Recent Advances in Cervical Cancer Management: A Review on Novel Prognostic Factors in Primary and Recurrent Tumors
Abstract
Simple Summary
Abstract
1. Introduction
- -
- macroscopic tumor site
- -
- tumor dimensions (measurements of horizontal extent and depth of invasion or tumor thickness)
- -
- maximum and minimum length of vaginal cuff and parametria in two dimensions
- -
- histological tumor type and tumor grade
- -
- coexistent pathology (squamous intraepithelial lesion, adenocarcinoma in situ, stratified mucin-producing intraepithelial lesion)
- -
- LVSI
- -
- minimum distance of uninvolved cervical stroma
- -
- extent of invasion (vaginal, uterine corpus, parametrial, adnexal, bladder, rectum involvement)
- -
- margin status (for invasive tumors and for precursor lesions)
- -
- lymph node status: sentinel lymph node status, total number of nodes retrieved, number of positive lymph nodes
- -
- pathologically confirmed distant metastases.
- -
- HPV dependent and independent status
- -
- Silva pattern of invasion
- -
- Ancillary studies (p16 immunohistochemistry; in-situ hybridization for HPV).
2. Materials and Methods
3. Prognostic Factors in Primary Neoplasm
3.1. HPV Status
- -
- HPV-dependent SILs (squamous intraepithelial lesions): high grade and low grade, respectively corresponding to CIN1 and CIN2-3 dysplasia
- -
- HPV-dependent AIS (adenocarcinoma in-situ) and its SMILE variant (stratified mucin producing intraepithelial lesion)
- -
- HPV-independent AIS: gastric-type AIS and ALEGH (atypical lobular endocervical glandular hyperplasia)
3.2. Grading of Cervical Cancer
3.2.1. Squamous Cell Carcinoma
- -
- the Broder’s system, based on the degree of keratinization, cytological atypia and mitotic activity;
- -
- the grading of invasive tumor front or the pattern-type of invasion (pushing versus infiltrative);
- -
- the typology of neoplastic cells and the presence/absence of keratinization (large-cell keratinizing, large-cell non-keratinizing, and small-cell non-keratinizing categories);
- -
- the WHO proposal that considers the degree of keratinization, nuclear pleomorphism, size of nucleoli and mitotic index.
3.2.2. Adenocarcinoma
3.3. Silva Pattern of Invasion for HPV-Associated Adenocarcinomas
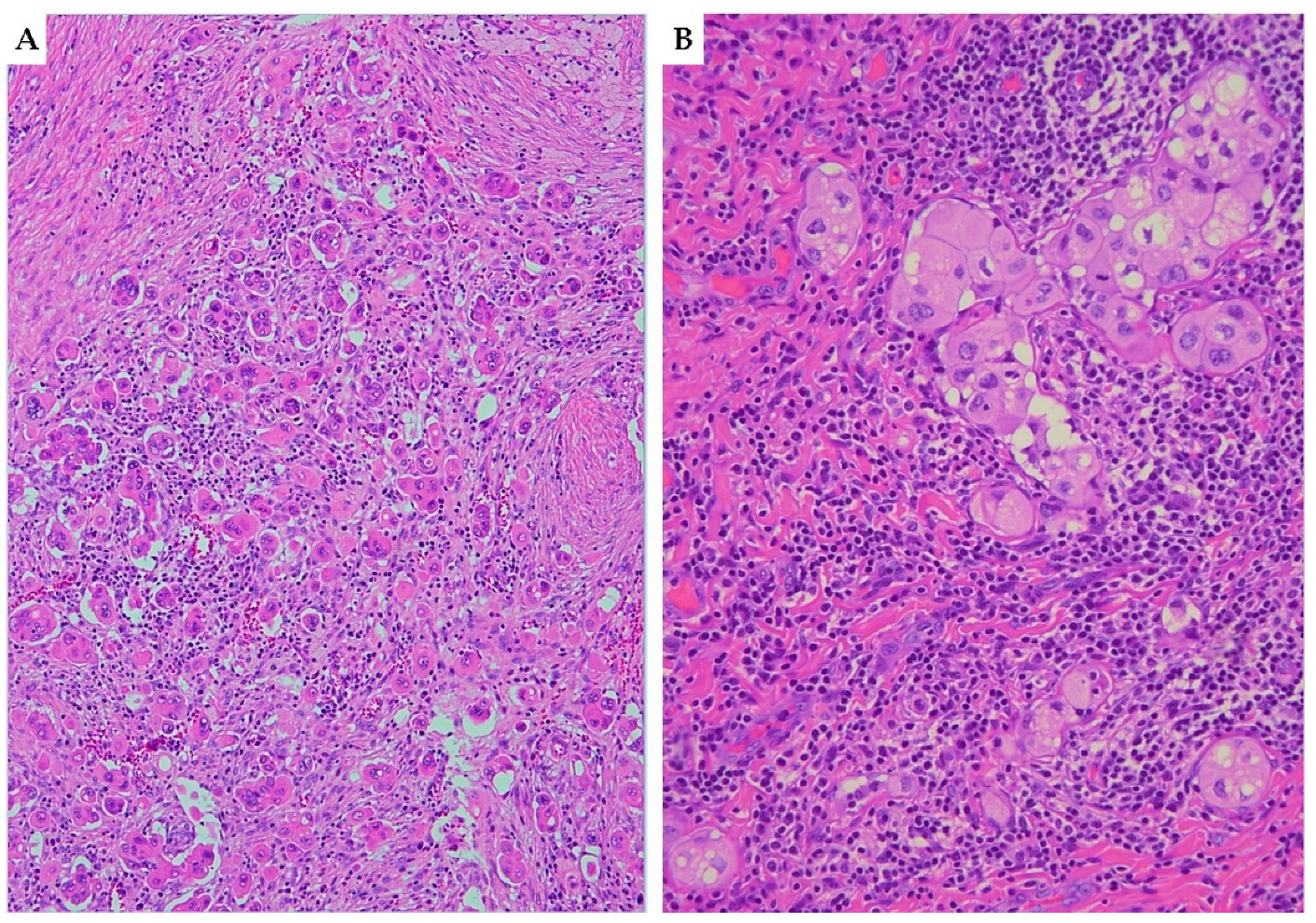
3.4. Lympho-Vascular Space Invasion (LVSI)
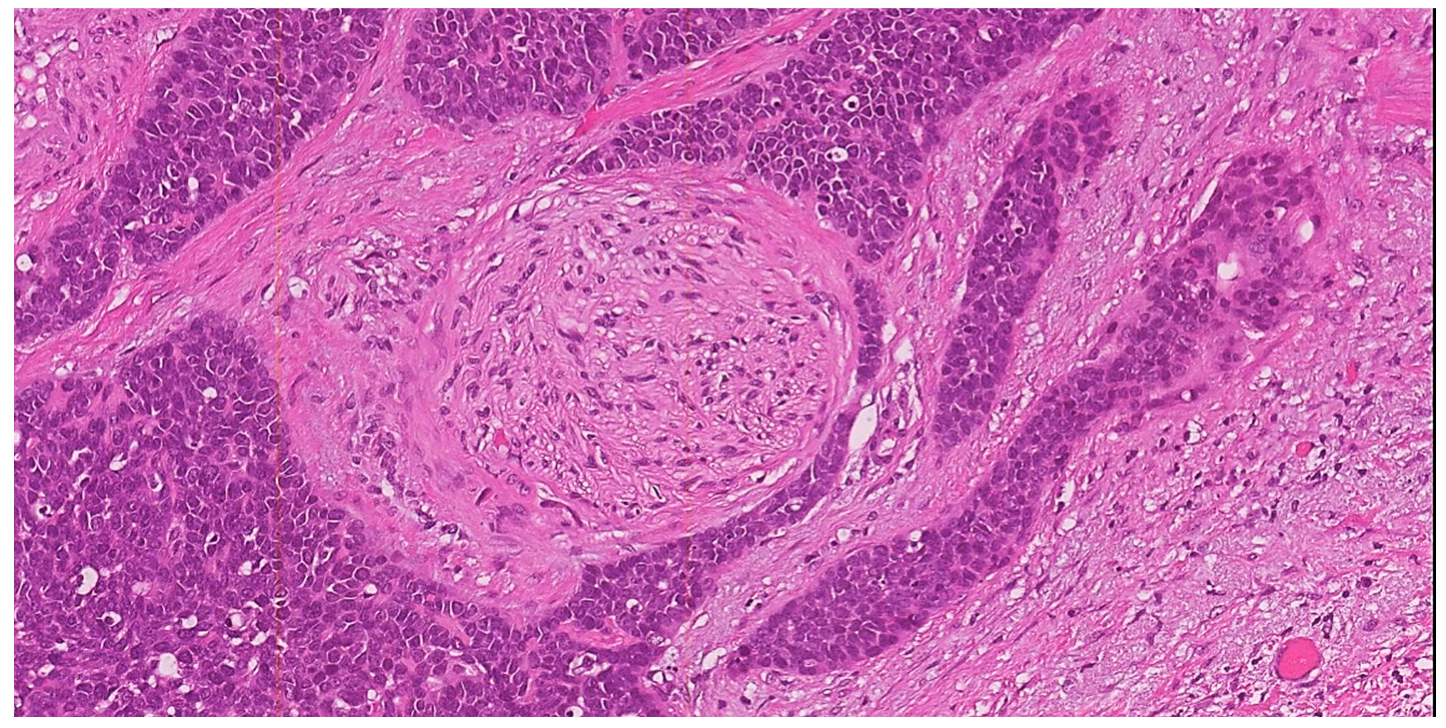
3.5. Perineural Invasion (PNI)
3.6. Depth of Stromal Invasion (DOI)
3.7. Maximum Horizontal Extent of Tumor
- -
- give a more complete picture of tumor extent (length and width);
- -
- appreciate tumor volume;
- -
- help future studies to further clarify its prognostic role.
3.8. Parametrial Involvement
3.9. Tumor-Free Distance (TFD)
3.10. Tumor-Infiltrating Lymphocytes (TILs)
3.11. Margin Status
4. Prognostic Factors in Advanced-Stage
4.1. Local Involvement: Endometrial, Adnexal and Vaginal Extension
4.2. Lymph Nodes Involvement
4.3. Distant Metastases
5. Molecular Markers and Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Female Genital Tumours, 5th ed.; WHO Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2020; Volume 4. [Google Scholar]
- Wang, M.; Yuan, B.; Zhou, Z.H.; Han, W.W. Clinicopathological characteristics and prognostic factors of cervical adenocarcinoma. Sci. Rep. 2021, 11, 7506. [Google Scholar] [CrossRef]
- Park, K.J.; Selinger, C.I.; Alvarado-Cabrero, I.; Duggan, M.A.; Kiyokawa, T.; Mills, A.M.; Ordi, J.; Otis, C.N.; Plante, M.; Stolnicu, S.; et al. Dataset for the Reporting of Carcinoma of the Cervix: Recommendations From the International Collaboration on Cancer Reporting (ICCR). Int. J. Gynecol. Pathol. 2022, 41 (Suppl. S1), S64–S89. [Google Scholar] [CrossRef]
- Zannoni, G.F.; Travaglino, A.; Raffone, A.; Arciuolo, D.; D’Alessandris, N.; Scaglione, G.; Tralongo, P.; Inzani, F.; Angelico, G.; Santoro, A. Depth of Stromal Invasion as the Most Prognostically Relevant Regression System in Locally Advanced Cervical Cancer after Neoadjuvant Treatment: A Systematic Review and Meta-Analysis Grading. Diagnostics 2021, 11, 1772. [Google Scholar] [CrossRef]
- Santoro, A.; Angelico, G.; Inzani, F.; Arciuolo, D.; Spadola, S.; Valente, M.; D’Alessandris, N.; Piermattei, A.; Fiorentino, V.; CIanfrini, F.; et al. Standard ultrastaging compared to one-step nucleic acid amplification (OSNA) for the detection of sentinel lymph node metastases in early stage cervical cancer. Int. J. Gynecol. Cancer 2020, 30, 1871–1877. [Google Scholar] [CrossRef]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef]
