Green Tea in Reproductive Cancers: Could Treatment Be as Simple?
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
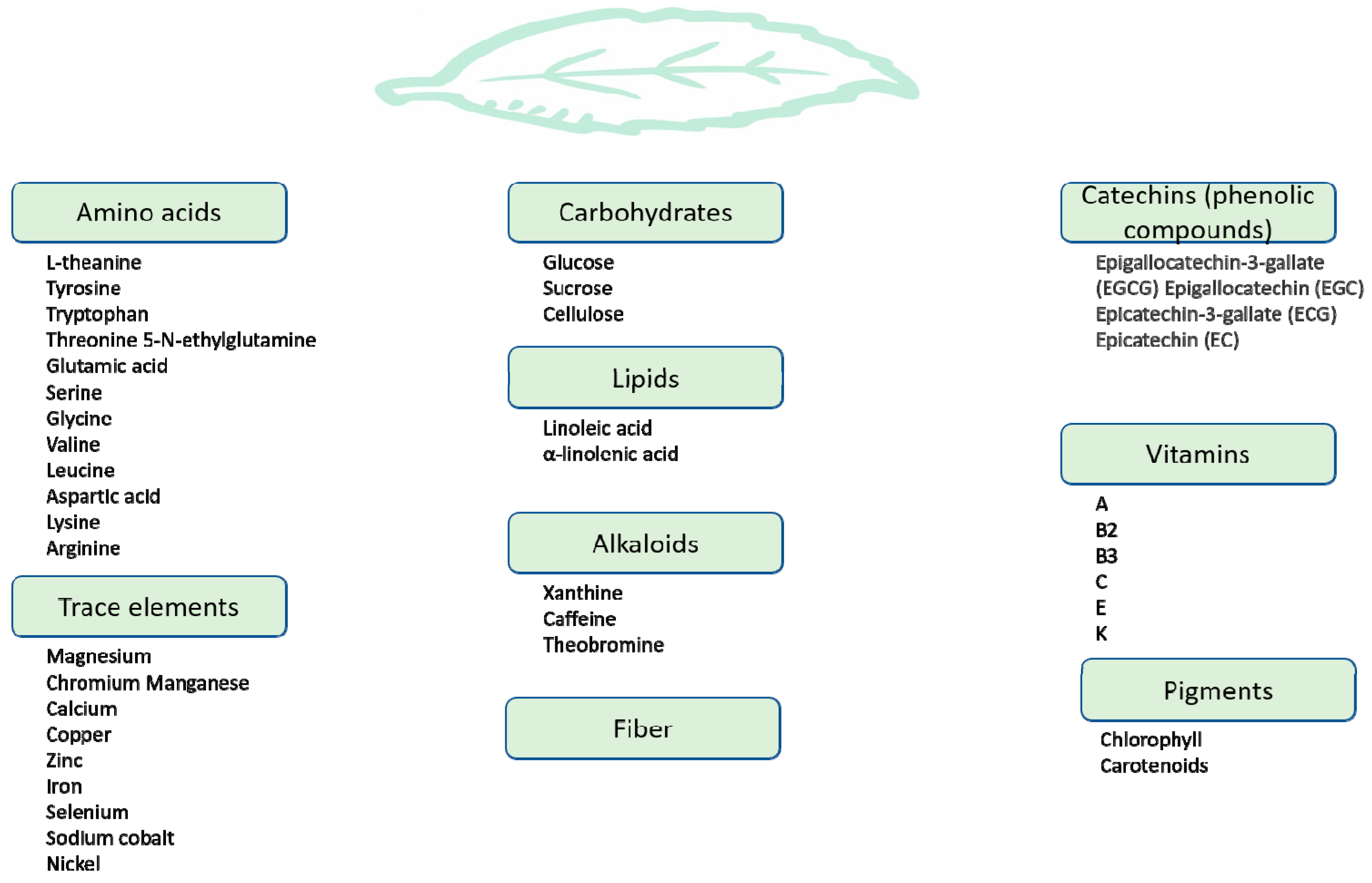
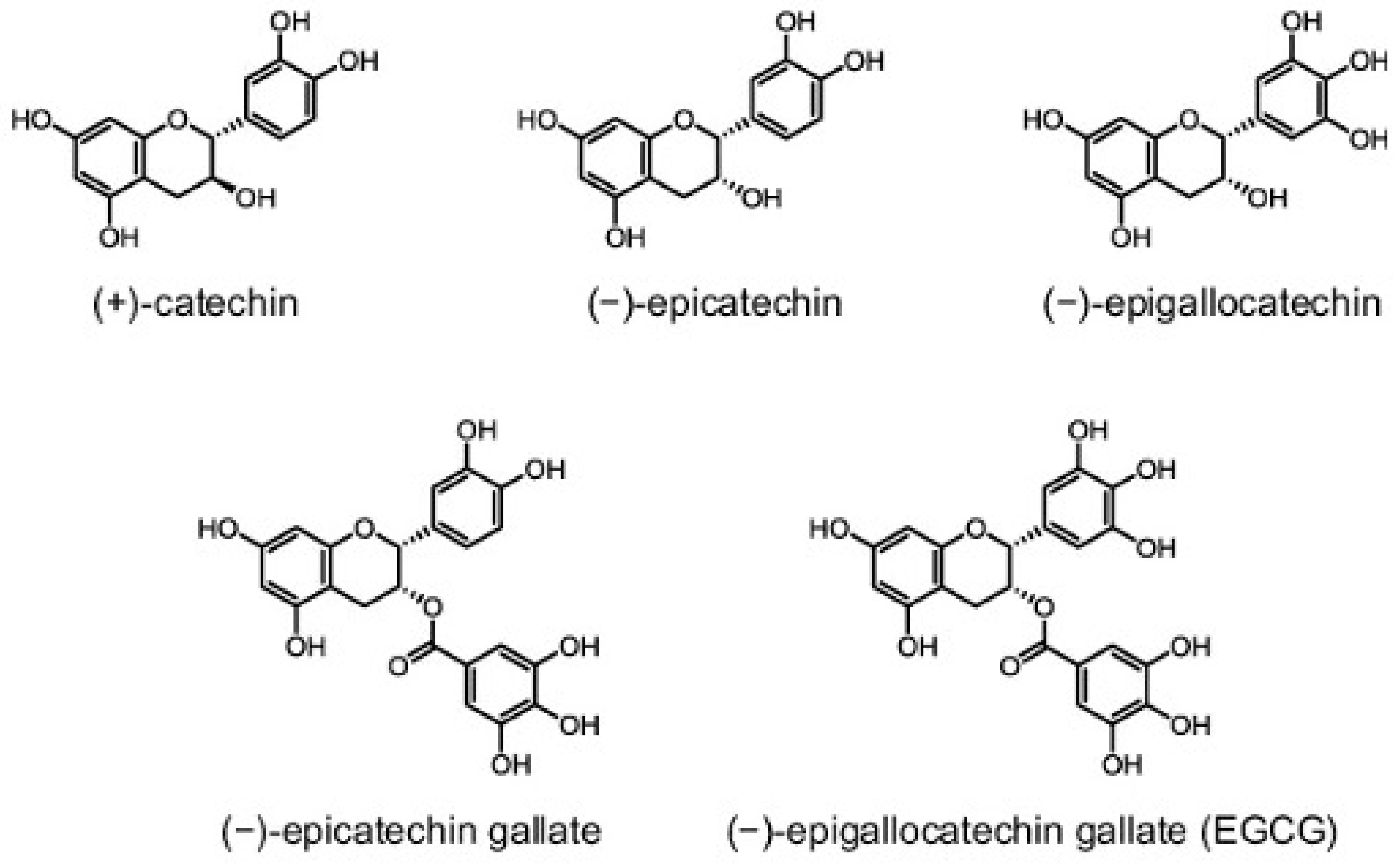
3. Green Tea: Chemistry and Bioavailability
4. Green Tea: Mechanisms of Actions
4.1. EGCG-Interacting Proteins
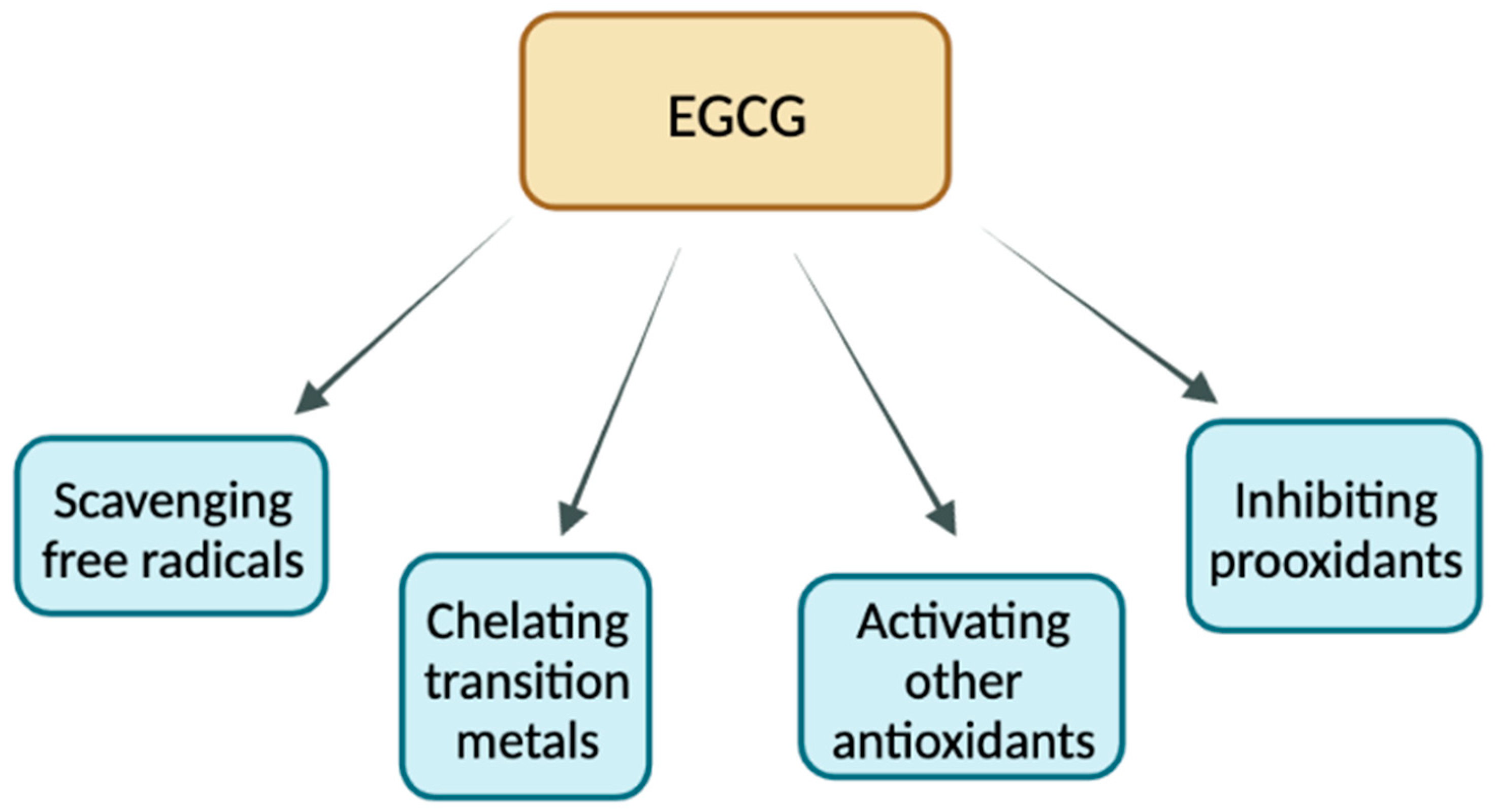
4.2. Antioxidant Properties of EGCG
4.3. ECCG and Lipid Peroxidation
4.4. Hypocholesterolemic Properties of EGCG
4.5. Pro-Oxidant Properties of EGCG
4.6. EGCG and Apoptosis
4.7. EGCG’s Role in Epigenetic Regulation
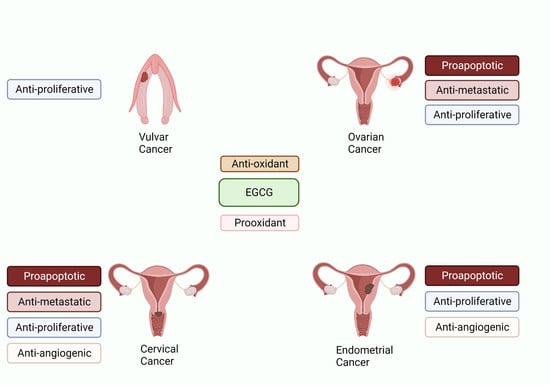
5. Role of Green Tea against Reproductive Cancers
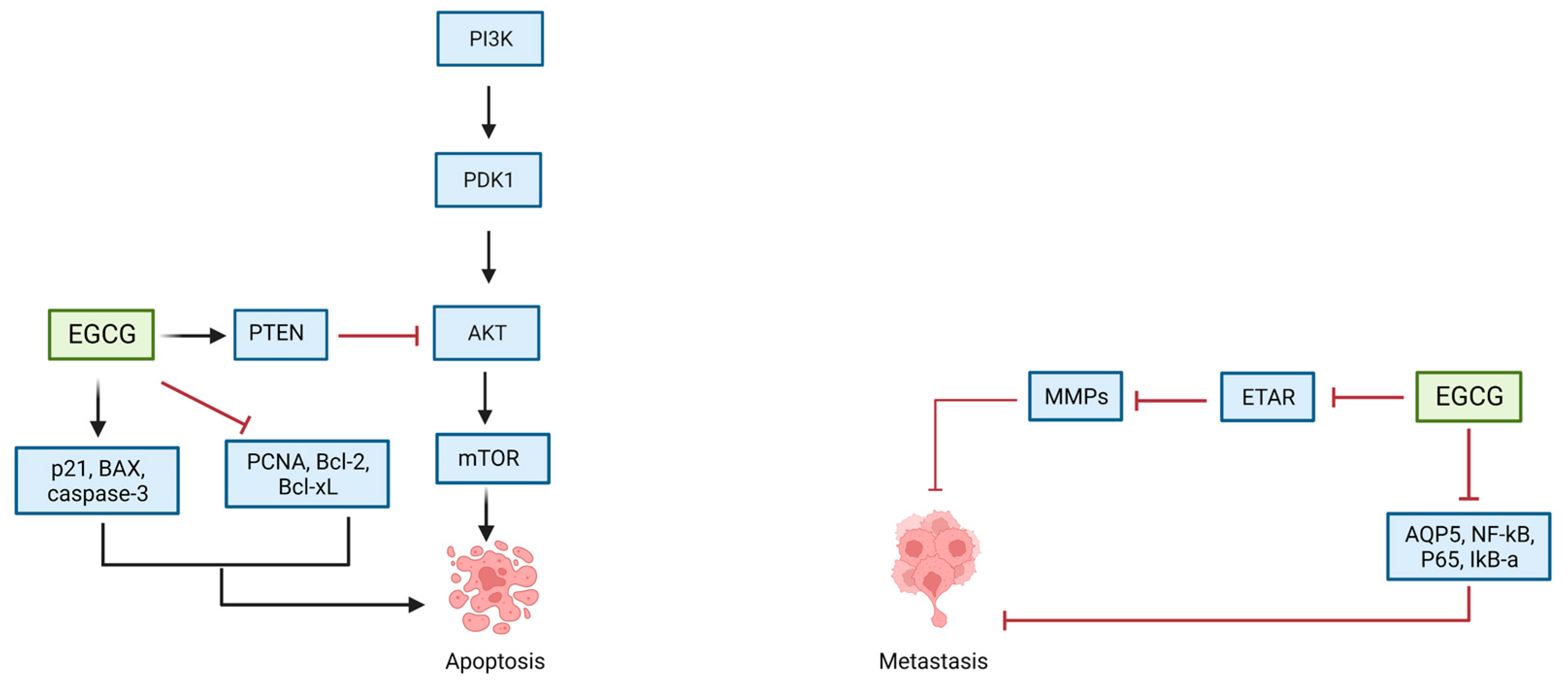
5.1. EGCG and Ovarian Cancer
| Study and Year | Cell Lines | Green Tea Extract/EGCG Concentrations | Molecular Targets | Mechanisms of Action | |
|---|---|---|---|---|---|
| Ovarian Cancer | Rao et al. (2010) [111] | SKOV-3 | 10–80 μg/mL | DNA fragmentation Cell morphological changes Cell cycle arrest | Inhibition of cellular proliferation Induction of apoptosis |
| Qin et al. (2020) [112] | SKOV-3, CAOV-3, NIH-OVCAR-3 | 0, 5, 10, 20, 40, and 80 μg/mL | Inhibiton of PTEN/AKT/Mtor signaling pathway Upregulation of caspase-3 and Bax Downregulation of Bcl-2 | Inhibition of cellular proliferation Induction of apoptosis | |
| Seung et al. (2004) [113] | SKOV-3, OVCAR-3, PA-1 | 6.25, 12.5, 25, 50, 100 μM | Cell cycle arrest in G1 phase Increased expression of p21WAF, Bax Decreased expression of PCNA, and Bcl-Xl | Inhibiton of cellular proliferation Induction of apoptosis | |
| Yan et al. (2012) [114] | SOV-3 | 20–100 μg/mL | Downregulation of p65, AQP5, NF-Κb | Inhibition of cellular proliferation Induction of apoptosis | |
| Spinella et al. (2006) [115] | HEY, OVCA-433 | 20–40 μM | Decreased expression of ETAR and ET-1 Reduction in ETAR-dependent signaling pathways | Inhibition of cellular proliferation Induction of apoptosis | |
| Cervical Cancer | Zou et al. (2010) [125] | HeLa, Me180, TCL1 | 1, 5, 10, 25, 50 μg/mL | Increased expression of p53 and p21 | Inhibition of cellular proliferation Induction of apoptosis |
| Singh et al. (2011) [126] | HeLa | 10, 15, 20 μg/mL | Cell cycle arrest in G1 phase Increase in reactive oxygen species, p53 and Bax expression, cytochrome c release Inhibition of AKT, NF-Κb and cyclin D1 | Inhibition of cellular proliferation Induction of apoptosis | |
| Kuhn et al. (2003) [127] | HeLa | 10 μM | Inhibition of ubiquitin/proteasome protein degradation Increase in p27 and Bax proteins | Induction of apoptosis | |
| Bonfili et al. (2011) [128] | HeLa | 0–140 μM | Inhibition of proteasome Accumulation of p53, p27 and IκB-α | Induction of apoptosis | |
| Li et al. (2007) [129] | HeLa | 2.5–20 μM | Inhibition of IGF-IR | Inhibition of cellular proliferation and anchorage independent cell transformation | |
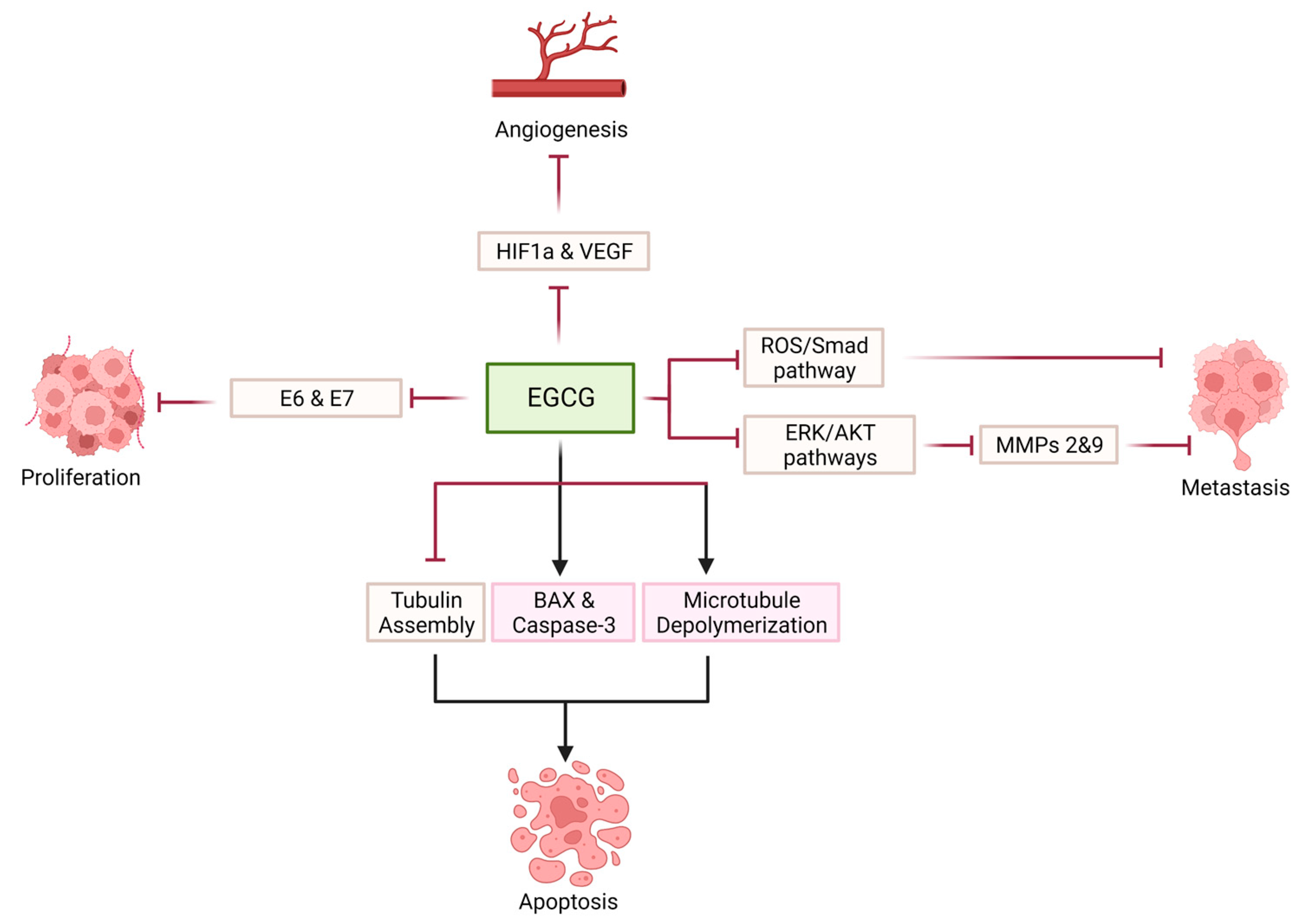
| Chakrabarty et al. (2015) [130] | HeLa | 10–50 μM | Depolymerization of cellular microtubules | Inhibition of cellular proliferation | |
| Ahn et al. (2003) [131] | CaSki | 35 μM | Cell cycle arrest at G1 | Inhibition of cellular proliferation Induction of apoptosis | |
| Qiao et al. (2009) [132] | CaSki, HeLa | 25, 50 μM | Inhibition of HPV E6 and E7 Decrease ER-α and aromatase expression | Inhibition of cellular proliferation | |
| Sah et al. (2004) [133] | HeLa, CaSki, SiHa | 5–50 μM | Inhibition of EGFR, ERK1/2 and AKT activation Increase in p53, p21, p27 Decrease in CDK2 kinase activity | Inhibition of cellular proliferation Induction of apoptosis | |
| Zhang et al. (2006) [134] | HeLa | 10–100 μmol/L | Inhibition of HIF-1 α protein accumulation VEGF expression Inhibition of PI3K/AKT and ERK1/2 signaling pathways | Inhibition of angiogenesis | |
| Tudoran et al. (2012) [135] | HeLa | 10 μM | Downregulation of PDFA and TGF-β2 Upregulation of IL-1 β Maintenance of cellular morphology | Inhibition of cellular proliferation Inhibition of angiogenesis Inhibition of metastasis | |
| Sharma et al. (2012) [136] | HeLa | 25 μM | Decreased expression of MMP-9 Increased expression of TIMP-1 | Inhibition of cellular proliferation Induction of apoptosis Inhibition of metastasis | |
| Roomi et al. (2010) [137] | HeLa, DoTc2-4510 | 10–100 μM | Inhibition of MMP-2 and MMP-9 expression | Inhibition of metastasis | |
| Panji et al. (2021) [138] | HeLa, SiHa | 0–100 μmol/L | Inhibition of TGF- β induced epithelial to mesenchymal transition | Inhibition of metastasis | |
| Endometrial Cancer | Manohar et al. (2013) [139] | Ishikawa | 100–150 μM | Decreased activation of ERK Upregulation of Bax and downregulation of bcl-2 Increase ROS and p38 activation | Inhibition of cellular proliferation Induction of apoptosis |
| Park et al. (2012) [140] | Ishikawa | 50, 100 μM | Cell cycle arrest Inhibition of MAPK and AKT signaling pathways Increase Bax/bcl ratio | Inhibition of cellular proliferation Induction of apoptosis | |
| Man et al. (2020) [141] | RL95-2, AN3 CA | 20, 40, 60 μM (Pro-EGCG) | Activation of p38 MAPK Inhibition of AKT/ERK signaling pathway | Inhibition of cellular proliferation Induction of apoptosis | |
| Wang et al. (2018) [142] | RL95-2, AN3 CA | 20, 40, 60 μM (Pro-EGCG) | Decrease VEGF by inhibiting PI3K/AKT/mTOR/HIF-1 α signaling pathway | Inhibition of angiogenesis | |
