Combinations of Anti-Angiogenic Agents and Immune Checkpoint Inhibitors in Renal Cell Carcinoma: Best Option?
Abstract
:Simple Summary
Abstract
1. Introduction
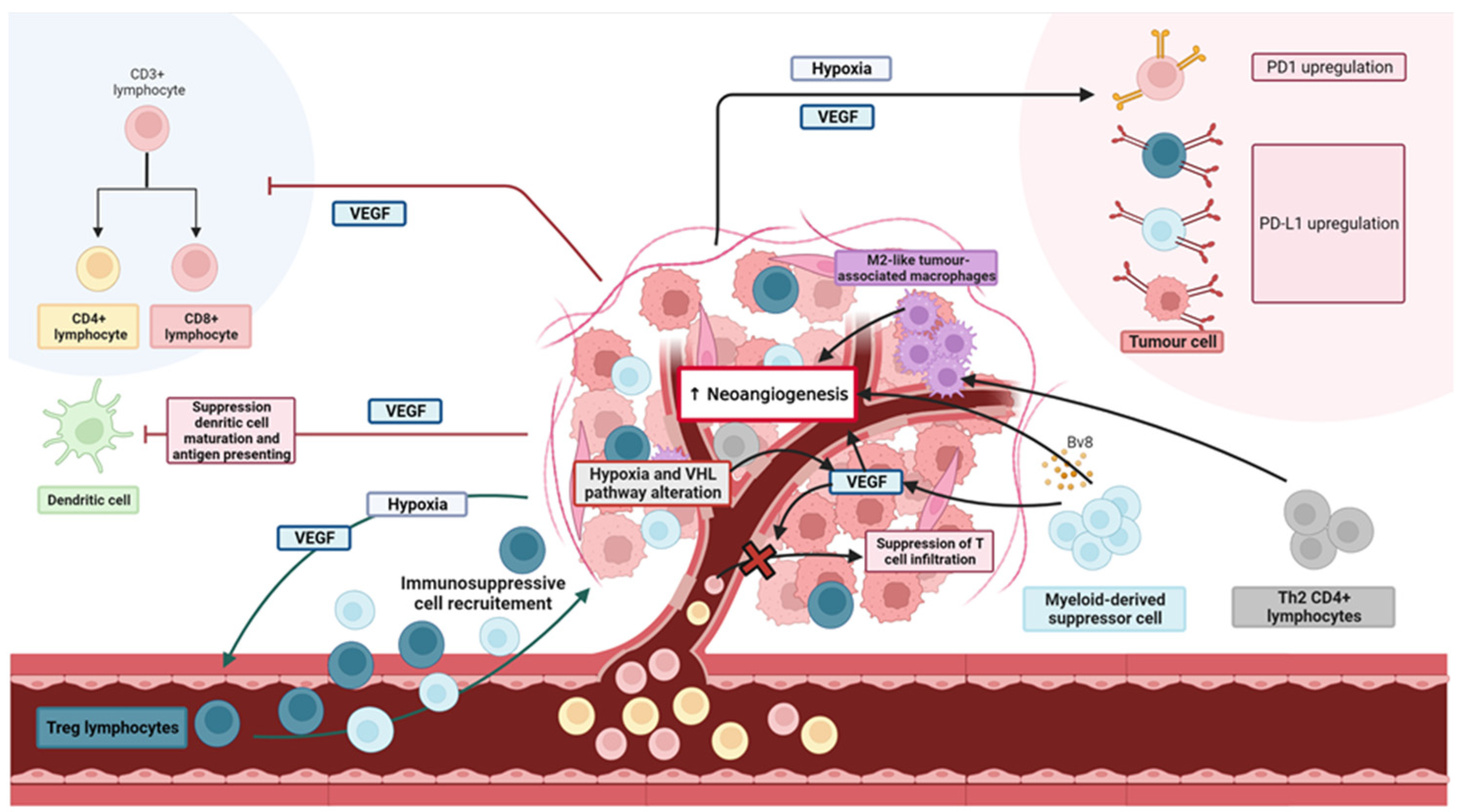
2. Rationale of and Preclinical Data on Monotherapy Efficacies: Two Principal Pathways Are Implicated in RCC Development
2.1. Anti-Angiogenesis
2.2. Immunogenicity
2.3. Rationale and Pre-Clinical Evidence for Synergy between TKIs and ICIs
3. Clinical Approach in First-Line Settings
3.1. The Past, Thus the Era of Monotherapy
3.2. The Present Era of Combination Therapy
4. Synergistic Toxicity
5. Selection Criteria: Therapeutic Objectives and Clinical Outcomes
6. What Is More Important? Any Response, a Long Response, or a Favourable Safety Profile? Some Suggestions Follow
- For patients with a rapidly progressive life-threatening disease, it is essential to avoid upfront progression. A TKI-ICI combination is preferred given the lower rate of refractory disease observed in those on lenvatinib plus pembrolizumab or cabozantinib plus nivolumab (at best 5% and 6% of those with progressive disease, respectively) [60,61];
- In contrast, durable responses are afforded by nivolumab plus ipilimumab (PFS 36% at 2 years and 31% at 4 years) [69]. Although this “plateau effect” may reflect the longer follow-up of the Checkmate 214 trial than other trials, an ICI–ICI combination seems to be a good option for patients of intermediate/poor IMDC status with no life-threatening lesion;
- For patients with brain metastases, cabozantinib afforded promising results, even in the absence of brain-directed local therapy [70]. The phase II CABRAMET Trial (NCT03967522) is currently recruiting patients with metastases to evaluate the intra- and extra-cranial effects of cabozantinib in a second-line real-world scenario;
- For patients with bone metastases, cabozantinib facilitated bone remodelling in preclinical studies, even when used as monotherapy [71], and was consistently better than sunitinib in patients with bone metastases in the Checkmate 9ER trial;
7. The Future: New Strategies
8. Discussion
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2022, 82, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Comandone, A.; Vana, F.; Comandone, T.; Tucci, M. Antiangiogenic Therapy in Clear Cell Renal Carcinoma (CCRC): Pharmacological Basis and Clinical Results. Cancers 2021, 13, 5896. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Glen, H.; Michaelson, M.D.; Molina, A.; Eisen, T.; Jassem, J.; Zolnierek, J.; Maroto, J.P.; Mellado, B.; et al. Lenvatinib, Everolimus, and the Combination in Patients with Metastatic Renal Cell Carcinoma: A Randomised, Phase 2, Open-Label, Multicentre Trial. Lancet Oncol. 2015, 16, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Albiges, L.; Bex, A.; Grünwald, V.; Porta, C.; Procopio, G.; Schmidinger, M.; Suárez, C.; de Velasco, G.; ESMO Guidelines Committee. Electronic address: Clinicalguidelines@esmo.org ESMO Clinical Practice Guideline Update on the Use of Immunotherapy in Early Stage and Advanced Renal Cell Carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network Comprehensive Molecular Characterization of Clear Cell Renal Cell Carcinoma. Nature 2013, 499, 43–49. [CrossRef]
- Ricketts, C.J.; De Cubas, A.A.; Fan, H.; Smith, C.C.; Lang, M.; Reznik, E.; Bowlby, R.; Gibb, E.A.; Akbani, R.; Beroukhim, R.; et al. The Cancer Genome Atlas Comprehensive Molecular Characterization of Renal Cell Carcinoma. Cell Rep. 2018, 23, 313–326.e5. [Google Scholar] [CrossRef]
- Mitchell, T.J.; Turajlic, S.; Rowan, A.; Nicol, D.; Farmery, J.H.R.; O’Brien, T.; Martincorena, I.; Tarpey, P.; Angelopoulos, N.; Yates, L.R.; et al. Timing the Landmark Events in the Evolution of Clear Cell Renal Cell Cancer: TRACERx Renal. Cell 2018, 173, 611–623.e17. [Google Scholar] [CrossRef]
- Sunela, K.L.; Kataja, M.J.; Kellokumpu-Lehtinen, P.-L.I. Changes in Symptoms of Renal Cell Carcinoma over Four Decades. BJU Int. 2010, 106, 649–653. [Google Scholar] [CrossRef]
