The Synthetic Collagen-Binding Peptide NIPEP-OSS Delays Mouse Myeloma Progression
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Maintenance
2.2. Animal Experimentation
2.3. Bioluminescence Imaging
2.4. p1NP Level Measurement
2.5. Bone Histomorphometric and Dexa Analysis for Spine
2.6. Statistical Analyses
3. Results
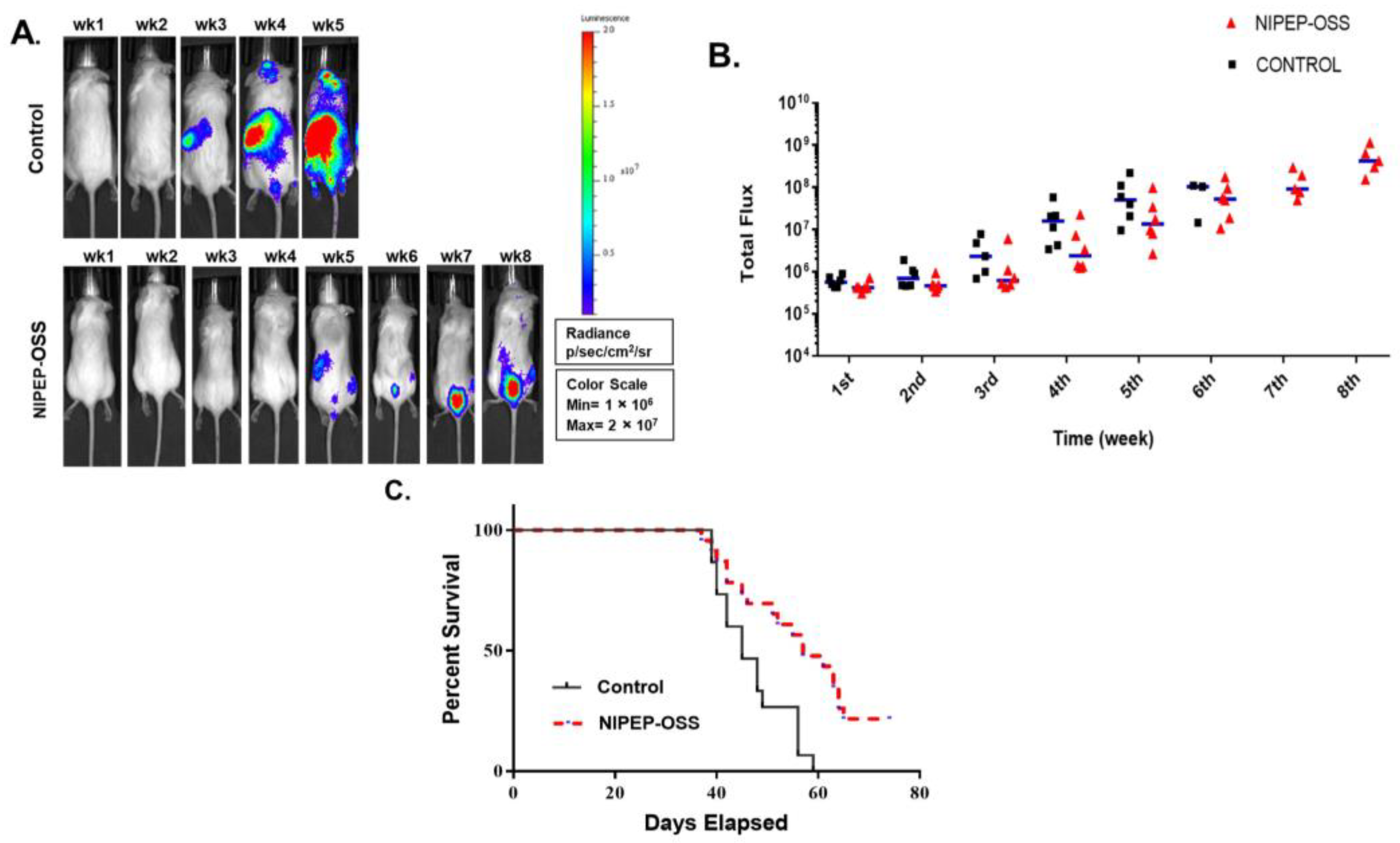
3.1. NIPEP-OSS Delays Myeloma Progression in the MM Model
3.2. NIPEP-OSS Increases Bone Minerals and Induces Bone Formation in MM Mice
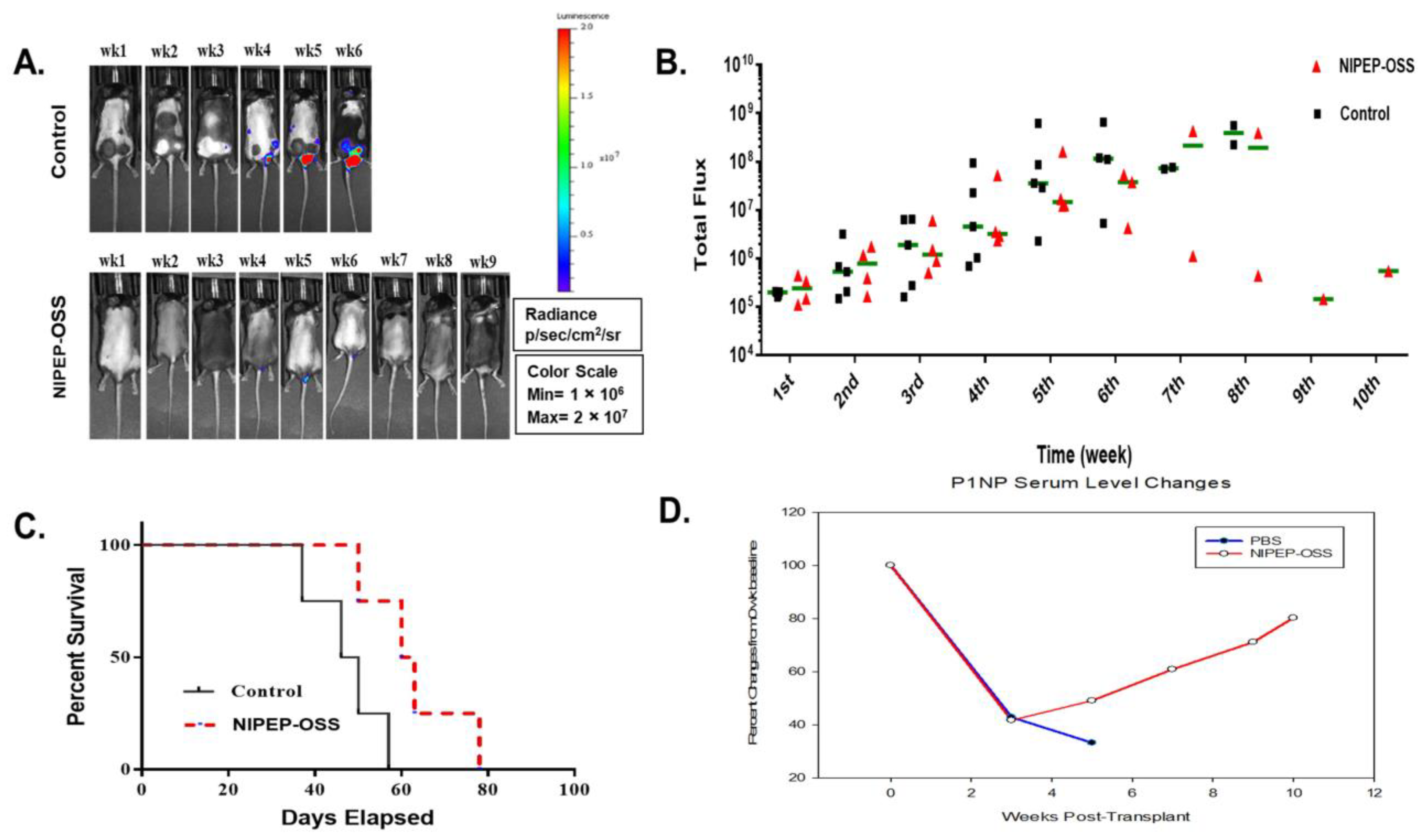
3.3. NIPEP-OSS Showed Similar Effects in C57BL/KaLwRij Model as in NSG MM Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, S.; Bano, M.C.; Cima, L.G.; Allcock, H.R.; Vacanti, J.P.; Vacanti, C.A.; Langer, R. Design of synthetic polymeric structures for cell transplantation and tissue engineering. Clin. Mater. 1993, 13, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Donzelli, E.; Salvade, A.; Mimo, P.; Vigano, M.; Morrone, M.; Papagna, R.; Carini, F.; Zaopo, A.; Miloso, M.; Baldoni, M.; et al. Mesenchymal stem cells cultured on a collagen scaffold: In vitro osteogenic differentiation. Arch. Oral Biol. 2007, 52, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Ishaug-Riley, S.L.; Crane-Kruger, G.M.; Yaszemski, M.J.; Mikos, A.G. Three-dimensional culture of rat calvarial osteoblasts in porous biodegradable polymers. Biomaterials 1998, 19, 1405–1412. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, U.J.; Blasioli, D.J.; Kim, H.J.; Kaplan, D.L. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials 2005, 26, 7082–7094. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Choo, J.E.; Choi, Y.S.; Park, J.B.; Min, D.S.; Lee, S.J.; Rhyu, H.K.; Jo, I.H.; Chung, C.P.; Park, Y.J. Assembly of collagen-binding peptide with collagen as a bioactive scaffold for osteogenesis in vitro and in vivo. Biomaterials 2007, 28, 4257–4267. [Google Scholar] [CrossRef]
- Dahl, T.; Veis, A. Electrostatic interactions lead to the formation of asymmetric collagen-phosphophoryn aggregates. Connect. Tissue Res. 2003, 44 (Suppl. 1), 206–213. [Google Scholar] [CrossRef]
- Fujisawa, R.; Kuboki, Y. Affinity of bone sialoprotein and several other bone and dentin acidic proteins to collagen fibrils. Calcif. Tissue Int. 1992, 51, 438–442. [Google Scholar] [CrossRef]
