Glucose-Regulated Protein 78 Is a Potential Serum and Imaging Marker for Early Detection of Ovarian Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1: Clinical Samples
2.2. Experiment 2: Preclinical Specimens
2.3. Experiment 3: Development of GRP78-Targeted Imaging Agents and Targeted-TVUS Imaging of Hens
2.4. Preparation of Ovarian Specimen for Biochemical Analysis
2.5. Immunohistochemistry
2.6. Immunoassay
2.7. One- and Two Dimensional (1-&2-D) Western Blot (WB)
2.8. Semi-Quantitative and Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
2.9. Statistical Analysis
3. Results
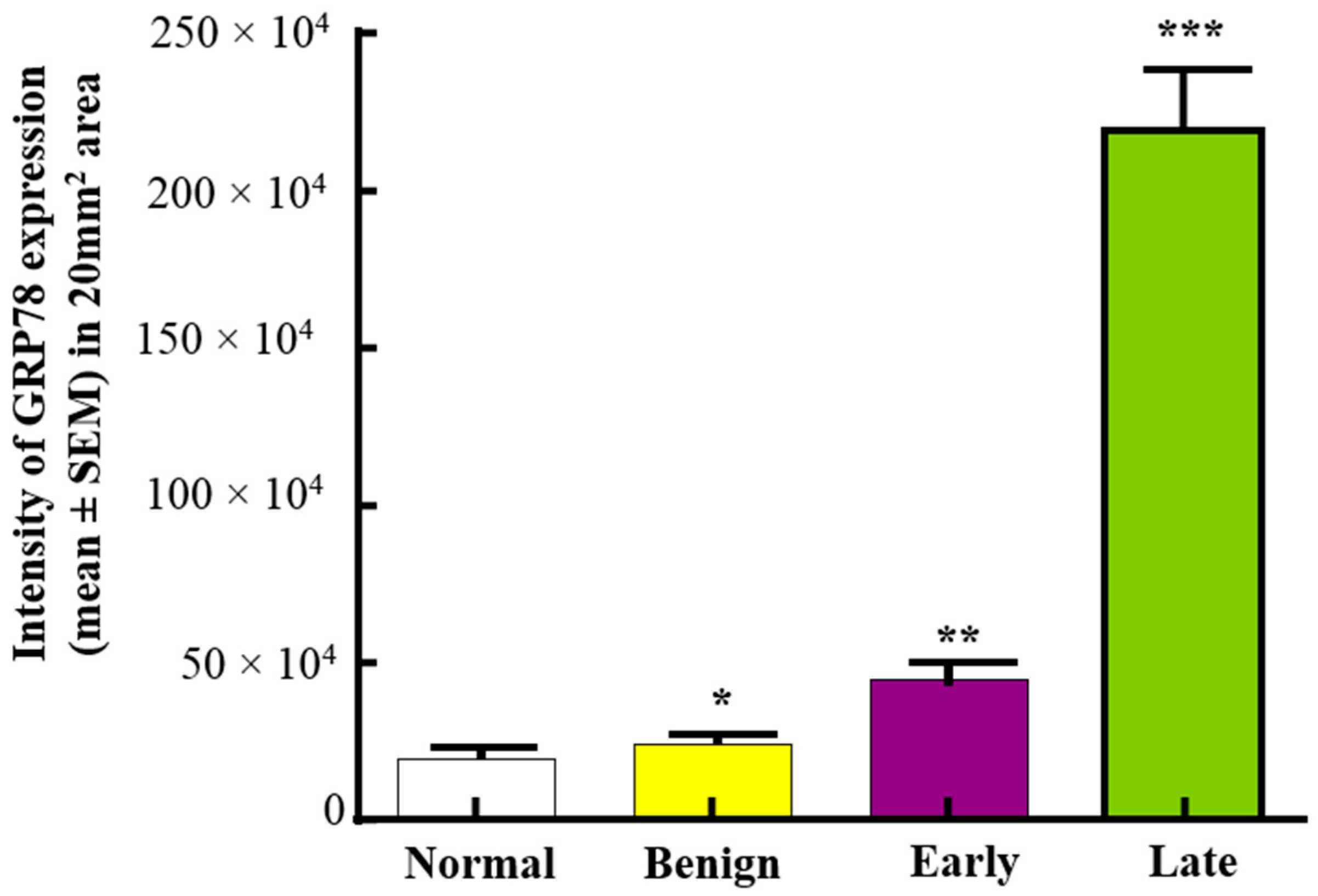
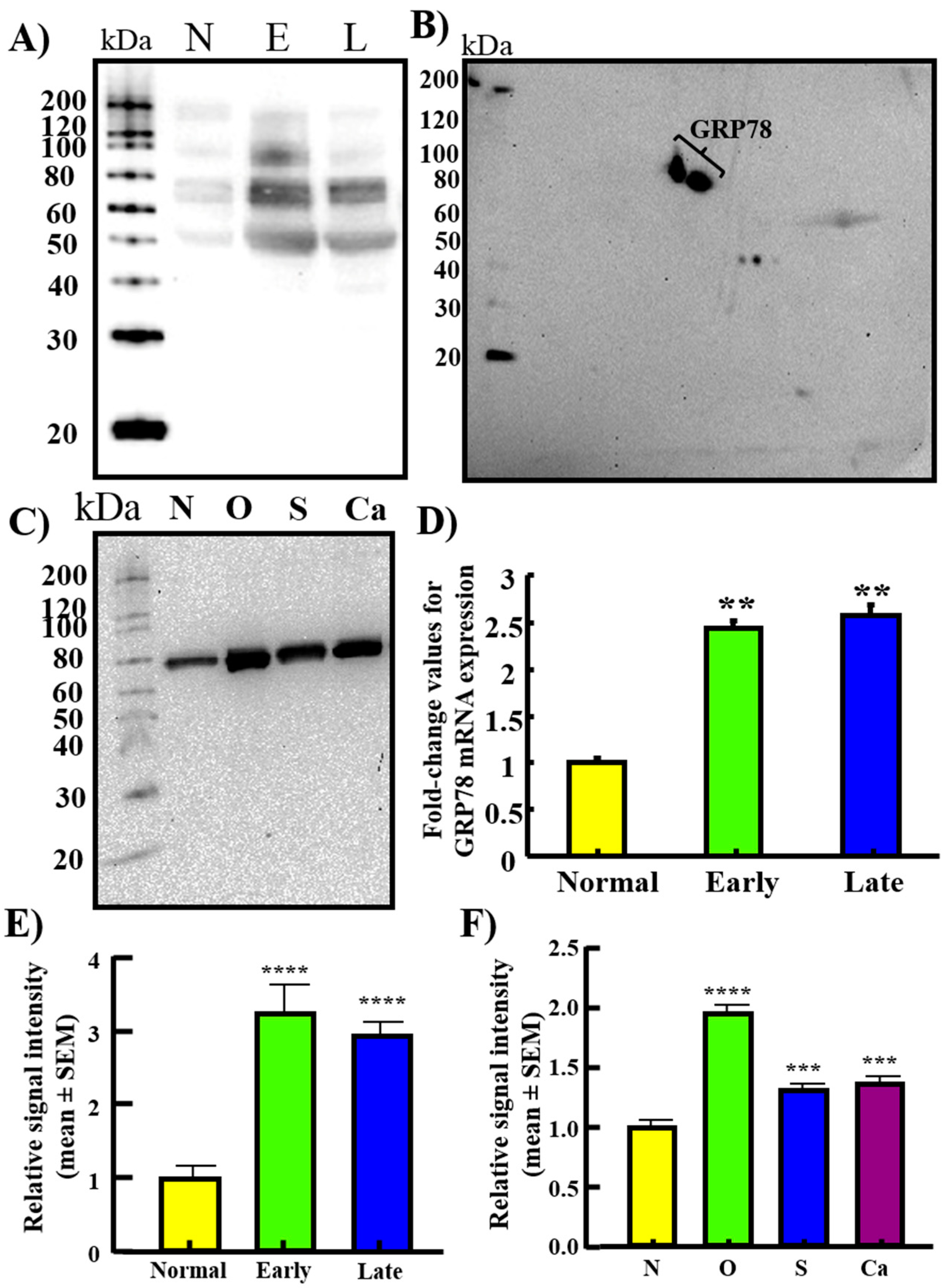
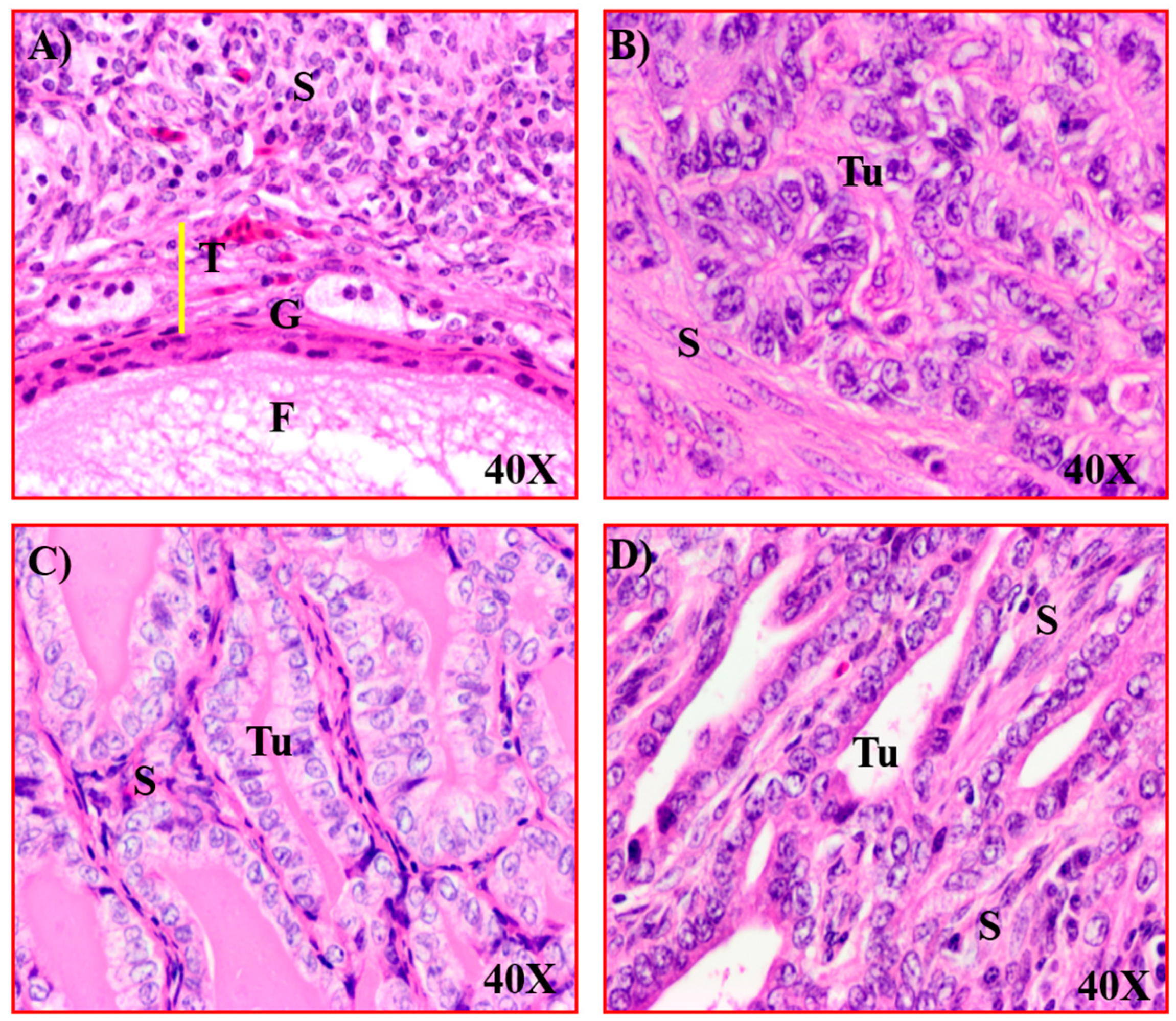
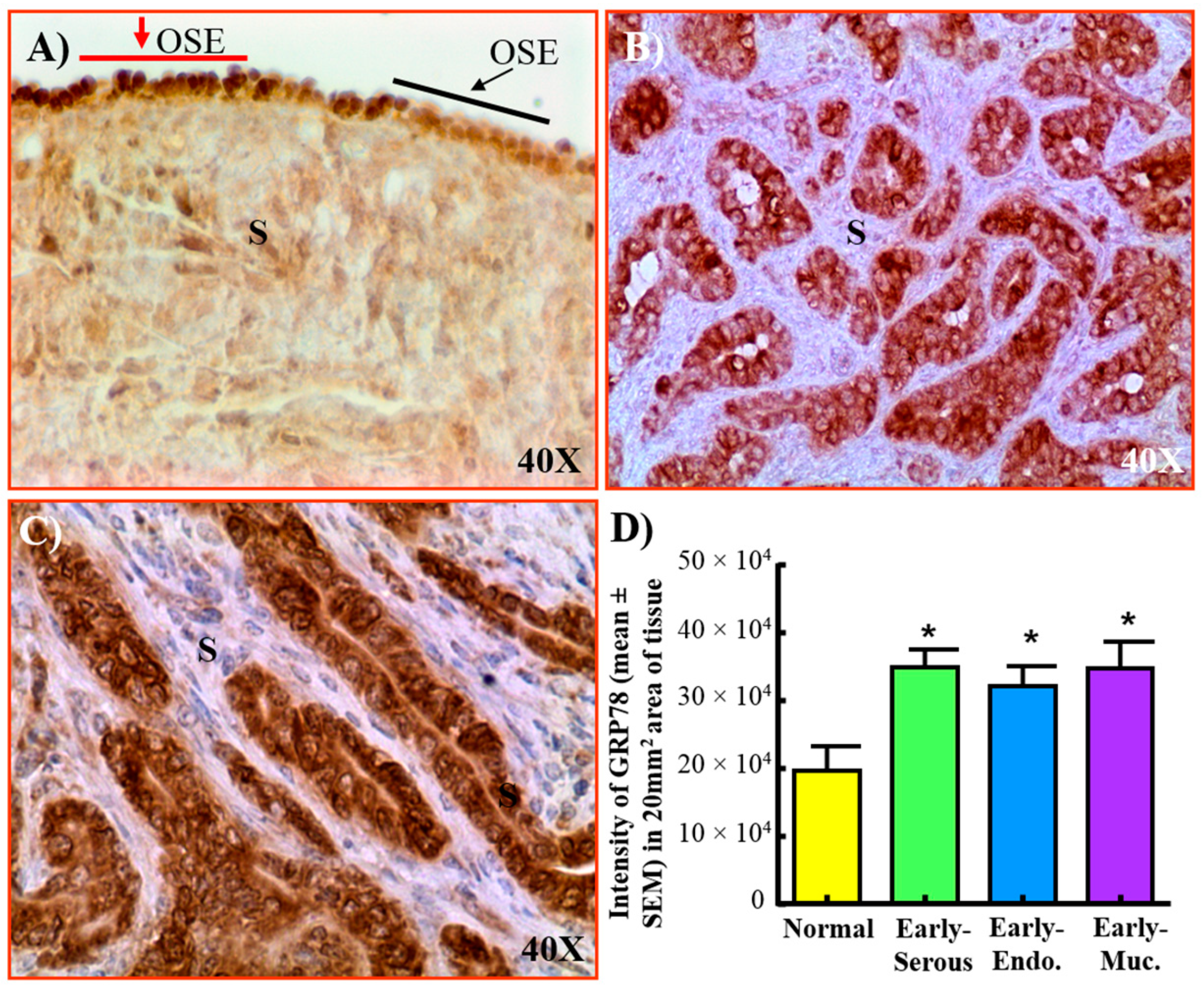
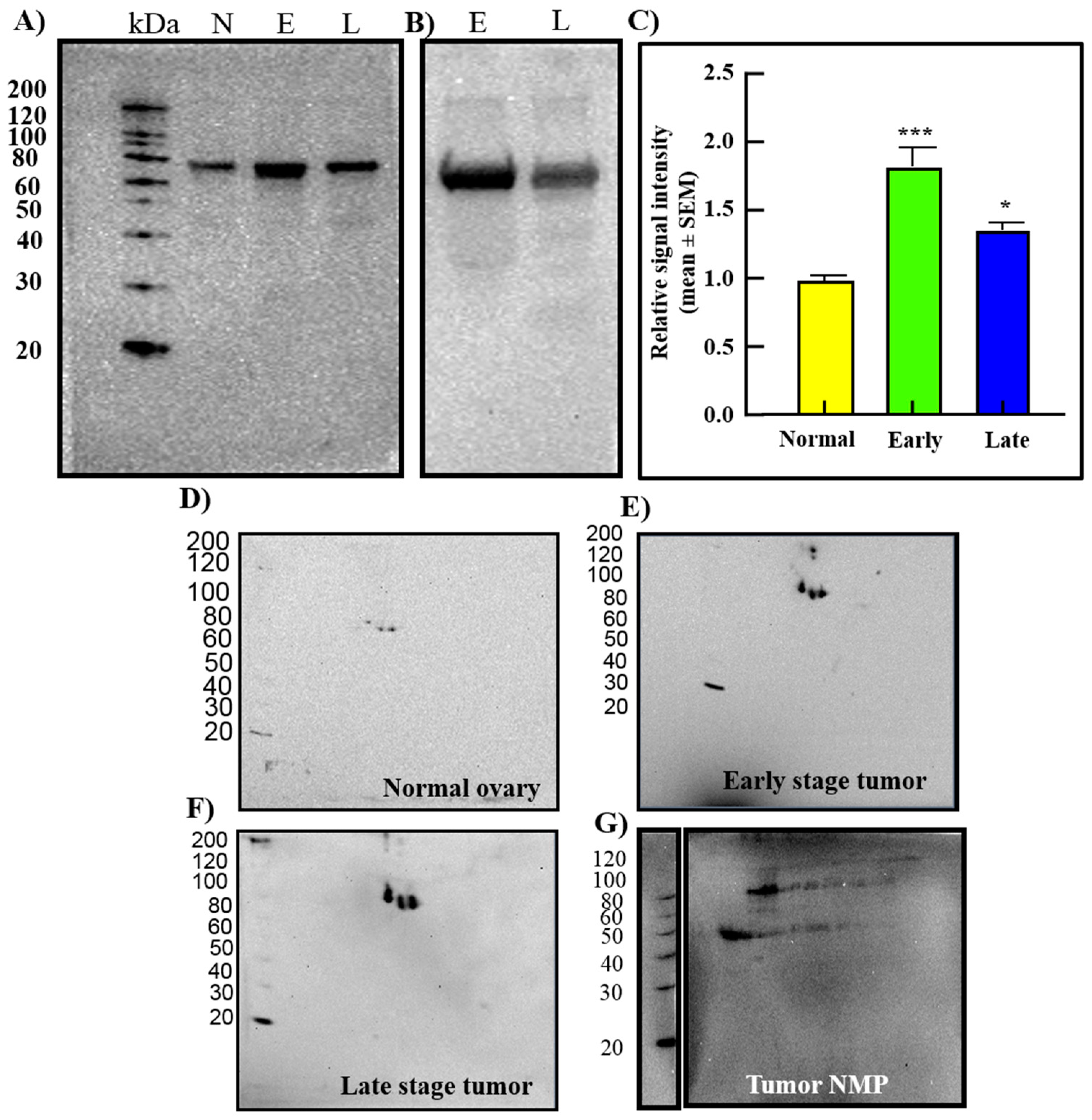
3.1. Expression of GRP78 in Normal Ovaries or Ovaries with Tumors in Patients
3.2. Changes in GRP78 Expression during OVCA Development in Hens
3.3. Serum Levels of GRP78
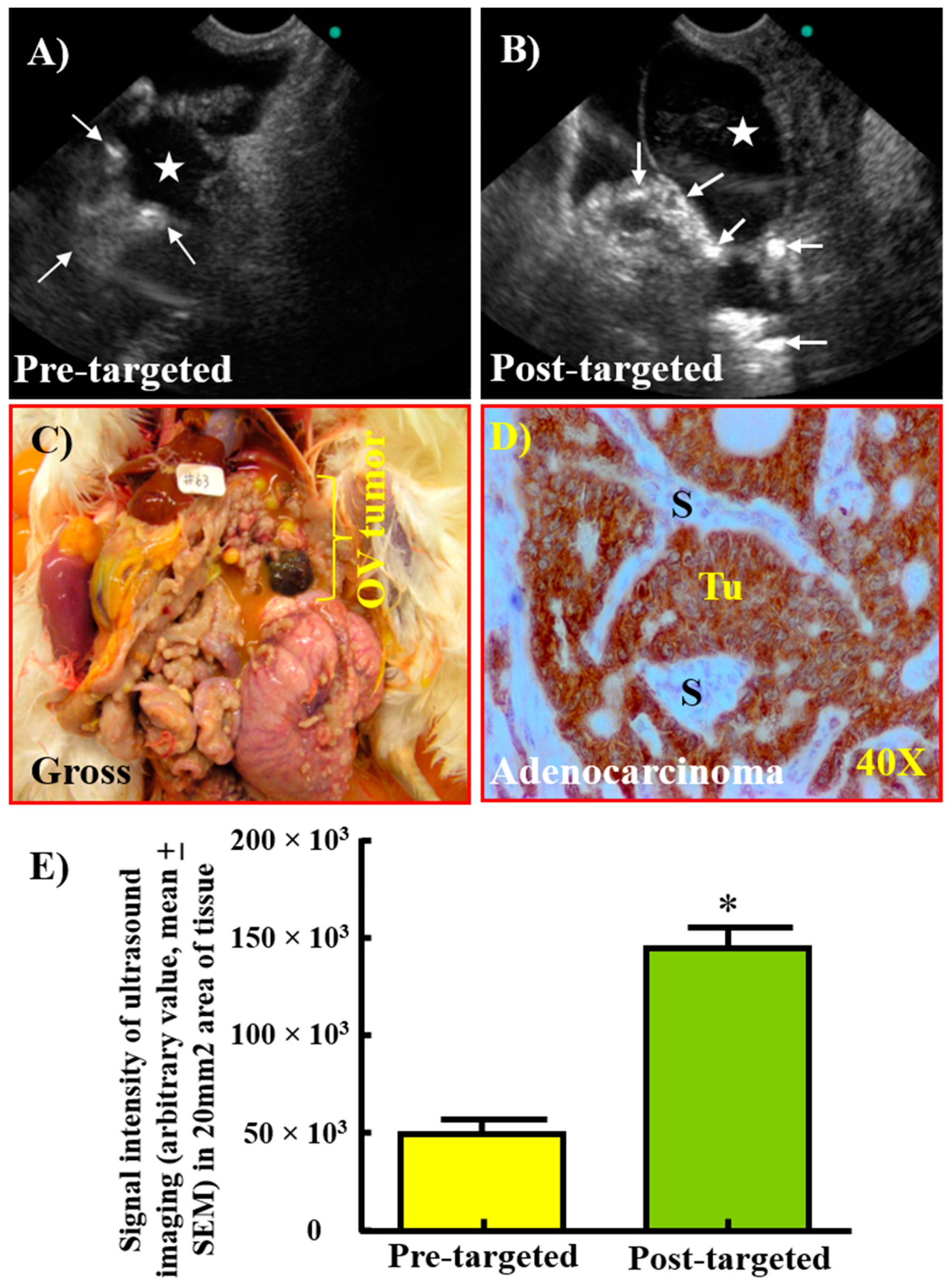
3.4. Enhancement in the Signal Intensity of TVUS Scanning by GRP78-Targeted Imaging Agents for the Detection of Ovarian Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surveillance, E.; End Results (SEER) Program. SEER*Stat Database: North American Association of Central Cancer Registries (NAACCR) Incidence Data-Cancer in North America (CiNA) Analytic File, 1995-2014; National Cancer Institute: Bethesda, MD, USA, 2016. [Google Scholar]
