Prostate Cancers Invisible on Multiparametric MRI: Pathologic Features in Correlation with Whole-Mount Prostatectomy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Patients
2.2. MR Imaging
2.3. Histology Processing
2.4. MR Image Analysis
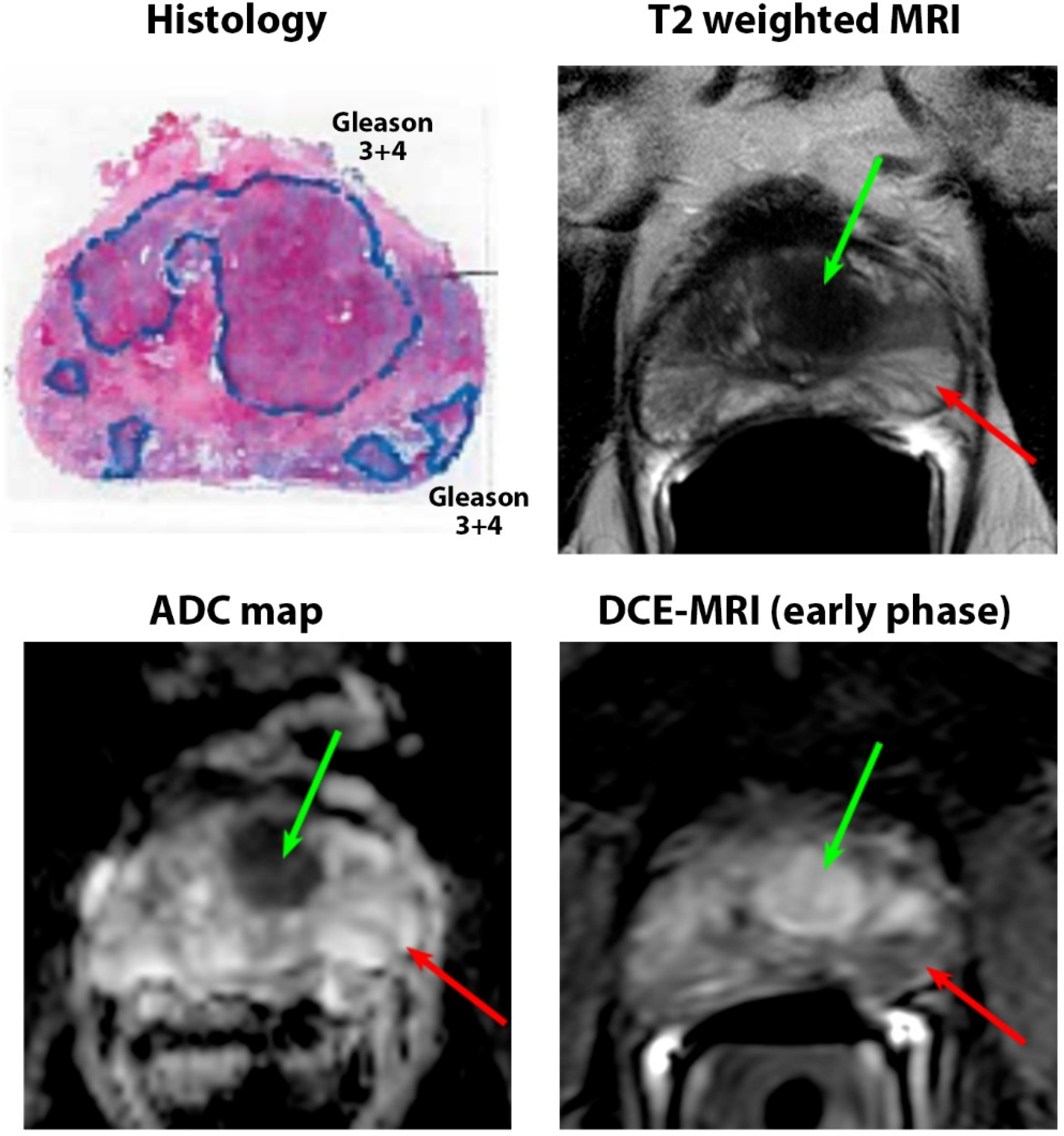
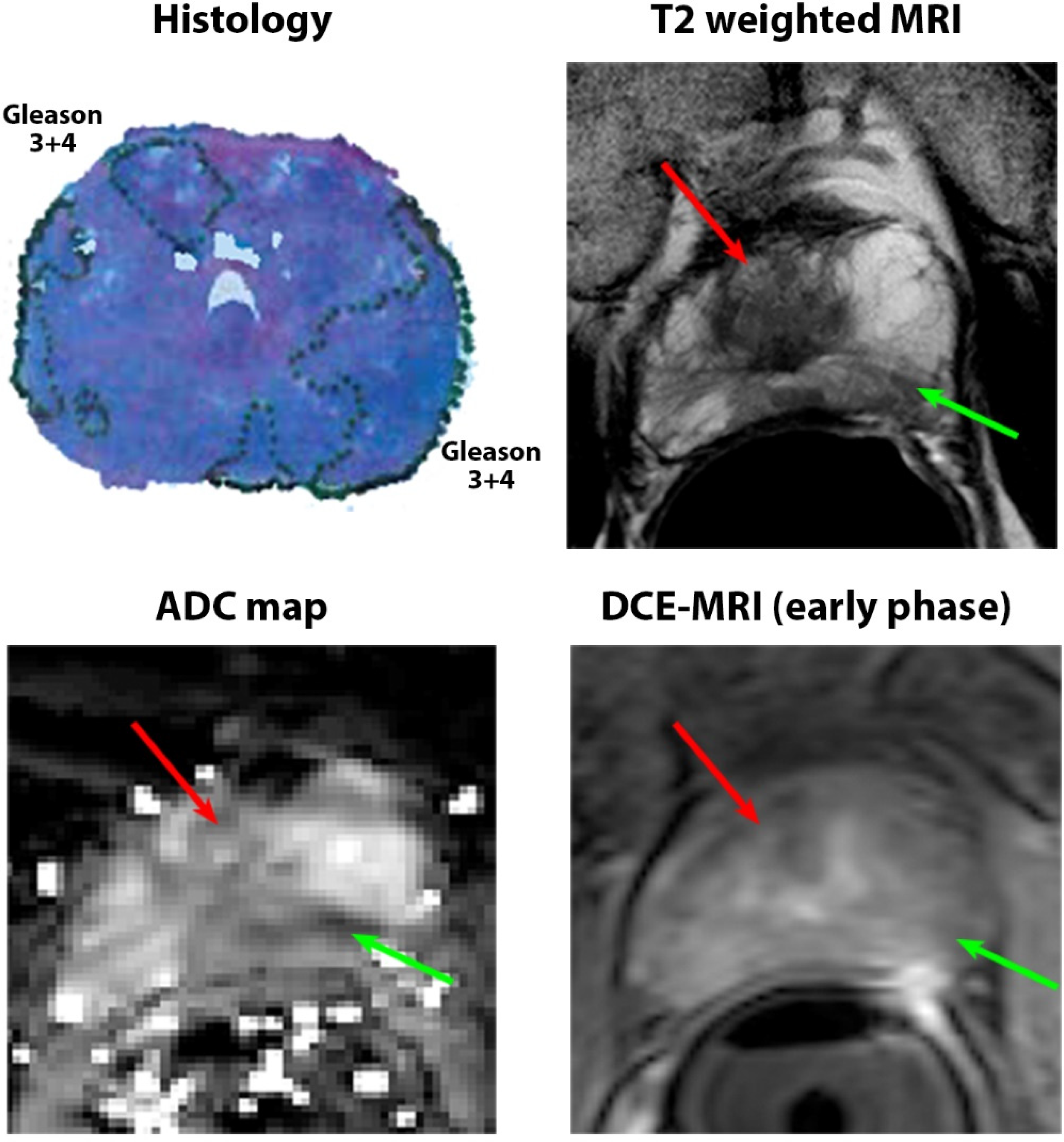
2.5. Pathology Image Analysis
2.6. Statistical Analysis
3. Results
3.1. Participant and Tumor Characteristics
3.2. Quantitative MRI Characteristics
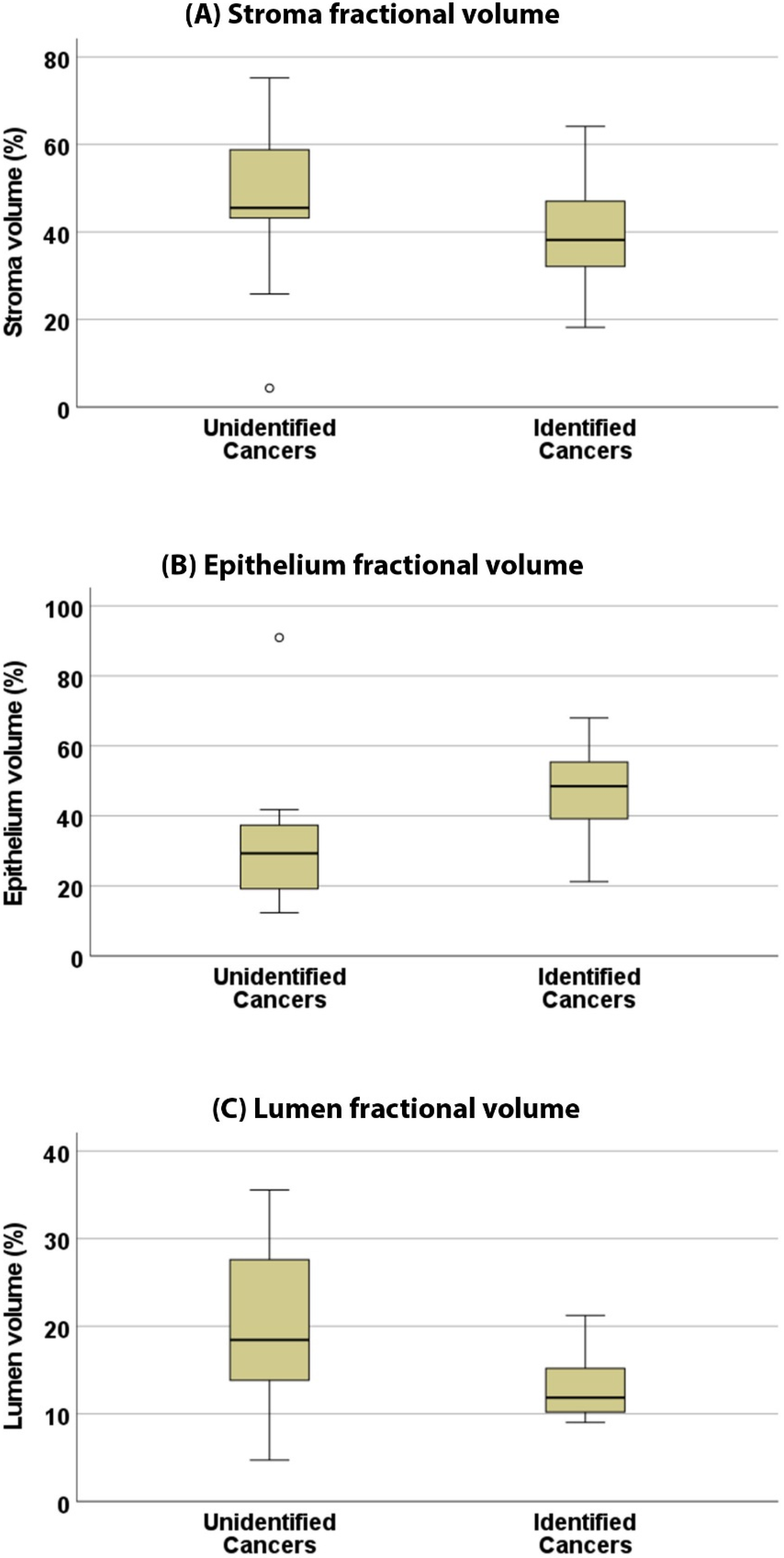
3.3. Pathologic Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Litwin, M.S.; Tan, H. The diagnosis and treatment of prostate cancer: A review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef]
- Panebianco, V.; Barchetti, F.; Sciarra, A.; Ciardi, A.; Indino, E.L.; Papalia, R.; Gallucci, M.; Tombolini, V.; Gentile, V.; Catalano, C. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: A randomized study. Urol. Oncol. 2015, 33, 17.e1–17.e17. [Google Scholar] [CrossRef]
- Woo, S.; Suh, C.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H. Diagnostic Performance of Prostate Imaging Reporting and Data System Version 2 for Detection of Prostate Cancer: A Systematic Review and Diagnostic Meta-analysis. Eur. Urol. 2017, 72, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Isebaert, S.; Van den Bergh, L.; Haustermans, K.; Joniau, S.; Lerut, E.; De Wever, L.; De Keyzer, F.; Budiharto, T.; Slagmolen, P.; Van Poppel, H.; et al. Multiparametric MRI for prostate cancer localization in correlation to whole-mount histopathology. J. Magn. Reson. Imaging 2013, 37, 1392–1401. [Google Scholar] [CrossRef]
- Moldovan, P.C.; Van den Broeck, T.; Sylvester, R.; Marconi, L.; Bellmunt, J.; van den Bergh, R.C.N.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; Fossati, N.; et al. What Is the Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in Excluding Prostate Cancer at Biopsy? A Systematic Review and Meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur. Urol. 2017, 72, 250–266. [Google Scholar] [CrossRef]
