Chemotherapeutic Activity of Pitavastatin in Vincristine Resistant B-Cell Acute Lymphoblastic Leukemia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Vincristine Resistance Stability
2.3. Cell Viability Studies
2.4. Cell Proliferation Assays
2.5. Co-Culture Experiments
2.6. Western Blot Analysis
2.7. Cellular Respiration
2.8. In Vivo Murine All Study
2.9. Statistical Analysis
3. Results
3.1. Development of a Vincristine-Resistant Cell Line
3.2. Stability of Vincristine-Resistant Cell Line
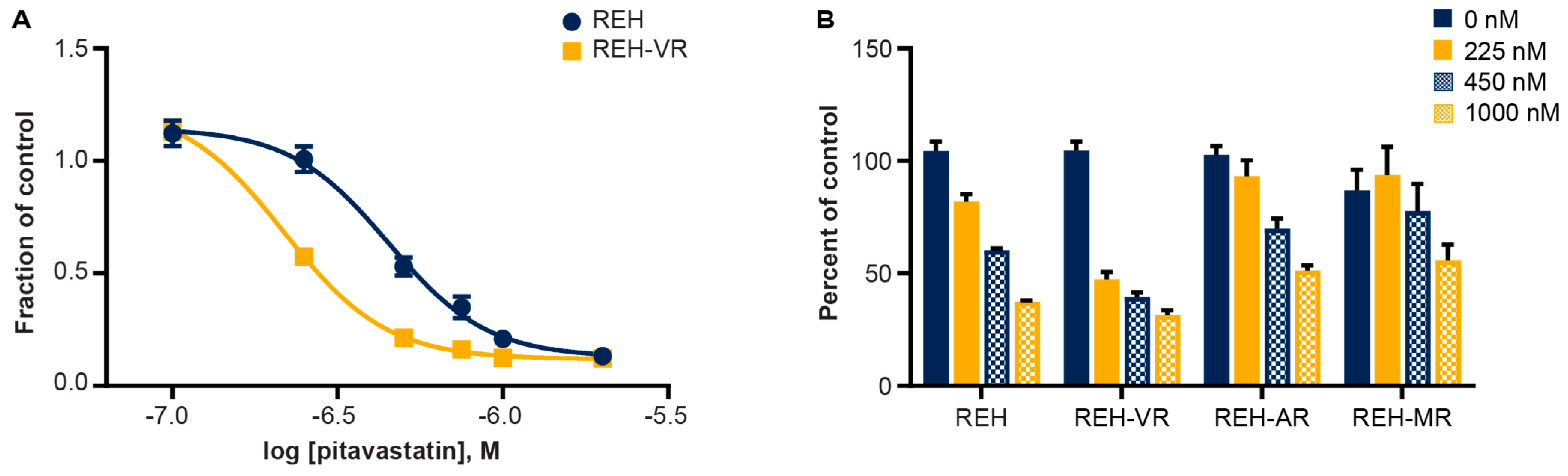
3.3. Pitavastatin Reduces Cell Proliferation in Vincristine-Resistant REH Cells
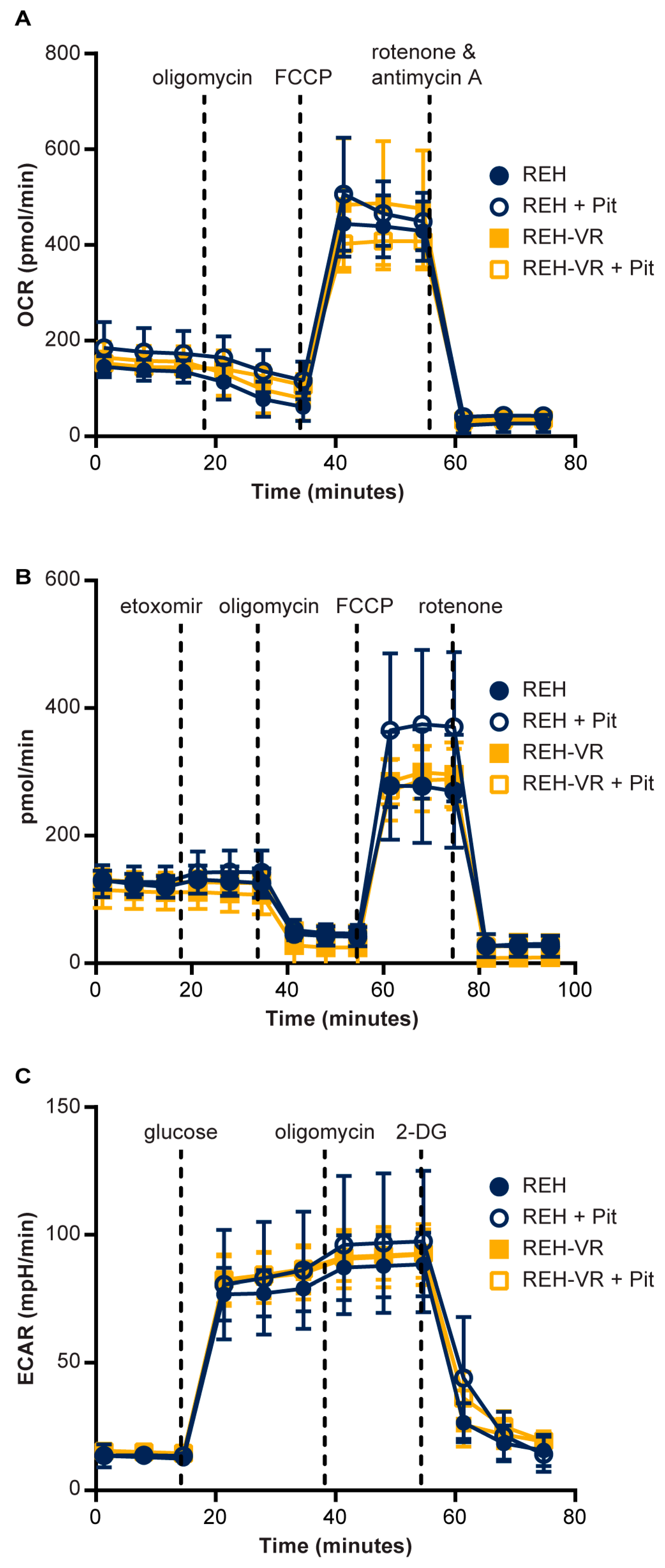
3.4. Effect of Pitavastatin on Mitochondrial Bioenergetics in Vincristine-Resistant REH Cells
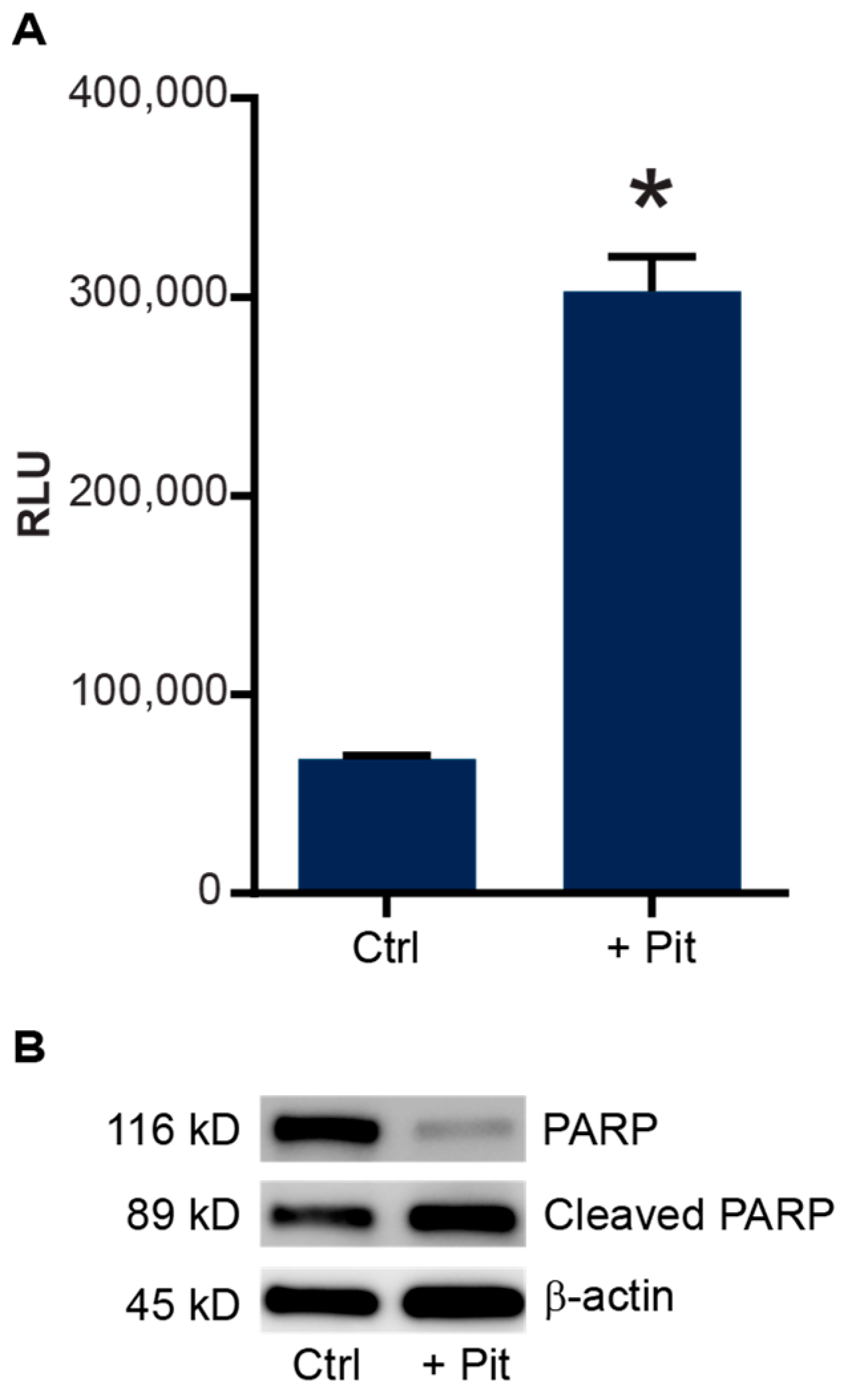
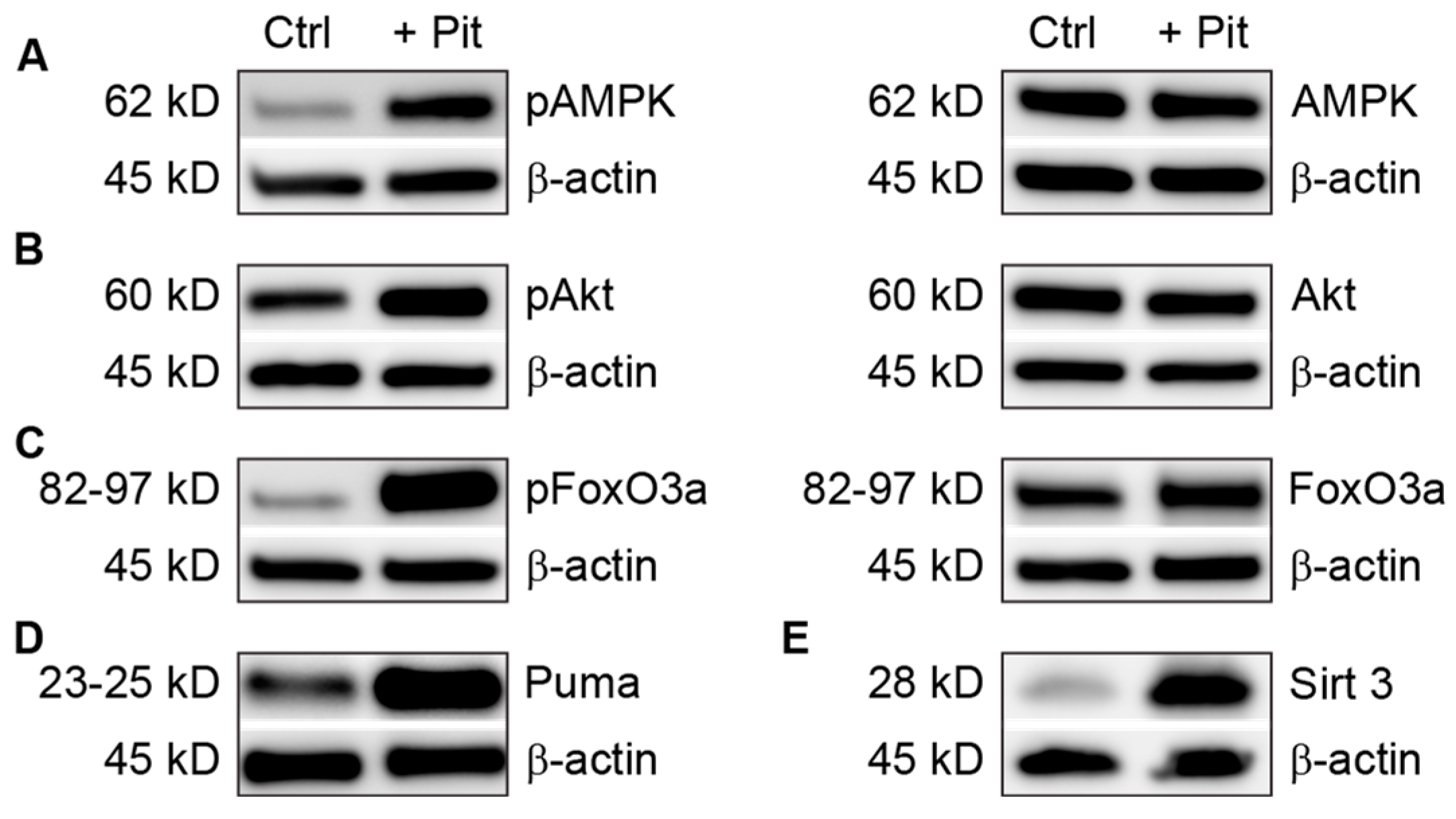
3.5. Pitavastatin Treatment Induces Apoptosis in Vincristine-Resistant Cells
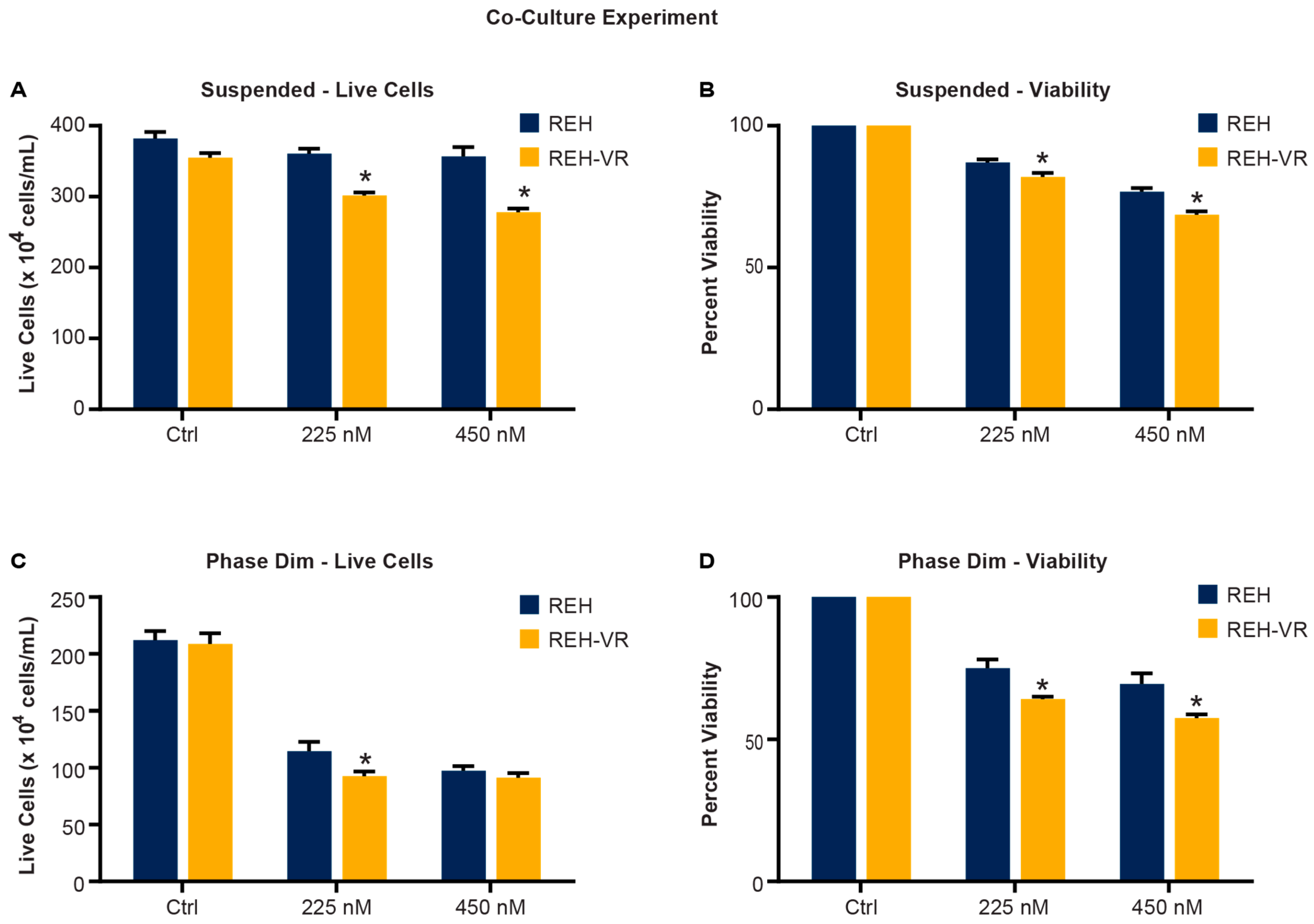
3.6. Pitavastatin Treatment Reduces the Proliferation of Leukemic Cells in a Co-Culture Model
3.7. Combination Treatment with Pitavastatin and Vincristine In Vitro
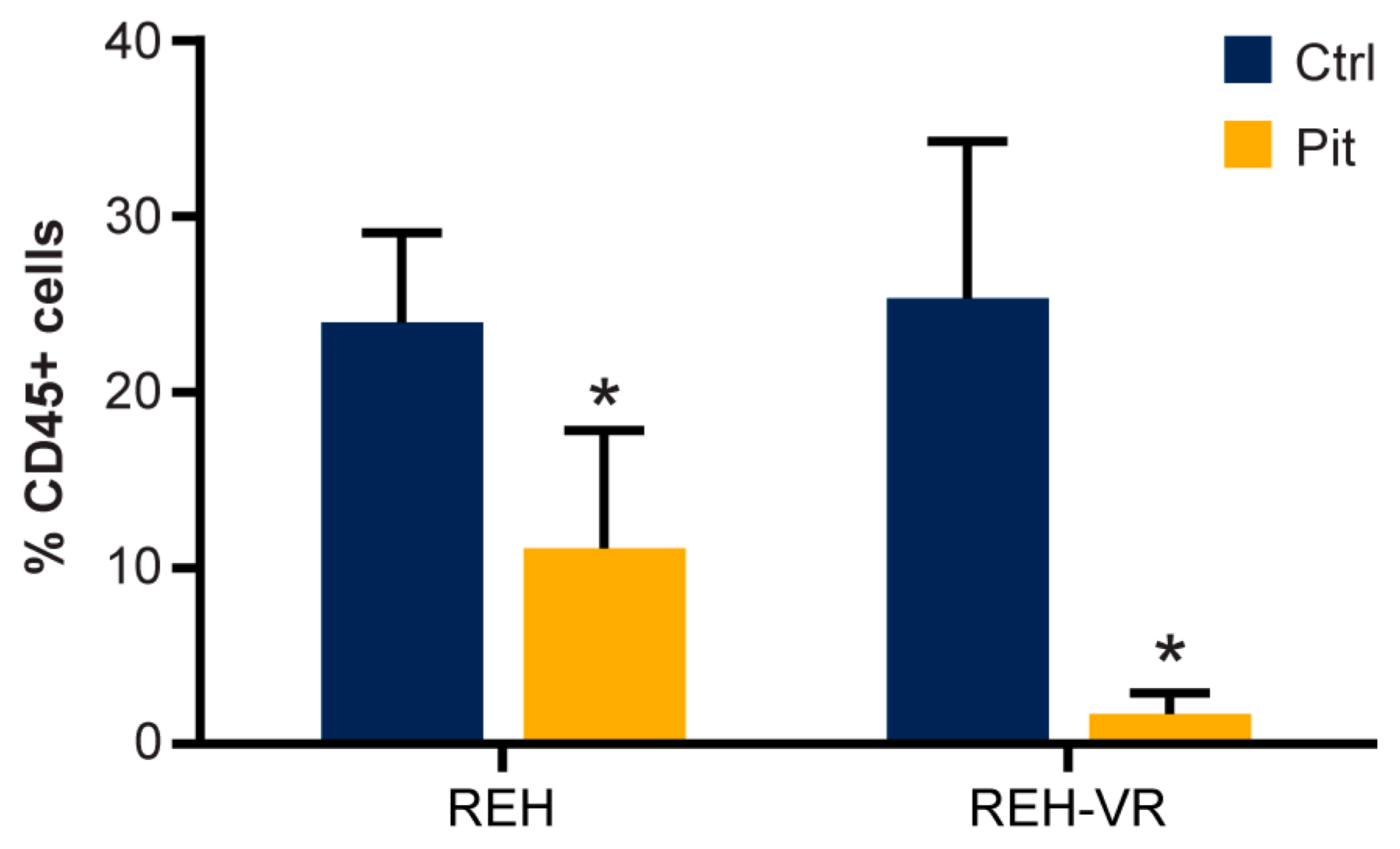
3.8. Pitavastatin Decreases the Number of Human CD45+ REH Cells in Mouse Bone Marrow
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delahaye, M.C.; Salem, K.I.; Pelletier, J.; Aurrand-Lions, M.; Mancini, S.J.C. Toward Therapeutic Targeting of Bone Marrow Leukemic Niche Protective Signals in B-Cell Acute Lymphoblastic Leukemia. Front. Oncol. 2020, 10, 606540. [Google Scholar] [CrossRef] [PubMed]
- Moses, B.S.; Slone, W.L.; Thomas, P.; Evans, R.; Piktel, D.; Angel, P.M.; Walsh, C.M.; Cantrell, P.S.; Rellick, S.L.; Martin, K.H.; et al. Bone marrow microenvironment modulation of acute lymphoblastic leukemia phenotype. Exp. Hematol. 2016, 44, 50–59, e51-52. [Google Scholar] [CrossRef] [PubMed]