- Ito, K.; Asano, T.; Yoshii, H.; Satoh, A.; Sumitomo, M.; Hayakawa, M. Impact of Thrombocytosis and C-Reactive Protein Elevation on the Prognosis for Patients with Renal Cell Carcinoma. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2006, 13, 1365–1370. [Google Scholar] [CrossRef]
- Karakiewicz, P.I.; Hutterer, G.C.; Trinh, Q.-D.; Jeldres, C.; Perrotte, P.; Gallina, A.; Tostain, J.; Patard, J.-J. C-Reactive Protein Is an Informative Predictor of Renal Cell Carcinoma-Specific Mortality: A European Study of 313 Patients. Cancer 2007, 110, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Roussel, E.; Kinget, L.; Verbiest, A.; Debruyne, P.R.; Baldewijns, M.; Van Poppel, H.; Albersen, M.; Beuselinck, B. C-Reactive Protein and Neutrophil-Lymphocyte Ratio Are Prognostic in Metastatic Clear-Cell Renal Cell Carcinoma Patients Treated with Nivolumab. Urol. Oncol. 2021, 39, 239.e17–239.e25. [Google Scholar] [CrossRef]
- Johnson, T.V.; Abbasi, A.; Owen-Smith, A.; Young, A.; Ogan, K.; Pattaras, J.; Nieh, P.; Marshall, F.F.; Master, V.A. Absolute Preoperative C-Reactive Protein Predicts Metastasis and Mortality in the First Year Following Potentially Curative Nephrectomy for Clear Cell Renal Cell Carcinoma. J. Urol. 2010, 183, 480–485. [Google Scholar] [CrossRef]
- Komai, Y.; Saito, K.; Sakai, K.; Morimoto, S. Increased Preoperative Serum C-Reactive Protein Level Predicts a Poor Prognosis in Patients with Localized Renal Cell Carcinoma. BJU Int. 2007, 99, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Silber, S.J.; Chen, C.Y.; Gould, F. Regression of Metastases after Nephrectomy for Renal Cell Carcinoma. Br. J. Urol. 1975, 47, 259–261. [Google Scholar] [CrossRef]
- Mohr, S.J.; Whitesel, J.A. Spontaneous Regression of Renal Cell Carcinoma Metastases after Preoperative Embolization of Primary Tumor and Subsequent Nephrectomy. Urology 1979, 14, 5–8. [Google Scholar] [CrossRef]
- Vizel, M.; Oster, M.W.; Austin, J.H. Spontaneous Regression of a Pulmonary Metastasis after Nephrectomy for Renal Cell Carcinoma. J. Surg. Oncol. 1979, 12, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Dreikorn, K.; Terwey, B.; Drings, P.; Horsch, R.; Palmtag, H.; Rössler, W. Complete Regression of Multiple Pulmonary Metastases in a Patient with Advanced Renal Cell Carcinoma Treated by Occlusion of the Renal Artery with Subsequent Radical Nephrectomy and Progesterone. Eur. Urol. 1983, 9, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.G.; Choyke, P.L.; Reiter, R.; Jaffe, G.S.; Alexander, R.B.; Linehan, W.M.; Rosenberg, S.A.; Walther, M.M. Regression of Metastatic Renal Cell Carcinoma after Cytoreductive Nephrectomy. J. Urol. 1993, 150, 463–466. [Google Scholar] [CrossRef]
- Thoroddsen, A.; Gudbjartsson, T.; Geirsson, G.; Agnarsson, B.A.; Magnusson, K. Spontaneous Regression of Pleural Metastases after Nephrectomy for Renal Cell Carcinoma--a Histologically Verified Case with Nine-Year Follow-Up. Scand. J. Urol. Nephrol. 2002, 36, 396–398. [Google Scholar] [CrossRef]
- Okazaki, A.; Kijima, T.; Schiller, P.; Ishikawa, N.; Fuchizawa, H.; Takei, K.; Suzuki, I.; Sakamoto, K.; Tsuzuki, T.; Kamai, T. Spontaneous Regression of Multiple Pulmonary Metastases Accompanied by Normalization of Serum Immune Markers Following Cytoreductive Nephrectomy in a Patient with Clear-Cell Renal Cell Carcinoma. IJU Case Rep. 2021, 4, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Larson, C.; Reid, T.R.; Carter, C.A. Case Series: Abscopal Benefit of Surgery in 3 Immunotherapy-Treated Patients With Unresectable Cancer. J. Investig. Med. High Impact Case Rep. 2018, 6, 2324709618786319. [Google Scholar] [CrossRef] [PubMed]