| Vulvar Cancer | Yap et al. (2021) [143] | HFK-HPV18, VIN cl.11 | 100 μM | Downregulation of E6 and E7 expression | Inhibition of cellular proliferation |
| Study and Year | Animal Model | Green Tea Extract/EGCG Concentrations | Molecular Targets | Mechanisms of Action | |
|---|---|---|---|---|---|
| Ovarian Cancer | Qin et al. (2020) [112] | Female BALB/c nude mice | 10, 30 or 50 mg/kg | Inhibition of PTEN/AKT/mTOR signaling pathway | Inhibition of ovarian tumor growth |
| Spinella et al. (2006) [115] | Female athymic (nu+/nu+) mice | 12.4 g/L | Decreased expression of ETAR and ET-1 | Inhibition of ovarian tumor growth | |
| Cervical Cancer | Roomi et al. (2015) [144] | Female nude mice | 0.5% supplementation with dietary mixture | Increase in extracellular matrix proteins | Inhibition of tumor growth |
| Roomi et al. (2015) [145] | Female athymic nude mice | 0.5% supplementation with dietary mixture | Decreased MMP-2 and MMP-9, Bcl-2 and VEGF expression | Inhibition of proliferation Inhibition of angiogenesis Inhibition of metastasis | |
| Endometrial Cancer | Man et al. (2020) [141] | Female athymic nude mice | 50 mg/kg | Inhibition of anti-apoptotic molecules NOD1 and NAIP | Inhibition of tumor growth |
| Study and Year | Type of Study | Cases/Size | Exposure vs. Control | OR (95% CI) | |
|---|---|---|---|---|---|
| Ovarian Cancer | Nagle et al. (2010) [122] | Case-control | 1271/1198 | Never vs. ≥1 cup/day | 0.82 (0.38–1.79) |
| Zhang et al. (2002) [119] | Case-control | 254/652 | Never or rarely vs. ≥1 time/day | 0.43 (0.30–0.63) | |
| Song et al. (2008) [120] | Case-control | 781/1263 | Never or rarely vs. ≥1 cup/day | 0.46 (0.26–0.84) | |
| Goodman et al. (2003) [121] | Case-control | 164/194 | Never vs. ≥1 cup/day | 0.90 (0.50–1.61) | |
| Endometrial Cancer | Gao et al. (2005) [146] | Case-control | 995/1087 | Never vs. ≥7 cups/day | 0.76 (0.60–0.95) |
| Kakuta et al. (2009) [147] | Case-control | 152/285 | 4 cups/week vs. ≥4 cups/day | 0.33 (0.15–0.75) | |
| Hirose et al. (2007) [148] | Case-control | 229/2425 | Never vs. ≥7 cups/day | 1.33 (0.75–2.35) | |
| Shimazu et al. (2008) [149] | Prospective cohort | 117/53,724 | 4 cups/week vs. ≥5 cups/day | 0.75 (0.44–1.30) | |
| Xu et al. (2007) [150] | Case-control | 1204/1212 | Never vs. ever green tea | 0.80 (0.60–0.90) |
| Study | Phase | Intervention vs. Control | Length of Intervention | Results | |
|---|---|---|---|---|---|
| Ovarian Cancer (FIGO stage III-IV serous or endometrioid ovarian cancer) | Trudel et al. [151] NCT00721890 | Phase II clinical trial N = 16 | Nonrandomized, single-arm, two-stage design. All treated with double-brewed green tea 500 mL (~639 mg/mL EGCG) for up to 18 months Primary outcome absence of recurrence at 18 months | Green tea intake for minimum less than 100 days; maximum more than 3 years | Absence of recurrence of ovarian cancer 5/16 women at 18 months Trial terminated |
| Cervical Intraepithelial Neoplasia (CIN) | Garcia et al. [152] NCT00303823 | Phase II clinical trial N = 98 50 treated and 48 control (41 in each group were analysed) | Randomized to Polyphenon E (800 mg of EGCG) vs. placebo Primary outcomes: HPV clearance or CIN1 resolution at 4 months | Polyphenon E or placebo intake for 16 weeks Follow up after 2 weeks of treatment completion | Clearance was similar. Progression of cervical lesion in 14.6% of women receiving intervention vs. 7.7% in those receiving placebo |
| Ahn et al. [153] | Clinical trial N = 51 27 treated and 39 control | Randomized to Polyphenon E and EGCG ointment or 200 mg capsule vs. untreated control group Primary outcomes: HPV DNA titers, histology or cytology | Polyphenol E ointment or 200 mg capsule or 200 mg EGCG capsule taken for 8 to 12 weeks | 69% (35/51 patients)response in group taking green tea extract vs. 10% (4/39 patients) response in control group | |
| Vulvar Usual type of vulvar Intraepithelial neoplasia (uVIN) | Yap et al. [154] | Phase II clinical trial N = 26 13 treated and 13 control | Randomized 1:1 to Sinecatechins 10% vs. placebo ointment Primary outcome was histological resolution of uVIN at 32 weeks | Sinecatechins ointment or placebo for 16 weeks Follow up at 2, 4, 8, 16, 32 and 52 weeks | 5/13 patients who received sinecatechins had complete clinical response and 8/13 had partial response |
5.2. EGCG and Cervical Cancer
5.3. EGCG and Endometrial Cancer
5.4. EGCG and Vulvar Cancer
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liang, Y.R.; Ye, Q.; Jin, J.; Liang, H.; Lu, J.L.; Du, Y.Y.; Dong, J.J. Chemical and instrumental assessment of green tea sensory preference. Int. J. Food Prop. 2008, 11, 258–272. [Google Scholar] [CrossRef]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Mokra, D.; Adamcakova, J.; Mokry, J. Green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG): A time for a new player in the treatment of respiratory diseases? Antioxidants 2022, 11, 1566. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Im, K.J.; Suh, S.I.; Jung, J.G. Protective effect of green tea polyphenol (-)-epigallocatechin gallate and other antioxidants on lipid peroxidation in gerbil brain homogenates. Phytother. Res. 2003, 17, 206–209. [Google Scholar] [CrossRef]