- Hansen, N.L.; Barrett, T.; Kesch, C.; Pepdjonovic, L.; Bonekamp, D.; O’Sullivan, R.; Distler, F.; Warren, A.; Samel, C.; Hadaschik, B.; et al. Multicentre evaluation of magnetic resonance imaging supported transperineal prostate biopsy in biopsy-naïve men with suspicion of prostate cancer. BJU Int. 2018, 122, 40–49. [Google Scholar] [CrossRef]
- Mohammadian Bajgiran, A.; Afshari Mirak, S.; Shakeri, S.; Felker, E.R.; Ponzini, D.; Ahuja, P.; Sisk, A.E.; Lu, D.S.; Raman, S.S. Characteristics of missed prostate cancer lesions on 3T multiparametric-MRI in 518 patients: Based on PI-RADSv2 and using whole-mount histopathology reference. Abdom. Radiol. 2019, 44, 1052–1061. [Google Scholar] [CrossRef]
- Borofsky, S.; George, A.K.; Gaur, S.; Bernardo, M.; Greer, M.D.; Mertan, F.V.; Taffel, M.; Moreno, V.; Merino, M.J.; Wood, B.J.; et al. What Are We Missing? False-Negative Cancers at Multiparametric MR Imaging of the Prostate. Radiology 2018, 286, 186–195. [Google Scholar] [CrossRef]
- Fütterer, J.J.; Briganti, A.; De Visschere, P.; Emberton, M.; Giannarini, G.; Kirkham, A.; Taneja, S.S.; Thoeny, H.; Villeirs, G.; Villers, A. Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. Eur. Urol. 2015, 68, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Schouten, M.G.; van der Leest, M.; Pokorny, M.; Hoogenboom, M.; Barentsz, J.O.; Thompson, L.C.; Futterer, J.J. Why and Where do We Miss Significant Prostate Cancer with Multi-parametric Magnetic Resonance Imaging followed by Magnetic Resonance-guided and Transrectal Ultrasound-guided Biopsy in Biopsy-naive Men? Eur. Urol. 2017, 71, 896–903. [Google Scholar] [CrossRef]
- Stabile, A.; Sorce, G.; Barletta, F.; Brembilla, G.; Mazzone, E.; Pellegrino, F.; Cannoletta, D.; Cirulli, G.O.; Gandaglia, G.; De Cobelli, F.; et al. Impact of prostate MRI central review over the diagnostic performance of MRI-targeted biopsy: Should we routinely ask for an expert second opinion? World J. Urol. 2023, 41, 3231–3237. [Google Scholar] [CrossRef] [PubMed]