- Howlader, N.; Krapcho, M.; Noone, A.M.; Miller, D.; Bishop, K.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; et al. SEER Cancer Statistics Review, 1975–2014. National Cancer Institute. Bethesda, MD. Available online: https://seer.cancer.gov/csr/1975_2014/ (accessed on 3 April 2017).
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Ries, L.A. Ovarian cancer. Survival and treatment differences by age. Cancer 1993, 71 (Suppl. S2), 524–529. [Google Scholar] [PubMed]
- Pinsky, P.F.; Yu, K.; Kramer, B.S.; Black, A.; Buys, S.S.; Partridge, E.; Gohagan, J.; Berg, C.D.; Prorok, P.C. Extended mortality results for ovarian cancer screening in the PLCO trial with median 15years follow-up. Gynecol. Oncol. 2016, 143, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Jaworek, J.; Leja-Szpak, A.; Nawrot-Porabka, K.; Szklarczyk, J.; Kot, M.; Pierzchalski, P.; Goralska, M.; Ceranowicz, P.; Warzecha, Z.; Dembinski, A.; et al. Effects of melatonin and its analogues on pancreatic inflammation, enzyme secretion, and tumorigenesis. Int. J. Mol. Sci. 2017, 18, 1014. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.; Rote, N.S.; Minium, J.; Kirwan, J.P. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 336–340. [Google Scholar] [CrossRef]
- Elssner, A.; Doseff, A.I.; Duncan, M.; Kotur, M.; Wewers, M.D. IL-16 is constitutively present in peripheral blood monocytes and spontaneously released during apoptosis. J. Immunol. 2004, 172, 7721–7725. [Google Scholar] [CrossRef]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef]