- Lokich, J. Spontaneous Regression of Metastatic Renal Cancer. Case Report and Literature Review. Am. J. Clin. Oncol. 1997, 20, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, H.; Teh, B.S.; Ren, H.; Chiang, S.; Tann, A.; Blanco, A.I.; Paulino, A.C.; Amato, R. Spontaneous Regression of Thoracic Metastases While Progression of Brain Metastases after Stereotactic Radiosurgery and Stereotactic Body Radiotherapy for Metastatic Renal Cell Carcinoma: Abscopal Effect Prevented by the Blood-Brain Barrier? Clin. Genitourin. Cancer 2012, 10, 196–198. [Google Scholar] [CrossRef]
- Melichar, B.; Vanecková, J.; Morávek, P.; Urminská, H.; Podhola, M. Spontaneous Regression of Renal Cell Carcinoma Lung Metastases in a Patient with Psoriasis. Acta Oncol. Stockh. Swed. 2009, 48, 925–927. [Google Scholar] [CrossRef]
- Hobohm, U. Fever Therapy Revisited. Br. J. Cancer 2005, 92, 421–425. [Google Scholar] [CrossRef]
- Zheng, W.; Qian, C.; Tang, Y.; Yang, C.; Zhou, Y.; Shen, P.; Chen, W.; Yu, S.; Wei, Z.; Wang, A.; et al. Manipulation of the Crosstalk between Tumor Angiogenesis and Immunosuppression in the Tumor Microenvironment: Insight into the Combination Therapy of Anti-Angiogenesis and Immune Checkpoint Blockade. Front. Immunol. 2022, 13, 1035323. [Google Scholar] [CrossRef]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing Cancer Immunotherapy Using Antiangiogenics: Opportunities and Challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef]
- Arroyo, A.G.; Iruela-Arispe, M.L. Extracellular Matrix, Inflammation, and the Angiogenic Response. Cardiovasc. Res. 2010, 86, 226–235. [Google Scholar] [CrossRef]
- Schmittnaegel, M.; Rigamonti, N.; Kadioglu, E.; Cassará, A.; Wyser Rmili, C.; Kiialainen, A.; Kienast, Y.; Mueller, H.-J.; Ooi, C.-H.; Laoui, D.; et al. Dual Angiopoietin-2 and VEGFA Inhibition Elicits Antitumor Immunity That Is Enhanced by PD-1 Checkpoint Blockade. Sci. Transl. Med. 2017, 9, eaak9670. [Google Scholar] [CrossRef]
- Geindreau, M.; Ghiringhelli, F.; Bruchard, M. Vascular Endothelial Growth Factor, a Key Modulator of the Anti-Tumor Immune Response. Int. J. Mol. Sci. 2021, 22, 4871. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Barreto, J.B.; Andreu, P.; Vasquez, L.; Tawfik, D.; Kolhatkar, N.; Coussens, L.M. CD4(+) T Cells Regulate Pulmonary Metastasis of Mammary Carcinomas by Enhancing Protumor Properties of Macrophages. Cancer Cell 2009, 16, 91–102. [Google Scholar] [CrossRef]
- Cao, H.; Ni, C.; Han, L.; Wang, R.; Blasig, R.; Haseloff, R.; Qin, Y.; Lan, J.; Lou, X.; Ma, P.; et al. Claudin-12 Deficiency Inhibits Tumor Growth by Impairing Transendothelial Migration of Myeloid-Derived Suppressor Cells. Cancer Res. 2022, 82, 2472–2484. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Bruno, A.; Noonan, D.M.; Mortara, L. Contribution to Tumor Angiogenesis From Innate Immune Cells Within the Tumor Microenvironment: Implications for Immunotherapy. Front. Immunol. 2018, 9, 527. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.D.; Seano, G.; Jain, R.K. Normalizing Function of Tumor Vessels: Progress, Opportunities, and Challenges. Annu. Rev. Physiol. 2019, 81, 505–534. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Jung, K.H.; Son, M.K.; Park, J.H.; Yan, H.H.; Fang, Z.; Kang, Y.W.; Han, B.; Lim, J.H.; Hong, S.-S. Tumor Vessel Normalization by the PI3K Inhibitor HS-173 Enhances Drug Delivery. Cancer Lett. 2017, 403, 339–353. [Google Scholar] [CrossRef]
- Rassy, E.; Flippot, R.; Albiges, L. Tyrosine Kinase Inhibitors and Immunotherapy Combinations in Renal Cell Carcinoma. Ther. Adv. Med. Oncol. 2020, 12, 1758835920907504. [Google Scholar] [CrossRef]
- Nair, S.; Boczkowski, D.; Moeller, B.; Dewhirst, M.; Vieweg, J.; Gilboa, E. Synergy between Tumor Immunotherapy and Antiangiogenic Therapy. Blood 2003, 102, 964–971. [Google Scholar] [CrossRef]
- Yasuda, S.; Sho, M.; Yamato, I.; Yoshiji, H.; Wakatsuki, K.; Nishiwada, S.; Yagita, H.; Nakajima, Y. Simultaneous Blockade of Programmed Death 1 and Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) Induces Synergistic Anti-Tumour Effect in Vivo. Clin. Exp. Immunol. 2013, 172, 500–506. [Google Scholar] [CrossRef]