- Nicolás, I.; Marimon, L.; Barnadas, E.; Saco, A.; Rodríguez-Carunchio, L.; Fusté, P.; Martí, C.; Rodriguez-Trujillo, A.; Torne, A.; Del Pino, M.; et al. HPV-negative tumors of the uterine cervix. Mod. Pathol. 2019, 32, 1189–1196. [Google Scholar] [CrossRef]
- Angelico, G.; Santoro, A.; Inzani, F.; Straccia, P.; Spadola, S.; Arciuolo, D.; Valente, M.; D’Alessandris, N.; Benvenuto, R.; Travaglino, A.; et al. An Emerging Anti-p16 Antibody-BC42 Clone as an Alternative to the Current E6H4 for Use in the Female Genital Tract Pathological Diagnosis: Our Experience and a Review on p16ink4a Functional Significance, Role in Daily-Practice Diagnosis, Prognostic Potential, and Technical Pitfalls. Diagnostics 2021, 11, 713. [Google Scholar]
- Talia, K.L.; Oliva, E.; Rabban, J.T.; Singh, N.; Stolnicu, S.; McCluggage, W.G. Grading of Endocervical Adenocarcinomas: Review of the Literature and Recommendations From the International Society of Gynecological Pathologists. Int. J. Gynecol. Pathol. 2021, 40 (Suppl. 1), S66–S74. [Google Scholar] [CrossRef]
- Cibula, D.; Pötter, R.; Chiva, L.; Planchamp, F.; Avall-Lundqvist, E.; Cibula, D.; Raspollini, M. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients With Cervical Cancer. Int. J. Gynecol. Cancer 2018, 472, 919–936. [Google Scholar]
- Bichel, P.; Jakobsen, A. Histopathologic grading and prognosis of uterine cervical carcinoma. Am. J. Clin. Oncol. 1985, 8, 247–254. [Google Scholar] [CrossRef]
- Eggen, T.; Arnes, M.; Moe, B.; Straume, B.; Ørbo, A. Prognosis of early cervical cancer (FIGO Stages IA2, IB, and IIA) in northern Norway predicted by malignancy grading score and objective morphometric image analysis. Int. J. Gynecol. Pathol. 2007, 26, 447–456. [Google Scholar] [CrossRef]
- Lindahl, B.; Ranstam, J.; Willen, R. Prospective malignancy grading of invasive squamous carcinoma of the uterine cervix. Prognostic significance in a long-term follow-up. Anticancer Res. 2007, 27, 2829–2832. [Google Scholar]
- Graflund, M.; Sorbe, B.; Hussein, A.; Bryne, M.; Karlsson, M. The prognostic value of histopathologic grading parameters and microvessel density in patients with early squamous cell carcinoma of the uterine cervix. Int. J. Gynecol. Cancer 2002, 12, 32–41. [Google Scholar] [CrossRef]
- Kristensen, G.B.; Abeler, V.M.; Risberg, B.; Tropé, C.; Bryne, M. Tumor size, depth of invasion, and grading of the invasive tumor front are the main prognostic factors in early squamous cell cervical carcinoma. Gynecol. Oncol. 1999, 74, 245–251. [Google Scholar] [CrossRef]
- Horn, L.-C.; Fischer, U.; Raptis, G.; Bilek, K.; Hentschel, B.; Richter, C.; Braumann, U.-D.; Einenkel, J. Pattern of invasion is of prognostic value in surgically treated cervical cancer patients. Gynecol. Oncol. 2006, 103, 906–911. [Google Scholar] [CrossRef]
- Horn, L.C.; Hentschel, B.; Braumann, U.D. Malignancy grading, pattern of invasion, and juxtatumoral stromal response (desmoplastic change) in squamous cell carcinoma of the uterine cervix. Int. J. Gynecol. Pathol. 2008, 27, 606–607. [Google Scholar] [CrossRef]
- Stendahl, U.; Eklund, G.; Willen, R. Invasive squamous cell carcinoma of the uterine cervix. IV. Analysis of a histopathologicmalignancy grading system and construction of a partial index. Acta Radiol. Oncol. 1981, 20, 289–294. [Google Scholar] [CrossRef]
- Jesinghaus, M.; Strehl, J.; Boxberg, M.; Wenzel, A.; Brühl, F.; Konukiewitz, B.; Schlitter, A.; Steiger, K.; Warth, A.; Schnelzer, A.; et al. Introducing a novel highly prognostic grading scheme based on tumor budding and cell nest size for squamous cell carcinoma of the uterine cervix. J. Pathol. Clin. Res. 2018, 4, 93–102. [Google Scholar] [CrossRef]
- Boxberg, M.; Jesinghaus, M.; Dorfner, C.; Mogler, C.; Drecoll, E.; Warth, A.; Steiger, K.; Bollwein, C.; Meyer, P.; Wolff, K.D.; et al. Tumour budding activity and cell nest size determine patient outcome in oral squamous cell carcinoma: Proposal for an adjusted grading system. Histopathology 2017, 70, 1125–1137. [Google Scholar] [CrossRef]
