cancer 2014, 120, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Lin, C.L.; Huang, W.Y.; Shangkuan, W.C.; Kang, B.H.; Chu, Y.H.; Lee, J.C.; Fan, H.C.; Kao, C.H. The use of adjunctive traditional chinese medicine therapy and survival outcome in patients with head and neck cancer: A nationwide population-based cohort study. QJM 2015, 108, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.T.; Liao, H.H.; Chiang, J.H.; Wu, M.Y.; Chen, B.C.; Chang, C.M.; Yeh, M.H.; Chang, T.T.; Sun, M.F.; Yeh, C.C.; et al. Complementary chinese herbal medicine therapy improves survival of patients with pancreatic cancer in taiwan: A nationwide population-based cohort study. Integr. Cancer 2018, 17, 411–422. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Non-CHM User (n = 5387) | CHM User (n = 5387) | p-Value |
|---|---|---|---|
| Age, mean ± SD (years) | 49.41 (10.12) | 49.35 (10.08) | 0.7591 |
| Age group, n (%) | 0.99 | ||
| 18–39 | 911 (16.91) | 911 (16.91) | |
| 40–59 | 3660 (67.94) | 3660 (67.94) | |
| ≥60 | 816 (15.15) | 816 (15.15) | |
| Urbanization level, n (%) | <0.0001 | ||
| 1 | 2002 (37.16) | 1790 (33.23) | |
| 2 | 1724 (32) | 1784 (33.12) | |
| 3 | 725 (13.46) | 879 (16.32) | |
| 4 | 936 (17.38) | 934 (17.34) | |
| CCI score, n (%) | 0.0006 | ||
| 0 | 5044 (96.63) | 5088 (94.45) | |
| 1 | 201 (3.73) | 214 (3.97) | |
| More than 2 | 142 (2.64) | 85 (1.58) | |
| Treatment, n (%) | |||
| Breast cancer surgery | 4619 (85.74) | 5107 (94.8) | <.0001 |
| Radiotherapy | 2666 (49.49) | 2770 (51.42) | 0.0451 |
| Chemotherapy | 3936(73.06) | 3958 (73.47) | 0.632 |
| Drugs, n (%) | |||
| Epirubicin | 2179 (40.45) | 2207 (40.97) | 0.583 |
| Fluorouracil | 3082 (57.21) | 3126 (58.03) | 0.391 |
| Cyclophosphamide | 3624 (67.27) | 3800 (70.54) | 0.0002 |
| Docetaxel | 1161 (21.55) | 909 (16.87) | <0.0001 |
| Paclitaxel | 782 (14.52) | 626 (11.62) | <0.0001 |
| Goserelin acetate | 67 (1.24) | 61 (1.13) | 0.5937 |
| Tamoxifen | 3514 (65.23) | 3769 (69.96) | <0.0001 |
| Anastrozole | 663 (12.31) | 878 (16.3) | <0.0001 |
| Letrozole | 771 (14.31) | 824 (15.3) | 0.1505 |
| Exemestane | 413 (7.67) | 405 (7.52) | 0.7711 |
| Toremifene | 52 (0.97) | 64 (1.19) | 0.2626 |
| Trastuzumab | 421 (7.82) | 356 (6.61) | 0.0155 |
| Characteristics | Non-CHM User | CHM User | Crude HR | Adjusted HR ‡ | ||||
|---|---|---|---|---|---|---|---|---|
| Event | Person Years | IR † | Event | Person Years | IR † | |||
| Total | 1141 | 27,576 | 41.38 | 456 | 33,488 | 13.62 | 0.33 (0.3–0.37) *** | 0.41 (0.37–0.46) *** |
| Age group | ||||||||
| 18–39 | 171 | 4879 | 34.92 | 75 | 6083 | 12.33 | 0.35 (0.27–0.46) *** | 0.4 (0.3–0.54) *** |