- Shrimali, R.K.; Yu, Z.; Theoret, M.R.; Chinnasamy, D.; Restifo, N.P.; Rosenberg, S.A. Antiangiogenic Agents Can Increase Lymphocyte Infiltration into Tumor and Enhance the Effectiveness of Adoptive Immunotherapy of Cancer. Cancer Res. 2010, 70, 6171–6180. [Google Scholar] [CrossRef] [PubMed]
- Manning, E.A.; Ullman, J.G.M.; Leatherman, J.M.; Asquith, J.M.; Hansen, T.R.; Armstrong, T.D.; Hicklin, D.J.; Jaffee, E.M.; Emens, L.A. A Vascular Endothelial Growth Factor Receptor-2 Inhibitor Enhances Antitumor Immunity through an Immune-Based Mechanism. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 3951–3959. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wong, M.K.; Yi, H.; Watkins, S.; Laird, A.D.; Wolf, S.F.; Gorelik, E. Combined Therapy of Local and Metastatic 4T1 Breast Tumor in Mice Using SU6668, an Inhibitor of Angiogenic Receptor Tyrosine Kinases, and the Immunostimulator B7.2-IgG Fusion Protein. Cancer Res. 2002, 62, 5727–5735. [Google Scholar] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Cella, D.; Reeves, J.; Hawkins, R.; Guo, J.; Nathan, P.; Staehler, M.; de Souza, P.; Merchan, J.R.; et al. Pazopanib versus Sunitinib in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2013, 369, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Escudier, B.; Tomczak, P.; Kaprin, A.; Szczylik, C.; Hutson, T.E.; Michaelson, M.D.; Gorbunova, V.A.; Gore, M.E.; Rusakov, I.G.; et al. Comparative Effectiveness of Axitinib versus Sorafenib in Advanced Renal Cell Carcinoma (AXIS): A Randomised Phase 3 Trial. Lancet Lond. Engl. 2011, 378, 1931–1939. [Google Scholar] [CrossRef]
- Cohen, H.T.; McGovern, F.J. Renal-Cell Carcinoma. N. Engl. J. Med. 2005, 353, 2477–2490. [Google Scholar] [CrossRef]
- Nanus, D.M.; Pfeffer, L.M.; Bander, N.H.; Bahri, S.; Albino, A.P. Antiproliferative and Antitumor Effects of Alpha-Interferon in Renal Cell Carcinomas: Correlation with the Expression of a Kidney-Associated Differentiation Glycoprotein. Cancer Res. 1990, 50, 4190–4194. [Google Scholar]
- Rosenberg, S.A.; Lotze, M.T.; Muul, L.M.; Leitman, S.; Chang, A.E.; Ettinghausen, S.E.; Matory, Y.L.; Skibber, J.M.; Shiloni, E.; Vetto, J.T. Observations on the Systemic Administration of Autologous Lymphokine-Activated Killer Cells and Recombinant Interleukin-2 to Patients with Metastatic Cancer. N. Engl. J. Med. 1985, 313, 1485–1492. [Google Scholar] [CrossRef]
- Oudard, S.; Beuselinck, B.; Decoene, J.; Albers, P. Sunitinib for the Treatment of Metastatic Renal Cell Carcinoma. Cancer Treat. Rev. 2011, 37, 178–184. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Oudard, S.; Negrier, S.; Szczylik, C.; Pili, R.; Bjarnason, G.A.; et al. Overall Survival and Updated Results for Sunitinib Compared with Interferon Alfa in Patients with Metastatic Renal Cell Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 3584–3590. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Davis, I.D.; Mardiak, J.; Szczylik, C.; Lee, E.; Wagstaff, J.; Barrios, C.H.; Salman, P.; Gladkov, O.A.; Kavina, A.; et al. Pazopanib in Locally Advanced or Metastatic Renal Cell Carcinoma: Results of a Randomized Phase III Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.M.G.; Tykodi, S.S.; Donskov, F.; Lee, J.-L.; Szczylik, C.; Malik, J.; Alekseev, B.Y.; Matveev, V.B.; Gafanov, R.A.; Tomczak, P.; et al. First-Line Pembrolizumab (Pembro) Monotherapy in Advanced Clear Cell Renal Cell Carcinoma (CcRCC): Updated Follow-up for KEYNOTE-427 Cohort A. Ann. Oncol. 2019, 30, v381–v382. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; McDermott, D.F.; Escudier, B.; Burotto, M.; Choueiri, T.K.; Hammers, H.J.; Barthélémy, P.; Plimack, E.R.; Porta, C.; George, S.; et al. Conditional Survival and Long-Term Efficacy with Nivolumab plus Ipilimumab versus Sunitinib in Patients with Advanced Renal Cell Carcinoma. Cancer 2022, 128, 2085–2097. [Google Scholar] [CrossRef] [PubMed]