- Jesinghaus, M.; Boxberg, M.; Konukiewitz, B.; Slotta-Huspenina, J.; Schlitter, A.M.; Steiger, K.; Specht, K.; Wieczorek, K.; Warth, A.; Schmidt, T.; et al. A novel grading system based on tumor budding and cell nest size is a strong predictor of patient outcome in esophageal squamous cell carcinoma. Am. J. Surg. Pathol. 2017, 41, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Rogers, A.; Winter, D.C.; Heeney, A.; Gibbons, D.; Lugli, A.; Puppa, G.; Sheahan, K. Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br. J. Cancer 2016, 115, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.B.; Schaeffer, D.F. Tumor budding as a standardized parameter in gastrointestinal carcinomas: More than just the colon. Mod. Pathol. 2018, 31, 862–872. [Google Scholar] [CrossRef]
- Huang, B.; Cai, J.; Xu, X.; Guo, S.; Wang, Z. High-grade tumor budding stratifies early-stage cervical cancer with recurrence risk. PLoS ONE 2016, 11, e0166311. [Google Scholar] [CrossRef]
- Zare, S.Y.; Aisagbonhi, O.; Hasteh, F.; Fadare, O. Independent validation of tumor budding activity and cell nest size as determinants of patient outcome in squamous cell carcinoma of the uterine cervix. Am. J. Surg. Pathol. 2020, 44, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, W.D.; Abdul-Karim, F.W.; Crum, C.; Fu, Y.S. Recommendations for the reporting of surgical specimens containing uterine cervical neoplasms. Mod. Pathol. 2000, 114, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, S.; Ioffe, O. Pathology of cervical cancer. Cancer J. 2003, 9, 335–347. [Google Scholar] [CrossRef]
- Nishio, S.; Mikami, Y.; Tokunaga, H.; Yaegashi, N.; Satoh, T.; Saito, M.; Okamoto, A.; Kasamatsu, T.; Miyamoto, T.; Shiozawa, T.; et al. Analysis of gastric type mucinous carcinoma of the uterine cervix—An aggressive tumor with a poor prognosis: A multi-institutional study. Gynecol. Oncol. 2019, 153, 13–19. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, C.; Fu, L.; Liu, Y.; Zhang, W.; Xu, T. Primary clear cell adenocarcinoma of the cervix: A clinical analysis of 18 cases without exposure to diethlystilbestrol. Obstet. Gynecol. Int. 2019, 2019, 9465375. [Google Scholar] [CrossRef]
- Horn, L.C.; Hentschel, B.; Bilek, K.; Richter, C.E.; Einenkel, J.; Leo, C. Mixed small cell carcinomas of the uterine cervix: Prognostic impact of focal neuroendocrine differentiation but not of Ki-67 labeling index. Ann. Diagn. Pathol. 2006, 10, 140–143. [Google Scholar] [CrossRef]
- Shi, H.; Ye, L.; Lu, W.; Lu, B. Grading of endocervical adenocarcinoma: A novel prognostic system based on tumor budding and cell cluster size. Mod. Pathol. 2022, 35, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Roma, A.A.; Diaz De Vivar, A.; Park, K.J.; Alvarado-Cabrero, I.; Rasty, G.; Chanona-Vilchis, J.G.; Mikami, Y.; Hong, S.R.; Teramoto, N.; Ali-Fehmi, R.; et al. Invasive endocervical adenocarcinoma: A new pattern-based classification system with important clinical significance. Am. J. Surg. Pathol. 2015, 39, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Piovesan, P.; Rey, A.; Atallah, D.; Haie-Meder, C.; Pautier, P.; Sideris, L.; Pomel, C.; Duvillard, P.; Castaigne, D. Prognostic value of lymphovascular space invasion determined with hematoxylin-eosin staining in early stage cervical carcinoma: Results of a multivariate analysis. Ann. Oncol. 2003, 14, 1511–1517. [Google Scholar] [CrossRef]
- Ho, C.M.; Chien, T.Y.; Huang, S.H.; Wu, C.J.; Shih, B.Y.; Chang, S.C. Multivariate analysis of the prognostic factors and outcomes in early cervical cancer patients undergoing radical hysterectomy. Gynecol. Oncol. 2004, 93, 458–464. [Google Scholar] [CrossRef]
- Singh, P.; Tripcony, L.; Nicklin, J. Analysis of prognostic variables, development of predictive models, and stratification of risk groups in surgically treated FIGO early-stage (IA-IIA) carcinoma cervix. Int. J. Gynecol. Cancer 2012, 22, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Sevin, B.U.; Lu, Y.; Bloch, D.A.; Nadji, M.; Koechli, O.R.; Averette, H.E. Surgically defined prognostic parameters in patients with early cervical carcinoma. A multivariate survival tree analysis. Cancer 1996, 78, 1438–1446. [Google Scholar] [CrossRef]
- Yahata, H.; Sonoda, K.; Inoue, S.; Yasutake, N.; Kodama, K.; Yagi, H.; Yasunaga, M.; Ohgami, T.; Onoyama, I.; Kaneki, E.; et al. Is Adjuvant Therapy Necessary for Patients with Intermediate-Risk Cervical Cancer after Open Radical Hysterectomy? Oncology 2020, 98, 853–858. [Google Scholar] [CrossRef]