- Moses, B.S.; Evans, R.; Slone, W.L.; Piktel, D.; Martinez, I.; Craig, M.D.; Gibson, L.F. Bone Marrow Microenvironment Niche Regulates miR-221/222 in Acute Lymphoblastic Leukemia. Mol. Cancer Res. 2016, 14, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Dalton, W.S.; Hazlehurst, L.; Shain, K.; Landowski, T.; Alsina, M. Targeting the bone marrow microenvironment in hematologic malignancies. Semin. Hematol. 2004, 41, 1–5. [Google Scholar] [CrossRef]
- Allegra, A.; Gioacchino, M.D.; Tonacci, A.; Petrarca, C.; Gangemi, S. Nanomedicine for Immunotherapy Targeting Hematological Malignancies: Current Approaches and Perspective. Nanomaterials 2021, 11, 2792. [Google Scholar] [CrossRef] [PubMed]
- Bolandi, S.M.; Pakjoo, M.; Beigi, P.; Kiani, M.; Allahgholipour, A.; Goudarzi, N.; Khorashad, J.S.; Eiring, A.M. A Role for the Bone Marrow Microenvironment in Drug Resistance of Acute Myeloid Leukemia. Cells 2021, 10, 2833. [Google Scholar] [CrossRef] [PubMed]
- Geldenhuys, W.J.; Nair, R.R.; Piktel, D.; Martin, K.H.; Gibson, L.F. The MitoNEET Ligand NL-1 Mediates Antileukemic Activity in Drug-Resistant B-Cell Acute Lymphoblastic Leukemia. J. Pharmacol. Exp. Ther. 2019, 370, 25–34. [Google Scholar] [CrossRef]
- Geldenhuys, W.J.; Piktel, D.; Moore, J.C.; Rellick, S.L.; Meadows, E.; Pinti, M.V.; Hollander, J.M.; Ammer, A.G.; Martin, K.H.; Gibson, L.F. Loss of the redox mitochondrial protein mitoNEET leads to mitochondrial dysfunction in B-cell acute lymphoblastic leukemia. Free Radic. Biol. Med. 2021, 175, 226–235. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, J.W.; He, X.R.; Jin, W.L.; He, X.Y. Statins: A repurposed drug to fight cancer. J. Exp. Clin. Cancer Res. 2021, 40, 241. [Google Scholar] [CrossRef]
- Lee, N.; Tilija Pun, N.; Jang, W.J.; Bae, J.W.; Jeong, C.H. Pitavastatin induces apoptosis in oral squamous cell carcinoma through activation of FOXO3a. J. Cell Mol. Med. 2020, 24, 7055–7066. [Google Scholar] [CrossRef] [PubMed]
- You, H.Y.; Zhang, W.J.; Xie, X.M.; Zheng, Z.H.; Zhu, H.L.; Jiang, F.Z. Pitavastatin suppressed liver cancer cells in vitro and in vivo. Onco. Targets Ther. 2016, 9, 5383–5388. [Google Scholar] [CrossRef] [PubMed]
