Is System xc− a Suitable Target for Tumour Detection and Response Assessment with Imaging?
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Redox Status
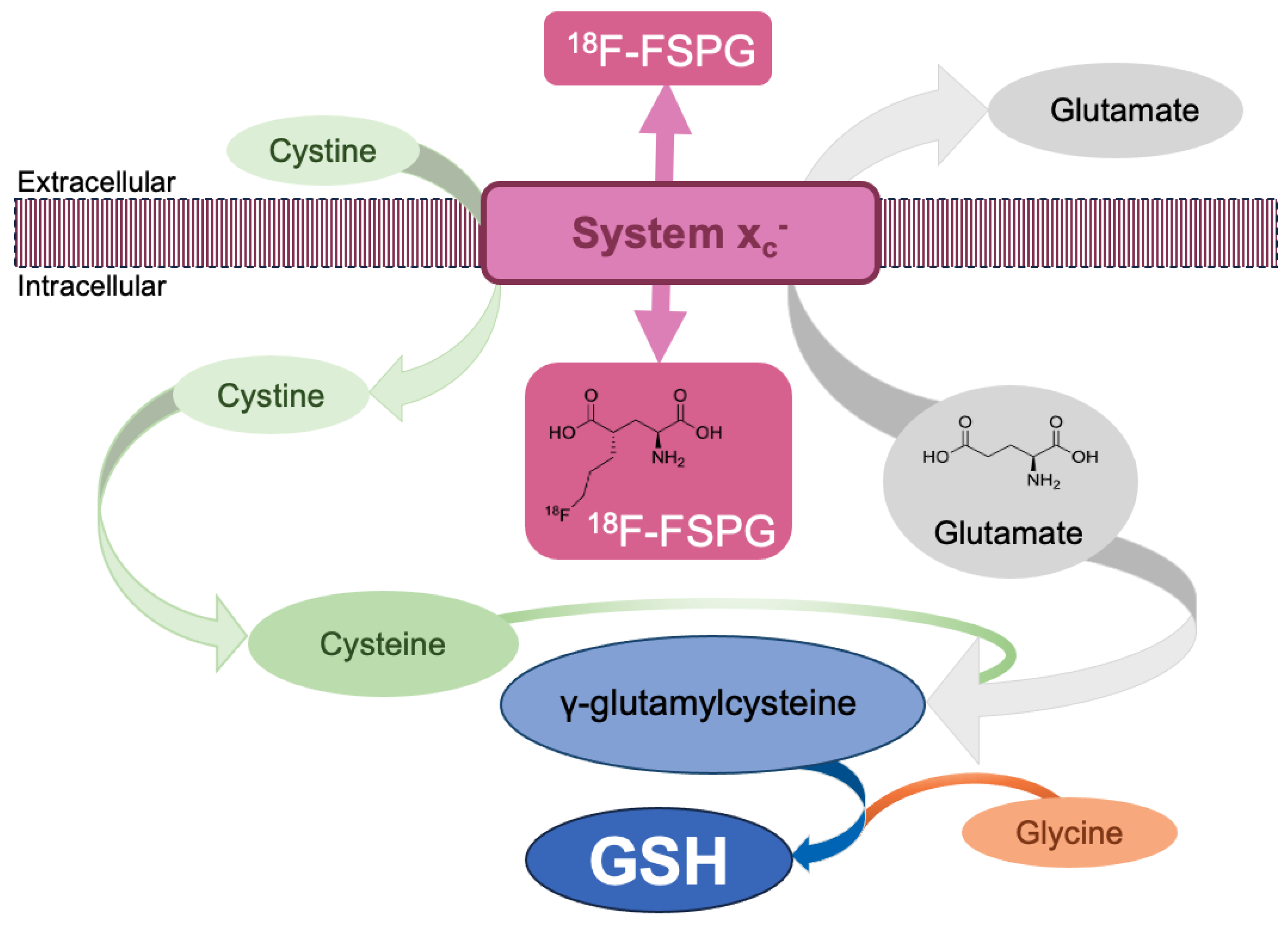
1.2. System xc−
1.3. Radiopharmaceuticals Imaging Oxidative Stress
1.4. Oxidative Stress and Treatment Resistance
1.5. Imaging Response to Therapy
2. Imaging System xc− Activity in Humans
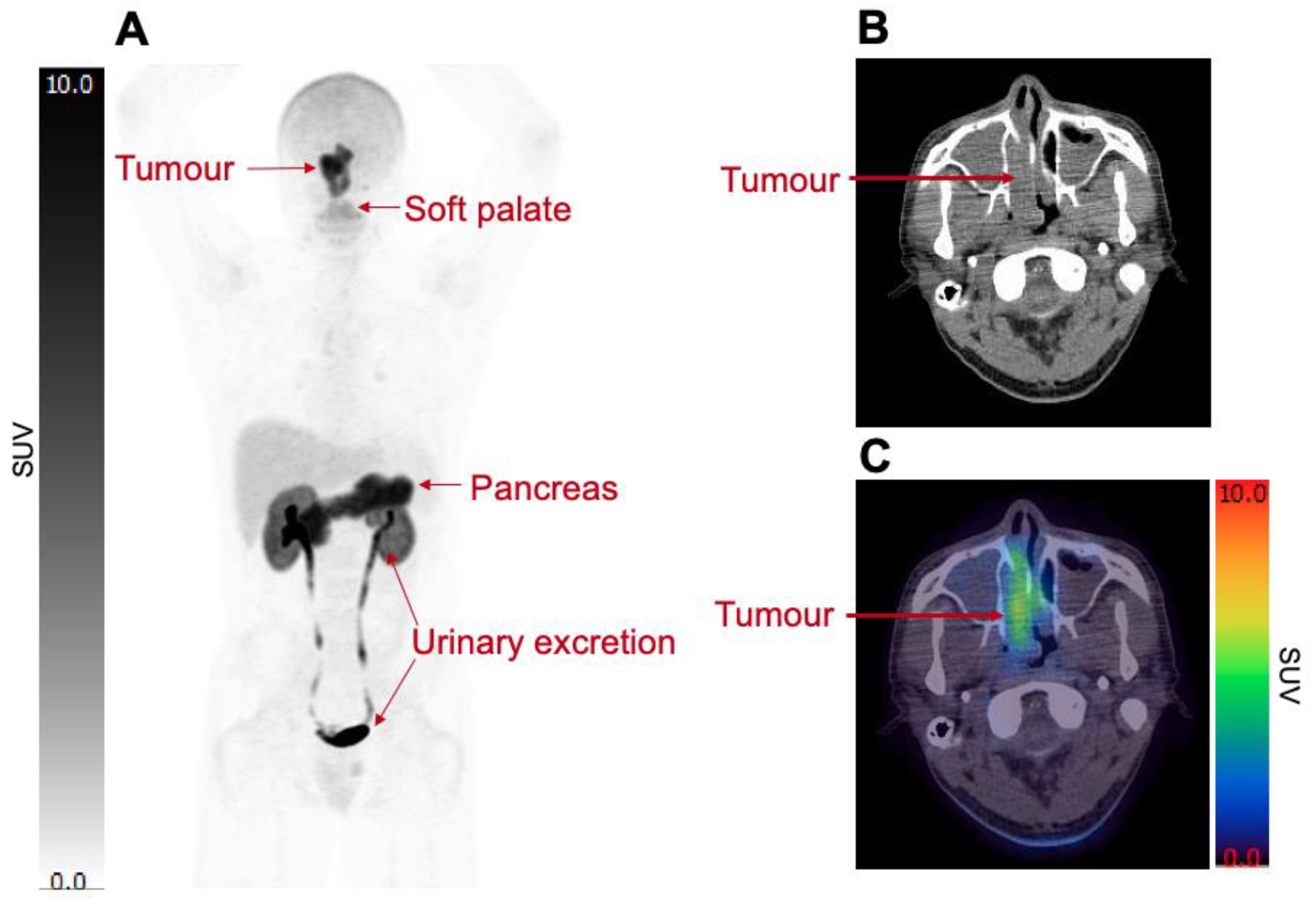
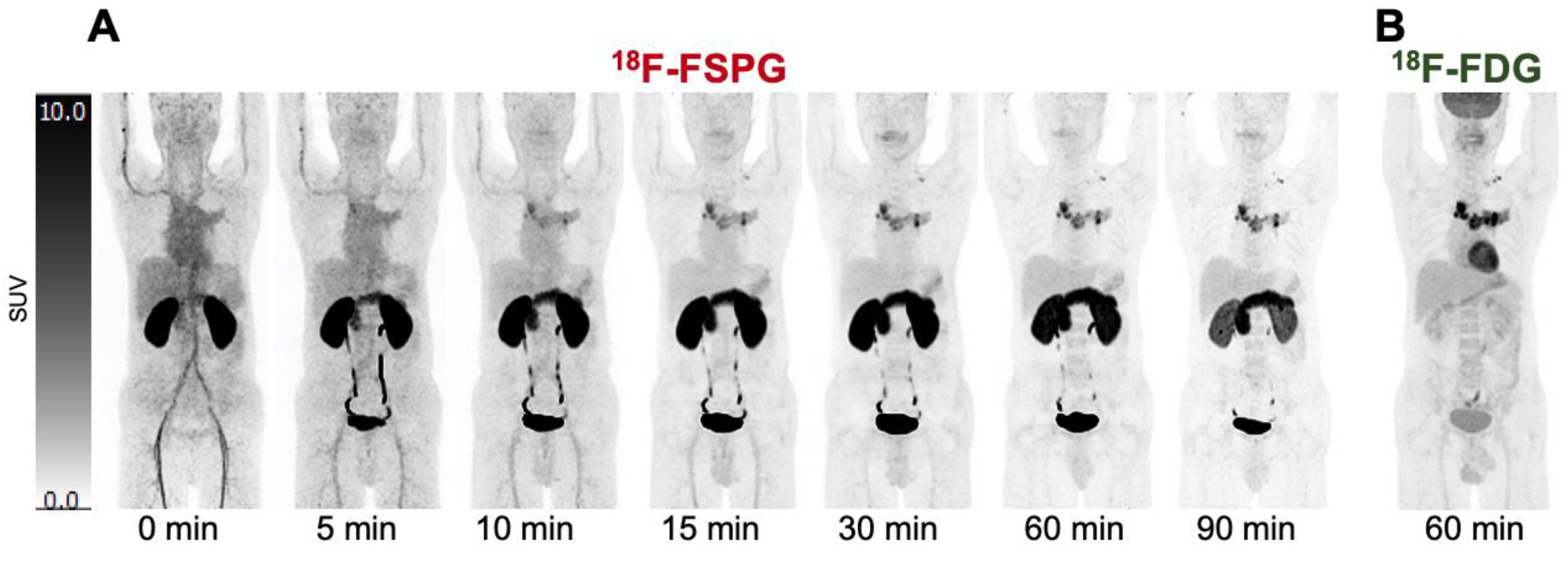
Biodistribution
3. Diagnostic Performance of 18F-FSPG in Cancer Patients
3.1. Comparison of 18F-FSPG PET/CT Imaging with 18F-FDG PET/CT
3.2. Comparison of 18F-FSPG PET/CT with Other Radiotracers Using PET/CT
3.3. Comparison of 18F-FSPG PET/CT with Standard-of-Care MRI
3.4. Histopathology Comparison