- Xu, R.; Yang, K.; Li, S.; Dai, M.; Chen, G. Effect of green tea consumption on blood lipids: A systematic review and meta-analysis of randomized controlled trials. Nutr. J. 2020, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. Epigallocatechin gallate for management of heavy metal-induced oxidative stress: Mechanisms of action, efficacy, and concerns. Int. J. Mol. Sci. 2021, 22, 4027. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhu, K.; Liu, Z.; Huang, J. Prooxidant effects of epigallocatechin-3-gallate in health benefits and potential adverse effect. Oxidative Med. Cell. Longev. 2020, 2020, 9723686. [Google Scholar] [CrossRef]
- Nakazato, T.; Ito, K.; Ikeda, Y.; Kizaki, M. Green tea component, catechin, induces apoptosis of human malignant B cells via production of reactive oxygen species. Clin. Cancer Res. 2005, 11, 6040–6049. [Google Scholar] [CrossRef]
- Khan, M.A.; Hussain, A.; Sundaram, M.K.; Alalami, U.; Gunasekera, D.; Ramesh, L.; Hamza, A.; Quraishi, U. (-)-Epigallocatechin-3-gallate reverses the expression of various tumor-suppressor genes by inhibiting DNA methyltransferases and histone deacetylases in human cervical cancer cells. Oncol. Rep. 2015, 33, 1976–1984. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Lin, Q.; Wang, Y.; Sun, H.; Wang, J.; Cui, G.; Cai, L.; Dong, X. Tea polyphenols induced apoptosis of breast cancer cells by suppressing the expression of Survivin. Sci. Rep. 2014, 4, 4416. [Google Scholar] [CrossRef]
- Ni, C.X.; Gong, H.; Liu, Y.; Qi, Y.; Jiang, C.L.; Zhang, J.P. Green tea consumption and the risk of liver cancer: A meta-analysis. Nutr. Cancer 2017, 69, 211–220. [Google Scholar] [CrossRef]
- Connors, S.K.; Chornokur, G.; Kumar, N.B. New insights into the mechanisms of green tea catechins in the chemoprevention of prostate cancer. Nutr. Cancer 2012, 64, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, C.E.; Wen, G.Y.; Xu, W.; Jia, J.H.; Rohan, L.; Corbo, C.; Di Maggio, V.; Jenkins, E.C., Jr.; Hillier, S. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob. Agents Chemother. 2008, 52, 962–970. [Google Scholar] [CrossRef]
- Hassan, S.T.S.; Sudomova, M. Molecular mechanisms of flavonoids against tumor gamma-herpesviruses and their correlated cancers-a focus on EBV and KSHV life cycles and carcinogenesis. Int. J. Mol. Sci. 2022, 24, 247. [Google Scholar] [CrossRef]
- Jones, C. Cervical cancer: Is herpes simplex virus type II a cofactor? Clin. Microbiol. Rev. 1995, 8, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Eliassen, E.; Lum, E.; Pritchett, J.; Ongradi, J.; Krueger, G.; Crawford, J.R.; Phan, T.L.; Ablashi, D.; Hudnall, S.D. Human herpesvirus 6 and malignancy: A review. Front. Oncol. 2018, 8, 512. [Google Scholar] [CrossRef]
- Ye, Y.; Yan, J.; Cui, J.; Mao, S.; Li, M.; Liao, X.; Tong, H. Dynamic changes in amino acids, catechins, caffeine and gallic acid in green tea during withering. J. Food Compost. Anal. 2018, 66, 98–108. [Google Scholar] [CrossRef]
- Cabrera, C.; Giménez, R.; López, M.C. Determination of tea components with antioxidant activity. J. Agric. Food Chem. 2003, 53, 4427–4435. [Google Scholar] [CrossRef]
- Roy, M.K.; Koide, M.; Rao, T.P.; Okubo, T.; Ogasawara, Y.; Juneja, L.R. ORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: Relationship between total polyphenol and individual catechin content. Int. J. Food Sci. Nutr. 2010, 61, 109–124. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed]
- Salah, N.; Miller, N.J.; Paganga, G.; Tijburg, L.; Bolwell, G.P.; Riceevans, C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch. Biochem. Biophys. 1995, 322, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Rice-evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Botten, D.; Fugallo, G.; Fraternali, F.; Molteni, C. Structural properties of green tea catechins. J. Phys. Chem. B 2015, 119, 12860–12867. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Szeto, Y.T. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef]
- Vinson, J.; Dabbagh, Y.; Serry, M.; Jang, J. Plant flavonoids, especially tea flavonols, are powerful antioxidants using an in vitro oxidation model for heart disease. J. Agric. Food Chem. 1995, 43, 2800–2802. [Google Scholar] [CrossRef]
- Vinson, J.A.; Dabbagh, Y.A. Tea phenols: Antioxidant effectiveness of teas, tea components, tea fractions and their binding with lipoproteins. Nutr. Res. 1998, 18, 1067–1075. [Google Scholar] [CrossRef]
- Brown, E.J.; Khodr, H.; Hider, C.R.; Rice-Evans, C.A. Structural dependence of flavonoid interactions with Cu2+ ions: Implications for their antioxidant properties. Biochem. J. 1998, 330, 1173–1178. [Google Scholar] [CrossRef]
- Braicu, C.; Pilecki, V.; Balacescu, O.; Irimie, A.; Neagoe, I.B. The relationships between biological activities and structure of flavan-3-ols. Int. J. Mol. Sci. 2011, 12, 9342–9353. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Neto, R.O.; dos Santos D’Almeida, C.T.; do Nascimento, T.P.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; Barros, F.A.R.d. Kombuchas from green and black teas have different phenolic profile, which impacts their antioxidant capacities, antibacterial and antiproliferative activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef]
- Komes, D.; Horžić, D.; Belščak, A.; Ganić, K.K.; Vulić, I. Green tea preparation and its influence on the content of bioactive compounds. Food Res. Int. 2010, 43, 167–176. [Google Scholar] [CrossRef]
