Are Aspects of Integrative Concepts Helpful to Improve Pancreatic Cancer Therapy?
Abstract
Simple Summary
Abstract
1. Introduction
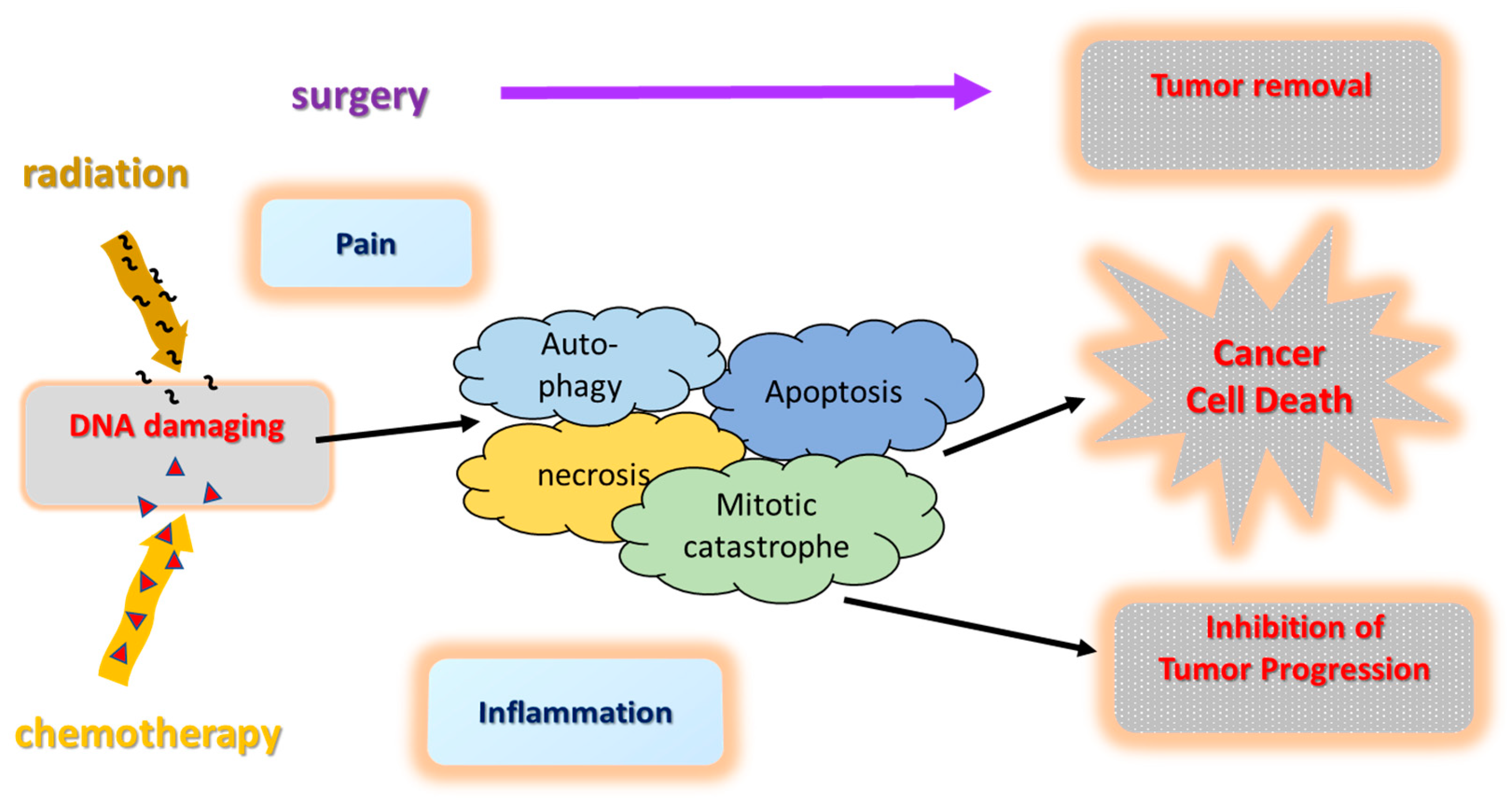
2. Standard Oncological Pancreatic Cancer Therapy
3. Targeted Therapy and Selective Precision Medicine
4. Anti-Tumor Mechanisms in PC Treatment
4.1. Anti-Stromal Effects
4.2. Immunomodulation
4.3. Induction of Apoptosis
4.4. Anti-Inflamation
5. Strategies to Overcome the Tumor Barrier
5.1. Nanomedicine
5.2. Hyperthermia
5.3. Electroporation
5.4. Intra-Tumoral Applications
5.5. Vaccines
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.; Schwarz, L.; Borbath, I.; Henry, A.; Van Laethem, J.L.; Malka, D.; Ducreux, M.; Conroy, T. An update on treatment options for pancreatic adenocarcinoma. Ther. Adv. Med. Oncol. 2019, 11, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Esch, T.; Brinkhaus, B. Neue Definitionen der Integrativen Medizin: Alter Wein in neuen Schläuchen? Complement. Med. Res 2020, 27, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Witt, C.M.; Balneaves, L.G.; Cardoso, M.J.; Cohen, L.; Greenlee, H.; Johnstone, P.; Kücük, Ö.; Mailman, J.; Mao, J.J. A Comprehensive Definition for Integrative Oncology. J. Natl. Cancer Inst. Monogr. 2017, 52, lgx012. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.M.; Gingras, M.C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.; Quinn, M.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef]
- Ghiorzo, P. Genetic predisposition to pancreatic cancer. World J. Gastroenterol. 2014, 20, 10778–10789. [Google Scholar] [CrossRef]
- S3-Leitlinie zum Exokrinen Pankreaskarzinom. 2021. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Pankreaskarzinom/Version_2/LL_Pankreaskarzinom_Langversion_2.0.pdf (accessed on 9 May 2022).