- Grünwald, V.; Voss, M.H.; Rini, B.I.; Powles, T.; Albiges, L.; Giles, R.H.; Jonasch, E. Axitinib plus Immune Checkpoint Inhibitor: Evidence- and Expert-Based Consensus Recommendation for Treatment Optimisation and Management of Related Adverse Events. Br. J. Cancer 2020, 123, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Rini, B.I.; Powles, T.; Atkins, M.B.; Escudier, B.; McDermott, D.F.; Suarez, C.; Bracarda, S.; Stadler, W.M.; Donskov, F.; Lee, J.L.; et al. Atezolizumab plus Bevacizumab versus Sunitinib in Patients with Previously Untreated Metastatic Renal Cell Carcinoma (IMmotion151): A Multicentre, Open-Label, Phase 3, Randomised Controlled Trial. Lancet Lond. Engl. 2019, 393, 2404–2415. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef]
- Motzer, R.; Alekseev, B.; Rha, S.-Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- EUpdate—Renal Cell Carcinoma Treatment Recommendations. Available online: https://www.esmo.org/guidelines/guidelines-by-topic/genitourinary-cancers/renal-cell-carcinoma/eupdate-renal-cell-carcinoma-treatment-recommendations (accessed on 11 December 2022).
- Amin, A.; Plimack, E.R.; Ernstoff, M.S.; Lewis, L.D.; Bauer, T.M.; McDermott, D.F.; Carducci, M.; Kollmannsberger, C.; Rini, B.I.; Heng, D.Y.C.; et al. Safety and Efficacy of Nivolumab in Combination with Sunitinib or Pazopanib in Advanced or Metastatic Renal Cell Carcinoma: The CheckMate 016 Study. J. Immunother. Cancer 2018, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Infante, J.R.; Hawkins, R.; Voss, M.H.; Perini, R.; Arkenau, T.; Voskoboynik, M.; Aimone, P.; Naeije, I.; Reising, A.; et al. A Phase I/II Study to Assess the Safety and Efficacy of Pazopanib and Pembrolizumab Combination Therapy in Patients with Advanced Renal Cell Carcinoma. Clin. Genitourin. Cancer 2021, 19, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Robbins, P.B.; Powles, T.; Albiges, L.; Haanen, J.B.; Larkin, J.; Mu, X.J.; Ching, K.A.; Uemura, M.; Pal, S.K.; et al. Avelumab plus Axitinib versus Sunitinib in Advanced Renal Cell Carcinoma: Biomarker Analysis of the Phase 3 JAVELIN Renal 101 Trial. Nat. Med. 2020, 26, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Motzer, R.J.; Rini, B.I.; Haanen, J.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Gravis-Mescam, G.; Uemura, M.; Lee, J.L.; et al. Updated Efficacy Results from the JAVELIN Renal 101 Trial: First-Line Avelumab plus Axitinib versus Sunitinib in Patients with Advanced Renal Cell Carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1030–1039. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Powles, T.B.; Albiges, L.; Burotto, M.; Szczylik, C.; Zurawski, B.; Riuz, E.Y.; Maruzzo, M.; Zaizar, A.S.; Fein, L.E. LBA8 Phase III Study of Cabozantinib (C) in Combination with Nivolumab (N) and Ipilimumab (I) in Previously Untreated Advanced Renal Cell Carcinoma (ARCC) of IMDC Intermediate or Poor Risk (COSMIC-313). Ann. Oncol. 2022, 33, S1430–S1431. [Google Scholar] [CrossRef]
- Rini, B.I.; Atkins, M.B.; Plimack, E.R.; Soulières, D.; McDermott, R.S.; Bedke, J.; Tartas, S.; Alekseev, B.; Melichar, B.; Shparyk, Y.; et al. Characterization and Management of Treatment-Emergent Hepatic Toxicity in Patients with Advanced Renal Cell Carcinoma Receiving First-Line Pembrolizumab plus Axitinib. Results from the KEYNOTE-426 Trial. Eur. Urol. Oncol. 2022, 5, 225–234. [Google Scholar] [CrossRef]
- Albiges, L.; Tannir, N.M.; Burotto, M.; McDermott, D.; Plimack, E.R.; Barthélémy, P.; Porta, C.; Powles, T.; Donskov, F.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib for First-Line Treatment of Advanced Renal Cell Carcinoma: Extended 4-Year Follow-up of the Phase III CheckMate 214 Trial. ESMO Open 2020, 5, e001079. [Google Scholar] [CrossRef]
- Hirsch, L.; Martinez Chanza, N.; Farah, S.; Xie, W.; Flippot, R.; Braun, D.A.; Rathi, N.; Thouvenin, J.; Collier, K.A.; Seront, E.; et al. Clinical Activity and Safety of Cabozantinib for Brain Metastases in Patients With Renal Cell Carcinoma. JAMA Oncol. 2021, 7, 1815–1823. [Google Scholar] [CrossRef]