- Westphalen, A.C.; McCulloch, C.E.; Anaokar, J.M.; Arora, S.; Barashi, N.S.; Barentsz, J.O.; Bathala, T.K.; Bittencourt, L.K.; Booker, M.T.; Braxton, V.G.; et al. Variability of the Positive Predictive Value of PI-RADS for Prostate MRI across 26 Centers: Experience of the Society of Abdominal Radiology Prostate Cancer Disease-focused Panel. Radiology 2020, 296, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.; Margolis, D.J.; Lu, D.Y.; King, K.G.; Huang, J.; Reiter, R.E.; Raman, S.S. Characteristics of Detected and Missed Prostate Cancer Foci on 3-T Multiparametric MRI Using an Endorectal Coil Correlated With Whole-Mount Thin-Section Histopathology. AJR Am. J. Roentgenol. 2015, 205, W87–W92. [Google Scholar] [CrossRef]
- Coker, M.A.; Glaser, Z.A.; Gordetsky, J.B.; Thomas, J.V.; Rais-Bahrami, S. Targets missed: Predictors of MRI-targeted biopsy failing to accurately localize prostate cancer found on systematic biopsy. Prostate Cancer Prostatic Dis. 2018, 21, 549–555. [Google Scholar] [CrossRef]
- Norris, J.M.; Carmona Echeverria, L.M.; Bott, S.R.J.; Brown, L.C.; Burns-Cox, N.; Dudderidge, T.; El-Shater Bosaily, A.; Frangou, E.; Freeman, A.; Ghei, M.; et al. What Type of Prostate Cancer Is Systematically Overlooked by Multiparametric Magnetic Resonance Imaging? An Analysis from the PROMIS Cohort. Eur. Urol. 2020, 78, 163–170. [Google Scholar] [CrossRef]
- Park, K.J.; Kim, M.-h.; Kim, J.K.; Cho, K.-S. Characterization and PI-RADS version 2 assessment of prostate cancers missed by prebiopsy 3-T multiparametric MRI: Correlation with whole-mount thin-section histopathology. Clin. Imaging 2019, 55, 174–180. [Google Scholar] [CrossRef]
- Rosenkrantz, A.B.; Mendrinos, S.; Babb, J.S.; Taneja, S.S. Prostate Cancer Foci Detected on Multiparametric Magnetic Resonance Imaging are Histologically Distinct From Those Not Detected. J. Urol. 2012, 187, 2032–2038. [Google Scholar] [CrossRef]
- van Houdt, P.J.; Ghobadi, G.; Schoots, I.G.; Heijmink, S.W.T.P.J.; de Jong, J.; van der Poel, H.G.; Pos, F.J.; Rylander, S.; Bentzen, L.; Haustermans, K.; et al. Histopathological Features of MRI-Invisible Regions of Prostate Cancer Lesions. J. Magn. Reson. Imaging 2020, 51, 1235–1246. [Google Scholar] [CrossRef]