- Hendershot, L.M.; Valentine, V.A.; Lee, A.S.; Morris, S.W.; Shapiro, D.N. Localization of the gene encoding human BiP/GRP78, the endoplasmic reticulum cognate of the HSP70 family, to chromosome 9q34. Genomics 1994, 20, 281–284. [Google Scholar] [CrossRef]
- Ames, B.N.; Gold, L.S.; Willett, W.C. The causes and prevention of cancer. Proc. Natl. Acad. Sci. USA 1995, 92, 5258–5265. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z. Glucose regulated protein 78: A critical link between tumor microenvironment and cancer hallmarks. Biochim. Biophys Acta 2012, 1826, 13–22. [Google Scholar] [CrossRef]
- Barua, A.; Bitterman, P.; Abramowicz, J.S.; Dirks, A.L.; Bahr, J.M.; Hales, D.B.; Bradaric, M.J.; Edassery, S.L.; Rotmensch, J.; Luborsky, J.L. Histopathology of ovarian tumors in laying hens: A preclinical model of human ovarian cancer. Int. J. Gynecol. Cancer 2009, 19, 531–539. [Google Scholar] [CrossRef]
- Rodriguez-Burford, C.; Barnes, M.N.; Berry, W.; Partridge, E.E.; Grizzle, W.E. Immunohistochemical expression of molecular markers in an avian model: A potential model for preclinical evaluation of agents for ovarian cancer chemoprevention. Gynecol. Oncol. 2001, 81, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Stammer, K.; Edassery, S.L.; Barua, A.; Bitterman, P.; Bahr, J.M.; Hales, D.B.; Luborsky, J.L. Selenium-Binding Protein 1 expression in ovaries and ovarian tumors in the laying hen, a spontaneous model of human ovarian cancer. Gynecol. Oncol. 