- Kamura, T.; Tsukamoto, N.; Tsuruchi, N.; Saito, T.; Matsuyama, T.; Akazawa, K.; Nakano, H. Multivariate analysis of the histopathologic prognostic factors of cervical cancer in patients undergoing radical hysterectomy. Cancer 1992, 69, 181–186. [Google Scholar] [CrossRef]
- Bosse, T.; Peters, E.E.; Creutzberg, C.L.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Mens, J.W.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Smit, V.T.; Nout, R.A. Substantial lymph-vascular space invasion (LVSI) is a significant risk factor for recurrence in endometrial cancer—A pooled analysis of PORTEC 1 and 2 trials. Eur. J. Cancer 2015, 51, 1742–1750. [Google Scholar] [CrossRef]
- Boothe, D.; Wolfson, A.; Christensen, M.; Francis, S.; Werner, T.L.; Gaffney, D.K. Lymphovascular Invasion in Endometrial Cancer: Prognostic Value and Implications on Adjuvant Radiation Therapy Use. Am. J. Clin. Oncol. 2019, 42, 549–554. [Google Scholar] [CrossRef]
- Restaino, S.; Tortorella, L.; Dinoi, G.; Zannoni, G.F.; Baroni, A.; Capasso, I.; Distefano, E.; Sozzi, G.; Chiantera, V.; Scambia, G.; et al. Semiquantitative evaluation of lymph-vascular space invasion in patients affected by endometrial cancer: Prognostic and clinical implications. Eur. J. Cancer 2021, 142, 29–37. [Google Scholar] [CrossRef]
- Ronsini, C.; Anchora, L.P.; Restaino, S.; Fedele, C.; Arciuolo, D.; Teodorico, E.; Bizzarri, N.; Zannoni, G.F.; Ferrandina, G.; Scambia, G.; et al. The role of semiquantitative evaluation of lympho-vascular space invasion in early stage cervical cancer patients. Gynecol. Oncol. 2021, 162, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Pol, F.J.; Zusterzeel, P.L.; van Ham, M.A.; Kuijpers, D.A.; Bulten, J.; Massuger, L.F. Satellite lymphovascular space invasion: An independent risk factor in early stage cervical cancer. Gynecol. Oncol. 2015, 138, 579–584. [Google Scholar] [CrossRef]
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer: A review of the literature. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef]
- Gil, Z.; Carlson, D.L.; Gupta, A.; Lee, N.; Hoppe, B.; Shah, J.P.; Kraus, D.H. Patterns and incidence of neural invasion in patients with cancers of the paranasal sinuses. Arch. Otolaryngol. Neck Surg. 2009, 135, 173–179. [Google Scholar] [CrossRef]
- Liebig, C.; Ayala, G.; Wilks, J.; Verstovsek, G.; Liu, H.; Agarwal, N.; Berger, D.H.; Albo, D. Perineural invasion is an independent predictor of outcome in colorectal cancer. J. Clin. Oncol. 2009, 27, 5131–5137. [Google Scholar] [CrossRef] [PubMed]
- Harnden, P.; Shelley, M.D.; Clements, H.; Coles, B.; Tyndale-Biscoe, R.S.; Naylor, B.; Mason, M.D. The prognostic significance of perineural invasion in prostatic cancer biopsies: A systematic review. Cancer 2007, 109, 13–24. [Google Scholar] [CrossRef]
- Sudo, T.; Murakami, Y.; Uemura, K.; Hayashidani, Y.; Hashimoto, Y.; Ohge, H.; Shimamoto, F.; Sueda, T. Prognostic impact of perineural invasion following pancreatoduodenectomy with lymphadenectomy for ampullary carcinoma. Dig. Dis. Sci. 2008, 53, 2281–2286. [Google Scholar] [CrossRef]
- Wan, T.; Tu, H.; Liu, L.; Huang, H.; Feng, Y.; Liu, J. Perineural Invasion Should Be Regarded as an Intermediate-Risk Factor for Recurrence in Surgically Treated Cervical Cancer: A Propensity Score Matching Study. Dis. Markers 2021, 2021, 1375123. [Google Scholar] [CrossRef]
- Gadducci, A.; Pistolesi, S.; Cosio, S.; Naccarato, A.G. Is Perineural Invasion a Novel Prognostic Factor Useful to Tailor Adjuvant Treatment in Patients Treated With Primary Surgery for Cervical and Vulvar Carcinoma? Anticancer Res. 2020, 40, 3031–3037. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Shi, Y.; Zhang, G.N. Perineural invasion as a prognostic factor for cervical cancer: A systematic review and meta-analysis. Arch Gynecol Obstet. 2015, 292, 13–19. [Google Scholar] [CrossRef]
- Cho, H.C.; Kim, H.; Cho, H.Y.; Kim, K.; No, J.H.; Kim, Y.B. Prognostic significance of perineural invasion in cervical cancer. Int. J. Gynecol. Pathol. 2013, 32, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Sedlis, A.; Bundy, B.N.; Rotman, M.Z.; Lentz, S.S.; Muderspach, L.I.; Zaino, R.J. A Randomized Trial of Pelvic Radiation Therapy versus No Further Therapy in Selected Patients with Stage IB Carcinoma of the Cervix after Radical Hysterectomy and Pelvic Lymphadenectomy: A Gynecologic Oncology Group Study. Gynecol. Oncol. 1999, 73, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Allam, M.; Feely, C.; Millan, D.; Nevin, J.; Davis, J.; Siddiqui, N. Depth of cervical stromal invasion as a prognostic factor after radical surgery for early stage cervical cancer. Gynecol. Oncol. 2004, 93, 637–641. [Google Scholar] [CrossRef]