- Sorce, G.; Stabile, A.; Lucianò, R.; Motterle, G.; Scuderi, S.; Barletta, F.; Pellegrino, F.; Cucchiara, V.; Gandaglia, G.; Fossati, N.; et al. Multiparametric magnetic resonance imaging of the prostate underestimates tumour volume of small visible lesions. BJU Int. 2022, 129, 201–207. [Google Scholar] [CrossRef]
- Sun, C.; Chatterjee, A.; Yousuf, A.; Antic, T.; Eggener, S.; Karczmar, G.S.; Oto, A. Comparison of T2-Weighted Imaging, DWI, and Dynamic Contrast-Enhanced MRI for Calculation of Prostate Cancer Index Lesion Volume: Correlation With Whole-Mount Pathology. Am. J. Roentgenol. 2018, 212, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Watson, G.; Myint, E.; Sved, P.; McEntee, M.; Bourne, R. Changes in Epithelium, Stroma, and Lumen Space Correlate More Strongly with Gleason Pattern and Are Stronger Predictors of Prostate ADC Changes than Cellularity Metrics. Radiology 2015, 277, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Gallan, A.J.; He, D.; Fan, X.; Mustafi, D.; Yousuf, A.; Antic, T.; Karczmar, G.S.; Oto, A. Revisiting quantitative multi-parametric MRI of benign prostatic hyperplasia and its differentiation from transition zone cancer. Abdom. Radiol. 2019, 44, 2233–2243. [Google Scholar] [CrossRef]
- Chatterjee, A.; Tokdemir, S.; Gallan, A.J.; Yousuf, A.; Antic, T.; Karczmar, G.S.; Oto, A. Multiparametric MRI Features and Pathologic Outcome of Wedge-Shaped Lesions in the Peripheral Zone on T2-Weighted Images of the Prostate. Am. J. Roentgenol. 2019, 212, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Turchan, W.T.; Fan, X.; Griffin, A.; Yousuf, A.; Karczmar, G.S.; Liauw, S.L.; Oto, A. Can Pre-treatment Quantitative Multi-parametric MRI Predict the Outcome of Radiotherapy in Patients with Prostate Cancer? Acad. Radiol. 2022, 29, 977–985. [Google Scholar] [CrossRef]
- Fan, X.; Medved, M.; River, J.N.; Zamora, M.; Corot, C.; Robert, P.; Bourrinet, P.; Lipton, M.; Culp, R.M.; Karczmar, G.S. New model for analysis of dynamic contrast-enhanced MRI data distinguishes metastatic from nonmetastatic transplanted rodent prostate tumors. Magn. Reson. Med. 2004, 51, 487–494. [Google Scholar] [CrossRef]
