Outcomes of Surgical Treatment for Extradural Benign Primary Spinal Tumors in Patients Younger than 25 Years: An Ambispective International Multicenter Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Treatment
2.3. Statistics
3. Results
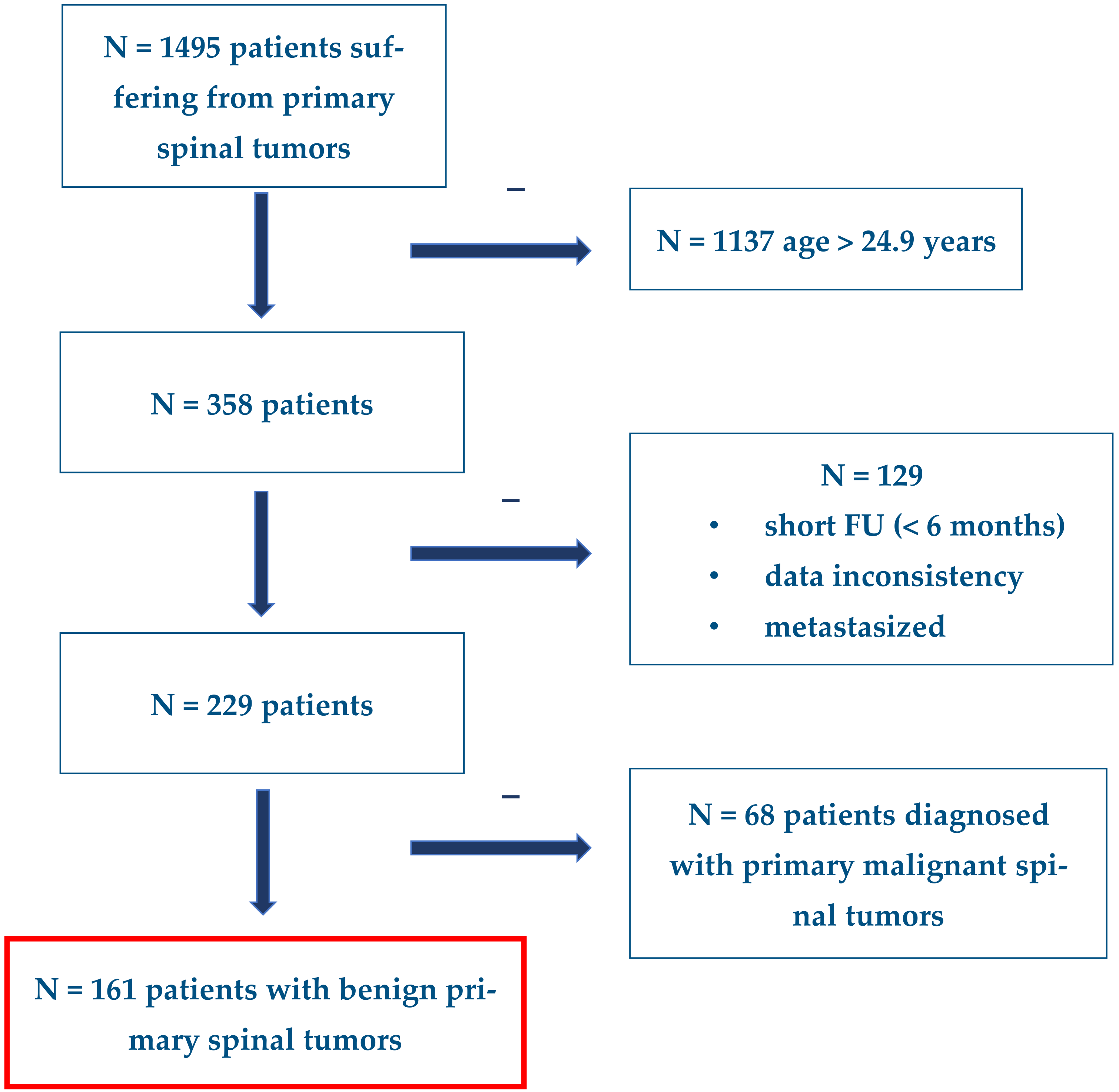
3.1. Patients
3.2. Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ravindra, V.M.; Eli, I.M.; Schmidt, M.H.; Brockmeyer, D.L. Primary osseous tumors of the pediatric spinal column: Review of pathology and surgical decision making. Neurosurg. Focus 2016, 41, E3. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.S.; Alston, R.D.; Eden, T.O.; Geraci, M.; Birch, J.M. The contrasting age-incidence patterns of bone tumours in teenagers and young adults: Implications for aetiology. Int. J. Cancer 2012, 131, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Bielack, S.; Abecassis, N.; Aro, H.T.; Bauer, S.; Biagini, R.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; Brennan, B.; et al. Bone sarcomas: ESMO-PaedCan-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv79–iv95. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Ro, J.Y. The 2020 WHO Classification of Tumors of Bone: An Updated Review. Adv. Anat. Pathol. 2021, 28, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.D. The evolving classification of soft tissue tumours–an update based on the new 2013 WHO classification. Histopathology 2014, 64, 2–11. [Google Scholar] [CrossRef]
- Boriani, S.; Weinstein, J.N.; Biagini, R. Primary bone tumors of the spine. Terminology and surgical staging. Spine 1997, 22, 1036–1044. [Google Scholar] [CrossRef]
- Chan, P.; Boriani, S.; Fourney, D.R.; Biagini, R.; Dekutoski, M.B.; Fehlings, M.G.; Ryken, T.C.; Gokaslan, Z.L.; Vrionis, F.D.; Harrop, J.S.; et al. An assessment of the reliability of the Enneking and Weinstein-Boriani-Biagini classifications for staging of primary spinal tumors by the Spine Oncology Study Group. Spine 2009, 34, 384–391. [Google Scholar] [CrossRef]