- Escudier, B.; Powles, T.; Motzer, R.J.; Olencki, T.; Arén Frontera, O.; Oudard, S.; Rolland, F.; Tomczak, P.; Castellano, D.; Appleman, L.J.; et al. Cabozantinib, a New Standard of Care for Patients With Advanced Renal Cell Carcinoma and Bone Metastases? Subgroup Analysis of the METEOR Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 765–772. [Google Scholar] [CrossRef]
- McDermott, D.F.; Choueiri, T.K.; Motzer, R.J.; Aren, O.R.; George, S.; Powles, T.; Donskov, F.; Harrison, M.R.; Rodriguez Cid, J.R.R.; Ishii, Y.; et al. CheckMate 214 Post-Hoc Analyses of Nivolumab plus Ipilimumab or Sunitinib in IMDC Intermediate/Poor-Risk Patients with Previously Untreated Advanced Renal Cell Carcinoma with Sarcomatoid Features. J. Clin. Oncol. 2019, 37, 4513. [Google Scholar] [CrossRef]
- Tannir, N.M.; Signoretti, S.; Choueiri, T.K.; McDermott, D.F.; Motzer, R.J.; George, S.; Powles, T.; Donskov, F.; Tykodi, S.S.; Pal, S.K.; et al. Efficacy and Safety of Nivolumab plus Ipilimumab (N+I) versus Sunitinib (S) for First-Line Treatment of Patients with Advanced Sarcomatoid Renal Cell Carcinoma (SRCC) in the Phase 3 CheckMate 214 Trial with Extended 5-Year Minimum Follow-Up. J. Clin. Oncol. 2022, 40, 352. [Google Scholar] [CrossRef]
- Motzer, R.J.; Nosov, D.; Eisen, T.; Bondarenko, I.; Lesovoy, V.; Lipatov, O.; Tomczak, P.; Lyulko, O.; Alyasova, A.; Harza, M.; et al. Tivozanib versus Sorafenib as Initial Targeted Therapy for Patients with Metastatic Renal Cell Carcinoma: Results from a Phase III Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 3791–3799. [Google Scholar] [CrossRef]
- Albiges, L.; Barthélémy, P.; Gross-Goupil, M.; Negrier, S.; Needle, M.N.; Escudier, B. TiNivo: Safety and Efficacy of Tivozanib-Nivolumab Combination Therapy in Patients with Metastatic Renal Cell Carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Choueiri, T.K.; McDermott, D.F.; Powles, T.; Vano, Y.-A.; Gupta, S.; Yao, J.; Han, C.; Ammar, R.; Papillon-Cavanagh, S.; et al. Biomarker Analysis from CheckMate 214: Nivolumab plus Ipilimumab versus Sunitinib in Renal Cell Carcinoma. J. Immunother. Cancer 2022, 10, e004316. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Abufaraj, M.; Mostafaei, H.; Quhal, F.; Fajkovic, H.; Remzi, M.; Karakiewicz, P.I.; Egawa, S.; Schmidinger, M.; Shariat, S.F.; et al. The Predictive Value of Programmed Death Ligand 1 in Patients with Metastatic Renal Cell Carcinoma Treated with Immune-Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Eur. Urol. 2021, 79, 783–792. [Google Scholar] [CrossRef]
- Zhu, J.; Armstrong, A.J.; Friedlander, T.W.; Kim, W.; Pal, S.K.; George, D.J.; Zhang, T. Biomarkers of Immunotherapy in Urothelial and Renal Cell Carcinoma: PD-L1, Tumor Mutational Burden, and Beyond. J. Immunother. Cancer 2018, 6, 4. [Google Scholar] [CrossRef]
- Rini, B.I.; Huseni, M.; Atkins, M.B.; McDermott, D.F.; Powles, T.B.; Escudier, B.; Banchereau, R.; Liu, L.-F.; Leng, N.; Fan, J.; et al. Molecular Correlates Differentiate Response to Atezolizumab (Atezo) + Bevacizumab (Bev) vs. Sunitinib (Sun): Results from a Phase III Study (IMmotion151) in Untreated Metastatic Renal Cell Carcinoma (MRCC). Ann. Oncol. 2018, 29, 724–725. [Google Scholar] [CrossRef]
- McDermott, D.F.; Huseni, M.A.; Atkins, M.B.; Motzer, R.J.; Rini, B.I.; Escudier, B.; Fong, L.; Joseph, R.W.; Pal, S.K.; Reeves, J.A.; et al. Clinical Activity and Molecular Correlates of Response to Atezolizumab Alone or in Combination with Bevacizumab versus Sunitinib in Renal Cell Carcinoma. Nat. Med. 2018, 24, 749–757. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Albiges, L.; Haanen, J.B.A.G.; Larkin, J.M.G.; Uemura, M.; Pal, S.K.; Gravis, G.; Campbell, M.T.; Penkov, K.; Lee, J.-L.; et al. Biomarker Analyses from JAVELIN Renal 101: Avelumab + Axitinib (A+Ax) versus Sunitinib (S) in Advanced Renal Cell Carcinoma (ARCC). J. Clin. Oncol. 2019, 37, 101. [Google Scholar] [CrossRef]