- Stoffel, E.M.; McKernin, S.E.; Brand, R.; Canto, M.; Goggins, M.; Moravek, C.; Nagarajan, A.; Petersen, G.M.; Simeone, D.M.; Yurgelun, M.; et al. Evaluating Susceptibility to Pancreatic Cancer: ASCO Provisional Clinical Opinion. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 153–164. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Djamgoz, M.B.A.; Jentzsch, V. Integrative Management of Pancreatic Cancer (PDAC): Emerging Complementary Agents and Modalities. Nutr. Cancer 2022, 74, 1139–1162. [Google Scholar] [CrossRef]
- Jentzsch, V.; Davis, J.A.A.; Djamgoz, M.B.A. Pancreatic Cancer (PDAC): Introduction of Evidence-Based Complementary Measures into Integrative Clinical Management. Cancers 2020, 12, 3096. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Carrato, A.; Falcone, A.; Ducreux, M.; Valle, J.W.; Parnaby, A.; Djazouli, K.; Alnwick-Allu, K.; Hutchings, A.; Palaska, C.; Parthenaki, I. A Systematic Review of the Burden of Pancreatic Cancer in Europe: Real-World Impact on Survival, Quality of Life and Costs. J. Gastrointest. Cancer 2015, 46, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Epstein, A.S. Palliative care and advance care planning for pancreas and other cancers. Chin. Clin. Oncol. 2017, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Moffat, G.T.; Epstein, A.S.; O’Reilly, E.M. Pancreatic cancer-A disease in need: Optimizing and integrating supportive care. Cancer 2019, 125, 3927–3935. [Google Scholar] [CrossRef] [PubMed]
- Lohse, I.; Wildermuth, E.; Brothers, S.P. Naturally occurring compounds as pancreatic cancer therapeutics. Oncotarget 2018, 9, 35448–35457. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, P.; Loh, W.M.; Gopinath, S.C.B.; Bonam, S.R.; Fareez, I.M.; Mac Guad, R.; Sim, M.S.; Wu, Y.S. Selective phytochemicals targeting pancreatic stellate cells as new anti-fibrotic agents for chronic pancreatitis and pancreatic cancer. Acta Pharm. Sinica. B 2020, 10, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Chone, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Khorana, A.A.; Mangu, P.B.; Berlin, J.; Engebretson, A.; Hong, T.S.; Maitra, A.; Mohile, S.G.; Mumber, M.; Schulick, R.; Shapiro, M.; et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 2324–2328. [Google Scholar] [CrossRef]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goere, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26 (Suppl. S5), v56–v68. [Google Scholar] [CrossRef]
- Sohal, D.P.S.; Kennedy, E.B.; Khorana, A.; Copur, M.S.; Crane, C.H.; Garrido-Laguna, I.; Krishnamurthi, S.; Moravek, C.; O’Reilly, E.M.; Philip, P.A.; et al. Metastatic Pancreatic Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Jo, J.H.; Lee, H.S.; Chung, M.J.; Bang, S.; Park, S.W.; Song, S.Y.; Park, J.Y. Comparison of efficacy and safety between standard-dose and modified-dose FOLFIRINOX as a first-line treatment of pancreatic cancer. World J. Gastrointest. Oncol. 2018, 10, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Palta, M.; Godfrey, D.; Goodman, K.A.; Hoffe, S.; Dawson, L.A.; Dessert, D.; Hall, W.A.; Herman, J.M.; Khorana, A.A.; Merchant, N.; et al. Radiation Therapy for Pancreatic Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2019, 9, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.M.; Der, C.J. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a031435. [Google Scholar] [CrossRef] [PubMed]
- Crowley, F.; Park, W.; O’Reilly, E.M. Targeting DNA damage repair pathways in pancreas cancer. Cancer Metastasis Rev. 2021, 40, 891–908. [Google Scholar] [CrossRef] [PubMed]
- Perkhofer, L.; Gout, J.; Roger, E.; Kude de Almeida, F.; Baptista Simões, C.; Wiesmüller, L.; Seufferlein, T.; Kleger, A. DNA damage repair as a target in pancreatic cancer: State-of-the-art and future perspectives. Gut 2021, 70, 606–617. [Google Scholar] [CrossRef]
- Yao, W.; Maitra, A.; Ying, H. Recent insights into the biology of pancreatic cancer. EBioMedicine 2020, 53, 102655. [Google Scholar] [CrossRef]
- Petersen, G.M. Familial pancreatic cancer. Semin. Oncol. 2016, 43, 548–553. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef]
- Kindler, H.L.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.O.; Hochhauser, D.; Arnold, D.; Oh, D.Y.; et al. Overall Survival Results From the POLO Trial: A Phase III Study of Active Maintenance Olaparib versus Placebo for Germline BRCA-Mutated Metastatic Pancreatic Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 3929–3939. [Google Scholar] [CrossRef]
- Zhu, H.; Wei, M.; Xu, J.; Hua, J.; Liang, C.; Meng, Q.; Zhang, Y.; Liu, J.; Zhang, B.; Yu, X.; et al. PARP inhibitors in pancreatic cancer: Molecular mechanisms and clinical applications. Mol. Cancer 2020, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Iyer, R.; Fountzilas, C. Poly(ADP-Ribose) Polymerase Inhibitors in Pancreatic Cancer: A New Treatment Paradigms and Future Implications. Cancers 2019, 11, 1980. [Google Scholar] [CrossRef] [PubMed]
- Slade, D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020, 34, 360–394. [Google Scholar] [CrossRef] [PubMed]
- Casolino, R.; Corbo, V.; Beer, P.; Hwang, C.I.; Paiella, S.; Silvestri, V.; Ottini, L.; Biankin, A.V. Germline Aberrations in Pancreatic Cancer: Implications for Clinical Care. Cancers 2022, 14, 3239. [Google Scholar] [CrossRef]
- Noordermeer, S.M.; van Attikum, H. PARP Inhibitor Resistance: A Tug-of-War in BRCA-Mutated Cells. Trends Cell Biol. 2019, 29, 820–834. [Google Scholar] [CrossRef]
- Jain, A.; Agostini, L.C.; McCarthy, G.A.; Chand, S.N.; Ramirez, A.; Nevler, A.; Cozzitorto, J.; Schultz, C.W.; Lowder, C.Y.; Smith, K.M.; et al. Poly (ADP) Ribose Glycohydrolase Can Be Effectively Targeted in Pancreatic Cancer. Cancer Res. 2019, 79, 4491–4502. [Google Scholar] [CrossRef]
- Lim, C.-S.; Im, K.; Lee, D.S.; Kwon, W.; Kim, J.R.; Han, Y.; Kim, S.-W.; Jang, J.-Y. The Implication of Cytogenetic Alterations in Pancreatic Ductal Adenocarcinoma and Intraductal Papillary Mucinous Neoplasm Identified by Fluorescence In Situ Hybridization and Their Potential Diagnostic Utility. Gut Liver 2020, 14, 509–520. [Google Scholar] [CrossRef]