- Seeram, N.P.; Henning, S.M.; Niu, Y.; Lee, R.; Scheuller, H.S.; Heber, D. Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. J. Agric. Food Chem. 2006, 54, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Yanagimoto, K.; Ochi, H.; Lee, K.G.; Shibamoto, T. Antioxidative activities of volatile extracts from green tea, oolong tea, and black tea. J. Agric. Food Chem. 2003, 51, 7396–7401. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Yang, C.S.; Chen, L.; Lee, M.J.; Balentine, D.; Kuo, M.C.; Schantz, S.P. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol. Biomark. Prev. 1998, 7, 351–354. [Google Scholar]
- Janle, E.M.; Morré, D.M.; Morré, D.J.; Zhou, Q.; Zhu, Y. Pharmacokinetics of green tea catechins in extract and sustained-release preparations. J. Diet. Suppl. 2008, 5, 248–263. [Google Scholar] [CrossRef]
- van het Hof, K.H.; Kivits, G.A.A.; Weststrate, J.A.; Tijburg, L.B.M. Bioavailability of catechins from tea: The effect of milk. Eur. J. Clin. Nutr. 1998, 52, 356–359. [Google Scholar] [CrossRef]
- Chow, H.S.; Cai, Y.; Alberts, D.S.; Hakim, I.; Dorr, R.; Shahi, F.; Crowell, J.A.; Yang, C.S.; Hara, Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol. Biomark. Prev. 2001, 10, 53–58. [Google Scholar]
- Clifford, M.N.; van der Hooft, J.J.J.; Crozier, A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am. J. Clin. Nutr. 2013, 98, 1619S–1630S. [Google Scholar] [CrossRef]
- Chu, K.O.; Pang, C.C. Pharmacokinetics and disposition of green tea catechins. In Pharmacokinetics and Adverse Effects of Drugs; Ntambwe, M., Ed.; IntechOpen: Rijeka, Croatia, 2018; Charpter 2. [Google Scholar]
- van der Hooft, J.J.J.; de Vos, R.C.H.; Mihaleva, V.; Bino, R.J.; Ridder, L.; de Roo, N.; Jacobs, D.M.; van Duynhoven, J.P.M.; Vervoort, J. Structural Elucidation and Quantification of Phenolic Conjugates Present in Human Urine after Tea Intake. Anal. Chem. 2012, 84, 7263–7271. [Google Scholar] [CrossRef]
- Del Rio, D.; Calani, L.; Scazzina, F.; Jechiu, L.; Cordero, C.; Brighenti, F. Bioavailability of catechins from ready-to-drink tea. Nutrition 2010, 26, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lee, M.-J.; Li, H.; Yang, C.S. Absorption, distribution, and elimination of tea polyphenols in rats. Drug Metab. Dispos. 1997, 25, 1045. [Google Scholar] [PubMed]
- Vaidyanathan, J.B.; Walle, T. Cellular uptake and efflux of the tea flavonoid (-)epicatechin-3-gallate in the human intestinal cell line Caco-2. J. Pharmacol. Exp. Ther. 2003, 307, 745. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Y.; Chow, M.S.S.; Zuo, Z. Investigation of intestinal absorption and disposition of green tea catechins by Caco-2 monolayer model. Int. J. Pharm. 2004, 287, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Lambert, J.D.; Lee, S.-H.; Sinko, P.J.; Yang, C.S. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (−)-epigallocatechin-3-gallate and its methyl metabolites. Biochem. Biophys. Res. Commun. 2003, 310, 222–227. [Google Scholar] [CrossRef]
- Song, Q.; Li, D.; Zhou, Y.; Yang, J.; Yang, W.; Zhou, G.; Wen, J. Enhanced uptake and transport of (+)-catechin and (-)-epigallocatechin gallate in niosomal formulation by human intestinal Caco-2 cells. Int. J. Nanomed. 2014, 9, 2157–2165. [Google Scholar] [CrossRef]
- Green, R.J.; Murphy, A.S.; Schulz, B.; Watkins, B.A.; Ferruzzi, M.G. Common tea formulations modulate in vitro digestive recovery of green tea catechins. Mol. Nutr. Food Res. 2007, 51, 1152–1162. [Google Scholar] [CrossRef]
- Peters, C.M.; Green, R.J.; Janle, E.M.; Ferruzzi, M.G. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res. Int. 2010, 43, 95–102. [Google Scholar] [CrossRef]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Kirkpatrick, N.J.; Polagruto, J.A.; Ensunsa, J.L.; Schmitz, H.H.; Keen, C.L. Food effects on the absorption and pharmacokinetics of cocoa flavanols. Life Sci. 2003, 73, 857–869. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Van Het Hof, K.H.; Tijburg, L.B.M.; Katan, M.B. Addition of milk does not affect the absorption of flavonols from tea in man. Free Radic. Res. 2001, 34, 297–300. [Google Scholar] [CrossRef]
- Lam, W.H.; Kazi, A.; Kuhn, D.J.; Chow, L.M.; Chan, A.S.; Dou, Q.P.; Chan, T.H. A potential prodrug for a green tea polyphenol proteasome inhibitor: Evaluation of the peracetate ester of (-)-epigallocatechin gallate [(-)-EGCG]. Bioorg. Med. Chem. 2004, 12, 5587–5593. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Sang, S.; Hong, J.; Kwon, S.J.; Lee, M.J.; Ho, C.T.; Yang, C.S. Peracetylation as a means of enhancing in vitro bioactivity and bioavailability of epigallocatechin-3-gallate. Drug Metab. Dispos. 2006, 34, 2111–2116. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Xu, H.; Man, G.C.; Zhang, T.; Chu, K.O.; Chu, C.Y.; Cheng, J.T.; Li, G.; He, Y.X.; Qin, L.; et al. Prodrug of green tea epigallocatechin-3-gallate (Pro-EGCG) as a potent anti-angiogenesis agent for endometriosis in mice. Angiogenesis 2013, 16, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.S.; Liu, G.; Renzetti, A.; Farshi, P.; Yang, H.; Soave, C.; Saed, G.; El-Ghoneimy, A.A.; El-Banna, H.A.; Foldes, R.; et al. Biological and mechanistic characterization of novel prodrugs of green tea polyphenol epigallocatechin gallate analogs in human leiomyoma cell lines. J. Cell. Biochem. 2016, 117, 2357–2369. [Google Scholar] [CrossRef]