| 40–59 | 741 | 18,929 | 39.15 | 288 | 22,641 | 12.72 | 0.33 (0.29–0.38) *** | 0.41 (0.35–0.47) *** |
| ≥60 | 229 | 3750 | 61.07 | 93 | 4765 | 19.52 | 0.33 (0.26–0.42) *** | 0.35 (0.27–0.45) *** |
| Urbanization level | ||||||||
| 1 | 348 | 10,701 | 32.52 | 138 | 11,097 | 12.44 | 0.39 (0.32–0.47) *** | 0.49 (0.4–0.6) *** |
| 2 | 368 | 8786 | 41.88 | 158 | 11,069 | 14.27 | 0.34 (0.28–0.41) *** | 0.38 (0.31–0.46) *** |
| 3 | 185 | 3512 | 52.68 | 71 | 5438 | 13.06 | 0.25 (0.19–0.33) *** | 0.33 (0.25–0.43) *** |
| 4 | 240 | 4577 | 52.43 | 89 | 5884 | 15.13 | 0.3 (0.23–0.38) *** | 0.39 (0.3–0.5) *** |
| CCI score | ||||||||
| 0 | 1012 | 26,100 | 38.77 | 416 | 31,838 | 13.07 | 0.34 (0.3–0.38) *** | 0.41 (0.37–0.46) *** |
| 1 | 70 | 937 | 74.73 | 21 | 1240 | 16.93 | 0.23 (0.14–0.37) *** | 0.29 (0.17–0.49) *** |
| More than 2 | 59 | 539 | 109.42 | 19 | 410 | 46.30 | 0.43 (0.26–0.73) ** | 0.42 (0.24–0.73) ** |
| Treatment | ||||||||
| Breast cancer surgery | ||||||||
| No | 367 | 2704 | 135.74 | 48 | 1903 | 25.22 | 0.23 (0.17–0.32) *** | 0.25 (0.18–0.34) *** |
| Yes | 774 | 24,872 | 31.12 | 408 | 31,585 | 12.92 | 0.41 (0.37–0.46) *** | 0.43 (0.38–0.49) *** |
| Radiotherapy | ||||||||
| No | 426 | 14,848 | 28.69 | 116 | 17,493 | 6.63 | 0.24 (0.2–0.3) *** | 0.29 (0.24–0.36) *** |
| Yes | 715 | 12,728 | 56.18 | 340 | 15,996 | 21.26 | 0.37 (0.33–0.43) *** | 0.46 (0.41–0.53) *** |
| Chemotherapy | ||||||||
| No | 177 | 7689 | 23.02 | 37 | 9584 | 3.86 | 0.18 (0.13–0.26) *** | 0.2 (0.14–0.28) *** |
| Yes | 964 | 19,887 | 48.87 | 419 | 23,904 | 17.53 | 0.36 (0.32–0.41) *** | 0.45 (0.4–0.5) *** |
| Drugs | ||||||||
| Epirubicin | ||||||||
| No | 567 | 17.185 | 32.99 | 182 | 20.655 | 8.81 | 0.27 (0.23–0.32) *** | 0.35 (0.3–0.42) *** |
| Yes | 574 | 10.391 | 55.24 | 274 | 12.834 | 21.35 | 0.38 (0.33–0.44) *** | 0.44 (0.38–0.51) *** |
| Fluorouracil | ||||||||
| No | 383 | 11.574 | 33.09 | 124 | 13.796 | 8.99 | 0.28 (0.23–0.34) *** | 0.35 (0.28–0.43) *** |
| Yes | 758 | 16.002 | 47.37 | 332 | 19.692 | 16.86 | 0.36 (0.31–0.4) *** | 0.42 (0.37–0.48) *** |
| Cyclophosphamide | ||||||||
| No | 332 | 8825 | 37.62 | 83 | 10.396 | 7.98 | 0.23 (0.18–0.29) *** | 0.29 (0.22–0.37) *** |
| Yes | 809 | 18.751 | 43.14 | 373 | 22.093 | 16.15 | 0.37 (0.33–0.42) *** | 0.44 (0.38–0.49) *** |
| Docetaxel | ||||||||
| No | 610 | 23.279 | 26.20 | 196 | 29.218 | 6.71 | 0.26 (0.22–0.31) *** | 0.31 (0.26–0.36) *** |
| Yes | 531 | 4297 | 123.57 | 260 | 4270 | 60.89 | 0.46 (0.39–0.53) *** | 0.52 (0.45–0.61) *** |
| Paclitaxel | ||||||||
| No | 722 | 24.227 | 29.80 | 228 | 30.102 | 7.57 | 0.26 (0.23–0.3) *** | 0.31 (0.27–0.36) *** |