- Enneking, W.F.; Spanier, S.S.; Goodman, M.A. A system for the surgical staging of musculoskeletal sarcoma. Clin. Orthop. Relat. Res. 1980, 153, 106–120. [Google Scholar] [CrossRef]
- Sciubba, D.M.; Hsieh, P.; McLoughlin, G.S.; Jallo, G.I. Pediatric tumors involving the spinal column. Neurosurg. Clin. N. Am. 2008, 19, 81–92. [Google Scholar] [CrossRef]
- Boriani, S.; Amendola, L.; Bandiera, S.; Simoes, C.E.; Alberghini, M.; Di Fiore, M.; Gasbarrini, A. Staging and treatment of osteoblastoma in the mobile spine: A review of 51 cases. Eur. Spine J. 2012, 21, 2003–2010. [Google Scholar] [CrossRef]
- Boriani, S.; Bandiera, S.; Casadei, R.; Boriani, L.; Donthineni, R.; Gasbarrini, A.; Pignotti, E.; Biagini, R.; Schwab, J.H. Giant cell tumor of the mobile spine: A review of 49 cases. Spine 2012, 37, E37–E45. [Google Scholar] [CrossRef]
- Garg, S.; Mehta, S.; Dormans, J.P. Modern surgical treatment of primary aneurysmal bone cyst of the spine in children and adolescents. J. Pediatr. Orthop. 2005, 25, 387–392. [Google Scholar] [CrossRef]
- Haupt, R.; Minkov, M.; Astigarraga, I.; Schafer, E.; Nanduri, V.; Jubran, R.; Egeler, R.M.; Janka, G.; Micic, D.; Rodriguez-Galindo, C.; et al. Langerhans cell histiocytosis (LCH): Guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr. Blood Cancer 2013, 60, 175-84. [Google Scholar] [CrossRef]
- Lam, S.; Reddy, G.D.; Mayer, R.; Lin, Y.; Jea, A. Eosinophilic granuloma/Langerhans cell histiocytosis: Pediatric neurosurgery update. Surg. Neurol. Int. 2015, 6, S435–S439. [Google Scholar] [CrossRef]
- McLoughlin, G.S.; Sciubba, D.M.; Wolinsky, J.P. Chondroma/Chondrosarcoma of the spine. Neurosurg. Clin. N. Am. 2008, 19, 57–63. [Google Scholar] [CrossRef]