- Vyas, S.; Sharma, M.; Sharma, P.D.; Singh, T.V. Design, semisynthesis, and evaluation of O-acyl derivatives of (-)-epigallocatechin-3-gallate as antitumor agents. J. Agric. Food Chem. 2007, 55, 6319–6324. [Google Scholar] [CrossRef] [PubMed]
- Landis-Piwowar, K.R.; Huo, C.; Chen, D.; Milacic, V.; Shi, G.; Chan, T.H.; Dou, Q.P. A novel prodrug of the green tea polyphenol (-)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer Res. 2007, 67, 4303–4310. [Google Scholar] [CrossRef]
- Chiou, Y.S.; Ma, N.J.; Sang, S.; Ho, C.T.; Wang, Y.J.; Pan, M.H. Peracetylated (-)-epigallocatechin-3-gallate (AcEGCG) potently suppresses dextran sulfate sodium-induced colitis and colon tumorigenesis in mice. J. Agric. Food Chem. 2012, 60, 3441–3451. [Google Scholar] [CrossRef]
- Chiou, Y.S.; Sang, S.; Cheng, K.H.; Ho, C.T.; Wang, Y.J.; Pan, M.H. Peracetylated (-)-epigallocatechin-3-gallate (AcEGCG) potently prevents skin carcinogenesis by suppressing the PKD1-dependent signaling pathway in CD34+ skin stem cells and skin tumors. Carcinogenesis 2013, 34, 1315–1322. [Google Scholar] [CrossRef]
- Meeran, S.M.; Patel, S.N.; Chan, T.H.; Tollefsbol, T.O. A novel prodrug of epigallocatechin-3-gallate: Differential epigenetic hTERT repression in human breast cancer cells. Cancer Prev. Res. 2011, 4, 1243–1254. [Google Scholar] [CrossRef]
- Sazuka, M.; Itoi, T.; Suzuki, Y.; Odani, S.; Koide, T.; Isemura, M. Evidence for the interaction between (-)-epigallocatechin gallate and human plasma proteins fibronectin, fibrinogen, and histidine-rich glycoprotein. Biosci. Biotechnol. Biochem. 1996, 60, 1317–1319. [Google Scholar] [CrossRef]
- Sazuka, M.; Imazawa, H.; Shoji, Y.; Mita, T.; Hara, Y.; Isemura, M. Inhibition of collagenases from mouse lung carcinoma cells by green tea catechins and black tea theaflavins. Biosci. Biotechnol. Biochem. 1997, 61, 1504–1506. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Demeule, M.; Brossard, M.; Pagé, M.; Gingras, D.; Béliveau, R. Matrix metalloproteinase inhibition by green tea catechins. Biochim. Biophys. Acta 2000, 1478, 51–60. [Google Scholar] [CrossRef]
- Ermakova, S.; Choi, B.Y.; Choi, H.S.; Kang, B.S.; Bode, A.M.; Dong, Z. The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J. Biol. Chem. 2005, 280, 16882–16890. [Google Scholar] [CrossRef] [PubMed]
- Palermo, C.M.; Westlake, C.A.; Gasiewicz, T.A. Epigallocatechin gallate inhibits aryl hydrocarbon receptor gene transcription through an indirect mechanism involving binding to a 90 kDa heat shock protein. Biochemistry 2005, 44, 5041–5052. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Henry, E.C.; Gasiewicz, T.A. (-)-Epigallocatechin-3-gallate is a novel Hsp90 inhibitor. Biochemistry 2009, 48, 336–345. [Google Scholar] [CrossRef]
- Ermakova, S.P.; Kang, B.S.; Choi, B.Y.; Choi, H.S.; Schuster, T.F.; Ma, W.Y.; Bode, A.M.; Dong, Z. (-)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein 78. Cancer Res. 2006, 66, 9260–9269. [Google Scholar] [CrossRef]
- Casas, C. GRP78 at the centre of the stage in cancer and neuroprotection. Front. Neurosci. 2017, 11, 177. [Google Scholar] [CrossRef]
- Qian, F.; Wei, D.; Zhang, Q.; Yang, S. Modulation of P-glycoprotein function and reversal of multidrug resistance by (-)-epigallocatechin gallate in human cancer cells. Biomed. Pharmacother. 2005, 59, 64–69. [Google Scholar] [CrossRef]
- Hayakawa, S.; Saeki, K.; Sazuka, M.; Suzuki, Y.; Shoji, Y.; Ohta, T.; Kaji, K.; Yuo, A.; Isemura, M. Apoptosis induction by epigallocatechin gallate involves its binding to Fas. Biochem. Biophys. Res. Commun. 2001, 285, 1102–1106. [Google Scholar] [CrossRef]
- Timmer, T.; de Vries, E.G.; de Jong, S. Fas receptor-mediated apoptosis: A clinical application? J. Pathol. 2002, 196, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Zhai, D.; Sareth, S.; Kitada, S.; Reed, J.C.; Pellecchia, M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003, 63, 8118–8121. [Google Scholar] [PubMed]
- Frenzel, A.; Grespi, F.; Chmelewskij, W.; Villunger, A. Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis 2009, 14, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, H.; Koga, K.; Fujimura, Y.; Yamada, K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004, 11, 380–381. [Google Scholar] [CrossRef]
- Urusova, D.V.; Shim, J.H.; Kim, D.J.; Jung, S.K.; Zykova, T.A.; Carper, A.; Bode, A.M.; Dong, Z. Epigallocatechin-gallate suppresses tumorigenesis by directly targeting Pin1. Cancer Prev. Res. 2011, 4, 1366–1377. [Google Scholar] [CrossRef]
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef]
- He, J.; Xu, L.; Yang, L.; Wang, X. Epigallocatechin gallate is the most effective catechin against antioxidant stress via hydrogen peroxide and radical scavenging activity. Med. Sci. Monit. 2018, 24, 8198–8206. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Frei, B.; Higdon, J.V. Antioxidant activity of tea polyphenols in vivo: Evidence from animal studies. J. Nutr. 2003, 133, 3275S–3284S. [Google Scholar] [CrossRef]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin gallate: A review of its beneficial properties to prevent metabolic syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef]