- Sammallahti, H.; Sarhadi, V.K.; Kokkola, A.; Ghanbari, R.; Rezasoltani, S.; Asadzadeh Aghdaei, H.; Puolakkainen, P.; Knuutila, S. Oncogenomic Changes in Pancreatic Cancer and Their Detection in Stool. Biomolecules 2022, 12, 652. [Google Scholar] [CrossRef]
- Martinez-Useros, J.; Martin-Galan, M.; Florez-Cespedes, M.; Garcia-Foncillas, J. Epigenetics of Most Aggressive Solid Tumors: Pathways, Targets and Treatments. Cancers 2021, 13, 3209. [Google Scholar] [CrossRef]
- Brown, W.S.; McDonald, P.C.; Nemirovsky, O.; Awrey, S.; Chafe, S.C.; Schaeffer, D.F.; Li, J.; Renouf, D.J.; Stanger, B.Z.; Dedhar, S. Overcoming Adaptive Resistance to KRAS and MEK Inhibitors by Co-targeting mTORC1/2 Complexes in Pancreatic Cancer. Cell Rep. Med. 2020, 1, 100131. [Google Scholar] [CrossRef]
- Strickler, J.H.; Satake, H.; George, T.J.; Yaeger, R.; Hollebecque, A.; Garrido-Laguna, I.; Schuler, M.; Burns, T.F.; Coveler, A.L.; Falchook, G.S.; et al. Sotorasib in KRAS p.G12C-Mutated Advanced Pancreatic Cancer. N. Engl. J. Med. 2023, 388, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Rémond, M.S.; Pellat, A.; Brezault, C.; Dhooge, M.; Coriat, R. Are targeted therapies or immunotherapies effective in metastatic pancreatic adenocarcinoma? ESMO Open 2022, 7, 100638. [Google Scholar] [CrossRef] [PubMed]
- Roa, F.J.; Peña, E.; Gatica, M.; Escobar-Acuña, K.; Saavedra, P.; Maldonado, M.; Cuevas, M.E.; Moraga-Cid, G.; Rivas, C.I.; Muñoz-Montesino, C. Therapeutic Use of Vitamin C in Cancer: Physiological Considerations. Front. Pharmacol. 2020, 11, 211. [Google Scholar] [CrossRef] [PubMed]
- Kanat, O.; Ertas, H. Shattering the castle walls: Anti-stromal therapy for pancreatic cancer. World J. Gastrointest. Oncol. 2018, 10, 202–210. [Google Scholar] [CrossRef]
- Bynigeri, R.R.; Jakkampudi, A.; Jangala, R.; Subramanyam, C.; Sasikala, M.; Rao, G.V.; Reddy, D.N.; Talukdar, R. Pancreatic stellate cell: Pandora’s box for pancreatic disease biology. World J. Gastroenterol. 2017, 23, 382–405. [Google Scholar] [CrossRef]
- McCarroll, J.A.; Phillips, P.A.; Santucci, N.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Vitamin A inhibits pancreatic stellate cell activation: Implications for treatment of pancreatic fibrosis. Gut 2006, 55, 79–89. [Google Scholar] [CrossRef]
- Froeling, F.E.; Feig, C.; Chelala, C.; Dobson, R.; Mein, C.E.; Tuveson, D.A.; Clevers, H.; Hart, I.R.; Kocher, H.M. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-β-catenin signaling to slow tumor progression. Gastroenterology 2011, 141, 1486–1497.e14. [Google Scholar] [CrossRef]
- Sarper, M.; Cortes, E.; Lieberthal, T.J.; Del Río Hernández, A. ATRA modulates mechanical activation of TGF-β by pancreatic stellate cells. Sci. Rep. 2016, 6, 27639. [Google Scholar] [CrossRef]
- Kocher, H.M.; Basu, B.; Froeling, F.E.M.; Sarker, D.; Slater, S.; Carlin, D.; deSouza, N.M.; De Paepe, K.N.; Goulart, M.R.; Hughes, C.; et al. Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat. Commun. 2020, 11, 4841. [Google Scholar] [CrossRef]
- Sherman, M.H.; Yu, R.T.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; Van Dyke, T.; Kozlov, S.; et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014, 159, 80–93. [Google Scholar] [CrossRef]
- Delitto, D.; Wallet, S.M.; Hughes, S.J. Targeting tumor tolerance: A new hope for pancreatic cancer therapy? Pharmacol. Ther. 2016, 166, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Wang, L.; Cheng, Y.G.; Zhang, G.Y.; Hu, S.Y.; Zhou, B.; Zhan, H.X. Immunotherapy for pancreatic cancer: A long and hopeful journey. Cancer Lett. 2018, 425, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Loef, M.; Walach, H. Quality of life in cancer patients treated with mistletoe: A systematic review and meta-analysis. BMC Complement. Med. Ther. 2020, 20, 227. [Google Scholar] [CrossRef] [PubMed]
- Oei, S.L.; Thronicke, A.; Schad, F. Mistletoe and Immunomodulation: Insights and Implications for Anticancer Therapies. Evid.-Based Complement. Altern. Med. 2019, 2019, 5893017. [Google Scholar] [CrossRef]
- Thronicke, A.; Schad, F.; Debus, M.; Grabowski, J.; Soldner, G. Viscum album L. Therapy in Oncology—An Update on Current Evidence. Complement. Med. Res. 2022, 29, 362–368. [Google Scholar] [CrossRef]
- Mansky, P.J.; Wallerstedt, D.B.; Sannes, T.S.; Stagl, J.; Johnson, L.L.; Blackman, M.R.; Grem, J.L.; Swain, S.M.; Monahan, B.P. NCCAM/NCI Phase 1 Study of Mistletoe Extract and Gemcitabine in Patients with Advanced Solid Tumors. Evid.-Based Complement. Altern. Med. 2013, 2013, 964592. [Google Scholar] [CrossRef]
- Troger, W.; Galun, D.; Reif, M.; Schumann, A.; Stankovic, N.; Milicevic, M. Viscum album [L.] extract therapy in patients with locally advanced or metastatic pancreatic cancer: A randomised clinical trial on overall survival. Eur. J. Cancer 2013, 49, 3788–3797. [Google Scholar] [CrossRef]
- Axtner, J.; Steele, M.; Kroz, M.; Spahn, G.; Matthes, H.; Schad, F. Health services research of integrative oncology in palliative care of patients with advanced pancreatic cancer. BMC Cancer 2016, 16, 579. [Google Scholar] [CrossRef]
- Wode, K.; Hök Nordberg, J.; Kienle, G.S.; Elander, N.O.; Bernhardson, B.M.; Sunde, B.; Sharp, L.; Henriksson, R.; Fransson, P. Efficacy of mistletoe extract as a complement to standard treatment in advanced pancreatic cancer: Study protocol for a multicentre, parallel group, double-blind, randomised, placebo-controlled clinical trial (MISTRAL). Trials 2020, 21, 783. [Google Scholar] [CrossRef]
- Thronicke, A.; Reinhold, T.; von Trott, P.; Matthes, H.; Schad, F. Cost-Effectiveness of Real-World Administration of Concomitant Viscum album L. Therapy for the Treatment of Stage IV Pancreatic Cancer. Evid.-Based Complement. Altern. Med. 2020, 2020, 3543568. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Aggarwal, B.B. “Spicing up” of the immune system by curcumin. J. Clin. Immunol. 2007, 27, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011, 68, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Amin, A.R.; Chen, Z.G.; Shin, D.M. New perspectives of curcumin in cancer prevention. Cancer Prev. Res. 2013, 6, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative preparation of curcumin for improved oral bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef]