- Disch, A.C.; Kleber, C.; Redemann, D.; Druschel, C.; Liljenqvist, U.; Schaser, K.D. Current surgical strategies for treating spinal tumors: Results of a questionnaire survey among members of the German Spine Society (DWG). Eur. J. Surg. Oncol. 2020, 46, 89–94. [Google Scholar] [CrossRef]
- Schaser, K.D.; Melcher, I.; Luzzati, A.; Disch, A.C. Bone sarcoma of the spine. Recent Results Cancer Res. 2009, 179, 141–167. [Google Scholar]
- Sciubba, D.M.; De la Garza Ramos, R.; Goodwin, C.R.; Abu-Bonsrah, N.; Bydon, A.; Witham, T.F.; Bettegowda, C.; Gokaslan, Z.L.; Wolinsky, J.P. Clinical, surgical, and molecular prognostic factors for survival after spinal sarcoma resection. Neurosurg. Focus 2016, 41, E9. [Google Scholar] [CrossRef]
- Charest-Morin, R.; Dirks, M.S.; Patel, S.; Boriani, S.; Luzzati, A.; Fehlings, M.G.; Fisher, C.G.; Dekutoski, M.B.; Williams, R.; Quraishi, N.A.; et al. Ewing Sarcoma of the Spine: Prognostic Variables for Survival and Local Control in Surgically Treated Patients. Spine 2018, 43, 622–629. [Google Scholar] [CrossRef]
- Dekutoski, M.B.; Clarke, M.J.; Rose, P.; Luzzati, A.; Rhines, L.D.; Varga, P.P.; Fisher, C.G.; Chou, D.; Fehlings, M.G.; Reynolds, J.J.; et al. Osteosarcoma of the spine: Prognostic variables for local recurrence and overall survival, a multicenter ambispective study. J. Neurosurg. Spine 2016, 25, 59–68. [Google Scholar] [CrossRef]
- Fisher, C.G.; Versteeg, A.L.; Dea, N.; Boriani, S.; Varga, P.P.; Dekutoski, M.B.; Luzzati, A.; Gokaslan, Z.L.; Willimas, R.P.; Reynolds, J.J.; et al. Surgical Management of Spinal Chondrosarcomas. Spine 2016, 41, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.G.; Goldschlager, T.; Boriani, S.; Varga, P.P.; Rhines, L.D.; Fehlings, M.G.; Luzzati, A.; Dekutoski, M.B.; Reynolds, J.J.; Chou, D.; et al. An evidence-based medicine model for rare and often neglected neoplastic conditions. J. Neurosurg. Spine 2014, 21, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Harrop, J.S.; Schmidt, M.H.; Boriani, S.; Shaffrey, C.I. Aggressive “benign” primary spine neoplasms: Osteoblastoma, aneurysmal bone cyst, and giant cell tumor. Spine 2009, 34, S39–S47. [Google Scholar] [CrossRef] [PubMed]