2008, 109, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Paris, E.A.; Bahr, J.M.; Bitterman, P.; Basu, S.; Abramowicz, J.S.; Barua, A. Incidence of malignant transformation in the oviductal fimbria in laying hens, a preclinical model of spontaneous ovarian cancer. PLoS ONE 2021, 16, e0255007. [Google Scholar] [CrossRef]
- Barua, A.; Abramowicz, J.S.; Bahr, J.M.; Bitterman, P.; Dirks, A.; Holub, K.A.; Sheiner, E.; Bradaric, M.J.; Edassery, S.L.; Luborsky, J.L. Detection of ovarian tumors in chicken by sonography: A step toward early diagnosis in humans? J. Ultrasound Med. 2007, 26, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Barua, A.; Yellapa, A.; Bahr, J.M.; Adur, M.K.; Utterback, C.W.; Bitterman, P.; Basu, S.; Sharma, S.; Abramowicz, J.S. Interleukin 16- (IL-16-) targeted ultrasound imaging agent improves detection of ovarian tumors in laying hens, a preclinical model of spontaneous ovarian cancer. Biomed. Res. Int. 2015, 2015, 567459. [Google Scholar] [CrossRef]
- Barua, A.; Bradaric, M.J.; Kebede, T.; Espionosa, S.; Edassery, S.L.; Bitterman, P.; Rotmensch, J.; Luborsky, J.L. Anti-tumor and anti-ovarian autoantibodies in women with ovarian cancer. Am. J. Reprod. Immunol. 2007, 57, 243–249. [Google Scholar] [CrossRef]