- Kanai, M. Therapeutic applications of curcumin for patients with pancreatic cancer. World J. Gastroenterol. 2014, 20, 9384–9391. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Kanai, M.; Otsuka, Y.; Otsuka, K.; Sato, M.; Nishimura, T.; Mori, Y.; Kawaguchi, M.; Hatano, E.; Kodama, Y.; Matsumoto, S.; et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother. Pharmacol. 2013, 71, 1521–1530. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Hubner, R.A.; Siveke, J.T.; Von Hoff, D.D.; Belanger, B.; de Jong, F.A.; Mirakhur, B.; Chen, L.T. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur. J. Cancer 2019, 108, 78–87. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Li, C.P.; Bodoky, G.; Dean, A.; Shan, Y.S.; Jameson, G.; Macarulla, T.; Lee, K.H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef] [PubMed]
- van der Horst, A.; Versteijne, E.; Besselink, M.G.H.; Daams, J.G.; Bulle, E.B.; Bijlsma, M.F.; Wilmink, J.W.; van Delden, O.M.; van Hooft, J.E.; Franken, N.A.P.; et al. The clinical benefit of hyperthermia in pancreatic cancer: A systematic review. Int. J. Hyperth. 2018, 34, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, G.; Sarti, D.; Casadei, V.; Milandri, C.; Dentico, P.; Mambrini, A.; Nani, R.; Fiorentini, C.; Guadagni, S. Modulated Electro-Hyperthermia as Palliative Treatment for Pancreatic Cancer: A Retrospective Observational Study on 106 Patients. Integr. Cancer Ther. 2019, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C., 2nd; Kwon, D.; Chalikonda, S.; Sellers, M.; Kotz, E.; Scoggins, C.; McMasters, K.M.; Watkins, K. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: Safety and efficacy. Ann. Surg. 2015, 262, 486–494; discussion 484–492. [Google Scholar] [CrossRef]
- Narayanan, G.; Hosein, P.J.; Beulaygue, I.C.; Froud, T.; Scheffer, H.J.; Venkat, S.R.; Echenique, A.M.; Hevert, E.C.; Livingstone, A.S.; Rocha-Lima, C.M.; et al. Percutaneous Image-Guided Irreversible Electroporation for the Treatment of Unresectable, Locally Advanced Pancreatic Adenocarcinoma. J. Vasc. Interv. Radiol. 2017, 28, 342–348. [Google Scholar] [CrossRef]
- Ruarus, A.H.; Vroomen, L.; Geboers, B.; van Veldhuisen, E.; Puijk, R.S.; Nieuwenhuizen, S.; Besselink, M.G.; Zonderhuis, B.M.; Kazemier, G.; de Gruijl, T.D.; et al. Percutaneous Irreversible Electroporation in Locally Advanced and Recurrent Pancreatic Cancer (PANFIRE-2): A Multicenter, Prospective, Single-Arm, Phase II Study. Radiology 2020, 294, 212–220. [Google Scholar] [CrossRef]
- Schad, F.; Atxner, J.; Buchwald, D.; Happe, A.; Popp, S.; Kroz, M.; Matthes, H. Intratumoral Mistletoe (Viscum album L) Therapy in Patients With Unresectable Pancreas Carcinoma: A Retrospective Analysis. Integr. Cancer Ther. 2014, 13, 332–340. [Google Scholar] [CrossRef]
- Steele, M.L.; Axtner, J.; Happe, A.; Kroz, M.; Matthes, H.; Schad, F. Use and safety of intratumoral application of European mistletoe (Viscum album L) preparations in Oncology. Integr. Cancer Ther. 2015, 14, 140–148. [Google Scholar] [CrossRef]
- Hirooka, Y.; Kasuya, H.; Ishikawa, T.; Kawashima, H.; Ohno, E.; Villalobos, I.B.; Naoe, Y.; Ichinose, T.; Koyama, N.; Tanaka, M.; et al. A Phase I clinical trial of EUS-guided intratumoral injection of the oncolytic virus, HF10 for unresectable locally advanced pancreatic cancer. BMC Cancer 2018, 18, 596. [Google Scholar] [CrossRef]
- Lee, J.C.; Shin, D.W.; Park, H.; Kim, J.; Youn, Y.; Kim, J.H.; Kim, J.; Hwang, J.H. Tolerability and safety of EUS-injected adenovirus-mediated double-suicide gene therapy with chemotherapy in locally advanced pancreatic cancer: A phase 1 trial. Gastrointest. Endosc. 2020, 92, 1044–1052.e1. [Google Scholar] [CrossRef]
- Asahara, S.; Takeda, K.; Yamao, K.; Maguchi, H.; Yamaue, H. Phase I/II clinical trial using HLA-A24-restricted peptide vaccine derived from KIF20A for patients with advanced pancreatic cancer. J. Transl. Med. 2013, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Hazama, S.; Ueno, T.; Matsui, H.; Shindo, Y.; Iida, M.; Yoshimura, K.; Yoshino, S.; Takeda, K.; Oka, M. A phase I clinical trial of vaccination with KIF20A-derived peptide in combination with gemcitabine for patients with advanced pancreatic cancer. J. Immunother. 2014, 37, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Picozzi, V.J.; Ko, A.H.; Wainberg, Z.A.; Kindler, H.; Wang-Gillam, A.; Oberstein, P.; Morse, M.A.; Zeh, H.J., 3rd; Weekes, C.; et al. Results from a Phase IIb, Randomized, Multicenter Study of GVAX Pancreas and CRS-207 Compared with Chemotherapy in Adults with Previously Treated Metastatic Pancreatic Adenocarcinoma (ECLIPSE Study). Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 5493–5502. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Wang-Gillam, A.; Picozzi, V.; Greten, T.F.; Crocenzi, T.; Springett, G.; Morse, M.; Zeh, H.; Cohen, D.; Fine, R.L.; et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1325–1333. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Magrì, A.; Germano, G.; Lorenzato, A.; Lamba, S.; Chilà, R.; Montone, M.; Amodio, V.; Ceruti, T.; Sassi, F.; Arena, S.; et al. High-dose vitamin C enhances cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaay8707. [Google Scholar] [CrossRef]
- Monti, D.A.; Mitchell, E.; Bazzan, A.J.; Littman, S.; Zabrecky, G.; Yeo, C.J.; Pillai, M.V.; Newberg, A.B.; Deshmukh, S.; Levine, M. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS ONE 2012, 7, e29794. [Google Scholar] [CrossRef]
- Welsh, J.L.; Wagner, B.A.; van’t Erve, T.J.; Zehr, P.S.; Berg, D.J.; Halfdanarson, T.R.; Yee, N.S.; Bodeker, K.L.; Du, J.; Roberts, L.J., II; et al. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): Results from a phase I clinical trial. Cancer Chemother. Pharmacol. 2013, 71, 765–775. [Google Scholar] [CrossRef]
- Polireddy, K.; Dong, R.; Reed, G.; Yu, J.; Chen, P.; Williamson, S.; Violet, P.C.; Pessetto, Z.; Godwin, A.K.; Fan, F.; et al. High Dose Parenteral Ascorbate Inhibited Pancreatic Cancer Growth and Metastasis: Mechanisms and a Phase I/IIa study. Sci. Rep. 2017, 7, 17188. [Google Scholar] [CrossRef]
- Alexander, M.S.; O’Leary, B.R.; Wilkes, J.G.; Gibson, A.R.; Wagner, B.A.; Du, J.; Sarsour, E.; Hwang, R.F.; Buettner, G.R.; Cullen, J.J. Enhanced Pharmacological Ascorbate Oxidation Radiosensitizes Pancreatic Cancer. Radiat. Res. 2019, 191, 43–51. [Google Scholar] [CrossRef]