- Stolnicu, S.; Hoang, L.; Almadani, N.; De Brot, L.; Bovolim, G.; Baiocchi, G.; Brito, M.J.; Karpathiou, G.; Ieni, A.; Fernandez, E.G.; et al. Horizontal tumor extent (HZTE) has limited prognostic significance in 2018 FIGO stage I endocervical adenocarcinoma (ECA): A retrospective study of 416 cases. J. Cancer Res. Clin. Oncol. 2022, 148, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.J.; Kim, K.R.; Song, D.E.; Ro, J.Y.; Kong, K.Y.; Lee, S.W.; Nam, J.H. Recognition of parametrial invasion, an important landmark when treating cervical cancer. Gynecol. Oncol. 2012, 124, 502–507. [Google Scholar] [CrossRef]
- Zreik, T.G.; Chambers, J.T.; Chambers, S.K. Parametrial involvement, regardless of nodal status: A poor prognostic factor for cervical cancer. Obstet. Gynecol. 1996, 87 Pt 1, 741–746. [Google Scholar] [CrossRef]
- van den Tillaart, S.A.; Trimbos, J.B.; Dreef, E.J.; Jordanova, E.S.; Fleuren, G.J. Patterns of parametrial involvement in radical hysterectomy specimens of cervical cancer patients. Int. J. Gynecol. Pathol. 2011, 30, 185–192. [Google Scholar] [CrossRef]
- Bizzarri, N.; Pedone Anchora, L.; Zannoni, G.F.; Carbone, V.; Bruno, M.; Fedele, C.; Gallotta, V.; Chiantera, V.; Avesani, G.; Gui, B.; et al. Validation of tumour-free distance as novel prognostic marker in early-stage cervical cancer: A retrospective, single-centre, cohort study. Br. J. Cancer 2021, 125, 561–568. [Google Scholar] [CrossRef]
- Tsukamoto, T. The studies on the spreading modus of the uterine cervical cancer: Especially a new proposal of the postoperative histological classification. Shinshu Med. J. 1966, 15, 354–383. [Google Scholar]
- Saatli, B.; Olgan, S.; Gorken, I.B.; Uslu, T.; Saygili, U.; Dicle, N.; Cingillioglu, B.; Gumurdulu, D.; Guzel, A.B.; Koyuncuoglu, M. Tumor-free distance from outermost layer of cervix is of prognostic value in surgically treated cervical cancer patients: A multicenter study. Arch. Gynecol. Obstet. 2014, 289, 1331–1335. [Google Scholar] [CrossRef]
- Cibula, D.; Slama, J.; Dostálek, L.; Fischerová, D.; Germanova, A.; Frühauf, F.; Dundr, P.; Nemejcova, K.; Jarkovsky, J.; Sebestova, S.; et al. Tumour-free distance: A novel prognostic marker in patients with early-stage cervical cancer treated by primary surgery. Br. J. Cancer 2021, 124, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Du, L.; Li, H.; Zhu, X.; Cui, L.; Li, X. Tumor-infiltrating lymphocytes: Warriors fight against tumors powerfully. Biomed. Pharmacother. 2020, 132, 110873. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, M.; Beduk Esen, C.S.; Ates Ozdemir, D.; Yildirim, S.; Yuce, D.; Usubutun, A.; Yildiz, F. Stromal or intraepithelial tumor-infiltrating lymphocytes: Which one has more prognostic significance in cervical cancer? Arch. Gynecol. Obstet. 2022, 1–12. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, A.X.J.; Chen, G.; Wu, Y.; Gu, W. Prognostic and therapeutic TILs of cervical cancer-Current advances and future perspectives. Mol. Ther. Oncolytics 2021, 22, 410–430. [Google Scholar] [CrossRef]
- Stevanović, S.; Draper, L.M.; Langhan, M.M.; Campbell, T.E.; Kwong, M.L.; Wunderlich, J.R.; Dudley, M.E.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J. Clin. Oncol. 2015, 33, 1543–1550. [Google Scholar] [CrossRef]
- Yin, H.; Guo, W.; Sun, X.; Li, R.; Feng, C.; Tan, Y. TILs and Anti-PD1 Therapy: An Alternative Combination Therapy for PDL1 Negative Metastatic Cervical Cancer. J. Immunol. Res. 2020, 2020, 8345235. [Google Scholar] [CrossRef]
- Ohno, A.; Iwata, T.; Katoh, Y.; Taniguchi, S.; Tanaka, K.; Nishio, H.; Nakamura, M.; Morisada, T.; Chen, G.; Saito, M.; et al. Tumor-infiltrating lymphocytes predict survival outcomes in patients with cervical cancer treated with concurrent chemoradiotherapy. Gynecol. Oncol. 2020, 159, 329–334. [Google Scholar] [CrossRef]
- Khanna, N.; Rauh, L.A.; Lachiewicz, M.P.; Horowitz, I.R. Margins for cervical and vulvar cancer. J. Surg. Oncol. 2016, 113, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.N.; Lee, H.; Hanson, E.; Berkowitz, R.S.; Crum, C.P. Influence of margin status and radiation on recurrence after radical hysterectomy in Stage IB cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 1501–1507. [Google Scholar] [CrossRef]