- Fujisawa, R.; Nodasaka, Y.; Kuboki, Y. Further characterization of interaction between bone sialoprotein (BSP) and collagen. Calcif. Tissue Int. 1995, 56, 140–144. [Google Scholar] [CrossRef]
- He, G.; George, A. Dentin matrix protein 1 immobilized on type I collagen fibrils facilitates apatite deposition in vitro. J. Biol. Chem. 2004, 279, 11649–11656. [Google Scholar] [CrossRef]
- Rocha, L.B.; Adam, R.L.; Leite, N.J.; Metze, K.; Rossi, M.A. Biomineralization of polyanionic collagen-elastin matrices during cavarial bone repair. J. Biomed. Mater. Res. A 2006, 79, 237–245. [Google Scholar] [CrossRef]
- Saito, T.; Arsenault, A.L.; Yamauchi, M.; Kuboki, Y.; Crenshaw, M.A. Mineral induction by immobilized phosphoproteins. Bone 1997, 21, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L. Matrix proteins and mineralization: An overview. Connect. Tissue Res. 1996, 35, 357–363. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.D.; Nanci, A. Osteopontin deposition in remodeling bone: An osteoblast mediated event. J. Bone Miner. Res. 1996, 11, 873–875. [Google Scholar] [CrossRef]
- McKee, M.D.; Nanci, A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: Ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc. Res. Tech. 1996, 33, 141–164. [Google Scholar] [CrossRef]
- McKee, M.D.; Nanci, A. Osteopontin: An interfacial extracellular matrix protein in mineralized tissues. Connect. Tissue Res. 1996, 35, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Lee, J.Y.; Suh, J.S.; Jin, Y.M.; Yu, Y.; Kim, H.Y.; Park, Y.J.; Chung, C.P.; Jo, I. Selective osteogenesis by a synthetic mineral inducing peptide for the treatment of osteoporosis. Biomaterials 2014, 35, 9747–9754. [Google Scholar] [CrossRef]
- Andersen, T.L.; Sondergaard, T.E.; Skorzynska, K.E.; Dagnaes-Hansen, F.; Plesner, T.L.; Hauge, E.M.; Plesner, T.; Delaisse, J.M. A physical mechanism for coupling bone resorption and formation in adult human bone. Am. J. Pathol. 2009, 174, 239–247. [Google Scholar] [CrossRef]
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef]
- Kong, Y.Y.; Yoshida, H.; Sarosi, I.; Tan, H.L.; Timms, E.; Capparelli, C.; Morony, S.; Oliveira-dos-Santos, A.J.; Van, G.; Itie, A.; et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999, 397, 315–323. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Khosla, S.; Dunstan, C.R.; Lacey, D.L.; Boyle, W.J.; Riggs, B.L. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J. Bone Miner. Res. 2000, 15, 2–12. [Google Scholar] [CrossRef]