- Alexander, M.S.; Wilkes, J.G.; Schroeder, S.R.; Buettner, G.R.; Wagner, B.A.; Du, J.; Gibson-Corley, K.; O’Leary, B.R.; Spitz, D.R.; Buatti, J.M.; et al. Pharmacologic Ascorbate Reduces Radiation-Induced Normal Tissue Toxicity and Enhances Tumor Radiosensitization in Pancreatic Cancer. Cancer Res. 2018, 78, 6838–6851. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.R.; Alexander, M.S.; Du, J.; Moose, D.L.; Henry, M.D.; Cullen, J.J. Pharmacological ascorbate inhibits pancreatic cancer metastases via a peroxide-mediated mechanism. Sci. Rep. 2020, 10, 17649. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.G.; Sclabas, G.M.; Fujioka, S.; Schmidt, C.; Peng, B.; Wu, T.; Tsao, M.S.; Evans, D.B.; Abbruzzese, J.L.; McDonnell, T.J.; et al. The function of multiple IkappaB: NF-kappaB complexes in the resistance of cancer cells to Taxol-induced apoptosis. Oncogene 2002, 21, 6510–6519. [Google Scholar] [CrossRef] [PubMed]
- Arlt, A.; Muerkoster, S.S.; Schafer, H. Targeting apoptosis pathways in pancreatic cancer. Cancer Lett. 2013, 332, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Zubair, H.; Azim, S.; Ahmad, A.; Khan, M.A.; Patel, G.K.; Singh, S.; Singh, A.P. Cancer Chemoprevention by Phytochemicals: Nature’s Healing Touch. Molecules 2017, 22, 395. [Google Scholar] [CrossRef]
- Bussing, A.; Schietzel, M. Apoptosis-inducing properties of Viscum album L. extracts from different host trees, correlate with their content of toxic mistletoe lectins. Anticancer Res. 1999, 19, 23–28. [Google Scholar]
- Seifert, G.; Jesse, P.; Laengler, A.; Reindl, T.; Luth, M.; Lobitz, S.; Henze, G.; Prokop, A.; Lode, H.N. Molecular mechanisms of mistletoe plant extract-induced apoptosis in acute lymphoblastic leukemia in vivo and in vitro. Cancer Lett. 2008, 264, 218–228. [Google Scholar] [CrossRef]
- Podlech, O.; Harter, P.N.; Mittelbronn, M.; Poschel, S.; Naumann, U. Fermented mistletoe extract as a multimodal antitumoral agent in gliomas. Evid.-Based Complement. Altern. Med. 2012, 2012, 501796. [Google Scholar] [CrossRef]
- Kim, M.S.; So, H.S.; Lee, K.M.; Park, J.S.; Lee, J.H.; Moon, S.K.; Ryu, D.G.; Chung, S.Y.; Jung, B.H.; Kim, Y.K.; et al. Activation of caspase cascades in Korean mistletoe (Viscum album var. coloratum) lectin-II-induced apoptosis of human myeloleukemic U937 cells. Gen. Pharmacol. 2000, 34, 349–355. [Google Scholar] [CrossRef]
- Szurpnicka, A.; Kowalczuk, A.; Szterk, A. Biological activity of mistletoe: In vitro and in vivo studies and mechanisms of action. Arch. Pharm. Res. 2020, 43, 593–629. [Google Scholar] [CrossRef]
- Xu, Q.; Zong, L.; Chen, X.; Jiang, Z.; Nan, L.; Li, J.; Duan, W.; Lei, J.; Zhang, L.; Ma, J.; et al. Resveratrol in the treatment of pancreatic cancer. Ann. N. Y. Acad. Sci. 2015, 1348, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Greenhough, A.; Smartt, H.J.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J. The COXIB experience: A look in the rearview mirror. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 265–290. [Google Scholar] [CrossRef] [PubMed]
- Cerella, C.; Sobolewski, C.; Dicato, M.; Diederich, M. Targeting COX-2 expression by natural compounds: A promising alternative strategy to synthetic COX-2 inhibitors for cancer chemoprevention and therapy. Biochem. Pharmacol. 2010, 80, 1801–1815. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Boffa, L.; Genzini, L.; Garella, D. Phytotherapeutics: An evaluation of the potential of 1000 plants. J. Clin. Pharm. Ther. 2010, 35, 11–48. [Google Scholar] [CrossRef]
- Hegde, P.; Maddur, M.S.; Friboulet, A.; Bayry, J.; Kaveri, S.V. Viscum album exerts anti-inflammatory effect by selectively inhibiting cytokine-induced expression of cyclooxygenase-2. PLoS ONE 2011, 6, e26312. [Google Scholar] [CrossRef]
- Kim, M.K.; Yun, K.J.; Lim, D.H.; Kim, J.; Jang, Y.P. Anti-Inflammatory Properties of Flavone di-C-Glycosides as Active Principles of Camellia Mistletoe, Korthalsella japonica. Biomol. Ther. 2016, 24, 630–637. [Google Scholar] [CrossRef]
- Saha, C.; Das, M.; Stephen-Victor, E.; Friboulet, A.; Bayry, J.; Kaveri, S.V. Differential Effects of Viscum album Preparations on the Maturation and Activation of Human Dendritic Cells and CD4+ T Cell Responses. Molecules 2016, 21, 912. [Google Scholar] [CrossRef]
- Gong, J.; Xie, J.; Bedolla, R.; Rivas, P.; Chakravarthy, D.; Freeman, J.W.; Reddick, R.; Kopetz, S.; Peterson, A.; Wang, H.; et al. Combined targeting of STAT3/NF-kappaB/COX-2/EP4 for effective management of pancreatic cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 1259–1273. [Google Scholar] [CrossRef]
- Kamal, M.M.; Akter, S.; Lin, C.N.; Nazzal, S. Sulforaphane as an anticancer molecule: Mechanisms of action, synergistic effects, enhancement of drug safety, and delivery systems. Arch. Pharmacal Res. 2020, 43, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Lozanovski, V.J.; Polychronidis, G.; Gross, W.; Gharabaghi, N.; Mehrabi, A.; Hackert, T.; Schemmer, P.; Herr, I. Broccoli sprout supplementation in patients with advanced pancreatic cancer is difficult despite positive effects-results from the POUDER pilot study. Investig. New Drugs 2020, 38, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Giordano, G.; Pancione, M.; Olivieri, N.; Parcesepe, P.; Velocci, M.; Di Raimo, T.; Coppola, L.; Toffoli, G.; D’Andrea, M.R. Nano albumin bound-paclitaxel in pancreatic cancer: Current evidences and future directions. World J. Gastroenterol. 2017, 23, 5875–5886. [Google Scholar] [CrossRef]
- Aras, A.; Khokhar, A.R.; Qureshi, M.Z.; Silva, M.F.; Sobczak-Kupiec, A.; Pineda, E.A.; Hechenleitner, A.A.; Farooqi, A.A. Targeting cancer with nano-bullets: Curcumin, EGCG, resveratrol and quercetin on flying carpets. Asian Pac. J. Cancer Prev. 2014, 15, 3865–3871. [Google Scholar] [CrossRef]
- Wei, Q.Y.; Xu, Y.M.; Lau, A.T.Y. Recent Progress of Nanocarrier-Based Therapy for Solid Malignancies. Cancers 2020, 12, 2783. [Google Scholar] [CrossRef]