- McCann, G.A.; Taege, S.K.; Boutsicaris, C.E.; Phillips, G.S.; Eisenhauer, E.L.; Fowler, J.M.; O’Malley, D.M.; Copeland, L.J.; Cohn, D.E.; Salani, R. The impact of close surgical margins after radical hysterectomy for early-stage cervical cancer. Gynecol. Oncol. 2013, 128, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Machida, H.; Mandelbaum, R.S.; Konishi, I.; Mikami, M. Validation of the 2018 FIGO cervical cancer staging system. Gynecol. Oncol. 2019, 152, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Matsuo, K.; Huang, Y.; Tergas, A.I.; Hou, J.Y.; Khoury-Collado, F.; St Clair, C.M.; Ananth, C.V.; Neugut, A.I.; Hershman, D.L. Prognostic Performance of the 2018 International Federation of Gynecology and Obstetrics Cervical Cancer Staging Guidelines. Obstet. Gynecol. 2019, 134, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.; Murali, R.; Park, K.J. Secondary Involvement of the Adnexa and Uterine Corpus by Carcinomas of the Uterine Cervix: A Detailed Morphologic Description. Int. J. Gynecol. Pathol. 2015, 34, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Gungor, T.; Altinkaya, S.O.; Ozat, M.; Akbay, S.; Mollamahmutoglu, L. Unusual form of superficial spreading squamous cell carcinoma of cervix involving the endometrium, bilateral tubes and ovaries: A case report with literature review. Arch. Gynecol. Obstet. 2011, 283, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Machida, H.; Blake, E.A.; Takiuchi, T.; Mikami, M.; Roman, L.D. Significance of uterine corpus tumor invasion in early-stage cervical cancer. Eur. J. Surg. Oncol. 2017, 43, 725–734. [Google Scholar] [CrossRef]
- Kim, M.J.; Chung, H.H.; Kim, J.W.; Park, N.H.; Song, Y.S.; Kang, S.B. Uterine corpus involvement as well as histologic type is an independent predictor of ovarian metastasis in uterine cervical cancer. J. Gynecol. Oncol. 2008, 19, 181–184. [Google Scholar] [CrossRef]
- He, F.; Li, W.; Liu, P.; Kang, S.; Sun, L.; Zhao, H.; Chen, X.; Yin, L.; Wang, L.; Chen, J.; et al. Influence of uterine corpus invasion on prognosis in stage IA2-IIB cervical cancer: A multicenter retrospective cohort study. Gynecol. Oncol. 2020, 158, 273–281. [Google Scholar] [CrossRef]
- Tabata, M.; Ichinoe, K.; Sakuragi, N.; Shiina, Y.; Yamaguchi, T.; Mabuchi, Y. Incidence of ovarian metastasis in patients with cancer of the uterine cervix. Gynecol. Oncol. 1987, 28, 255–261. [Google Scholar] [CrossRef]
- Shimada, M.; Kigawa, J.; Nishimura, R.; Yamaguchi, S.; Kuzuya, K.; Nakanishi, T.; Suzuki, M.; Kita, T.; Iwasaka, T.; Terakawa, N. Ovarian metastasis in carcinoma of the uterine cervix. Gynecol. Oncol. 2006, 101, 234–237. [Google Scholar] [CrossRef]
- Casey, L.; Singh, N. Metastases to the ovary arising from endometrial, cervical and fallopian tube cancer: Recent advances. Histopathology 2020, 76, 37–51. [Google Scholar] [CrossRef]
- Chen, N.J. Vagina invasion by cervical carcinoma. Acta Med. Okayama 1984, 38, 305–313. [Google Scholar] [PubMed]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri: 2021 update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. S1), 28–44. [Google Scholar] [CrossRef]
- Gurram, L.; Patil, R.; Chopra, S.; Maheshwari, A.; Shylasree, T.S.; Gupta, S.; Ghosh, J.; Gulia, S.; Ghadi, Y.; Dheera, A.; et al. Evaluation of outcomes in patients of cervical Cancer with lower one third vaginal involvement: A single institutional experience. Gynecol. Oncol. 2020, 159, 359–364. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Lehne, B.; Bhatia, R.; Lyons, D.; Paraskevaidis, E.; Li, J.V.; et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci. Rep. 2015, 5, 16865. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zheng, Z.; Zhu, J.; Hu, K.; Hou, X.; Shen, J.; Lian, X.; Sun, S.; Miao, Z.; Shen, J.; et al. Radiotherapy for Vaginal Recurrences of Cervical Cancer in Patients After Prior Surgery: Analysis of Effect and Prognostic Factors. Front. Oncol. 2021, 11, 744871. [Google Scholar] [CrossRef] [PubMed]
- Guani, B.; Mahiou, K.; Crestani, A.; Cibula, D.; Buda, A.; Gaillard, T.; Mathevet, P.; Kocian, R.; Sniadecki, M.; Wydra, D.G.; et al. Clinical impact of low-volume lymph node metastases in early-stage cervical cancer: A comprehensive meta-analysis. Gynecol. Oncol. 2022, 164, 446–454. [Google Scholar] [CrossRef]
