Immune-Related Thyroiditis in Patients with Advanced Lung Cancer Treated with Immune Checkpoint Inhibitors: Imaging Features and Clinical Implications
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
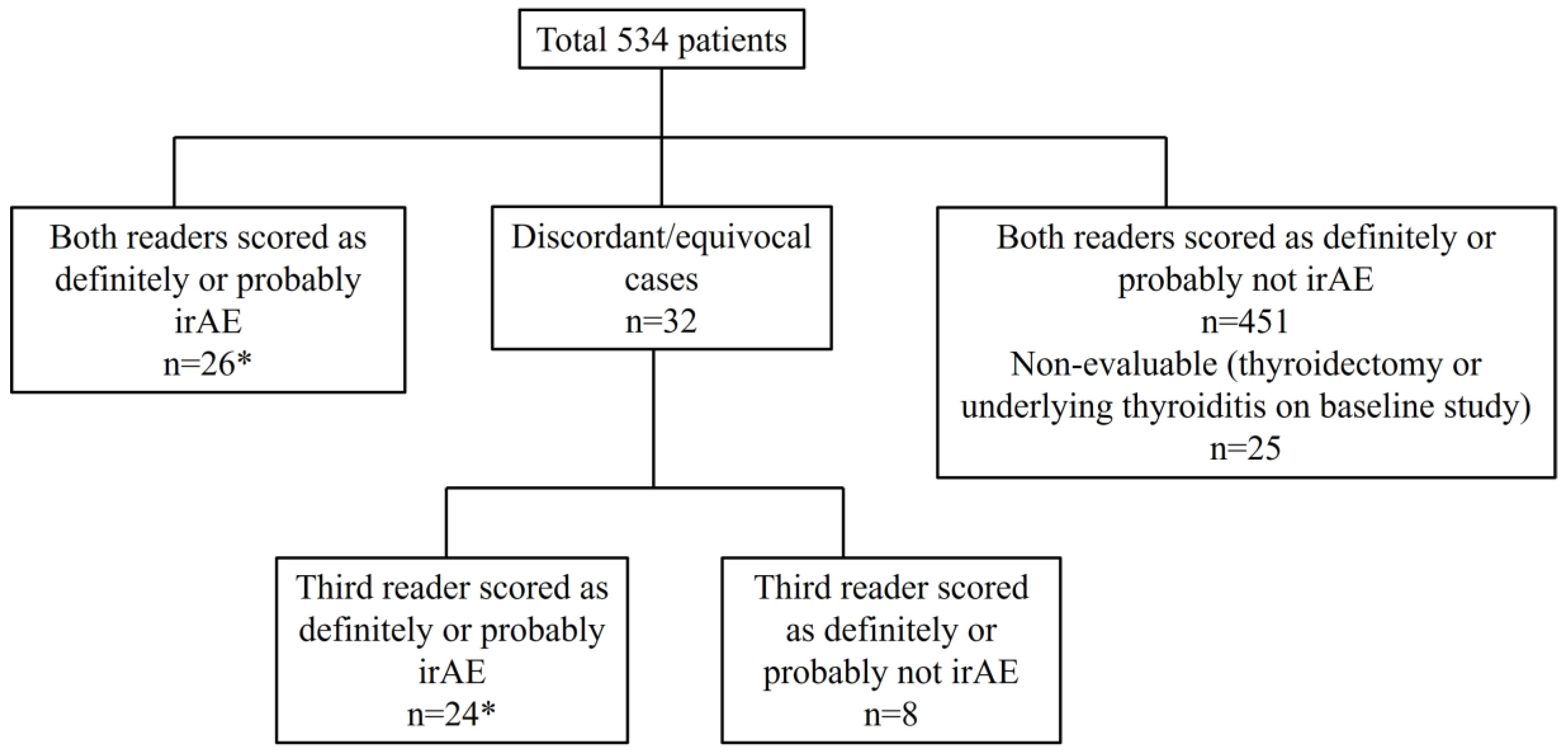
2.1. Patients
2.2. Imaging Review
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the Patients
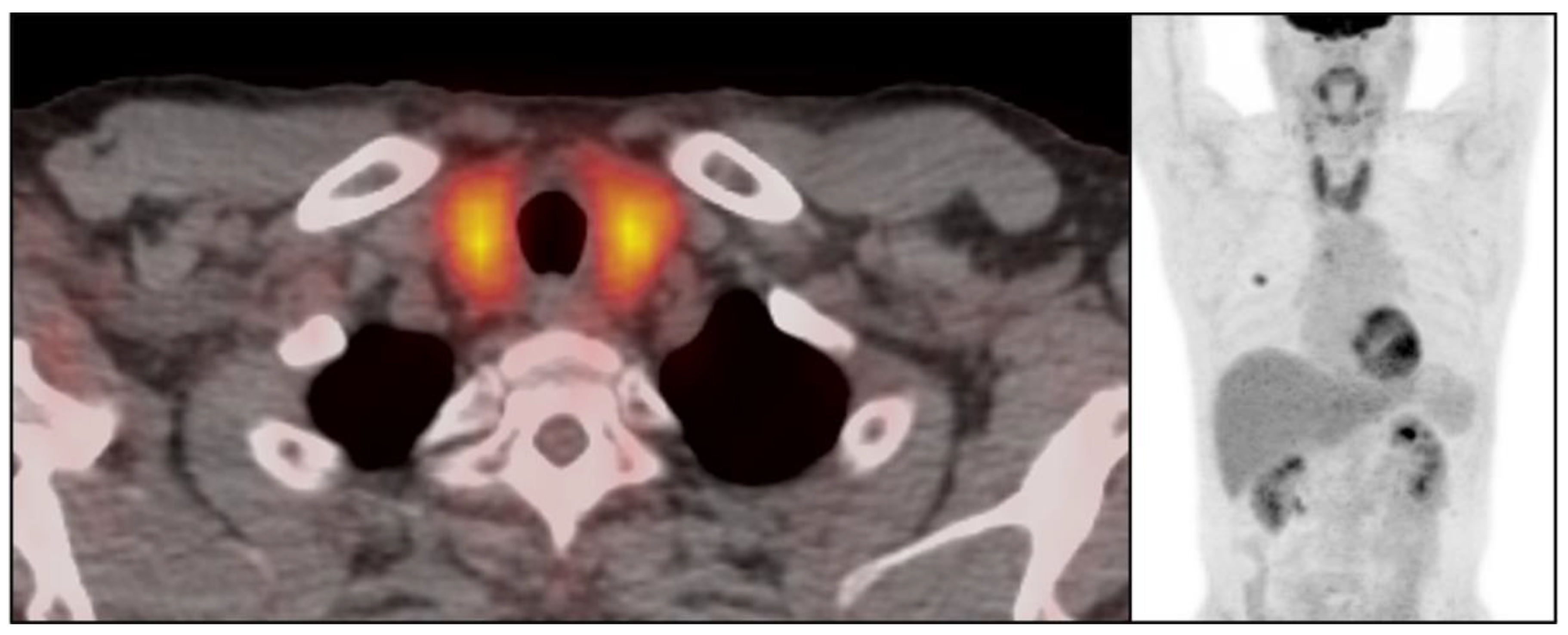
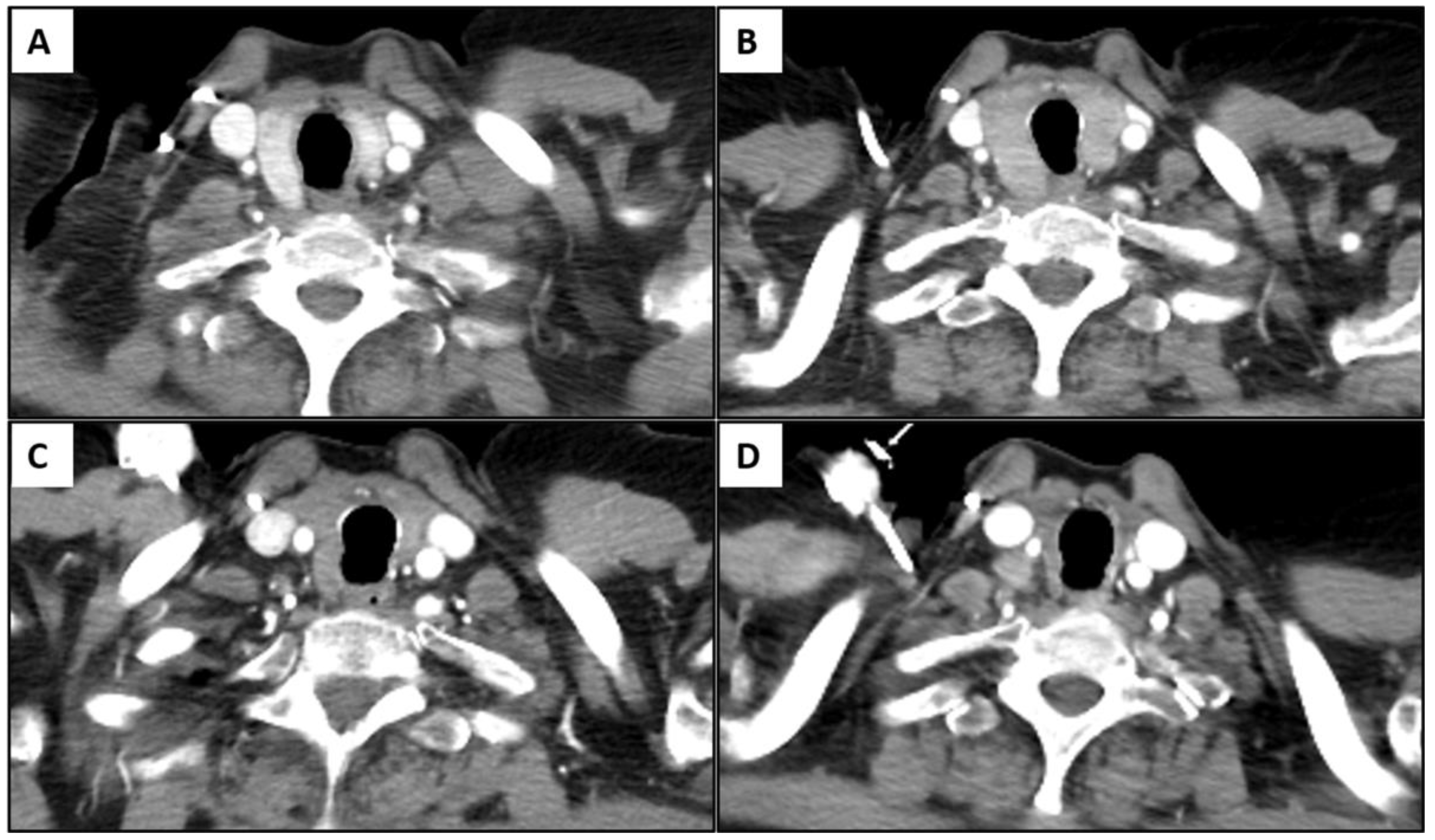
3.2. Immune-Related Thyroiditis Based on Imaging Findings
3.3. Immune-Related Thyroiditis Based on Abnormal Serum TSH
3.4. Clinically and Radiologically Evident Immune-Related Thyroiditis
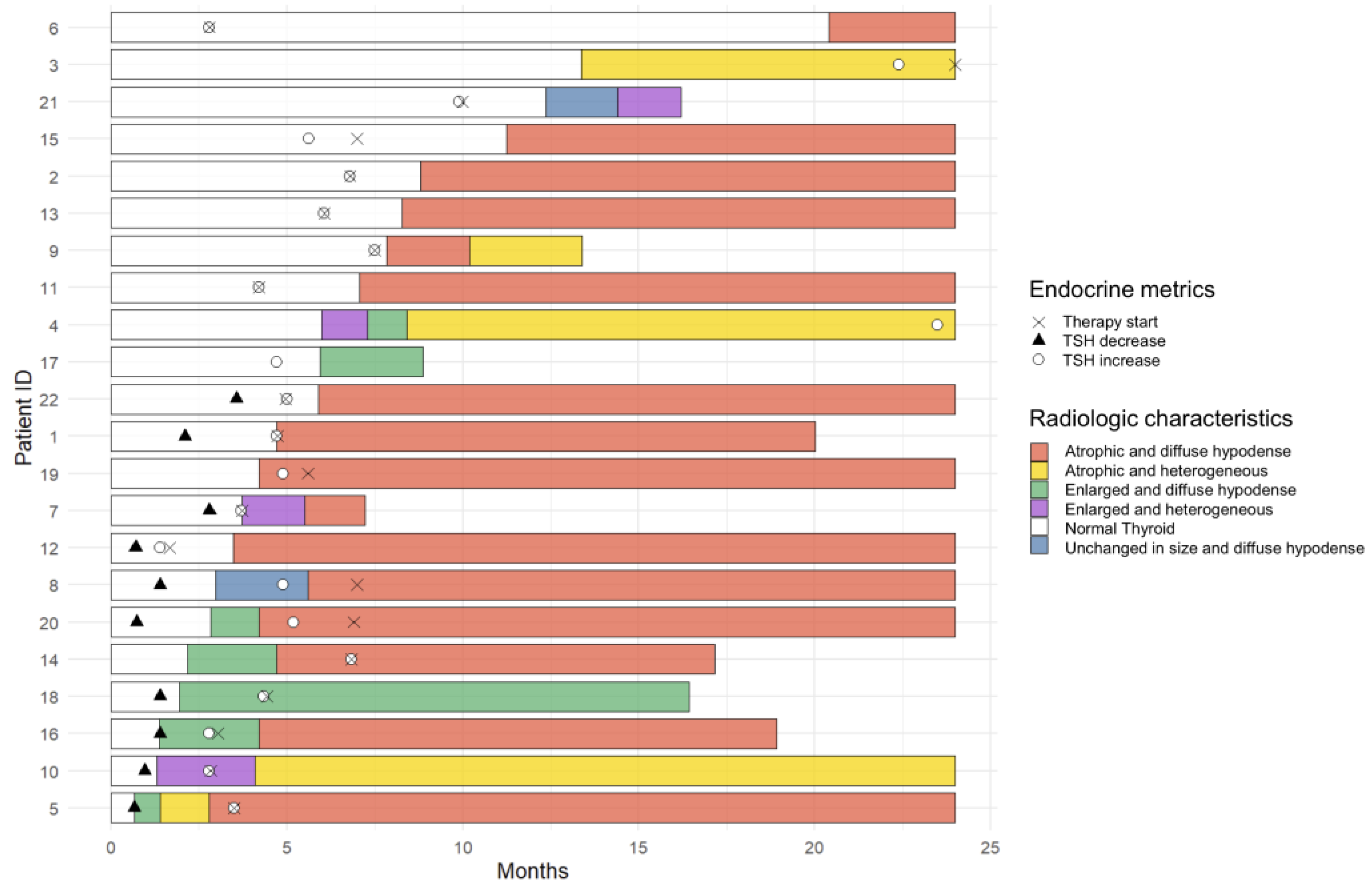
3.5. Temporal Changes in the CT Findings Correlating with TSH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antonia, S.J.; Borghaei, H.; Ramalingam, S.S.; Horn, L.; De Castro Carpeno, J.; Pluzanski, A.; Burgio, M.A.; Garassino, M.; Chow, L.Q.M.; Gettinger, S.; et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: A pooled analysis. Lancet. Oncol. 2019, 20, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, Y.; Ng, C.S.; Hwu, P.; Hwu, W.J. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR. Am. J. Roentgenol. 2011, 197, W992–W1000. [Google Scholar] [CrossRef] [PubMed]
- Tirumani, S.H.; Ramaiya, N.H.; Keraliya, A.; Bailey, N.D.; Ott, P.A.; Hodi, F.S.; Nishino, M. Radiographic Profiling of Immune-Related Adverse Events in Advanced Melanoma Patients Treated with Ipilimumab. Cancer Immunol. Res. 2015, 3, 1185–1192. [Google Scholar] [CrossRef]