- Ducy, P.; Schinke, T.; Karsenty, G. The osteoblast: A sophisticated fibroblast under central surveillance. Science 2000, 289, 1501–1504. [Google Scholar] [CrossRef]
- Teitelbaum, S.L.; Ross, F.P. Genetic regulation of osteoclast development and function. Nat. Reviews. Genet. 2003, 4, 638–649. [Google Scholar] [CrossRef]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cells Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef]
- Martin, T.J. Historically significant events in the discovery of RANK/RANKL/OPG. World J. Orthop. 2013, 4, 186–197. [Google Scholar] [CrossRef]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast Differentiation and Bone Matrix Formation In Vivo and In Vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef]
- Eghbali-Fatourechi, G.Z.; Lamsam, J.; Fraser, D.; Nagel, D.; Riggs, B.L.; Khosla, S. Circulating osteoblast-lineage cells in humans. N. Engl. J. Med. 2005, 352, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Esteve, F.R.; Roodman, G.D. Pathophysiology of myeloma bone disease. Best Pract. Res. Clin. Haematol. 2007, 20, 613–624. [Google Scholar] [CrossRef]
- Kyle, R.A.; Gertz, M.A.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin. Proc. 2003, 78, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Yaccoby, S. Advances in the understanding of myeloma bone disease and tumour growth. Br. J. Haematol. 2010, 149, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhyan, R.K.; Gerasimov, U.V. Bone marrow osteogenic stem cells: In vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987, 20, 263–272. [Google Scholar] [CrossRef]
- Digirolamo, C.M.; Stokes, D.; Colter, D.; Phinney, D.G.; Class, R.; Prockop, D.J. Propagation and senescence of human marrow stromal cells in culture: A simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br. J. Haematol. 1999, 107, 275–281. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Stenslokken, K.O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef]