- Barua, A.; Qureshi, T.; Bitterman, P.; Bahr, J.M.; Basu, S.; Abramowicz, J.S. Molecular targeted imaging of vascular endothelial growth factor receptor (VEGFR)-2 and anti-NMP autoantibodies detect ovarian tumor at early stage. Cancer Res. 2007, 72 (Suppl. S8), 2455. [Google Scholar] [CrossRef]
- Yu, E.; Lee, H.; Oh, W.; Yu, B.; Moon, H.; Lee, I. Morphological and biochemical analysis of anti-nuclear matrix protein antibodies in human sera. J. Korean Med. Sci. 1999, 14, 27–33. [Google Scholar] [CrossRef]
- Penumatsa, K.; Edassery, S.L.; Barua, A.; Bradaric, M.J.; Luborsky, J.L. Differential expression of aldehyde dehydrogenase 1a1 (ALDH1) in normal ovary and serous ovarian tumors. J. Ovarian Res. 2010, 3, 28. [Google Scholar] [CrossRef]
- Yellapa, A.; Bahr, J.M.; Bitterman, P.; Abramowicz, J.S.; Edassery, S.L.; Penumatsa, K.; Basu, S.; Rotmensch, J.; Barua, A. Association of interleukin 16 with the development of ovarian tumor and tumor-associated neoangiogenesis in laying hen model of spontaneous ovarian cancer. Int. J. Gynecol. Cancer 2012, 22, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Bahr, J.M.; Yellapa, A.; Bitterman, P.; Abramowicz, J.S.; Edassery, S.L.; Basu, S.; Rotmensch, J.; Barua, A. Expression of leukocyte inhibitory immunoglobulin-like transcript 3 receptors by ovarian tumors in laying hen model of spontaneous ovarian cancer. Transl. Oncol. 2012, 5, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Le Naour, F.; Brichory, F.; Misek, D.E.; Brechot, C.; Hanash, S.M.; Beretta, L. A distinct repertoire of autoantibodies in hepatocellular carcinoma identified by proteomic analysis. Mol. Cell Proteom. 