- Meng, Q.; Velalar, C.N.; Ruan, R. Effects of epigallocatechin-3-gallate on mitochondrial integrity and antioxidative enzyme activity in the aging process of human fibroblast. Free Radic. Biol. Med. 2008, 44, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhao, B.; Li, M.; Shen, S.; Xin, W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim. Biophys. Acta 1996, 1304, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Suzuki-Sugihara, N.; Kishimoto, Y.; Saita, E.; Taguchi, C.; Kobayashi, M.; Ichitani, M.; Ukawa, Y.; Sagesaka, Y.M.; Suzuki, E.; Kondo, K. Green tea catechins prevent low-density lipoprotein oxidation via their accumulation in low-density lipoprotein particles in humans. Nutr. Res. 2016, 36, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Yee, W.L.; Wang, Q.; Agdinaoay, T.; Dang, K.; Chang, H.; Grandinetti, A.; Franke, A.A.; Theriault, A. Green tea catechins decrease apolipoprotein B-100 secretion from HepG2 cells. Mol. Cell. Biochem. 2002, 229, 85–92. [Google Scholar] [CrossRef]
- Li, L.; Stillemark-Billton, P.; Beck, C.; Boström, P.; Andersson, L.; Rutberg, M.; Ericsson, J.; Magnusson, B.; Marchesan, D.; Ljungberg, A.; et al. Epigallocatechin gallate increases the formation of cytosolic lipid droplets and decreases the secretion of apoB-100 VLDL. J. Lipid Res. 2006, 47, 67–77. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, W.; Owusu, L.; Wu, D.; Xin, Y. Epigallocatechin-3-gallate induces the apoptosis of hepatocellular carcinoma LM6 cells but not non-cancerous liver cells. Int. J. Mol. Med. 2015, 35, 117–124. [Google Scholar] [CrossRef]
- Zhao, J.; Blayney, A.; Liu, X.; Gandy, L.; Jin, W.; Yan, L.; Ha, J.-H.; Canning, A.J.; Connelly, M.; Yang, C.; et al. EGCG binds intrinsically disordered N-terminal domain of p53 and disrupts p53-MDM2 interaction. Nat. Commun. 2021, 12, 986. [Google Scholar] [CrossRef]
- Huang, C.Y.; Han, Z.; Li, X.; Xie, H.H.; Zhu, S.S. Mechanism of EGCG promoting apoptosis of MCF-7 cell line in human breast cancer. Oncol. Lett. 2017, 14, 3623–3627. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.H.; Zhan, W.H.; Li, Z.R.; Wang, Z.; He, Y.L.; Peng, J.S.; Cai, S.R.; Ma, J.P.; Zhang, C.H. (-)-Epigallocatechin-3-gallate inhibits growth of gastric cancer by reducing VEGF production and angiogenesis. World J. Gastroenterol. 2007, 13, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Qanungo, S.; Das, M.; Haldar, S.; Basu, A. Epigallocatechin-3-gallate induces mitochondrial membrane depolarization and caspase-dependent apoptosis in pancreatic cancer cells. Carcinogenesis 2005, 26, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.P.; Kuo, S.C.; Huang, W.W.; Yang, J.S.; Lai, K.C.; Chen, H.J.; Lin, K.L.; Chiu, Y.J.; Huang, L.J.; Chung, J.G. (-)-Epigallocatechin gallate induced apoptosis in human adrenal cancer NCI-H295 cells through caspase-dependent and caspase-independent pathway. Anticancer Res. 2009, 29, 1435–1442. [Google Scholar] [PubMed]
- Lin, H.Y.; Hou, S.C.; Chen, S.C.; Kao, M.C.; Yu, C.C.; Funayama, S.; Ho, C.T.; Way, T.D. (-)-Epigallocatechin gallate induces Fas/CD95-mediated apoptosis through inhibiting constitutive and IL-6-induced JAK/STAT3 signaling in head and neck squamous cell carcinoma cells. J. Agric. Food Chem. 2012, 60, 2480–2489. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Haldar, S. Combinatorial effect of epigallocatechin-3-gallate and TRAIL on pancreatic cancer cell death. Int. J. Oncol. 2009, 34, 281–286. [Google Scholar] [CrossRef]
- Kuo, P.L.; Lin, C.C. Green tea constituent (-)-epigallocatechin-3-gallate inhibits Hep G2 cell proliferation and induces apoptosis through p53-dependent and Fas-mediated pathways. J. Biomed. Sci. 2003, 10, 219–227. [Google Scholar] [CrossRef]
- Brusselmans, K.; De Schrijver, E.; Heyns, W.; Verhoeven, G.; Swinnen, J.V. Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid synthase in intact cells and selectively induces apoptosis in prostate cancer cells. Int. J. Cancer 2003, 106, 856–862. [Google Scholar] [CrossRef]
- Deb, G.; Shankar, E.; Thakur, V.S.; Ponsky, L.E.; Bodner, D.R.; Fu, P.; Gupta, S. Green tea-induced epigenetic reactivation of tissue inhibitor of matrix metalloproteinase-3 suppresses prostate cancer progression through histone-modifying enzymes. Mol. Carcinog. 2019, 58, 1194–1207. [Google Scholar] [CrossRef]
- Borutinskaitė, V.; Virkšaitė, A.; Gudelytė, G.; Navakauskienė, R. Green tea polyphenol EGCG causes anti-cancerous epigenetic modulations in acute promyelocytic leukemia cells. Leuk. Lymphoma 2018, 59, 469–478. [Google Scholar] [CrossRef]
- Yamada, S.; Tsukamoto, S.; Huang, Y.; Makio, A.; Kumazoe, M.; Yamashita, S.; Tachibana, H. Epigallocatechin-3-O-gallate up-regulates microRNA-let-7b expression by activating 67-kDa laminin receptor signaling in melanoma cells. Sci. Rep. 2016, 6, 19225. [Google Scholar] [CrossRef]
- Wang, H.; Bian, S.; Yang, C.S. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1α. Carcinogenesis 2011, 32, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Society, A.C. Key Statistics for Ovarian Cancer. Available online: https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html#references (accessed on 31 December 2022).
- CDC. Ovarian Cancer Statistics. Available online: cdc.gov/cancer/ovarian/statistics/index.htm (accessed on 6 June 2022).