- Glodkowska-Mrowka, E.; Mrowka, P.; Basak, G.W.; Niesiobedzka-Krezel, J.; Seferynska, I.; Wlodarski, P.K.; Jakobisiak, M.; Stoklosa, T. Statins inhibit ABCB1 and ABCG2 drug transporter activity in chronic myeloid leukemia cells and potentiate antileukemic effects of imatinib. Exp. Hematol. 2014, 42, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.; Kim, T.Y.; Min, H.J.; Kim, M.; Kang, M.S.; Huh, J.Y.; Kim, Y.; Lee, D.S. Synergistic killing effect of imatinib and simvastatin on imatinib-resistant chronic myelogenous leukemia cells. Anticancer Drugs 2013, 24, 20–31. [Google Scholar] [CrossRef]
- Chao, M.W.; Lai, M.J.; Liou, J.P.; Chang, Y.L.; Wang, J.C.; Pan, S.L.; Teng, C.M. The synergic effect of vincristine and vorinostat in leukemia in vitro and in vivo. J. Hematol. Oncol. 2015, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, D.; Morales, C.; Cooke, L.S.; Manziello, A.; Mount, D.W.; Persky, D.O.; Fisher, R.I.; Miller, T.P.; Qi, W. Alisertib added to rituximab and vincristine is synthetic lethal and potentially curative in mice with aggressive DLBCL co-overexpressing MYC and BCL2. PLoS ONE 2014, 9, e95184. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Huang, L.; Wang, D.; Wang, J.; Jiang, L.; Zhou, K.; Yang, Y.; Xu, D.; Zhou, J. Successful engraftment of human acute lymphoblastic leukemia cells in NOD/SCID mice via intrasplenic inoculation. Cancer Biol. Ther. 2012, 13, 1158–1164. [Google Scholar] [CrossRef]
- Nair, R.R.; Piktel, D.; Hathaway, Q.A.; Rellick, S.L.; Thomas, P.; Saralkar, P.; Martin, K.H.; Geldenhuys, W.J.; Hollander, J.M.; Gibson, L.F. Pyrvinium Pamoate Use in a B cell Acute Lymphoblastic Leukemia Model of the Bone Tumor Microenvironment. Pharm. Res. 2020, 37, 43. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Chen, A.; Teli, M.; Kondo, R.; Jalali, A.; Fan, Y.; Liu, S.; Zhao, X.; Siegel, A.; et al. Pitavastatin slows tumor progression and alters urine-derived volatile organic compounds through the mevalonate pathway. FASEB J. 2019, 33, 13710–13721. [Google Scholar] [CrossRef]