- Vano, Y.-A.; Elaidi, R.; Bennamoun, M.; Chevreau, C.; Borchiellini, D.; Pannier, D.; Maillet, D.; Gross-Goupil, M.; Tournigand, C.; Laguerre, B.; et al. Nivolumab, Nivolumab-Ipilimumab, and VEGFR-Tyrosine Kinase Inhibitors as First-Line Treatment for Metastatic Clear-Cell Renal Cell Carcinoma (BIONIKK): A Biomarker-Driven, Open-Label, Non-Comparative, Randomised, Phase 2 Trial. Lancet Oncol. 2022, 23, 612–624. [Google Scholar] [CrossRef] [PubMed]
- Labriola, M.K.; Zhu, J.; Gupta, R.T.; McCall, S.; Jackson, J.; Kong, E.F.; White, J.R.; Cerqueira, G.; Gerding, K.; Simmons, J.K.; et al. Characterization of Tumor Mutation Burden, PD-L1 and DNA Repair Genes to Assess Relationship to Immune Checkpoint Inhibitors Response in Metastatic Renal Cell Carcinoma. J. Immunother. Cancer 2020, 8, e000319. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Parikh, R.A.; Tsao-Wei, D.D.; Groshen, S.G.; Li, M.; Appleman, L.J.; Tagawa, S.T.; Nanus, D.M.; Molina, A.M.; Kefauver, C.; et al. Phase II Randomized Double Blind Trial of Axitinib (Axi) +/- PF-04518600, an OX40 Antibody (PFOX) after PD1/PDL1 Antibody (IO) Therapy (Tx) in Metastatic Renal Cell Carcinoma (MRCC). J. Clin. Oncol. 2022, 40, 4529. [Google Scholar] [CrossRef]
| Study | Treatment Arms | Patients (No.) | Primary Outcomes | IMDC Group (%) | Previous Nephrectomy (%) | Sarcomatoid Features (%) | Bone Metastasis Status | Liver Metastasis Status | PD-L1 Expression ≥ 1% (Score) |
|---|---|---|---|---|---|---|---|---|---|
| CheckMate 214 [54] | IPI + NIVO vs. SUN | 425 vs. 422 | PFS OS and ORR (intermediate/poor risk patients) (IRC) | Favourable: 23 Intermediate: 61 Poor: 16 | 82 | 17 | 20 | 18 | 23 * |
| KEYNOTE-426 [59] | AXI + PEMBRO vs. SUN | 432 vs. 429 | PFS (BICR) and OS in an ITT population | Favourable: 32 Intermediate: 55 Poor: 13 | 83 | 18 | 24 | 15 | 59 ** |
| CheckMate 9ER [60] | CABO + NIVO vs. SUN | 323 vs. 328 | PFS (BICR) in an ITT population | Favourable: 23 Intermediate: 58 Poor: 19 | 69 | 11 | 24 | 23 | 26 * |
| CLEAR [61] | PEMBRO + LENVA vs. SUN | 335 vs. 357 | PFS (IRC) in an ITT population | Favourable: 31 Intermediate: 52 Poor: 9 | 73 | 8 | 24 | 17 | 30 ** |
| COSMIC-313 [67] | NIVO + IPI + CABO vs. NIVO + IPI | 428 vs. 427 | PFS (BICR) | Intermediate: 75 Poor: 25 | 65 | NA | 17 | 20 | 64 *** |
| Study | Treatment Arms | Patients (No.) | IMDC Group | Follow-Up (Months); Median | PFS (Months); Median | OS (Months); Median | Complete Response (%) |
|---|---|---|---|---|---|---|---|
| CheckMate 214 [54] | IPI + NIVO vs. SUN | 425 vs. 422 | Intermediate and poor | 67.7 | 11.2 vs. 8.3 HR 0.74 95% CI [0.62–0.88] p < 0.0004 | 48.1 vs. 26.6 HR 0.65 95% CI [0.54–0.78] p < 0.0001 | 10.4 vs. 1.4 * |
| KEYNOTE-426 [59] | AXI + PEMBRO vs. SUN | 432 vs. 429 | All | 42.8 | 15.7 vs. 11.1 HR 0.68 95% CI [0.58–0.80] p < 0.0001 | 45.7 vs. 40.1 HR 0.73 95 CI [0.60–0.88] p < 0.001 | 10.0 vs. 3.5 ** |
| CheckMate 9ER [60] | CABO + NIVO vs. SUN | 323 vs. 328 | All | 23.5 | 16.6 vs. 8.3 HR 0.56 [95% CI 0.46–0.68] p < 0.0001 | 37.7 vs. 34.3 HR 0.70 95% CI [0.55–0.90] p = 0.004 | 12 vs. 5 ** |
| CLEAR [61] | PEMBRO + LENVA vs. SUN | 335 vs. 357 | All | 26.6 | 23.9 vs. 9.2 HR 0.39 [0.32–0.49] p < 0.001 | NR vs. NR HR 0.66 [0.49–0.88] p = 0.005 | 16.1 vs. 4.2 * |
| COSMIC-313 [67] | NIVO + IPI + CABO vs. NIVO + IPI | 428 vs. 427 | All | 20.2 | NR vs. 11.3, HR 0.73 [0.57–0.94] p < 0.013 | NR | 3 vs. 3 ** |
| Study | Grade 3–4 AEs | Principal TRAEs (All Grades) in the Experimental Arms | Principal TRAEs (Grades 3–4) in the Experimental Arms | Dose Reduction | Drug Interruption | Drug Discontinuation |
|---|---|---|---|---|---|---|
| CheckMate 214 [54] | IPI + NIVO vs. SUN | Fatigue: 38% Pruritus: 29.3% Diarrhoea: 28.3% Rash: 22.7% Nausea: 20.1% | Lipase increase: 10.6% Hepatic: 8.6% Endocrine: 6.9% Gastrointestinal: 4.9% Skin: 3.8% | NA | IPI: 27.1% (85.3% because of AEs); NIVO: 58.3% (65.8% because of AEs) | 21.6% |
| KEYNOTE-426 [59] | AXI + PEMBRO vs. SUN | Diarrhoea: 49% Hypertension: 41.7% Hypothyroidism: 31.5% Fatigue: 30.3% PPE: 27.7% | Hypertension: 21.2% ALT increase: 12.1% Diarrhoea: 7.2% PPE: 5.1% Proteinuria: 2.6% | AXI: 20% dose reduction because of drug-related AEs PEMBRO: NA | Any treatment: 69.9% PEMBRO and AXI 35.7% PEMBRO: 50.3% AXI: 63.9% | PEMBRO or AXI: 25.9% PEMBRO: 18.6% Both: 6.3% |