| Yes | 419 | 3349 | 125.12 | 228 | 3387 | 67.32 | 0.5 (0.43–0.59) *** | 0.55 (0.47–0.65) *** |
| Goserelin acetate | ||||||||
| No | 1133 | 27.222 | 40.89 | 440 | 33.156 | 13.27 | 0.33 (0.29–0.37) *** | 0.4 (0.36–0.45) *** |
| Yes | 28 | 354 | 79.19 | 16 | 332 | 48.14 | 0.59 (0.32–1.09) | 0.49 (0.22–1.07) |
| Tamoxifen | ||||||||
| No | 526 | 8274 | 63.57 | 147 | 9402 | 15.64 | 0.26 (0.22–0.31) *** | 0.34 (0.28–0.41) *** |
| Yes | 615 | 19.302 | 31.86 | 309 | 24.087 | 12.83 | 0.4 (0.35–0.46) *** | 0.45 (0.39–0.52) *** |
| Anastrozole | ||||||||
| No | 895 | 23.944 | 37.38 | 299 | 27.694 | 10.80 | 0.3 (0.26–0.34) *** | 0.37 (0.32–0.42) *** |
| Yes | 246 | 3632 | 67.74 | 157 | 5795 | 27.09 | 0.39 (0.32–0.47) *** | 0.5 (0.4–0.61) *** |
| Letrozole | ||||||||
| No | 898 | 23.522 | 38.18 | 295 | 28.283 | 10.43 | 0.28 (0.25–0.32) *** | 0.35 (0.3–0.39) *** |
| Yes | 243 | 4054 | 59.94 | 161 | 5206 | 30.93 | 0.49 (0.4–0.6) *** | 0.6 (0.49–0.74) *** |
| Exemestane | ||||||||
| No | 962 | 25.296 | 38.03 | 337 | 30.880 | 10.91 | 0.29 (0.26–0.33) *** | 0.37 (0.32–0.42) *** |
| Yes | 179 | 2280 | 78.49 | 119 | 2609 | 45.62 | 0.55 (0.44–0.7) *** | 0.58 (0.46–0.74) *** |
| Toremifene | ||||||||
| No | 1123 | 27.243 | 41.22 | 447 | 33.027 | 13.53 | 0.33 (0.3–0.37) *** | 0.41 (0.36–0.45) *** |
| Yes | 18 | 333 | 54.05 | 9 | 461 | 19.50 | 0.35 (0.16–0.78) ** | 0.11 (0.02–0.48) ** |
| Trastuzumab | ||||||||
| No | 934 | 26.041 | 35.87 | 347 | 31.897 | 10.88 | 0.31 (0.27–0.35) *** | 0.39 (0.34–0.44) *** |
| Yes | 207 | 1535 | 134.90 | 109 | 1591 | 68.50 | 0.46 (0.37–0.58) *** | 0.48 (0.38–0.61) *** |
| Characteristics | N | Mortality | HR (95% CI) | |
|---|---|---|---|---|
| No. of Event | Crude | Adjusted † | ||
| Non-CHM users | 5387 | 1141 | 1 (reference) | 1 (reference) |
| CHM users | ||||
| 30–90 days | 3209 | 288 | 0.36 (0.31–0.40) *** | 0.44 (0.38–0.50) *** |
| 90–180 days | 1233 | 106 | 0.33 (0.27–0.41) *** | 0.41 (0.33–0.50) *** |
| >180 days | 945 | 62 | 0.26 (0.20–0.33) *** | 0.31 (0.24–0.40) *** |
| p for trend | <0.0001 | <0.0001 | ||
| Annual Average CHM Dose (g) | N | Mortality | HR (95% CI) | |
|---|---|---|---|---|
| No. of Event | Crude | Adjusted † | ||
| Non-CHM users | 5387 | 1141 | 1 (reference) | 1 (reference) |
| CHM users | ||||
| <35.1 (g)/year | 1346 | 140 | 0.42 (0.35–0.50) *** | 0.50 (0.42–0.60) *** |
| 35.1–67.2 (g)/year | 1346 | 123 | 0.36 (0.30–0.44) *** | 0.43 (0.35–0.51) *** |
| 67.2–147 (g)/year | 1340 | 113 | 0.33 (0.27–0.40) *** | 0.39 (0.32–0.48) *** |
| >147 (g)/year | 1355 | 80 | 0.23 (0.18–0.29) *** | 0.30 (0.24–0.38) *** |