- Nishino, M.; Ramaiya, N.H.; Awad, M.M.; Sholl, L.M.; Maattala, J.A.; Taibi, M.; Hatabu, H.; Ott, P.A.; Armand, P.F.; Hodi, F.S. PD-1 Inhibitor-Related Pneumonitis in Advanced Cancer Patients: Radiographic Patterns and Clinical Course. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 6051–6060. [Google Scholar] [CrossRef] [PubMed]
- Alessandrino, F.; Sahu, S.; Nishino, M.; Adeni, A.E.; Tirumani, S.H.; Shinagare, A.B.; Awad, M.M. Frequency and imaging features of abdominal immune-related adverse events in metastatic lung cancer patients treated with PD-1 inhibitor. Abdom. Radiol. 2019, 44, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Barroso-Sousa, R.; Barry, W.T.; Garrido-Castro, A.C.; Hodi, F.S.; Min, L.; Krop, I.E.; Tolaney, S.M. Incidence of Endocrine Dysfunction Following the Use of Different Immune Checkpoint Inhibitor Regimens: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 173–182. [Google Scholar] [CrossRef]
- Delivanis, D.A.; Gustafson, M.P.; Bornschlegl, S.; Merten, M.M.; Kottschade, L.; Withers, S.; Dietz, A.B.; Ryder, M. Pembrolizumab-Induced Thyroiditis: Comprehensive Clinical Review and Insights Into Underlying Involved Mechanisms. J. Clin. Endocrinol. Metab. 2017, 102, 2770–2780. [Google Scholar] [CrossRef]
- Ryder, M.; Callahan, M.; Postow, M.A.; Wolchok, J.; Fagin, J.A. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: A comprehensive retrospective review from a single institution. Endocr. Relat. Cancer 2014, 21, 371–381. [Google Scholar] [CrossRef]