- Gasbarrini, A.; Cappuccio, M.; Bandiera, S.; Amendola, L.; van Urk, P.; Boriani, S. Osteoid osteoma of the mobile spine: Surgical outcomes in 81 patients. Spine 2011, 36, 2089–2093. [Google Scholar] [CrossRef]
- Mesfin, A.; Boriani, S.; Gambarotti, M.; Bandiera, S.; Gasbarrini, A. Can Osteoblastoma Evolve to Malignancy? A Challenge in the Decision-Making Process of a Benign Spine Tumor. World Neurosurg. 2020, 136, 150–156. [Google Scholar] [CrossRef]
- Charest-Morin, R.; Fisher, C.G.; Varga, P.P.; Gokaslan, Z.L.; Rhines, L.D.; Reynolds, J.J.; Dekutoski, M.B.; Quraishi, N.A.; Bilsky, M.H.; Fehlings, M.G.; et al. En Bloc Resection Versus Intralesional Surgery in the Treatment of Giant Cell Tumor of the Spine. Spine 2017, 42, 1383–1390. [Google Scholar] [CrossRef]
- Traina, F.; Errani, C.; Toscano, A.; Pungetti, C.; Fabbri, D.; Mazzotti, A.; Donati, D.; Faldini, C. Current concepts in the biopsy of musculoskeletal tumors. JBJS 2015, 97, e7. [Google Scholar] [CrossRef]
- Fisher, C.G.; Saravanja, D.D.; Dvorak, M.F.; Rampersaud, Y.R.; Clarkson, P.W.; Hurlbert, J.; Fox, R.; Zhang, H.; Lewis, S.; Riaz, S.; et al. Surgical management of primary bone tumors of the spine: Validation of an approach to enhance cure and reduce local recurrence. Spine 2011, 36, 830–836. [Google Scholar] [CrossRef]
- Boriani, S.; Cecchinato, R.; Cuzzocrea, F.; Bandiera, S.; Gambarotti, M.; Gasbarrini, A. Denosumab in the treatment of giant cell tumor of the spine. Preliminary report, review of the literature and protocol proposal. Eur. Spine J. 2020, 29, 257–271. [Google Scholar] [CrossRef]
| Variable | n (%) | |