- Trudel, D.; Labbe, D.P.; Bairati, I.; Fradet, V.; Bazinet, L.; Tetu, B. Green tea for ovarian cancer prevention and treatment: A systematic review of the in vitro, in vivo and epidemiological studies. Gynecol. Oncol. 2012, 126, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: Biological and therapeutic significance. Semin. Cancer Biol. 2019, 59, 147–160. [Google Scholar] [CrossRef]
- Dobbin, Z.C.; Landen, C.N. The importance of the PI3K/AKT/MTOR pathway in the progression of ovarian cancer. Int. J. Mol. Sci. 2013, 14, 8213–8227. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Wang, K.L.; Chen, H.Y.; Chiang, Y.F.; Hsia, S.M. Protective effects of epigallocatechin gallate (EGCG) on endometrial, breast, and ovarian cancers. Biomolecules 2020, 10, 1481. [Google Scholar] [CrossRef]
- Rao, S.D.; Pagidas, K. Epigallocatechin-3-gallate, a natural polyphenol, inhibits cell proliferation and induces apoptosis in human ovarian cancer cells. Anticancer Res. 2010, 30, 2519–2523. [Google Scholar]
- Qin, J.; Fu, M.; Wang, J.; Huang, F.; Liu, H.; Huangfu, M.; Yu, D.; Liu, H.; Li, X.; Guan, X.; et al. PTEN/AKT/mTOR signaling mediates anticancer effects of epigallocatechin-3-gallate in ovarian cancer. Oncol. Rep. 2020, 43, 1885–1896. [Google Scholar] [CrossRef]
- Huh, S.W.; Bae, S.M.; Kim, Y.W.; Lee, J.M.; Namkoong, S.E.; Lee, I.P.; Kim, S.H.; Kim, C.K.; Ahn, W.S. Anticancer effects of (−)-epigallocatechin-3-gallate on ovarian carcinoma cell lines. Gynecol. Oncol. 2004, 94, 760–768. [Google Scholar] [CrossRef]
- Yan, C.; Yang, J.; Shen, L.; Chen, X. Inhibitory effect of Epigallocatechin gallate on ovarian cancer cell proliferation associated with aquaporin 5 expression. Arch. Gynecol. Obstet. 2012, 285, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Spinella, F.; Rosano, L.; Di Castro, V.; Decandia, S.; Albini, A.; Nicotra, M.R.; Natali, P.G.; Bagnato, A. Green tea polyphenol epigallocatechin-3-gallate inhibits the endothelin axis and downstream signaling pathways in ovarian carcinoma. Mol. Cancer Ther. 2006, 5, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.; Rao, S.; Waye, M.M.Y.; Ng, T.B.; Wang, C.C. Autophagy signals orchestrate chemoresistance of gynecological cancers. Biochim. et Biophys. Acta (BBA)-Rev. Cancer 2021, 1875, 188525. [Google Scholar] [CrossRef] [PubMed]
- Panji, M.; Behmard, V.; Zare, Z.; Malekpour, M.; Nejadbiglari, H.; Yavari, S.; Nayerpour Dizaj, T.; Safaeian, A.; Bakhshi, A.; Abazari, O.; et al. Synergistic effects of green tea extract and paclitaxel in the induction of mitochondrial apoptosis in ovarian cancer cell lines. Gene 2021, 787, 145638. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, P.; Wang, P.; Yang, C.S.; Wang, X.; Feng, Q. EGCG Enhances Cisplatin Sensitivity by Regulating Expression of the Copper and Cisplatin Influx Transporter CTR1 in Ovary Cancer. PLoS ONE 2015, 10, e0125402. [Google Scholar] [CrossRef]
- Zhang, M.; Binns, C.W.; Lee, A.H. Tea consumption and ovarian cancer risk: A case-control study in China. Cancer Epidemiol. Biomark. Prev. 2002, 11, 713–718. [Google Scholar]
- Song, Y.J.; Kristal, A.R.; Wicklund, K.G.; Cushing-Haugen, K.L.; Rossing, M.A. Coffee, tea, colas, and risk of epithelial ovarian cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 712–716. [Google Scholar] [CrossRef]
- Goodman, M.T.; Tung, K.H.; McDuffie, K.; Wilkens, L.R.; Donlon, T.A. Association of caffeine intake and CYP1A2 genotype with ovarian cancer. Nutr. Cancer 2003, 46, 23–29. [Google Scholar] [CrossRef]
- Nagle, C.M.; Olsen, C.M.; Bain, C.J.; Whiteman, D.C.; Green, A.C.; Webb, P.M. Tea consumption and risk of ovarian cancer. Cancer Causes Control 2010, 21, 1485–1491. [Google Scholar] [CrossRef]
- Butler, L.M.; Wu, A.H. Green and black tea in relation to gynecologic cancers. Mol. Nutr. Food Res. 2011, 55, 931–940. [Google Scholar] [CrossRef]
- Zhang, M.; Lee, A.H.; Binns, C.W.; Xie, X. Green tea consumption enhances survival of epithelial ovarian cancer. Int. J. Cancer 2004, 112, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Liu, H.; Feugang, J.M.; Hao, Z.; Chow, H.H.; Garcia, F. Green tea compound in chemoprevention of cervical cancer. Int. J. Gynecol. Cancer 2010, 20, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, R.; Bhui, K.; Tyagi, S.; Mahmood, Z.; Shukla, Y. Tea polyphenols induce apoptosis through mitochondrial pathway and by inhibiting nuclear factor-kappaB and Akt activation in human cervical cancer cells. Oncol. Res. 2011, 19, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.J.; Burns, A.C.; Kazi, A.; Dou, Q.P. Direct inhibition of the ubiquitin-proteasome pathway by ester bond-containing green tea polyphenols is associated with increased expression of sterol regulatory element-binding protein 2 and LDL receptor. Biochim. Biophys. Acta 2004, 1682, 1–10. [Google Scholar] [CrossRef]
- Bonfili, L.; Cuccioloni, M.; Mozzicafreddo, M.; Cecarini, V.; Angeletti, M.; Eleuteri, A.M. Identification of an EGCG oxidation derivative with proteasome modulatory activity. Biochimie 2011, 93, 931–940. [Google Scholar] [CrossRef]
- Li, M.; He, Z.; Ermakova, S.; Zheng, D.; Tang, F.; Cho, Y.Y.; Zhu, F.; Ma, W.Y.; Sham, Y.; Rogozin, E.A.; et al. Direct inhibition of insulin-like growth factor-I receptor kinase activity by (-)-epigallocatechin-3-gallate regulates cell transformation. Cancer Epidemiol. Biomark. Prev. 2007, 16, 598–605. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Ganguli, A.; Das, A.; Nag, D.; Chakrabarti, G. Epigallocatechin-3-gallate shows anti-proliferative activity in HeLa cells targeting tubulin-microtubule equilibrium. Chem. Biol. Interact. 2015, 242, 380–389. [Google Scholar] [CrossRef]
- Ahn, W.S.; Huh, S.W.; Bae, S.M.; Lee, I.P.; Lee, J.M.; Namkoong, S.E.; Kim, C.K.; Sin, J.I. A major constituent of green tea, EGCG, inhibits the growth of a human cervical cancer cell line, CaSki cells, through apoptosis, G(1) arrest, and regulation of gene expression. DNA Cell Biol. 2003, 22, 217–224. [Google Scholar] [CrossRef]
- Qiao, Y.; Cao, J.; Xie, L.; Shi, X. Cell growth inhibition and gene expression regulation by (-)-epigallocatechin-3-gallate in human cervical cancer cells. Arch. Pharm. Res. 2009, 32, 1309–1315. [Google Scholar] [CrossRef]
- Sah, J.F.; Balasubramanian, S.; Eckert, R.L.; Rorke, E.A. Epigallocatechin-3-gallate inhibits epidermal growth factor receptor signaling pathway. Evidence for direct inhibition of ERK1/2 and AKT kinases. J. Biol. Chem. 2004, 279, 12755–12762. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, X.; Lu, Q.; Zhang, Z.; Rao, J.; Le, A.D. Green tea extract and (-)-epigallocatechin-3-gallate inhibit hypoxia- and serum-induced HIF-1alpha protein accumulation and VEGF expression in human cervical carcinoma and hepatoma cells. Mol. Cancer Ther. 2006, 5, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Tudoran, O.; Soritau, O.; Balacescu, O.; Balacescu, L.; Braicu, C.; Rus, M.; Gherman, C.; Virag, P.; Irimie, F.; Berindan-Neagoe, I. Early transcriptional pattern of angiogenesis induced by EGCG treatment in cervical tumour cells. J. Cell. Mol. Med. 2012, 16, 520–530. [Google Scholar] [CrossRef]
- Sharma, C.; Nusri, Q.E.A.; Begum, S.; Javed, E.; Rizvi, T.A.; Hussain, A. (-)-Epigallocatechin-3-gallate induces apoptosis and inhibits invasion and migration of human cervical cancer cells. Asian Pac. J. Cancer Prev. 2012, 13, 4815–4822. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Monterrey, J.C.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. In vitro modulation of MMP-2 and MMP-9 in human cervical and ovarian cancer cell lines by cytokines, inducers and inhibitors. Oncol. Rep. 2010, 23, 605–614. [Google Scholar] [CrossRef]
- Panji, M.; Behmard, V.; Zare, Z.; Malekpour, M.; Nejadbiglari, H.; Yavari, S.; Nayerpour Dizaj, T.; Safaeian, A.; Maleki, N.; Abbasi, M.; et al. Suppressing effects of green tea extract and Epigallocatechin-3-gallate (EGCG) on TGF-beta- induced Epithelial-to-mesenchymal transition via ROS/Smad signaling in human cervical cancer cells. Gene 2021, 794, 145774. [Google Scholar] [CrossRef] [PubMed]
- Manohar, M.; Fatima, I.; Saxena, R.; Chandra, V.; Sankhwar, P.L.; Dwivedi, A. (−)-Epigallocatechin-3-gallate induces apoptosis in human endometrial adenocarcinoma cells via ROS generation and p38 MAP kinase activation. J. Nutr. Biochem. 2013, 24, 940–947. [Google Scholar] [CrossRef]
- Park, S.B.; Bae, J.W.; Kim, J.M.; Lee, S.G.; Han, M. Antiproliferative and apoptotic effect of epigallocatechin-3-gallate on Ishikawa cells is accompanied by sex steroid receptor downregulation. Int. J. Mol. Med. 2012, 30, 1211–1218. [Google Scholar] [CrossRef]