- Langer, D.L.; van der Kwast, T.H.; Evans, A.J.; Sun, L.; Yaffe, M.J.; Trachtenberg, J.; Haider, M.A. Intermixed normal tissue within prostate cancer: Effect on MR imaging measurements of apparent diffusion coefficient and T2—sparse versus dense cancers. Radiology 2008, 249, 900–908. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lin, G.; Hong, J.-H.; Lin, Y.-P.; Chen, F.-H.; Ng, S.-H.; Wang, C.-C. Diffusion radiomics analysis of intratumoral heterogeneity in a murine prostate cancer model following radiotherapy: Pixelwise correlation with histology. J. Magn. Reson. Imaging 2017, 46, 483–489. [Google Scholar] [CrossRef]
- Chatterjee, A.; Oto, A. Future Perspectives in Multiparametric Prostate MR Imaging. Magn. Reson. Imaging Clin. 2019, 27, 117–130. [Google Scholar] [CrossRef]
- Turkbey, B.; Merino, M.J.; Shih, J.H.; Wood, B.J.; Pinto, P.A.; Choyke, P.L.; Shah, V.P.; Pang, Y.; Bernardo, M.; Xu, S.; et al. Is apparent diffusion coefficient associated with clinical risk scores for prostate cancers that are visible on 3-T MR images? Radiology 2011, 258, 488–495. [Google Scholar] [CrossRef]
- Chatterjee, A.; Devaraj, A.; Matthew, M.; Szasz, T.; Antic, T.; Karczmar, G.; Oto, A. Performance of T2 maps in the detection of prostate cancer. Acad. Radiol. 2019, 26, 15–21. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Chatterjee, A.; Fan, X.; Wang, S.; Eggener, S.; Yousuf, A.; Antic, T.; Oto, A.; Karczmar, G.S. Feasibility of Dynamic Contrast-Enhanced Magnetic Resonance Imaging Using Low-Dose Gadolinium: Comparative Performance With Standard Dose in Prostate Cancer Diagnosis. Investig. Radiol. 2018, 53, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Miyai, K.; Mikoshi, A.; Hamabe, F.; Nakanishi, K.; Ito, K.; Tsuda, H.; Shinmoto, H. Histological differences in cancer cells, stroma, and luminal spaces strongly correlate with in vivo MRI-detectability of prostate cancer. Mod. Pathol. 2019, 32, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- Langer, D.L.; van der Kwast, T.H.; Evans, A.J.; Plotkin, A.; Trachtenberg, J.; Wilson, B.C.; Haider, M.A. Prostate tissue composition and MR measurements: Investigating the relationships between ADC, T2, K(trans), v(e), and corresponding histologic features. Radiology 2010, 255, 485–494. [Google Scholar] [CrossRef]