- Mohan, M.; Samant, R.S.; Yoon, D.; Buros, A.F.; Branca, A.; Montgomery, C.O.; Nicholas, R.; Suva, L.J.; Morello, R.; Thanendrarajan, S.; et al. Extensive Remineralization of Large Pelvic Lytic Lesions Following Total Therapy Treatment in Patients with Multiple Myeloma. J. Bone Miner. Res. 2017, 32, 1261–1266. [Google Scholar] [CrossRef]
- Mehdi, S.H.; Morris, C.A.; Lee, J.A.; Yoon, D. An Improved Animal Model of Multiple Myeloma Bone Disease. Cancers 2021, 13, 4277. [Google Scholar] [CrossRef]
- Zangari, M.; Suva, L.J. The effects of proteasome inhibitors on bone remodeling in multiple myeloma. Bone 2016, 86, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Pandey, S.C.; Kapoor, P.; Dingli, D.; Hayman, S.R.; Leung, N.; et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia 2014, 28, 1122–1128. [Google Scholar] [CrossRef]
- Ring, E.S.; Lawson, M.A.; Snowden, J.A.; Jolley, I.; Chantry, A.D. New agents in the Treatment of Myeloma Bone Disease. Calcif. Tissue Int. 2018, 102, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Silbermann, R.; Roodman, G.D. Myeloma bone disease: Pathophysiology and management. J. Bone Oncol. 2013, 2, 59–69. [Google Scholar] [CrossRef]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Dimopoulos, M.A. Myeloma bone disease: From biology findings to treatment approaches. Blood 2019, 133, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Radl, J.; De Glopper, E.D.; Schuit, H.R.; Zurcher, C. Idiopathic paraproteinemia. II. Transplantation of the paraprotein-producing clone from old to young C57BL/KaLwRij mice. J. Immunol. 1979, 122, 609–613. [Google Scholar] [CrossRef]
- Radl, J. Animal model of human disease. Benign monoclonal gammopathy (idiopathic paraproteinemia). Am. J. Pathol. 1981, 105, 91–93. [Google Scholar] [PubMed]
- Mehdi, S.H.; Nafees, S.; Mehdi, S.J.; Morris, C.A.; Mashouri, L.; Yoon, D. Animal Models of Multiple Myeloma Bone Disease. Front. Genet. 2021, 12, 640954. [Google Scholar] [CrossRef]
- Asosingh, K.; Radl, J.; Van Riet, I.; Van Camp, B.; Vanderkerken, K. The 5TMM series: A useful in vivo mouse model of human multiple myeloma. Hematol. J. 2000, 1, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Morgan, G.; Dimopoulos, M.A.; Drake, M.T.; Lentzsch, S.; Raje, N.; Sezer, O.; Garcia-Sanz, R.; Shimizu, K.; Turesson, I.; et al. International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J. Clin. Oncol. 2013, 31, 2347–2357. [Google Scholar] [CrossRef]