- De Filette, J.; Jansen, Y.; Schreuer, M.; Everaert, H.; Velkeniers, B.; Neyns, B.; Bravenboer, B. Incidence of Thyroid-Related Adverse Events in Melanoma Patients Treated with Pembrolizumab. J. Clin. Endocrinol. Metab. 2016, 101, 4431–4439. [Google Scholar] [CrossRef]
- Eshghi, N.; Garland, L.L.; Nia, E.; Betancourt, R.; Krupinski, E.; Kuo, P.H. (18)F-FDG PET/CT Can Predict Development of Thyroiditis Due to Immunotherapy for Lung Cancer. J. Nucl. Med. Technol. 2018, 46, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hodi, F.S.; Giobbie-Hurder, A.; Ott, P.A.; Buchbinder, E.I.; Haq, R.; Tolaney, S.; Barroso-Sousa, R.; Zhang, K.; Donahue, H.; et al. Characterization of Thyroid Disorders in Patients Receiving Immune Checkpoint Inhibition Therapy. Cancer Immunol. Res. 2017, 5, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Hatabu, H.; Ricciuti, B.; Aijazi, S.J.; Awad, M.M.; Nishino, M. Immune-related adverse events on body CT in patients with small-cell lung cancer treated with immune-checkpoint inhibitors. Eur. J. Radiol. 2020, 132, 109275. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet. Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Joshua, A.M.; Kefford, R.; Joseph, R.W.; Wolchok, J.D.; Hodi, F.S.; Hamid, O.; Weber, J.S.; Gangadhar, T.C.; Dronca, R.S.; et al. Association of immune-related thyroid disorders with pembrolizumab (pembro, MK-3475) in patients (pts) with advanced melanoma treated in KEYNOTE-001. J. Clin. Oncol. 2015, 33, 9050. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Abid, H.; Khavandi, M.; Siddiqui, N.; Panjawatanan, P.; Patel, A. Incidence and Risk of Thyroid Dysfunction in Advanced or Metastatic Non-small Cell Lung Cancer Patients Treated with Pembrolizumab: A Meta-analysis. Cureus 2019, 11, e5997. [Google Scholar] [CrossRef]