- Chatterjee, A.; Mercado, C.; Bourne, R.M.; Yousuf, A.; Hess, B.; Antic, T.; Eggener, S.; Oto, A.; Karczmar, G.S. Validation of Prostate Tissue Composition by Using Hybrid Multidimensional MRI: Correlation with Histologic Findings. Radiology 2022, 302, 368–377. [Google Scholar] [CrossRef]
- Chatterjee, A.; Bourne, R.M.; Wang, S.; Devaraj, A.; Gallan, A.J.; Antic, T.; Karczmar, G.S.; Oto, A. Diagnosis of Prostate Cancer with Noninvasive Estimation of Prostate Tissue Composition by Using Hybrid Multidimensional MR Imaging: A Feasibility Study. Radiology 2018, 287, 864–873. [Google Scholar] [CrossRef]
- Sabouri, S.; Fazli, L.; Chang, S.D.; Savdie, R.; Jones, E.C.; Goldenberg, S.L.; Black, P.C.; Kozlowski, P. MR measurement of luminal water in prostate gland: Quantitative correlation between MRI and histology. J. Magn. Reson. Imaging 2017, 46, 861–869. [Google Scholar] [CrossRef]
- Bailey, C.; Bourne, R.M.; Siow, B.; Johnston, E.W.; Brizmohun Appayya, M.; Pye, H.; Heavey, S.; Mertzanidou, T.; Whitaker, H.; Freeman, A.; et al. VERDICT MRI validation in fresh and fixed prostate specimens using patient-specific moulds for histological and MR alignment. NMR Biomed. 2019, 32, e4073. [Google Scholar] [CrossRef]
- Sadinski, M.; Karczmar, G.; Peng, Y.; Wang, S.; Jiang, Y.; Medved, M.; Yousuf, A.; Antic, T.; Oto, A. Pilot Study of the Use of Hybrid Multidimensional T2-Weighted Imaging–DWI for the Diagnosis of Prostate Cancer and Evaluation of Gleason Score. Am. J. Roentgenol. 2016, 207, 592–598. [Google Scholar] [CrossRef]