- Madamsetty, V.S.; Pal, K.; Keshavan, S.; Caulfield, T.R.; Dutta, S.K.; Wang, E.; Fadeel, B.; Mukhopadhyay, D. Development of multi-drug loaded PEGylated nanodiamonds to inhibit tumor growth and metastasis in genetically engineered mouse models of pancreatic cancer. Nanoscale 2019, 11, 22006–22018. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, A.; Desai, P.; Chenreddy, S.; Modi, J.; Thio, A.; Khamas, W.; Ann, D.; Wang, J.; Prabhu, S. Novel nano-drug combination therapeutic regimen demonstrates significant efficacy in the transgenic mouse model of pancreatic ductal adenocarcinoma. Am. J. Cancer Res. 2018, 8, 2005–2019. [Google Scholar]
- Desai, P.; Thakkar, A.; Ann, D.; Wang, J.; Prabhu, S. Loratadine self-microemulsifying drug delivery systems (SMEDDS) in combination with sulforaphane for the synergistic chemoprevention of pancreatic cancer. Drug Deliv. Transl. Res. 2019, 9, 641–651. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, X.; Zhou, Y.; Shi, S.; Liang, C.; Yu, X.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; et al. Exosomes derived from immunogenically dying tumor cells as a versatile tool for vaccination against pancreatic cancer. Biomaterials 2022, 280, 121306. [Google Scholar] [CrossRef]
- Ruivo, C.F.; Adem, B.; Silva, M.; Melo, S.A. The Biology of Cancer Exosomes: Insights and New Perspectives. Cancer Res. 2017, 77, 6480–6488. [Google Scholar] [CrossRef] [PubMed]
- Batista, I.A.; Melo, S.A. Exosomes and the Future of Immunotherapy in Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 567. [Google Scholar] [CrossRef] [PubMed]
- Ruarus, A.; Vroomen, L.; Puijk, R.; Scheffer, H.; Meijerink, M. Locally Advanced Pancreatic Cancer: A Review of Local Ablative Therapies. Cancers 2018, 10, 16. [Google Scholar] [CrossRef]
- Maiettini, D.; Mauri, G.; Varano, G.; Bonomo, G.; Della Vigna, P.; Rebonato, A.; Orsi, F. Pancreatic ablation: Minimally invasive treatment options. Int. J. Hyperth. 2019, 36, 53–58. [Google Scholar] [CrossRef]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, J.; Shukla, H.D.; Soman, S.; Samanta, S.; Singh, P.; Kamlapurkar, S.; Saeed, A.; Amin, N.P.; Vujaskovic, Z. Immunotherapy, Radiotherapy, and Hyperthermia: A Combined Therapeutic Approach in Pancreatic Cancer Treatment. Cancers 2018, 10, 469. [Google Scholar] [CrossRef]
- Rubinsky, B.; Onik, G.; Mikus, P. Irreversible electroporation: A new ablation modality–clinical implications. Technol. Cancer Res. Treat. 2007, 6, 37–48. [Google Scholar] [CrossRef]
- van Veldhuisen, E.; van den Oord, C.; Brada, L.J.; Walma, M.S.; Vogel, J.A.; Wilmink, J.W.; Del Chiaro, M.; van Lienden, K.P.; Meijerink, M.R.; van Tienhoven, G.; et al. Locally Advanced Pancreatic Cancer: Work-Up, Staging, and Local Intervention Strategies. Cancers 2019, 11, 976. [Google Scholar] [CrossRef]
- Imran, K.M.; Nagai-Singer, M.A.; Brock, R.M.; Alinezhadbalalami, N.; Davalos, R.V.; Allen, I.C. Exploration of Novel Pathways Underlying Irreversible Electroporation Induced Anti-Tumor Immunity in Pancreatic Cancer. Front. Oncol. 2022, 12, 853779. [Google Scholar] [CrossRef]
- Geboers, B.; Timmer, F.E.F.; Ruarus, A.H.; Pouw, J.E.E.; Schouten, E.A.C.; Bakker, J.; Puijk, R.S.; Nieuwenhuizen, S.; Dijkstra, M.; van den Tol, M.P.; et al. Irreversible Electroporation and Nivolumab Combined with Intratumoral Administration of a Toll-Like Receptor Ligand, as a Means of In Vivo Vaccination for Metastatic Pancreatic Ductal Adenocarcinoma (PANFIRE-III). A Phase-I Study Protocol. Cancers 2021, 13, 3902. [Google Scholar] [CrossRef]
- Yan, B.M.; Van Dam, J. Endoscopic ultrasound-guided intratumoural therapy for pancreatic cancer. Can. J. Gastroenterol. 2008, 22, 405–410. [Google Scholar] [CrossRef]
- Beuth, J.; Ko, H.L.; Schneider, H.; Tawadros, S.; Kasper, H.U.; Zimst, H.; Schierholz, J.M. Intratumoral application of standardized mistletoe extracts down regulates tumor weight via decreased cell proliferation, increased apoptosis and necrosis in a murine model. Anticancer Res. 2006, 26, 4451–4456. [Google Scholar] [PubMed]
- Rostock, M.; Huber, R.; Greiner, T.; Fritz, P.; Scheer, R.; Schueler, J.; Fiebig, H.H. Anticancer activity of a lectin-rich mistletoe extract injected intratumorally into human pancreatic cancer xenografts. Anticancer Res. 2005, 25, 1969–1975. [Google Scholar]
- Engeland, C.E.; Ungerechts, G. Immuntherapie mit onkolytischen Viren: Wenn Viren Turmorzellen zum “Platzen” bringen. Dtsch. Ärzteblatt Int. 2019, 116, 10. [Google Scholar] [CrossRef]
- Banerjee, K.; Kumar, S.; Ross, K.A.; Gautam, S.; Poelaert, B.; Nasser, M.W.; Aithal, A.; Bhatia, R.; Wannemuehler, M.J.; Narasimhan, B.; et al. Emerging trends in the immunotherapy of pancreatic cancer. Cancer Lett. 2018, 417, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Salman, B.; Zhou, D.; Jaffee, E.M.; Edil, B.H.; Zheng, L. Vaccine therapy for pancreatic cancer. Oncoimmunology 2013, 2, e26662. [Google Scholar] [CrossRef]
- Middleton, G.; Silcocks, P.; Cox, T.; Valle, J.; Wadsley, J.; Propper, D.; Coxon, F.; Ross, P.; Madhusudan, S.; Roques, T.; et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): An open-label, randomised, phase 3 trial. Lancet Oncol. 2014, 15, 829–840. [Google Scholar] [CrossRef]
- Yang, J.; Shangguan, J.; Eresen, A.; Li, Y.; Wang, J.; Zhang, Z. Dendritic cells in pancreatic cancer immunotherapy: Vaccines and combination immunotherapies. Pathol. Res. Pract. 2019, 215, 152691. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, L.; Shang, K.; Liu, F.; Che, J.; Li, H.; Cao, B. Long non-coding RNA H19, a novel therapeutic target for pancreatic cancer. Mol. Med. 2020, 26, 30. [Google Scholar] [CrossRef]
- Jiang, J.; Zhou, H.; Ni, C.; Hu, X.; Mou, Y.; Huang, D.; Yang, L. Immunotherapy in pancreatic cancer: New hope or mission impossible? Cancer Lett. 2019, 445, 57–64. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, G.; Tang, T.Y.; Gao, X.; Liang, T.B. Personalized pancreatic cancer therapy: From the perspective of mRNA vaccine. Mil. Med. Res. 2022, 9, 53. [Google Scholar] [CrossRef] [PubMed]


| No | Study ID Estimated Completion | Population | Design | Interventions | Outcomes | Locations |
|---|---|---|---|---|---|---|
| 1 | NCT04241276 2024 | 170 PC | Multicentric Phase 2, RCT 2 armed | Oral ATRA in combination with gemcitabine and nab-paclitaxel/only gemcitabine and nab-paclitaxel | PFS; RR; OS; AEs; surgical resection rate; QL | UK |