- Hong, A.R.; Yoon, J.H.; Kim, H.K.; Kang, H.-C. MON-575 Clinical Implication of Sonographic Evaluation for Immune Checkpoint Inhibitor-Related Thyroiditis: A Case Series Study. J. Endocr. Soc. 2019, 3, MON-575. [Google Scholar] [CrossRef]
- Chaudhary, V.; Bano, S. Thyroid ultrasound. Indian J. Endocrinol. Metab. 2013, 17, 219–227. [Google Scholar] [CrossRef]
- Iravani, A.; Galligan, A.; Lasocki, A.; Wallace, R.; Weppler, A.; Au Yeung, G.; Akhurst, T.; Sachithanandan, N.; Chiang, C.; Sandhu, S.; et al. FDG PET in the evaluation of immune-related hypophysitis and thyroiditis following combination ipilimumab and nivolumab in advanced melanoma. J. Nucl. Med. 2020, 61, 482. [Google Scholar]
- Han, Y.M.; Kim, Y.C.; Park, E.K.; Choe, J.G. Diagnostic value of CT density in patients with diffusely increased FDG uptake in the thyroid gland on PET/CT images. AJR. Am. J. Roentgenol. 2010, 195, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Reis, M.; Zhou, Y. Correlation Between Computed Tomography Density and Functional Status of the Thyroid Gland. J. Comput. Assist. Tomogr. 2016, 40, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Angell, T.E.; Min, L.; Wieczorek, T.J.; Hodi, F.S. Unique Cytologic Features of Thyroiditis Caused by Immune Checkpoint Inhibitor Therapy for Malignant Melanoma. Genes Dis. 2018, 5, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Kotwal, A.; Gustafson, M.P.; Bornschlegl, S.; Kottschade, L.; Delivanis, D.A.; Dietz, A.B.; Gandhi, M.; Ryder, M. Immune Checkpoint Inhibitor-Induced Thyroiditis Is Associated with Increased Intrathyroidal T Lymphocyte Subpopulations. Thyroid Off. J. Am. Thyroid Assoc. 