- Terpos, E.; Dimopoulos, M.A.; Sezer, O.; Roodman, D.; Abildgaard, N.; Vescio, R.; Tosi, P.; Garcia-Sanz, R.; Davies, F.; Chanan-Khan, A.; et al. The use of biochemical markers of bone remodeling in multiple myeloma: A report of the International Myeloma Working Group. Leukemia 2010, 24, 1700–1712. [Google Scholar] [CrossRef]
- Terpos, E.; Zamagni, E.; Lentzsch, S.; Drake, M.T.; Garcia-Sanz, R.; Abildgaard, N.; Ntanasis-Stathopoulos, I.; Schjesvold, F.; de la Rubia, J.; Kyriakou, C.; et al. Treatment of multiple myeloma-related bone disease: Recommendations from the Bone Working Group of the International Myeloma Working Group. Lancet. Oncol. 2021, 22, e119–e130. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P.; Grimaux, M.; Demiaux, B.; Preaudat, C.; Seguin, P.; Delmas, P.D. Measurement of serum osteocalcin with a human-specific two-site immunoradiometric assay. J. Bone Miner. Res. 1992, 7, 1389–1398. [Google Scholar] [CrossRef]
- Arron, J.R.; Choi, Y. Bone versus immune system. Nature 2000, 408, 535–536. [Google Scholar] [CrossRef]
- Gnoni, A.; Brunetti, O.; Longo, V.; Calabrese, A.; Argentiero, A.L.; Calbi, R.; Solimando Antonio, G.; Licchetta, A. Immune system and bone microenvironment: Rationale for targeted cancer therapies. Oncotarget 2020, 11, 480–487. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehdi, S.H.; Gentry, A.C.; Lee, J.-Y.; Chung, C.-P.; Yoon, D. The Synthetic Collagen-Binding Peptide NIPEP-OSS Delays Mouse Myeloma Progression. Cancers 2023, 15, 2473. https://doi.org/10.3390/cancers15092473
Mehdi SH, Gentry AC, Lee J-Y, Chung C-P, Yoon D. The Synthetic Collagen-Binding Peptide NIPEP-OSS Delays Mouse Myeloma Progression. Cancers. 2023; 15(9):2473. https://doi.org/10.3390/cancers15092473
Chicago/Turabian StyleMehdi, Syed Hassan, Austin C. Gentry, Jue-Yeon Lee, Chong-Pyoung Chung, and Donghoon Yoon. 2023. "The Synthetic Collagen-Binding Peptide NIPEP-OSS Delays Mouse Myeloma Progression" Cancers 15, no. 9: 2473. https://doi.org/10.3390/cancers15092473
APA StyleMehdi, S. H., Gentry, A. C., Lee, J.-Y., Chung, C.-P., & Yoon, D. (2023). The Synthetic Collagen-Binding Peptide NIPEP-OSS Delays Mouse Myeloma Progression. Cancers, 15(9), 2473. https://doi.org/10.3390/cancers15092473