- Zhong, J.F.; Zhan, Y.; Anderson, W.F.; Zhao, Y. Murine hematopoietic stem cell distribution and proliferation in ablated and nonablated bone marrow transplantation. Blood 2002, 100, 3521–3526. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zheng, S.H.; Yang, W.G.; Yang, C.; Yuan, W.T. Targeting colon cancer stem cells with novel blood cholesterol drug pitavastatin. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1226–1233. [Google Scholar] [PubMed]
- Chen, Y.H.; Chen, Y.C.; Lin, C.C.; Hsieh, Y.P.; Hsu, C.S.; Hsieh, M.C. Synergistic Anticancer Effects of Gemcitabine with Pitavastatin on Pancreatic Cancer Cell Line MIA PaCa-2 in vitro and in vivo. Cancer Manag. Res. 2020, 12, 4645–4665. [Google Scholar] [CrossRef] [PubMed]
- Slone, W.L.; Moses, B.S.; Evans, R.; Piktel, D.; Martin, K.H.; Petros, W.; Craig, M.; Gibson, L.F. Modeling Chemotherapy Resistant Leukemia In Vitro. J. Vis. Exp. 2016, 108, e53645. [Google Scholar] [CrossRef] [PubMed]
- Rellick, S.L.; Hu, G.; Piktel, D.; Martin, K.H.; Geldenhuys, W.J.; Nair, R.R.; Gibson, L.F. Co-culture model of B-cell acute lymphoblastic leukemia recapitulates a transcription signature of chemotherapy-refractory minimal residual disease. Sci. Rep. 2021, 11, 15840. [Google Scholar] [CrossRef]
- Saito, Y. Pitavastatin: An overview. Atheroscler. Suppl. 2011, 12, 271–276. [Google Scholar] [CrossRef]
- Otahal, A.; Aydemir, D.; Tomasich, E.; Minichsdorfer, C. Delineation of cell death mechanisms induced by synergistic effects of statins and erlotinib in non-small cell lung cancer cell (NSCLC) lines. Sci. Rep. 2020, 10, 959. [Google Scholar] [CrossRef]
- Hu, T.; Shen, H.; Huang, H.; Yang, Z.; Zhou, Y.; Zhao, G. Cholesterol-lowering drug pitavastatin targets lung cancer and angiogenesis via suppressing prenylation-dependent Ras/Raf/MEK and PI3K/Akt/mTOR signaling. Anticancer Drugs 2020, 31, 377–384. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Z.; Zhang, M. Downregulation of survivin expression and elevation of caspase-3 activity involved in pitavastatin-induced HepG 2 cell apoptosis. Oncol. Rep. 2007, 18, 383–387. [Google Scholar] [CrossRef]