- Oerther, B.; Engel, H.; Nedelcu, A.; Schlett, C.L.; Grimm, R.; von Busch, H.; Sigle, A.; Gratzke, C.; Bamberg, F.; Benndorf, M. Prediction of upgrade to clinically significant prostate cancer in patients under active surveillance: Performance of a fully automated AI-algorithm for lesion detection and classification. Prostate 2023, 83, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Giannini, V.; Mazzetti, S.; Defeudis, A.; Stranieri, G.; Calandri, M.; Bollito, E.; Bosco, M.; Porpiglia, F.; Manfredi, M.; De Pascale, A.; et al. A Fully Automatic Artificial Intelligence System Able to Detect and Characterize Prostate Cancer Using Multiparametric MRI: Multicenter and Multi-Scanner Validation. Front. Oncol. 2021, 11, 718155. [Google Scholar] [CrossRef] [PubMed]
- Hötker, A.M.; Mazaheri, Y.; Aras, Ö.; Zheng, J.; Moskowitz, C.S.; Gondo, T.; Matsumoto, K.; Hricak, H.; Akin, O. Assessment of Prostate Cancer Aggressiveness by Use of the Combination of Quantitative DWI and Dynamic Contrast-Enhanced MRI. AJR Am. J. Roentgenol. 2016, 206, 756–763. [Google Scholar] [CrossRef]
- Chatterjee, A.; Antic, T.; Gallan, A.J.; Paner, G.P.; Lin, L.I.K.; Karczmar, G.S.; Oto, A. Histological validation of prostate tissue composition measurement using hybrid multi-dimensional MRI: Agreement with pathologists’ measures. Abdom. Radiol. 2022, 47, 801–813. [Google Scholar] [CrossRef] [PubMed]


| Imaging Sequence | Pulse Sequence | FOV (mm) | Scan Matrix Size | In Plane Resolution (mm) | TE (ms) | TR (ms) | Slice Thickness (mm) | Flip Angle (°) |
|---|---|---|---|---|---|---|---|---|
| Axial T2W | SE-TSE | 160 × 160 | 400 × 400 | 0.4 × 0.4 | 115 | 8230 | 3 | 90 |
| Multi-echo T2W (T2 mapping) | SE-TSE | 160 × 160 | 212 × 212 | 0.75 × 0.75 | 30, 60, 90, 120, 150, 180, 210, 240, 270 | 7850 | 3 | 90 |
| DWI a | SE-EPI | 180 × 180 | 120 × 120 | 1.5 × 1.5 | 80 | 6093 | 3 | 90 |
| DCE-MRI b | T1-FFE | 250 × 385 | 200 × 308 | 1.25 × 1.25 | 3.3 | 4.8 | 3.5 | 10 |
| Gleason Score | Identified Cancers | Unidentified Cancers | All Cancers |
|---|---|---|---|
| 3 + 3 | 20 | 11 | 31 |
| 3 + 4 | 59 | 8 | 67 |
| 4 + 3 | 13 | - | 13 |
| 4 + 5 | 4 | - | 4 |
| Overall | 96 | 19 | 115 |
| Relative density | |||
| Relative density category | Identified Cancers + | Unidentified Cancers | All Cancers |
| Significantly less glandular density than surrounding benign tissue (Category 1) | 0 | 1 | 1 |