2020, 30, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.C.; Cabanillas, M.E.; Waguespack, S.G.; Hu, M.I.; Thosani, S.; Lavis, V.R.; Busaidy, N.L.; Subudhi, S.K.; Diab, A.; Dadu, R. Immune-Related Thyroiditis with Immune Checkpoint Inhibitors. Thyroid Off. J. Am. Thyroid Assoc. 2018, 28, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Fatourechi, V.; Aniszewski, J.P.; Fatourechi, G.Z.; Atkinson, E.J.; Jacobsen, S.J. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J. Clin. Endocrinol. Metab. 2003, 88, 2100–2105. [Google Scholar] [CrossRef]
- Caron, P. Thyroiditis and SARS-CoV-2 pandemic: A review. Endocrine 2021, 72, 326–331. [Google Scholar] [CrossRef]
- Lisco, G.; De Tullio, A.; Jirillo, E.; Giagulli, V.A.; De Pergola, G.; Guastamacchia, E.; Triggiani, V. Thyroid and COVID-19: A review on pathophysiological, clinical and organizational aspects. J. Endocrinol. Investig. 2021, 44, 1801–1814. [Google Scholar] [CrossRef]

| CT Positive for Thyroiditis (n = 50) | CT Negative for Thyroiditis (n = 484) | All Patients (n = 534) | p Value | ||
|---|---|---|---|---|---|
| Age | Median (years) (range) | 63 (39–91) | 66 (25–92) | 65 (25–92) | 0.22 |
| Sex | Male | 20 | 229 | 249 | 0.37 |
| Female | 30 | 255 | 285 | ||
| Smoking | Never | 3 | 73 | 76 | 0.089 |
| Current/Former | 47 | 411 | 458 | ||
| Pathology | Adenocarcinoma | 39 | 373 | 412 | 1.00 |