- Braamskamp, M.J.; Stefanutti, C.; Langslet, G.; Drogari, E.; Wiegman, A.; Hounslow, N.; Kastelein, J.J.; Group, P.S. Efficacy and Safety of Pitavastatin in Children and Adolescents at High Future Cardiovascular Risk. J. Pediatr. 2015, 167, 338–343.e335. [Google Scholar] [CrossRef]
- Adams, S.P.; Alaeiilkhchi, N.; Wright, J.M. Pitavastatin for lowering lipids. Cochrane Database Syst. Rev. 2020, 6, CD012735. [Google Scholar] [CrossRef]
- Wu, L.; Chatla, S.; Lin, Q.; Chowdhury, F.A.; Geldenhuys, W.; Du, W. Quinacrine-CASIN combination overcomes chemoresistance in human acute lymphoid leukemia. Nat. Commun. 2021, 12, 6936. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Geldenhuys, W.J.; Piktel, D.; Sadana, P.; Gibson, L.F. Novel compounds that target lipoprotein lipase and mediate growth arrest in acute lymphoblastic leukemia. Bioorg. Med. Chem. Lett. 2018, 28, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.S.; Schiller, G.J.; Heffner, L.T.; Stock, W.; Rao, A.V.; Roboz, G.J.; Westervelt, P.; Wieduwilt, M.J.; Yang, J.; Prasad, L.; et al. Plasma Vincristine Levels Are 100-Fold Higher with Marqibo® (Vincristine Sulfate LIPOSOME Injection) in Place of Standard Vincristine in Combination Chemotherapy of Patients ≥ 60 Years Old with Newly Diagnosed Acute Lymphoblastic Leukemia (ALL). Blood 2015, 126, 2491. [Google Scholar] [CrossRef]
- van der Heijden, L.T.; Uittenboogaard, A.; Nijstad, A.L.; Gebretensae, A.; Kaspers, G.J.L.; Beijnen, J.H.; Huitema, A.D.R.; Rosing, H. A sensitive liquid chromatographic-mass spectrometry method for the quantification of vincristine in whole blood collected with volumetric absorptive microsampling. J. Pharm. Biomed. Anal. 2023, 225, 115232. [Google Scholar] [CrossRef] [PubMed]