| CheckMate 9ER [60] | CABO + NIVO vs. SUN | Diarrhoea: 56.9% PPE: 38.1% Hypothyroidism: 33.4% Hypertension: 30.3% Fatigue: 26.9% | Hypertension: 10.9% PPE: 7.5% Hyponatremia: 6.9% Diarrhoea: 5.6% Lipase increase: 5.3% Hypophosphoremia: 5.3% | CABO: 59.4% | Any treatment: 89.4% NIVO: 73.1% CABO: 81.9% | CABO or NIVO: 23.4% CABO: 7.2% NIVO: 9.7% Both: 5.0% |
| CLEAR [61] | PEMBRO + LENVA vs. SUN | Diarrhoea: 61.4% Hypertension: 55.4% Hypothyroidism: 47.2% Decreased appetite: 40.3% Fatigue: 40.1% | Hypertension: 27.6% Lipase increase: 12.8% Diarrhoea: 9.7% Weight decrease: 8% Proteinuria: 7.7% | LENVA: 68.8% | LENVA or PEMBRO: 78.4% LENVA: 73.0% PEMBRO: 55.1% Both: 39.2% | LENVA or PEMBRO: 37.2% LENVA: 25.6% PEMBRO: 28.7% 13.4% |
| COSMIC-313 [67] | NIVO + IPI + CABO vs. NIVO + IPI | ALT elevation: 46% AST elevation: 44% Diarrhoea: 41% PPE: 28% Hypothyroidism: 24% Hypertension: 23% | ALT elevation: 26% AST elevation: 20% Lipase increase: 9% Hypertension: 8% | CABO: 54% | Any treatment: 90% | Any treatment: 45% CABO or placebo: 28% NIVO: 26% IPI: 30% All: 12% |
| Study Name | Main Characteristics | Population | Experimental Arm | Comparator Arm | Primary Endpoint | Recruitment Status | Study Number |
|---|---|---|---|---|---|---|---|
| Escalation strategy | |||||||
| MK6482-012 | First line | 1431 | Belzutifan + pembrolizumab + lenvatinib and pembrolizumab + quavonlimab + lenvatinib | Pembrolizumab + lenvatinib | PFS, OS | Recruiting | NCT04736706 |
| PDIGREE | First line (int/poor IMDC) According to the response after 4 cycles of nivolumab + ipilimumab | 1046 | Non-CR/NonPD cabozantinib + nivolumab CR: Nivolumab PD: Cabozantinib | NonCR/Non-PD CR: Nivolumab PD: Cabozantinib | OS | Recruiting | NCT03793166 |
| COSMIC-313 | First line (int/poor IMDC) | 855 | Nivolumab + Ipilimumab + Cabozantinib | Nivolumab + Ipilimumab | PFS | Active, not recruiting | NCT03937219 |
| De-escalation strategy | |||||||
| MOIO | First line | 646 | Standard dose intensity ICI | Reduced dose intensity ICI every 3 months | PFS | Recruiting | NCT05078047 |
| SPICI | First line (fav/int with one IMDC fav criteria only) With OR at 12 Months with PD1/ICI + TKI | 372 | Treatment Pause | Treatment continuation | PFR | Not yet recruiting | NCT05219318 |
| Rechallenge | |||||||
| CONTACT-03 | Post-anti PD(L)1 | 523 | Cabozantinib + atezolizumab | Cabozantinib | PFS, OS | Active, not recruiting | NCT04338269 |
| TiNivo-2 | Second/Third line after ICI | 326 | Tivozanib + Nivolumab | Tivozanib | PFS | Recruiting | NCT04987203 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granet-Vaissiere, E.; Lefort, F.; Domblides, C.; Larroquette, M.; Ravaud, A.; Bernhard, J.-C.; Gross-Goupil, M. Combinations of Anti-Angiogenic Agents and Immune Checkpoint Inhibitors in Renal Cell Carcinoma: Best Option? Cancers 2023, 15, 1048. https://doi.org/10.3390/cancers15041048
Granet-Vaissiere E, Lefort F, Domblides C, Larroquette M, Ravaud A, Bernhard J-C, Gross-Goupil M. Combinations of Anti-Angiogenic Agents and Immune Checkpoint Inhibitors in Renal Cell Carcinoma: Best Option? Cancers. 2023; 15(4):1048. https://doi.org/10.3390/cancers15041048
Chicago/Turabian StyleGranet-Vaissiere, Estelle, Félix Lefort, Charlotte Domblides, Mathieu Larroquette, Alain Ravaud, Jean-Christophe Bernhard, and Marine Gross-Goupil. 2023. "Combinations of Anti-Angiogenic Agents and Immune Checkpoint Inhibitors in Renal Cell Carcinoma: Best Option?" Cancers 15, no. 4: 1048. https://doi.org/10.3390/cancers15041048
APA StyleGranet-Vaissiere, E., Lefort, F., Domblides, C., Larroquette, M., Ravaud, A., Bernhard, J.-C., & Gross-Goupil, M. (2023). Combinations of Anti-Angiogenic Agents and Immune Checkpoint Inhibitors in Renal Cell Carcinoma: Best Option? Cancers, 15(4), 1048. https://doi.org/10.3390/cancers15041048