| Somewhat less glandular density than surrounding benign tissue (Category 2) | 0 | 3 | 3 |
| Similar glandular density to surrounding benign tissue (Category 3) | 4 | 3 | 7 |
| Somewhat higher glandular density than surrounding benign tissue (Category 4) | 12 | 9 | 21 |
| Significantly higher glandular density than surrounding benign tissue (Category 5) | 9 | 3 | 12 |
| Overall | 25 | 19 | 44 |
| Absolute density | |||
| Absolute density category (% of cancer tissue) | Identified Cancers + | Unidentified Cancers | All Cancers |
| Highly sparse (0–25%) (Category 1) | 0 | 1 | 1 |
| Sparse (25–50%) (Category 2) | 2 | 7 | 9 |
| Dense (50–75%) (Category 3) | 16 | 7 | 23 |
| Highly dense (75–100%) (Category 4) | 7 | 4 | 11 |
| Overall | 25 | 19 | 44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, A.; Gallan, A.; Fan, X.; Medved, M.; Akurati, P.; Bourne, R.M.; Antic, T.; Karczmar, G.S.; Oto, A. Prostate Cancers Invisible on Multiparametric MRI: Pathologic Features in Correlation with Whole-Mount Prostatectomy. Cancers 2023, 15, 5825. https://doi.org/10.3390/cancers15245825
Chatterjee A, Gallan A, Fan X, Medved M, Akurati P, Bourne RM, Antic T, Karczmar GS, Oto A. Prostate Cancers Invisible on Multiparametric MRI: Pathologic Features in Correlation with Whole-Mount Prostatectomy. Cancers. 2023; 15(24):5825. https://doi.org/10.3390/cancers15245825
Chicago/Turabian StyleChatterjee, Aritrick, Alexander Gallan, Xiaobing Fan, Milica Medved, Pranadeep Akurati, Roger M. Bourne, Tatjana Antic, Gregory S. Karczmar, and Aytekin Oto. 2023. "Prostate Cancers Invisible on Multiparametric MRI: Pathologic Features in Correlation with Whole-Mount Prostatectomy" Cancers 15, no. 24: 5825. https://doi.org/10.3390/cancers15245825
APA StyleChatterjee, A., Gallan, A., Fan, X., Medved, M., Akurati, P., Bourne, R. M., Antic, T., Karczmar, G. S., & Oto, A. (2023). Prostate Cancers Invisible on Multiparametric MRI: Pathologic Features in Correlation with Whole-Mount Prostatectomy. Cancers, 15(24), 5825. https://doi.org/10.3390/cancers15245825