2002, 1, 197–203. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Yellapa, A.; Bitterman, P.; Sharma, S.; Guirguis, A.S.; Bahr, J.M.; Basu, S.; Abramowicz, J.S.; Barua, A. Interleukin 16 expression changes in association with ovarian malignant transformation. Am. J. Obstet. Gynecol. 2014, 210, 272e1-10. [Google Scholar] [CrossRef]
- Barua, A.; Yellapa, A.; Bahr, J.M.; Abramowicz, J.S.; Edassery, S.L.; Basu, S.; Rotmensch, J.; Bitterman, P. Expression of death receptor 6 by ovarian tumors in laying hens, a preclinical model of spontaneous ovarian cancer. Transl. Oncol. 2012, 5, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.M.; Su, J.; Dong, H.; Wei, M.; Cui, M.H. Expression and role of glucose-regulated protein 78 in ovarian serous adenocarcinoma. Zhonghua Yi Xue Za Zhi 2013, 93, 659–662. [Google Scholar]
- Shin, B.K.; Wang, H.; Yim, A.M.; le Naour, F.; Brichory, F.; Jang, J.H.; Zhao, R.; Puravs, E.; Tra, J.; Michael, C.W.; et al. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J. Biol. Chem. 2003, 278, 7607–7616. [Google Scholar] [CrossRef]
- Mintz, P.J.; Kim, J.; Do, K.A.; Wang, X.; Zinner, R.G.; Cristofanilli, M.; Arap, M.A.; Hong, W.K.; Troncoso, P.; Logothetis, C.J.; et al. Fingerprinting the circulating repertoire of antibodies from cancer patients. Nat. Biotechnol. 2003, 21, 57–63. [Google Scholar] [CrossRef]