- Nijstad, A.L.; Chu, W.Y.; de Vos-Kerkhof, E.; Enters-Weijnen, C.F.; van de Velde, M.E.; Kaspers, G.J.L.; Barnett, S.; Veal, G.J.; Lalmohamed, A.; Zwaan, C.M.; et al. A Population Pharmacokinetic Modelling Approach to Unravel the Complex Pharmacokinetics of Vincristine in Children. Pharm. Res. 2022, 39, 2487–2495. [Google Scholar] [CrossRef]
- Sethi, V.S.; Jackson, D.V., Jr.; White, D.R.; Richards, F., 2nd; Stuart, J.J.; Muss, H.B.; Cooper, M.R.; Spurr, C.L. Pharmacokinetics of vincristine sulfate in adult cancer patients. Cancer Res. 1981, 41, 3551–3555. [Google Scholar]
- Sethi, V.S.; Kimball, J.C. Pharmacokinetics of vincristine sulfate in children. Cancer Chemother. Pharmacol. 1981, 6, 111–115. [Google Scholar] [CrossRef]
- Guerra, F.; Arbini, A.A.; Moro, L. Mitochondria and cancer chemoresistance. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 686–699. [Google Scholar] [CrossRef]
- Emmings, E.; Mullany, S.; Chang, Z.; Landen, C.N., Jr.; Linder, S.; Bazzaro, M. Targeting Mitochondria for Treatment of Chemoresistant Ovarian Cancer. Int. J. Mol. Sci. 2019, 20, 229. [Google Scholar] [CrossRef]
- Steinbach, D.; Legrand, O. ABC transporters and drug resistance in leukemia: Was P-gp nothing but the first head of the Hydra? Leukemia 2007, 21, 1172–1176. [Google Scholar] [CrossRef]
- Fu, Y.; Ricciardiello, F.; Yang, G.; Qiu, J.; Huang, H.; Xiao, J.; Cao, Z.; Zhao, F.; Liu, Y.; Luo, W.; et al. The Role of Mitochondria in the Chemoresistance of Pancreatic Cancer Cells. Cells 2021, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhang, Y.; Gu, J.; Feng, P.; Wang, Y. Pharmacokinetic Properties of Single- and Multiple-Dose Pitavastatin Calcium Tablets in Healthy Chinese Volunteers. Curr. Ther. Res. Clin. Exp. 2015, 77, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Egawa, T.; Toda, K.; Nemoto, Y.; Ono, M.; Akisaw, N.; Saibara, T.; Hayashi, Y.; Hiroi, M.; Enzan, H.; Onishi, S. Pitavastatin ameliorates severe hepatic steatosis in aromatase-deficient (Ar-/-) mice. Lipids 2003, 38, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, N.; Liu, P.; Xie, X. AMPK and Cancer. Exp. Suppl. 2016, 107, 203–226. [Google Scholar] [CrossRef]