|---|---|---|
| Diagnosis (n = 161) | GCT | 16 (9.9) |
| OO | 45 (28.0) | |
| OBL | 53 (32.9) | |
| OCH | 4 (2.5) | |
| ABC | 32 (19.9) | |
| LCH | 2 (1.2) | |
| SCH | 9 (5.6) | |
| Gender (n = 161) | Female | 66 (41.0) |
| Male | 95 (59.0) | |
| Age at time of diagnosis (years) (n = 161) | 17.0 ± 4.7 | |
| Age at time of surgery (years) (n = 161) | 17.8 ± 5.3 | |
| Pain at Diagnosis (n = 161) | No | 5 (3.1) |
| Yes | 156 (96.9) | |
| Pathologic Fracture at Diagnosis (n = 161) | No | 143 (88.8) |
| Yes | 18 (11.2) | |
| Previous Spine Tumor Operation (n = 161) | No | 143 (88.8) |
| Yes | 18 (11.2) | |
| Preoperative Frankel and ASIA Score * (n = 158) | A | 2 (1.3) |
| B | 0 (0.0) | |
| C | 6 (3.8) | |
| D | 22 (13.9) | |
| E | 128 (81.0) | |
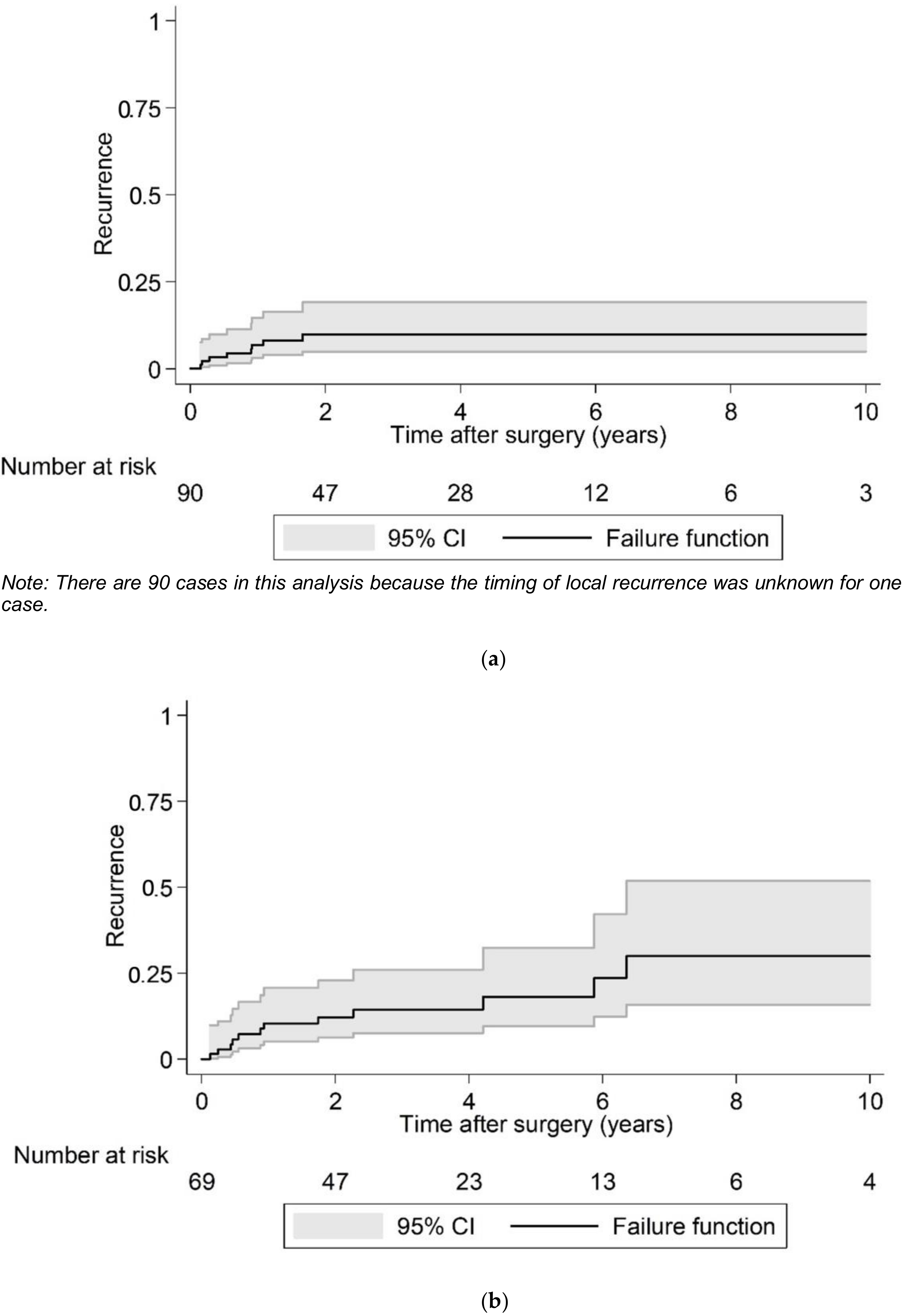
| Local recurrence over 10 years postoperative (n = 160) | No | 139 (86.9) |
| Yes | 21 (13.1) | |
| Survival over 10 years postoperative (n = 160) | Alive | 158 (98.8) |
| Dead | 2 (1.3) | |
| Variable | n (%) | |
|---|---|---|
| Tumor Size (cm) | Tumor Volume Ellipsoid Body (cm3) * (n = 132) | 4.2 (1.0, 9.4) |
| <5 | 81 (61.4) | |
| ≥5 | 51 (38.6) | |
| Spinal level (n = 161) | Mobile | 142 (88.2) |
| Fixed | 19 (11.8) | |
| Level by Cervical, Thoracic, Lumbar, Sacral (n = 152) | Cervical | 48 (31.6) |
| Thoracic | 49 (32.2) | |
| Lumbar | 42 (27.6) | |
| Sacral | 13 (8.6) | |
| Number of Vertebral Levels Spanned by the Tumor (n = 161) | 1 | 121 (75.2) |
| ≥2 | 40 (24.8) | |
| Tumor Grade (Enneking Classification) (n = 161) | S1 | 14 (8.7) |
| S2 | 73 (45.3) | |
| S3 | 74 (46.0) | |
| Variable | n (%) | |
| Preoperative Embolization (n = 152) | No | 104 (68.4) |
| Yes | 48 (31.6) | |
| Surgical Approach (n = 161) | Anterior | 11 (6.8) |
| Posterior | 126 (78.3) | |
| Anterior/Posterior | 4 (2.5) | |
| Posterior/Anterior | 17 (10.6) | |
| Posterior/Anterior/Posterior | 1 (0.6) | |