| p for trend | <0.0001 | <0.0001 | ||
| Characteristics | Mortality | HR (95% CI) | |
|---|---|---|---|
| No. of Event | Crude | Adjusted † | |
| Follow-up period:≤2 years | |||
| Non-CHM users | 440 | 1 (reference) | 1 (reference) |
| CHM users | |||
| 30–90 days | 136 | 0.43 (0.35–0.52) *** | 0.51 (0.42–0.62) *** |
| 90–180 days | 52 | 0.42 (0.31–0.56) *** | 0.45 (0.34–0.61) *** |
| >180 days | 24 | 0.25 (0.17–0.38) *** | 0.29 (0.19–0.44) *** |
| Follow-up period: 2–5 years | |||
| Non-CHM users | 137 | 1 (reference) | 1 (reference) |
| CHM users | |||
| 30–90 days | 91 | 0.82 (0.63–1.07) | 0.88 (0.68–1.15) |
| 90–180 days | 37 | 0.83 (0.58–1.20) | 0.82 (0.57–1.19) |
| >180 days | 28 | 0.87 (0.58–1.31) | 0.82 (0.55–1.24) |
| Follow-up period: >5 years | |||
| Non-CHM users | 25 | 1 (reference) | 1 (reference) |
| CHM users | |||
| 30–90 days | 16 | 0.70 (0.37–1.31) | 0.60 (0.31–1.51) |
| 90–180 days | 6 | 0.69 (0.28–1.67) | 0.58 (0.22–1.57) |
| >180 days | 8 | 1.28 (0.58–2.83) | 0.75 (0.32–1.78) |
| CHM Prescription | Mortality | HR (95% CI) | ||
|---|---|---|---|---|
| N | No. of Event | Crude | Adjusted † | |
| Non-CHM user | 5387 | 1141 | 1 (reference) | 1 (reference) |
| Single constituent | ||||
| Rhizoma Rhei | 3049 | 278 | 0.36 (0.31–0.41) *** | 0.42 (0.37–0.48) *** |
| Radix Scutellaria | 3958 | 327 | 0.32 (0.28–0.36) *** | 0.40 (0.36–0.46) *** |
| Rhizoma Coptidis | 2644 | 215 | 0.31 (0.27–0.36) *** | 0.39 (0.34–0.45) *** |
| Compounds | ||||
| SHXXT | 489 | 33 | 0.25 (0.18–0.36) *** | 0.32 (0.22–0.45) *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winardi, D.; Wu, C.-H.; Chiang, J.-H.; Chen, Y.-H.; Hsieh, C.-L.; Yang, J.-C.; Wu, Y.-C. The Use of San-Huang-Xie-Xin-Tang Reduces the Mortality Rate among Breast Cancer Patients. Cancers 2023, 15, 1213. https://doi.org/10.3390/cancers15041213
Winardi D, Wu C-H, Chiang J-H, Chen Y-H, Hsieh C-L, Yang J-C, Wu Y-C. The Use of San-Huang-Xie-Xin-Tang Reduces the Mortality Rate among Breast Cancer Patients. Cancers. 2023; 15(4):1213. https://doi.org/10.3390/cancers15041213
Chicago/Turabian StyleWinardi, Daniel, Chieh-Hsin Wu, Jen-Huai Chiang, Yung-Hsiang Chen, Ching-Liang Hsieh, Juan-Cheng Yang, and Yang-Chang Wu. 2023. "The Use of San-Huang-Xie-Xin-Tang Reduces the Mortality Rate among Breast Cancer Patients" Cancers 15, no. 4: 1213. https://doi.org/10.3390/cancers15041213
APA StyleWinardi, D., Wu, C.-H., Chiang, J.-H., Chen, Y.-H., Hsieh, C.-L., Yang, J.-C., & Wu, Y.-C. (2023). The Use of San-Huang-Xie-Xin-Tang Reduces the Mortality Rate among Breast Cancer Patients. Cancers, 15(4), 1213. https://doi.org/10.3390/cancers15041213