- Finkel, T.; Deng, C.X.; Mostoslavsky, R. Recent progress in the biology and physiology of sirtuins. Nature 2009, 460, 587–591. [Google Scholar] [CrossRef]
- Ma, J.; Liu, B.; Yu, D.; Zuo, Y.; Cai, R.; Yang, J.; Cheng, J. SIRT3 deacetylase activity confers chemoresistance in AML via regulation of mitochondrial oxidative phosphorylation. Br. J. Haematol. 2019, 187, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, N.; Hadden, T.J.; Rishi, A.K. Akt, FoxO and regulation of apoptosis. Biochim. Biophys. Acta 2011, 1813, 1978–1986. [Google Scholar] [CrossRef]
- Hare, I.; Gencheva, M.; Evans, R.; Fortney, J.; Piktel, D.; Vos, J.A.; Howell, D.; Gibson, L.F. In Vitro Expansion of Bone Marrow Derived Mesenchymal Stem Cells Alters DNA Double Strand Break Repair of Etoposide Induced DNA Damage. Stem Cells Int. 2016, 2016, 8270464. [Google Scholar] [CrossRef]
- Evans, R.; Martin, K.H.; Moses, B.S.; Slone, W.L.; Hare, I.; Piktel, D.; Thomas, P.; Gibson, L.F. Modeling the Bone Marrow Microenvironment’s Influence on Leukemic Disease. Transl. Biomed. 2015, 6, 14. [Google Scholar] [CrossRef]
- Bruce, A.; Evans, R.; Mezan, R.; Shi, L.; Moses, B.S.; Martin, K.H.; Gibson, L.F.; Yang, Y. Three-Dimensional Microfluidic Tri-Culture Model of the Bone Marrow Microenvironment for Study of Acute Lymphoblastic Leukemia. PLoS ONE 2015, 10, e0140506. [Google Scholar] [CrossRef] [PubMed]
- Aldawood, A.M.; Kinkade, Z.; Rosado, F.G.; Esan, O.A.; Gibson, L.F.; Vos, J.A. A Novel Method to Assess Bone Marrow Purity is Useful in Determining Blast Percentage by Flow Cytometry in Acute Myeloid Leukemia and Myelodysplasia. Ann. Hematol. Oncol. 2015, 2, 1038. [Google Scholar] [PubMed]