- Man, G.C.W.; Wang, J.; Song, Y.; Wong, J.H.; Zhao, Y.; Lau, T.S.; Leung, K.T.; Chan, T.H.; Wang, H.; Kwong, J.; et al. Therapeutic potential of a novel prodrug of green tea extract in induction of apoptosis via ERK/JNK and Akt signaling pathway in human endometrial cancer. BMC Cancer 2020, 20, 964. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Lu, J.L.; Liang, Y.R.; Li, Q.S. Suppressive Effects of EGCG on Cervical Cancer. Molecules 2018, 23, 2334. [Google Scholar] [CrossRef]
- Yap, J.K.W.; Kehoe, S.T.; Woodman, C.B.J.; Dawson, C.W. The Major Constituent of Green Tea, Epigallocatechin-3-Gallate (EGCG), Inhibits the Growth of HPV18-Infected Keratinocytes by Stimulating Proteasomal Turnover of the E6 and E7 Oncoproteins. Pathogens 2021, 10, 459. [Google Scholar] [CrossRef]
- Roomi, M.W.; Cha, J.; Kalinovsky, T.; Roomi, N.; Niedzwiecki, A.; Rath, M. Effect of a nutrient mixture on the localization of extracellular matrix proteins in HeLa human cervical cancer xenografts in female nude mice. Exp. Ther. Med. 2015, 10, 901–906. [Google Scholar] [CrossRef]
- Roomi, M.W.; Kalinovsky, T.; Cha, J.; Roomi, N.W.; Niedzwiecki, A.; Rath, M. Effects of a nutrient mixture on immunohistochemical localization of cancer markers in human cervical cancer HeLa cell tumor xenografts in female nude mice. Exp. Ther. Med. 2015, 9, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xiang, Y.B.; Xu, W.H.; Shao, C.X.; Ruan, Z.X.; Cheng, J.R.; Shu, X.O.; Gao, Y.T. Green tea consumption and the risk of endometrial cancer: A population-based case-control study in urban Shanghai. Zhonghua Liu Xing Bing Xue Za Zhi 2005, 26, 323–327. [Google Scholar] [PubMed]
- Kakuta, Y.; Nakaya, N.; Nagase, S.; Fujita, M.; Koizumi, T.; Okamura, C.; Niikura, H.; Ohmori, K.; Kuriyama, S.; Tase, T.; et al. Case-control study of green tea consumption and the risk of endometrial endometrioid adenocarcinoma. Cancer Causes Control 2009, 20, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Niwa, Y.; Wakai, K.; Matsuo, K.; Nakanishi, T.; Tajima, K. Coffee consumption and the risk of endometrial cancer: Evidence from a case-control study of female hormone-related cancers in Japan. Cancer Sci. 2007, 98, 411–415. [Google Scholar] [CrossRef]
- Shimazu, T.; Inoue, M.; Sasazuki, S.; Iwasaki, M.; Kurahashi, N.; Yamaji, T.; Tsugane, S.; JPHC Study Group. Coffee consumption and risk of endometrial cancer: A prospective study in Japan. Int. J. Cancer 2008, 123, 2406–2410. [Google Scholar] [CrossRef]
- Xu, W.H.; Dai, Q.; Xiang, Y.B.; Long, J.R.; Ruan, Z.X.; Cheng, J.R.; Zheng, W.; Shu, X.O. Interaction of soy food and tea consumption with CYP19A1 genetic polymorphisms in the development of endometrial cancer. Am. J. Epidemiol. 2007, 166, 1420–1430. [Google Scholar] [CrossRef]
- Trudel, D.; Labbé, D.P.; Araya-Farias, M.; Doyen, A.; Bazinet, L.; Duchesne, T.; Plante, M.; Grégoire, J.; Renaud, M.-C.; Bachvarov, D. A two-stage, single-arm, phase II study of EGCG-enriched green tea drink as a maintenance therapy in women with advanced stage ovarian cancer. Gynecol. Oncol. 2013, 131, 357–361. [Google Scholar] [CrossRef]
- Garcia, F.A.; Cornelison, T.; Nuño, T.; Greenspan, D.L.; Byron, J.W.; Hsu, C.-H.; Alberts, D.S.; Chow, H.-H.S. Results of a phase II randomized, double-blind, placebo-controlled trial of Polyphenon E in women with persistent high-risk HPV infection and low-grade cervical intraepithelial neoplasia. Gynecol. Oncol. 2014, 132, 377–382. [Google Scholar] [CrossRef]
- Ahn, W.S.; Yoo, J.; Huh, S.W.; Kim, C.K.; Lee, J.M.; Namkoong, S.E.; Bae, S.M.; Lee, I.P. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur. J. Cancer Prev. 2003, 12, 383–390. [Google Scholar] [CrossRef]
- Yap, J.; Slade, D.; Goddard, H.; Dawson, C.; Ganesan, R.; Velangi, S.; Sahu, B.; Kaur, B.; Hughes, A.; Luesley, D. Sinecatechins ointment as a potential novel treatment for usual type vulval intraepithelial neoplasia: A single-centre double-blind randomised control study. BJOG 2021, 128, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cervical Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 31 December 2022).
- CDC. What Are the Risk Factors for Cervical Cancer? Available online: https://www.cdc.gov/cancer/cervical/basic_info/risk_factors.htm (accessed on 31 December 2022).
- Gonzalez, S.L.; Stremlau, M.; He, X.; Basile, J.R.; Munger, K. Degradation of the retinoblastoma tumor suppressor by the human papillomavirus type 16 E7 oncoprotein is important for functional inactivation and is separable from proteasomal degradation of E7. J. Virol. 2001, 75, 7583–7591. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sampath, A.; Raychaudhuri, P.; Bagchi, S. Both Rb and E7 are regulated by the ubiquitin proteasome pathway in HPV-containing cervical tumor cells. Oncogene 2001, 20, 4740–4749. [Google Scholar] [CrossRef] [PubMed]
- Kilic, U.; Sahin, K.; Tuzcu, M.; Basak, N.; Orhan, C.; Elibol-Can, B.; Kilic, E.; Sahin, F.; Kucuk, O. Enhancement of Cisplatin sensitivity in human cervical cancer: Epigallocatechin-3-gallate. Front. Nutr. 2014, 1, 28. [Google Scholar] [CrossRef]
- Yokoyama, M.; Noguchi, M.; Nakao, Y.; Ysunaga, M.; Yamasaki, F.; Iwasaka, T. Antiproliferative effects of the major tea polyphenol, (-)-epigallocatechin gallate and retinoic acid in cervical adenocarcinoma. Gynecol. Oncol. 2008, 108, 326–331. [Google Scholar] [CrossRef]
- Alshatwi, A.A.; Periasamy, V.S.; Athinarayanan, J.; Elango, R. Synergistic anticancer activity of dietary tea polyphenols and bleomycin hydrochloride in human cervical cancer cell: Caspase-dependent and independent apoptotic pathways. Chem. Biol. Interact. 2016, 247, 1–10. [Google Scholar] [CrossRef]
- Chen, X.; Tong, R.; Liu, B.; Liu, H.; Feng, X.; Ding, S.; Lei, Q.; Tang, G.; Wu, J.; Fang, W. Duo of (–)-epigallocatechin-3-gallate and doxorubicin loaded by polydopamine coating ZIF-8 in the regulation of autophagy for chemo-photothermal synergistic therapy. Biomater. Sci. 2020, 8, 1380–1393. [Google Scholar] [CrossRef]
- CDC. What Are the Risk Factors? Available online: https://www.cdc.gov/cancer/uterine/basic_info/risk_factors.htm (accessed on 31 December 2022).
- Banno, K.; Yanokura, M.; Iida, M.; Masuda, K.; Aoki, D. Carcinogenic mechanisms of endometrial cancer: Involvement of genetics and epigenetics. J. Obstet. Gynaecol. Res. 2014, 40, 1957–1967. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, H.; Zhang, M.; Xiong, H.; Jiang, Q. Knocking down FAM83B inhibits endometrial cancer cell proliferation and metastasis by silencing the PI3K/AKT/mTOR pathway. Biomed. Pharmacother. 2019, 115, 108939. [Google Scholar] [CrossRef]
- Wang, J.; Man, G.C.W.; Chan, T.H.; Kwong, J.; Wang, C.C. A prodrug of green tea polyphenol (-)-epigallocatechin-3-gallate (Pro-EGCG) serves as a novel angiogenesis inhibitor in endometrial cancer. Cancer Lett. 2018, 412, 10–20. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, H.; Zhou, J.G.; Ma, Y.; Wu, T.; Ma, H. Green tea, black tea consumption and risk of endometrial cancer: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2016, 293, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.P.; Li, H.; Qiu, Y.L.; Zhou, G.M.; Ma, J. Tea consumption and risk of endometrial cancer: A meta analysis. Am. J. Obstet. Gynecol. 2009, 201, 605.e1–605.e8. [Google Scholar] [CrossRef] [PubMed]
- McCann, S.E.; Yeh, M.; Rodabaugh, K.; Moysich, K.B. Higher regular coffee and tea consumption is associated with reduced endometrial cancer risk. Int. J. Cancer 2009, 124, 1650–1653. [Google Scholar] [CrossRef] [PubMed]
- Society, A.C. Key Statistics for Vulvar Vancer. Available online: https://www.cancer.org/cancer/vulvar-cancer/about/key-statistics.html#:~:text=In%20the%20United%20States%2C%20women,will%20die%20of%20this%20cancer (accessed on 31 December 2022).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parish, M.; Massoud, G.; Hazimeh, D.; Segars, J.; Islam, M.S. Green Tea in Reproductive Cancers: Could Treatment Be as Simple? Cancers 2023, 15, 862. https://doi.org/10.3390/cancers15030862
Parish M, Massoud G, Hazimeh D, Segars J, Islam MS. Green Tea in Reproductive Cancers: Could Treatment Be as Simple? Cancers. 2023; 15(3):862. https://doi.org/10.3390/cancers15030862
Chicago/Turabian StyleParish, Maclaine, Gaelle Massoud, Dana Hazimeh, James Segars, and Md Soriful Islam. 2023. "Green Tea in Reproductive Cancers: Could Treatment Be as Simple?" Cancers 15, no. 3: 862. https://doi.org/10.3390/cancers15030862
APA StyleParish, M., Massoud, G., Hazimeh, D., Segars, J., & Islam, M. S. (2023). Green Tea in Reproductive Cancers: Could Treatment Be as Simple? Cancers, 15(3), 862. https://doi.org/10.3390/cancers15030862