| Other | 11 | 111 | 122 | ||
| ECOG PS | 0-1 | 40 | 400 | 440 | 0.69 |
| ≥2 | 10 | 84 | 94 | ||
| ICI regimen | PD-1/PD-L1 monotherapy | 46 | 457 | 503 | 0.51 |
| PD-1/CTLA-4 combination therapy | 4 | 27 | 31 | ||
| Line of therapy | 1st line | 13 | 141 | 154 | 0.74 |
| ≥2nd line | 37 | 343 | 380 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Hata, A.; Hatabu, H.; Ricciuti, B.; Awad, M.; Nishino, M. Immune-Related Thyroiditis in Patients with Advanced Lung Cancer Treated with Immune Checkpoint Inhibitors: Imaging Features and Clinical Implications. Cancers 2023, 15, 649. https://doi.org/10.3390/cancers15030649
Park H, Hata A, Hatabu H, Ricciuti B, Awad M, Nishino M. Immune-Related Thyroiditis in Patients with Advanced Lung Cancer Treated with Immune Checkpoint Inhibitors: Imaging Features and Clinical Implications. Cancers. 2023; 15(3):649. https://doi.org/10.3390/cancers15030649
Chicago/Turabian StylePark, Hyesun, Akinori Hata, Hiroto Hatabu, Biagio Ricciuti, Mark Awad, and Mizuki Nishino. 2023. "Immune-Related Thyroiditis in Patients with Advanced Lung Cancer Treated with Immune Checkpoint Inhibitors: Imaging Features and Clinical Implications" Cancers 15, no. 3: 649. https://doi.org/10.3390/cancers15030649
APA StylePark, H., Hata, A., Hatabu, H., Ricciuti, B., Awad, M., & Nishino, M. (2023). Immune-Related Thyroiditis in Patients with Advanced Lung Cancer Treated with Immune Checkpoint Inhibitors: Imaging Features and Clinical Implications. Cancers, 15(3), 649. https://doi.org/10.3390/cancers15030649