- Chen, W.C.; Hu, G.; Hazlehurst, L.A. Contribution of the bone marrow stromal cells in mediating drug resistance in hematopoietic tumors. Curr. Opin. Pharmacol. 2020, 54, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Toth, R.K.; Tran, J.D.; Muldong, M.T.; Nollet, E.A.; Schulz, V.V.; Jensen, C.C.; Hazlehurst, L.A.; Corey, E.; Durden, D.; Jamieson, C.; et al. Hypoxia-induced PIM kinase and laminin-activated integrin alpha6 mediate resistance to PI3K inhibitors in bone-metastatic CRPC. Am. J. Clin. Exp. Urol. 2019, 7, 297–312. [Google Scholar]
- Nair, R.R.; Tolentino, J.; Hazlehurst, L.A. The bone marrow microenvironment as a sanctuary for minimal residual disease in CML. Biochem. Pharmacol. 2010, 80, 602–612. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piktel, D.; Moore, J.C.; Nesbit, S.; Sprowls, S.A.; Craig, M.D.; Rellick, S.L.; Nair, R.R.; Meadows, E.; Hollander, J.M.; Geldenhuys, W.J.; et al. Chemotherapeutic Activity of Pitavastatin in Vincristine Resistant B-Cell Acute Lymphoblastic Leukemia. Cancers 2023, 15, 707. https://doi.org/10.3390/cancers15030707
Piktel D, Moore JC, Nesbit S, Sprowls SA, Craig MD, Rellick SL, Nair RR, Meadows E, Hollander JM, Geldenhuys WJ, et al. Chemotherapeutic Activity of Pitavastatin in Vincristine Resistant B-Cell Acute Lymphoblastic Leukemia. Cancers. 2023; 15(3):707. https://doi.org/10.3390/cancers15030707
Chicago/Turabian StylePiktel, Debbie, Javohn C. Moore, Sloan Nesbit, Samuel A. Sprowls, Michael D. Craig, Stephanie L. Rellick, Rajesh R. Nair, Ethan Meadows, John M. Hollander, Werner J. Geldenhuys, and et al. 2023. "Chemotherapeutic Activity of Pitavastatin in Vincristine Resistant B-Cell Acute Lymphoblastic Leukemia" Cancers 15, no. 3: 707. https://doi.org/10.3390/cancers15030707
APA StylePiktel, D., Moore, J. C., Nesbit, S., Sprowls, S. A., Craig, M. D., Rellick, S. L., Nair, R. R., Meadows, E., Hollander, J. M., Geldenhuys, W. J., Martin, K. H., & Gibson, L. F. (2023). Chemotherapeutic Activity of Pitavastatin in Vincristine Resistant B-Cell Acute Lymphoblastic Leukemia. Cancers, 15(3), 707. https://doi.org/10.3390/cancers15030707