- Buckley, S.L.; Tritz, D.M.; Van Le, L.; Higgins, R.; Sevin, B.U.; Ueland, F.R.; DePriest, P.D.; Gallion, H.H.; Bailey, C.L.; Kryscio, R.J.; et al. Lymph node metastases and prognosis in patients with stage IA2 cervical cancer. Gynecol. Oncol. 1996, 63, 4–9. [Google Scholar] [CrossRef]
- Gien, L.T.; Covens, A. Lymph node assessment in cervical cancer: Prognostic and therapeutic implications. J. Surg. Oncol. 2009, 99, 242–247. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, K.; Liu, A.; Shen, J.; Hou, X.; Lian, X.; Sun, S.; Yan, J.; Zhang, F. Patterns of lymph node metastasis in locally advanced cervical cancer. Medicine 2016, 95, e4814. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Park, H.; Kim, M.S.; Kang, J.H.; Paik, E.S.; Lee, Y.Y.; Kim, T.J.; Lee, J.W.; Kim, B.G.; Bae, D.S.; et al. Pretreatment Lymph Node Metastasis as a Prognostic Significance in Cervical Cancer: Comparison between Disease Status. Cancer Res. Treat. 2020, 52, 516–523. [Google Scholar] [CrossRef]
- Du, R.; Li, L.; Ma, S.; Tan, X.; Zhong, S.; Wu, M. Lymph nodes metastasis in cervical cancer: Incidences, risk factors, consequences and imaging evaluations. Asia Pac. J. Clin. Oncol. 2018, 14, e380–e385. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tao, X.; Wu, X.; Jiang, H.; Xia, H. Number of Removed Pelvic Lymph Nodes as a Prognostic Marker in FIGO Stage IB1 Cervical Cancer with Negative Lymph Nodes. J. Minim. Invasive Gynecol. 2020, 27, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Kim, T.H.; Lee, M.; Kim, H.S.; Chung, H.H.; Lee, T.S.; Jeon, H.W.; Kim, J.W.; Park, N.H.; Song, Y.S. Lymph Node Ratio Is a Strong Prognostic Factor in Patients with Early-Stage Cervical Cancer Undergoing Minimally Invasive Radical Hysterectomy. Yonsei Med. J. 2021, 62, 231–239. [Google Scholar] [CrossRef]
- Aslan, K.; Meydanli, M.M.; Oz, M.; Tohma, Y.A.; Haberal, A.; Ayhan, A. The prognostic value of lymph node ratio in stage IIIC cervical cancer patients triaged to primary treatment by radical hysterectomy with systematic pelvic and para-aortic lymphadenectomy. J. Gynecol. Oncol. 2020, 31, e1. [Google Scholar] [CrossRef]
- Fleming, N.D.; Frumovitz, M.; Schmeler, K.M.; dos Reis, R.; Munsell, M.F.; Eifel, P.J.; Soliman, P.T.; Nick, A.M.; Westin, S.N.; Ramirez, P.T. Significance of lymph node ratio in defining risk category in node-positive early stage cervical cancer. Gynecol. Oncol. 2015, 136, 48–53. [Google Scholar] [CrossRef]
- Cui, H.; Huang, Y.; Wen, W.; Li, X.; Xu, D.; Liu, L. Prognostic value of lymph node ratio in cervical cancer: A meta-analysis. Medicine 2022, 101, e30745. [Google Scholar] [CrossRef] [PubMed]
- Llueca, A.; Escrig, J.; Gil-Moreno, A.; Benito, V.; Hernández, A.; Díaz-Feijoo, B.; Spain-Gynecologic Oncology Group (GOG) Working Group. The extent of aortic lymphadenectomy in locally advanced cervical cancer impacts on survival. J. Gynecol. Oncol. 2021, 32, e4. [Google Scholar] [CrossRef]
- Kwon, J.; Eom, K.Y.; Kim, I.A.; Kim, J.S.; Kim, Y.B.; No, J.H.; Kim, K. Prognostic Value of Log Odds of Positive Lymph Nodes after Radical Surgery Followed by Adjuvant Treatment in High-Risk Cervical Cancer. Cancer Res. Treat. 2016, 48, 632–640. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Cheng, X. Advances in diagnosis and treatment of metastatic cervical cancer. J. Gynecol. Oncol. 2016, 27, e43. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Peng, F. Patterns of metastases in cervical cancer: A population-based study. Int. J. Clin. Exp. Pathol. 2020, 13, 1615–1623. [Google Scholar]
- Yin, Z.; Tang, H.; Li, L.; Ni, J.; Yuan, S.; Lou, H.; Chen, M. Impact of sites versus number of metastases on survival of patients with organ metastasis from newly diagnosed cervical cancer. Cancer Manag. Res. 2019, 11, 7759–7766. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, M.H.; Kim, B.J.; Park, S.I.; Ryu, S.Y.; Cho, C.K. Prognostic importance of the site of recurrence in patients with metastatic recurrent cervical cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, S.D.; Balakrishnan, V.; Oon, C.E.; Kaur, G. Key Molecular Events in Cervical Cancer Development. Medicina 2019, 55, 384. [Google Scholar] [CrossRef]