- Davidson, D.J.; Haskell, C.; Majest, S.; Kherzai, A.; Egan, D.A.; Walter, K.A.; Schneider, A.; Gubbins, E.F.; Solomon, L.; Chen, Z.; et al. Kringle 5 of human plasminogen induces apoptosis of endothelial and tumor cells through surface-expressed glucose-regulated protein 78. Cancer Res. 2005, 65, 4663–4672. [Google Scholar] [CrossRef]
- Cali, G.; Insabato, L.; Conza, D.; Bifulco, G.; Parrillo, L.; Mirra, P.; Fiory, F.; Miele, C.; Raciti, G.A.; di Jeso, B.; et al. GRP78 mediates cell growth and invasiveness in endometrial cancer. J. Cell Physiol. 2014, 229, 1417–1426. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas. Available online: https://gdc.cancer.gov/ (accessed on 25 January 2023).
- Grossman, R.L.; Heath, A.P.; Ferretti, V.; Varmus, H.E.; Lowy, D.R.; Kibbe, W.A.; Staudt, L.M. Toward a Shared Vision for Cancer Genomic Data. N. Engl. J. Med. 2016, 375, 1109–1112. [Google Scholar] [CrossRef]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 25 January 2023).
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Calaf, G.M.; Urzua, U.; Termini, L.; Aguayo, F. Oxidative stress in female cancers. Oncotarget 2018, 9, 23824–23842. [Google Scholar] [CrossRef] [PubMed]
- Machelon, V.; Emilie, D. Production of ovarian cytokines and their role in ovulation in the mammalian ovary. Eur. Cytokine Netw. 1997, 8, 137–143. [Google Scholar] [PubMed]
- Ni, M.; Zhang, Y.; Lee, A.S. Beyond the endoplasmic reticulum: Atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem. J. 2011, 434, 181–188. [Google Scholar] [CrossRef]