| Other | 2 (1.2) | |
| Fixation Used (n = 161) | Anterior | 5 (3.1) |
| Posterior | 72 (44.7) | |
| Both | 9 (5.6) | |
| None | 75 (46.6) | |
| Neurology Sacrificed: Cord (n = 159) | No | 159 (100.0) |
| Yes | 0 (0.0) | |
| Neurology Sacrificed: Cauda Equina (n = 159) | No | 159 (100.0) |
| Yes | 0 (0.0) | |
| Neurology Sacrificed: Nerve Roots (n = 159) | No | 144 (90.6) |
| Yes | 15 (9.4) | |
| Pathology result from the surgical specimen (n = 152) | Wide or marginal | 40 (26.3) |
| Intralesional | 112 (73.7) | |
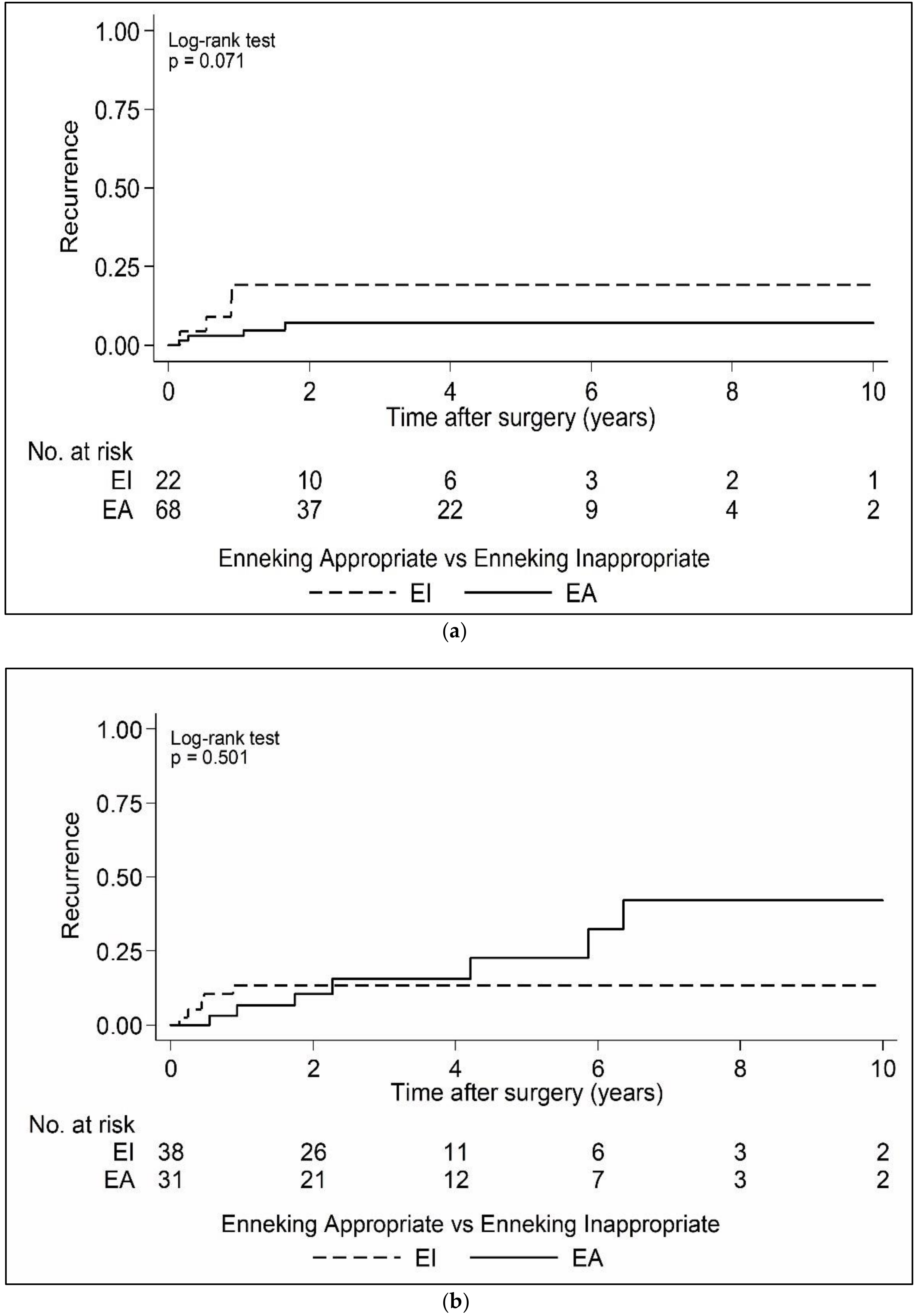
| Enneking appropriateness (n = 161) | EA | 100 (62.1) |
| EI | 61 (37.9) | |
| Adjuvant therapy (n = 160) | No | 150 (93.8) |
| Yes | 10 (6.3) | |
| Timing of chemotherapy (n = 161) | Preop | 0 (0.0) |
| Postop | 4 (2.5) | |
| Both | 0 (0.0) | |
| Neither (no chemo) | 156 (96.9) | |
| Timing unknown | 1 (0.6) | |
| Timing of radiation therapy (n = 161) | Preop | 0 (0.0) |
| Postop | 8 (5.0) | |
| Both | 0 (0.0) | |
| Neither (no radiation) | 152 (94.4) | |
| Timing unknown | 1 (0.6) | |
| Type of Radiation Therapy given (n = 9) | Conventional | 7 (77.8) |
| IMRT | 0 (0.0) | |
| Radiosurgery | 0 (0.0) | |
| Proton Beam | 0 (0.0) | |
| Unknown | 2 (22.2) | |
| Variable | n (%) | Variable | n (%) | p |
|---|---|---|---|---|
| Diagnosis | ||||
| (n = 92) | (n = 69) | |||
| Osteoid osteoma | 45 (48.9) | Giant cell tumor | 16 (23.2) | |
| Osteochondroma | 4 (4.3) | Osteoblastoma | 53 (76.8) | |
| ABC | 32 (34.8) | Tumor grade (n = 69) | ||
| LCH | 2 (2.2) | S2 | 16 (23.2) | |
| Schwannoma | 9 (9.8) | S3 | 53 (76.8) | |
| Enneking appropriateness | ||||
| (n = 92) | (n = 69) | |||
| EA | 69 (75.0) | EA | 31 (44.9) | >0.05 |
| EI | 23 (25.0) | EI | 38 (55.1) | |
| Local recurrence over 10 years postoperative | ||||
| (n = 91) | (n = 69) | |||
| No | 82 (90.1) | No | 57 (82.6) | |