| 2a | NCT03541486 2030 | 60 PC neoplasms | Phase 2 2 armed | Vit C infusions in combination with Ctx and Radio standard therapy/only standard therapy | OS; RR; PFS; Tox | USA |
| 2b | NCT03146962 2023 | 50 PC, colorectal and lung cancer | Multicentric Phase 2 3 armed | (A) High-Dose Vit C IV 2–4 weeks prior surgery; (B) 6 months; (C) 1–2 weeks prior to and following radioembolization of hepatic metastases | RR; Tox; PFS; VitC levels | USA |
| 2c | NCT03410030 2022 | 36 PC stage IV | Phase 1/2 1 armed | Vit C infusions in combination with Nanoparticles Paclitaxel + Cisplatin + Gemcitabine | Tox; OS; PFS; QL; pain | USA |
| 2d | NCT01852890 2024 | 16 PC neoplasms | Phase 1 1 armed | Dose-escalation of Vit C infusions in combination with gemcitabine and radiotherapy | AEs during radiation; progression; OS; AEs post-treatment | USA |
| 2e | NCT02905578 2025 | 65 PC neoplasms | Phase 2, RCT 2 armed | Vit C infusions in combination with gemcitabine and nab-paclitaxel/only Ctx standard therapy | OS; RR; PFS; AEs | USA |
| 2f | NCT03146962 2023 | 78 PC, colorectal and lung cancer | Phase 2 1 armed | High dose Vit C IV infusions in patients with solid tumor malignancies | Anti-tumor activity; disease control; max tolerated Vit C dose; PFS; AEs | USA |
| 3a | NCT03520790 2025 | 112 PC stage IV | Phase 1/2 RCT Placebo 2 armed | Paricalcitol (IV or orally) in combination with gemcitabine and Nab-Paclitaxel/Placebo | AEs; OS; RR; PFS | USA |
| 3b | NCT03331562 2020 | 24 PC | Phase 2, RCT Multicentric Placebo 2 armed | Paricalcitol IV combined with Pembrolizumab/Placebo | Progression; Tox; OS; mutations (sequencing); Vit D receptor binding sites | USA |
| 3c | NCT02930902 2022 | 24 PC (resectable) | Phase 1 2 armed | Paricalcitol IV and pembro-zumab without and with Ctx | Tox; AEs; resection rate; disease free survival; OS | USA |
| 3d | NCT03883919 2022 | 20 PC stage IV | Phase 1 Pilot 1 armed | Paricalcitol in combination with liposomal Irinotecan Plus | Tox, RR, PFS; OS; CA19-9 PC-tumormarker; duration of response | USA |
| 3e | NCT03415854 2023 | 14 PC (untreated) | Phase 2 1 armed | Paricalcitol in combination with cisplatin, paclitaxel, and gemcitabine | RR; CA19-9 PC-tumormarker; biomarker (Paricalcitol, Ctx) | USA |
| 3f | NCT03138720 2023 | 24 PC (untreated) | Phase 2 1 armed | Paricalcitol in combination with cisplatin, paclitaxel, and gemcitabine | CA19-9 PC-tumormarker; RR; OS | USA |
| 3g | NCT05365893 2023 | 20 PC (resectable) | Early phase 1 2 armed | Paricalcitol IV + hydroxychloroquine + Losartan in combination with Ctx and surgery/only Ctx and surgery | AEs; feasibility | USA |
| 3h | NCT04617067 2024 | 43 PC advanced | Phase 2 1 armed | Paricalcitol (orally) in combination with gemcitabine and Nab-Paclitaxel | PFS; OS; TTF; RR; AEs | Ireland |
| 4a | NCT02948309 2022 | 290 PC inoperable | Phase 3 RCT Placebo 2 armed | Additional sc application of mistletoe-extracts/placebo: sc isotonic saline solution | OS; QL; BMI; corticosteroid consumption; number required visits, AEs; pain | Sweden |
| 4b | EudraCT2014-002386-30 2020 | 290 locally advanced or metastatic PC | Multicentric RCT 2 armed | Additional sc application of mistletoe-extracts/only standard therapy | OS; fatigue; QL; pain; body weight; AEs | Bulgaria Serbia |
| 4c | NCT03051477 2022 | 56 advanced solid tumors | Phase 1 1 armed | IV infusions of mistletoe-extracts; dose-escalation | Tox; AEs; maximum tolerated dose; tumor marker kinetics | USA |
| 5a | NCT01077427 2021 | 336 PC resected | Phase 3 RCT 2 armed | Regional hyperthermia in combination with gem-and capecitabine/only Ctx | PFS; OS; Tox; QL | Germany |
| 5b | NCT02439593 2021 | 78 PC advanced | Phase 2 RCT 2 armed | Regional hyperthermia in combination with Ctx and radiation/only Ctx and radiation | OS; PFS; Progression; AEs | Switzerland |
| 5c | NCT03251365 2024 | 42 PC resectable | Phase 2/3 RCT 2 armed | Hyperthermic intra-abdominal and gemcitabine/only gemcitabine | Morbidity; OS | Spain |
| 5d | NCT02862015 2019 | 100 PC metastatic | Multicentric Phase 2, RCT 2 armed | Whole body hyperthermia combined with Ctx/only Ctx | QL; opioid use; pain; AEs | Korea |
| 5e | NCT04467593 2022 | 14 PC stage IV | 2 armed | Whole body hyperthermia only/combined with Ctx | AEs; CA19-9 and CEA levels | Belgium |
| 5f | NCT04858009 2026 | 40 PC (metastatic) | Phase 2 1 armed | Hyperthermic intra-peritonal combined with Ctx | OS; disease control; recurrence; morbidity | USA |
| 5g | NCT04889742 2028 | 110 recurrent cancer | 1 armed | Local hyperthermia combined with re-irradiation | Tumor recurrence; OS; PFS; OS; QL | Germany |
| 6a | NCT02343835 2021 | 20 PC (inoperable) | RCT 2 armed | Nanoknife IRE/no treatment | Immune response (intra-tumoral and systemic) | China |
| 6b | NCT03614910 2022 | 30 PC (advanced, inoperable) | 1 armed | Nanoknife IRE | OS; PFS; RR; complications; CA19-9; Pain | USA |
| 6c | NCT04310553 2020 | 240 PC (advanced) | Multicentric 1 armed | Nanoknife IRE | OS; time to progress; PFS; RR; disease control rate; QL | China |
| 6d | NCT02791503 2022 | 74 PC (neoplasm) | RCT 2 armed | Nanoknife IRE in combination with Ctx/Stereotactic Body Radiotherapy in combination with Ctx | OS; PFS; AEs; pain; cost-effectiveness; QL; immune status; CA 19-9 | The Netherlands |
| 6e | NCT04093141 2024 | 30 PC (inoperable) | 1 armed | IRE after Ctx | 2-year-survival proportion; OS; PFS; progression; complications; QL | Denmark |
| 6f | NCT02041936 2022 | 12 PC (inoperable) | 1 armed | Nanoknife IRE | AEs; pain; QL | USA |
| 6g | NCT04276857 2026 | 27 PC (advanced) | 1 armed | Nanoknife IRE after Ctx | PFS; OS; QL; rate of IRE; complications; cost-effectiveness | Canada |
| 6h | NCT02343835 2021 | 20 PC (advanced) | RCT 2 armed | Nanoknife IRE/no intervention | Immune responses, between non-ablated and ablated PC; OS; PFS | China |
| 6i | NCT02898649 2019 | 100 PC (advanced) | 1 armed | Nanoknife IRE after standard therapy without response | OS; safety; progression; tumor size; pain; CA19-9 | Korea |
| 6j | NCT03105921 2021 | 20 PC (untreated) | 1 armed | Nanoknife IRE | R0 resection rate | France |
| 6k | NCT03257150 2022 | 47 PC (inoperable) | Phase 1/2 1 armed | Nanoknife IRE via laparotomy surgery | AEs; OS; PFS | Canada |