- Barker, S.; Weinfeld, M.; Zheng, J.; Li, L.; Murray, D. Identification of mammalian proteins cross-linked to DNA by ionizing radiation. J. Biol. Chem. 2005, 280, 33826–33838. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information, U.S. National Library of Medicine. Endoplasmic Reticulum Chaperone BiP Precursor [Gallus Gallus]. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 26 January 2023).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paris, E.A.; Bahr, J.M.; Abramowicz, J.S.; Basu, S.; Barua, A. Glucose-Regulated Protein 78 Is a Potential Serum and Imaging Marker for Early Detection of Ovarian Cancer. Cancers 2023, 15, 1140. https://doi.org/10.3390/cancers15041140
Paris EA, Bahr JM, Abramowicz JS, Basu S, Barua A. Glucose-Regulated Protein 78 Is a Potential Serum and Imaging Marker for Early Detection of Ovarian Cancer. Cancers. 2023; 15(4):1140. https://doi.org/10.3390/cancers15041140
Chicago/Turabian StyleParis, Elizabeth A., Janice M. Bahr, Jacques S. Abramowicz, Sanjib Basu, and Animesh Barua. 2023. "Glucose-Regulated Protein 78 Is a Potential Serum and Imaging Marker for Early Detection of Ovarian Cancer" Cancers 15, no. 4: 1140. https://doi.org/10.3390/cancers15041140
APA StyleParis, E. A., Bahr, J. M., Abramowicz, J. S., Basu, S., & Barua, A. (2023). Glucose-Regulated Protein 78 Is a Potential Serum and Imaging Marker for Early Detection of Ovarian Cancer. Cancers, 15(4), 1140. https://doi.org/10.3390/cancers15041140