- de Maria, S.; Santoro, A.; Fuggetta, M.P.; Rocchetti, R.; Cottarelli, A.; Lanzilli, G.; Stiuso, P.; Angelico, G.; Spadola, S.; Franco Zannoni, G.; et al. A possible interplay between HR-HPV and stemness in tumor development: An in vivo investigation of CD133 as a putative marker of cancer stem cell in HPV18-infected KB cell line. Apmis 2020, 128, 637–646. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Albert Einstein College of Medicine; Analytical Biological Services. Integrated genomic and molecular characterization of cervical cancer. Nature 2017, 543, 378–384. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakayama, K.; Minamoto, T.; Ishibashi, T.; Ohnishi, K.; Yamashita, H.; Ono, R.; Sasamori, H.; Razia, S.; Hossain, M.M.; et al. Lynch Syndrome-Related Clear Cell Carcinoma of the Cervix: A Case Report. Int. J. Mol. Sci. 2018, 19, 979. [Google Scholar] [CrossRef] [PubMed]
- Contos, G.; Baca, Y.; Xiu, J.; Brown, J.; Holloway, R.; Korn, W.; Herzog, T.; Jones, N.; Winer, I. Assessment of immune biomarkers and establishing a triple negative phenotype in gynecologic cancers. Gynecol. Oncol. 2021, 163, 312–319. [Google Scholar] [CrossRef]
- Ji, X.; Sui, L.; Song, K.; Lv, T.; Zhao, H.; Yao, Q. PD-L1, PARP1 and MMRs as potential therapeutic biomarkers for neuroendocrine cervical cancer. Cancer Med. 2021, 10, 4743–4751. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N. Pembrolizumab for persistent, reccurent or metastatic cervical cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef]
| Squamous Cell Carcinoma | ||
|---|---|---|
| Established Prognostic Factor | Novel Prognostic Factor | Uncertain Prognostic Utility |
| HPV status | Tumor-budding/Cell nest size | Grading |
| Depth of stomal invasion LVSI Parametrial extension Margin status | Tumor-free distance (TFD) Perineural Invasion (PNI) | Horizontal extension |
| TILS | ||
| Adenocarcinoma | ||
|---|---|---|
| Established Prognostic Factor | Novel Prognostic Factor | Uncertain Prognostic Utility |
| HPV status | Tumor-budding/Cell nest size | Grading Neuroendocrine differentiation Horizontal extension |
| Silva pattern of invasion | ||
| Depth of stomal invasion LVSI | Tumor-free distance (TFD) | |
| Parametrial extension Margin status | Perineural Invasion (PNI) | |
| TILS | ||
| Special histologic types (gastric-type, clear cell, mesonephric, micropapillary, signet ring, invasive stratified mucinous carcinoma) | ||
| Squamous Cell Carcinoma/Adenocarcinoma | ||
|---|---|---|
| Established Prognostic Factor | Novel Prognostic Factor | Uncertain Prognostic Utility |
| Size metastasis Number of metastatic lymph nodes | Metastatic Lymph Node Ratio | Endometrial extension |
| Location of metastatic lymph nodes | Log Odds of Positive LNs (LODDs) | Adnexal extension |
| Vaginal extension | ||
| Distant metastases | ||
| Molecular Marker with Prognostic Utility |
|---|
| TCGA molecular subgroups keratin-low/keratin-high squamous cell carcinomas adenocarcinoma-rich endometrial-like |
| Therapeutic targets |
| Microsatellite instability |
| ERBB3 (HER3) |
| BCAR4 |
| PDLI1 |
| PDL2 |
| Deregulated pathways Wnt PI3K/AKT/mTOR VEGF EGFR Notch Hedgeog |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, A.; Inzani, F.; Angelico, G.; Arciuolo, D.; Bragantini, E.; Travaglino, A.; Valente, M.; D’Alessandris, N.; Scaglione, G.; Sfregola, S.; et al. Recent Advances in Cervical Cancer Management: A Review on Novel Prognostic Factors in Primary and Recurrent Tumors. Cancers 2023, 15, 1137. https://doi.org/10.3390/cancers15041137
Santoro A, Inzani F, Angelico G, Arciuolo D, Bragantini E, Travaglino A, Valente M, D’Alessandris N, Scaglione G, Sfregola S, et al. Recent Advances in Cervical Cancer Management: A Review on Novel Prognostic Factors in Primary and Recurrent Tumors. Cancers. 2023; 15(4):1137. https://doi.org/10.3390/cancers15041137
Chicago/Turabian StyleSantoro, Angela, Frediano Inzani, Giuseppe Angelico, Damiano Arciuolo, Emma Bragantini, Antonio Travaglino, Michele Valente, Nicoletta D’Alessandris, Giulia Scaglione, Stefania Sfregola, and et al. 2023. "Recent Advances in Cervical Cancer Management: A Review on Novel Prognostic Factors in Primary and Recurrent Tumors" Cancers 15, no. 4: 1137. https://doi.org/10.3390/cancers15041137
APA StyleSantoro, A., Inzani, F., Angelico, G., Arciuolo, D., Bragantini, E., Travaglino, A., Valente, M., D’Alessandris, N., Scaglione, G., Sfregola, S., Piermattei, A., Cianfrini, F., Roberti, P., & Zannoni, G. F. (2023). Recent Advances in Cervical Cancer Management: A Review on Novel Prognostic Factors in Primary and Recurrent Tumors. Cancers, 15(4), 1137. https://doi.org/10.3390/cancers15041137