| 6l | EudraCT2020-004623-17 | 12 PC (metastatic) | Phase 2 1 armed | Nanoknife IRE + Nivolumab | AEs; OS; PFS; tumor response; QL | Denmark |
| 6m | NCT04612530 2023 | 18 PC | Phase 1 RCT 3 armed | (A) Nivolumab (B) IRE + Nivolumab (C) IRE + Nivolumab + Toll-Like Receptor 9 (intra tumoral) | AEs; OS; PFS; immunomodulation; tumor response; QL | The Netherlands |
| 6n | NCT03899649 2023 | 532 PC (stage III) | Registry Multicentric 2 armed | Nanoknife IRE/standard therapy | OS | USA |
| 6o | NCT03899636 2023 | 528 PC (stage III, inoperable) | Phase 3, RCT Multicentric 2 armed | Nanoknife IRE + FOLFIRINOX/only FOLFIRINOX | OS | USA |
| 6p | NCT05170802 2023 | 30 PC | Registry 1 armed | Nanoknife IRE via laparotomy surgery | AEs; OS; PFS | USA |
| 7a | NCT03252808 2035 | 36 PC (inoperable) stage III and IV | Multicentric Phase 1, RCT 3 armed | Oncolytic virus (HF10 intra-tumoral) in combination with Gem + Nab-paclitaxel/Tegafur (TS-1) | Tox; AE; RR; PFS | Japan |
| 7b | NCT02705196 2025 | 55 PC | Phase 1/2 2 armed | Oncolytic virus (LOAd703 intra-tumoral) in combination with Gem + Nab-paclitaxel+/-atezolizumab | Tox; RR; OS | USA |
| 7c | NCT03225989 2024 | 50 PC, biliary, ovarian, and colorectal cancer | Phase 1/2 1 armed | Oncolytic virus (LOAd703 intra-tumoral) in combination with standard care Ctx | Tox; AEs; tolerability; tumor size; OS; time to progression; PFS; immune activation | Sweden |
| 7d | NCT03740256 2038 | 39 cancer patients with solid tumors | Phase 1 1 armed | Oncolytic virus (CAdVEC intra-tumoral) in combination with HER2 specific CAR-T cells | Tox; AEs; RR; PFS; OS | USA |
| 7e | NCT04637698 2022 | 25 PC advanced/metastatic | Phase 1/2 1 armed | Oncolytic virus OH2 intratumoral injection after first-line therapy failed | RR; AEs; disease control; duration of response; PFS; QL | China |
| 7f | NCT04226066 2022 | 69 PC, stomach or liver cancer (advanced malignant) | Phase 1/2 2 armed | Recombinant oncolytic virus T601 injection after other options failed. dose-escalation/in combination with 5-FC | AEs; RR; disease control; PFS; pharmacokinetics of T601; 5-FC-determination in blood | China |
| 7g | NCT05361954 2024 | 36 cancer patients with solid tumors | Phase 1 1 armed | Oncolytic virus (STI-1386 intra-tumoral) | AEs; tolerability; disease control; pharmacokinetics; immune activation | USA |
| 7h | NCT05076760 2025 | 18 cancer patients with solid tumors | Phase 1 1 armed | Oncolytic virus (MEM-288 intra-tumoral) | Tox; AEs; RR; PFS; OS | USA |
| Method | Study | Relevant Findings/Clinical Evidence | Reference |
|---|---|---|---|
| Nanomedicine | 861 advanced PC, RCT; nab-paclitaxel plus gemcitabine versus gemcitabine. | nab-paclitaxel plus gemcitabine significantly improved overall survival, progression-free survival, and response rate. | [69] |
| 16 PC patients, Phase 1; dose-escalation with nanoparticle-based curcumin preparations. | Safety and pharmacokinetics analyses revealed higher curcumin plasma levels without increased toxicity. | [70] | |
| 417 PC patients; RCT Phase 3: irinotecan encapsulated in lipid bilayer liposomes combined with fluorouracil [nal-IRI+5-FU/LV] versus 5-FU/LV. | The survival benefits of nal-IRI+5-FU/LV] versus 5-FU/LV maintained over an extended follow-up, and prognostic markers of survival ≥1 year were identified. | [71,72] NAPOLI | |
| Hyperthermia | Systematic review analysis, 1293 articles with a total of 395 PC patients. | Possible benefits of hyperthermia: improved tumor response, reduced adverse effect rate, prolonged overall survival. | [73] |
| Observational study on 106 PC patients treated with or without electro-hyperthermia. | Modified electro-hyperthermia may improve tumor response and survival of PC patients. | [74] | |
| Irreversible electropolation [IRE] | 200 locally advanced (stage III) PC patients. | The addition of IRE to radiation and chemotherapy appeared to be safe and seemed to prolong overall survival compared with historical controls. | [75,76] |
| 50 unresectable advanced and recurrent PC patients; multicenter, Phase 2. | IRE revealed an acceptable safety profile and seems to prolong overall survival compared with standard of care. | [77] PANFIRE | |
| Intratumoral injection (IT) | Observational study on 123 cancer patients including 59 PC receiving IT of mistletoe preparations. | IT of mistletoe preparations appeared to be safe. | [78,79] |
| 12 locally advanced PC patients received IT of oncolytic virus HF 10; open-label; Phase 1. | IT of oncolytic virus HF10 in combination with erlotinib and gemcitabine were safe and well tolerated. | [80] | |
| 11 locally advanced PC patients received IT with a replication-competent adenovirus (Ad5-DS); open-label; Phase 1. | A combination of IT Ad5-DS and gemcitabine is safe and well tolerated. | [81] | |
| Vaccines | 29 resp. 9 advanced PC patients, Phase 1/2 dose-escalation study with KIF20A-66 (an epitope peptide of a member of the kinesin super family). | KIF20A-66 peptide vaccination was well tolerated, and overall survival seemed prolonged compared to the historical controls. | [82,83] |
| 303 metastatic PC, RCT, multicenter, Phase 2 with mesothelin vaccine (CRS-207) + GVAX + cyclophosphamide (Cy). | Cy/GVAX followed by CRS-207 (as third-line therapy for PC) significantly improved overall survival as compared with Cy/GVAX alone. The combination of Cy/GVAX + CRS-207 did not improve survival over chemotherapy. | [84,85] ECLIPSE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oei, S.L.; Schad, F. Are Aspects of Integrative Concepts Helpful to Improve Pancreatic Cancer Therapy? Cancers 2023, 15, 1116. https://doi.org/10.3390/cancers15041116
Oei SL, Schad F. Are Aspects of Integrative Concepts Helpful to Improve Pancreatic Cancer Therapy? Cancers. 2023; 15(4):1116. https://doi.org/10.3390/cancers15041116
Chicago/Turabian StyleOei, Shiao Li, and Friedemann Schad. 2023. "Are Aspects of Integrative Concepts Helpful to Improve Pancreatic Cancer Therapy?" Cancers 15, no. 4: 1116. https://doi.org/10.3390/cancers15041116
APA StyleOei, S. L., & Schad, F. (2023). Are Aspects of Integrative Concepts Helpful to Improve Pancreatic Cancer Therapy? Cancers, 15(4), 1116. https://doi.org/10.3390/cancers15041116