| Yes | 9 (9.9) | Yes | 12 (17.4) | >0.05 |
| Survival over 10 years postoperative | ||||
| (n = 91) | (n = 69) | |||
| Alive | 91 (100.0) | Alive | 67 (97.1) | |
| Dead | 0 (0.0) | Dead | 2 (2.9) | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Disch, A.C.; Boriani, S.; Lazary, A.; Rhines, L.D.; Luzzati, A.; Gokaslan, Z.L.; Fisher, C.G.; Fehlings, M.G.; Clarke, M.J.; Chou, D.; et al. Outcomes of Surgical Treatment for Extradural Benign Primary Spinal Tumors in Patients Younger than 25 Years: An Ambispective International Multicenter Study. Cancers 2023, 15, 650. https://doi.org/10.3390/cancers15030650
Disch AC, Boriani S, Lazary A, Rhines LD, Luzzati A, Gokaslan ZL, Fisher CG, Fehlings MG, Clarke MJ, Chou D, et al. Outcomes of Surgical Treatment for Extradural Benign Primary Spinal Tumors in Patients Younger than 25 Years: An Ambispective International Multicenter Study. Cancers. 2023; 15(3):650. https://doi.org/10.3390/cancers15030650
Chicago/Turabian StyleDisch, Alexander C., Stefano Boriani, Aron Lazary, Laurence D. Rhines, Alessandro Luzzati, Ziya L. Gokaslan, Charles G. Fisher, Michael G. Fehlings, Michelle J. Clarke, Dean Chou, and et al. 2023. "Outcomes of Surgical Treatment for Extradural Benign Primary Spinal Tumors in Patients Younger than 25 Years: An Ambispective International Multicenter Study" Cancers 15, no. 3: 650. https://doi.org/10.3390/cancers15030650
APA StyleDisch, A. C., Boriani, S., Lazary, A., Rhines, L. D., Luzzati, A., Gokaslan, Z. L., Fisher, C. G., Fehlings, M. G., Clarke, M. J., Chou, D., Germscheid, N. M., Schaser, K.-D., Reynolds, J. J., & The AO Spine Knowledge Forum Tumor. (2023). Outcomes of Surgical Treatment for Extradural Benign Primary Spinal Tumors in Patients Younger than 25 Years: An Ambispective International Multicenter Study. Cancers, 15(3), 650. https://doi.org/10.3390/cancers15030650