3.5. Heterogeneity of 18F-FSPG Retention
3.6. Further Confounds of Imaging Redox Status
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- McCormick, P.N.; Greenwood, H.E.; Glaser, M.; Maddocks, O.D.K.; Gendron, T.; Sander, K.; Gowrishankar, G.; Hoehne, A.; Zhang, T.; Shuhendler, A.J.; et al. Assessment of Tumor Redox Status through (S)-4-(3-[18F]Fluoropropyl)-l-Glutamic Acid Positron Emission Tomography Imaging of System Xc- Activity. Cancer Res. 2018, 79, 853–863. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Valle, N.R.-D.; Huang, P. Redox Regulation of Cell Survival. Antioxid. Redox Sign. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Heiden, M.G.V.; DeBerardinis, R.J. Understanding the Intersections between Metabolism and Cancer Biology. Cell 2017, 168, 657–669. [Google Scholar] [CrossRef]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.L.; Deme, J.C.; Kolokouris, D.; Kuteyi, G.; Biggin, P.C.; Lea, S.M.; Newstead, S. Molecular Basis for Redox Control by the Human Cystine/Glutamate Antiporter System Xc−. Nat. Commun. 2021, 12, 7147. [Google Scholar] [CrossRef] [PubMed]
- Lewerenz, J.; Hewett, S.J.; Huang, Y.; Lambros, M.; Gout, P.W.; Kalivas, P.W.; Massie, A.; Smolders, I.; Methner, A.; Pergande, M.; et al. The Cystine/Glutamate Antiporter System Xc− in Health and Disease: From Molecular Mechanisms to Novel Therapeutic Opportunities. Antioxid. Redox Sign. 2013, 18, 522–555. [Google Scholar] [CrossRef]
- Lim, J.K.M.; Delaidelli, A.; Minaker, S.W.; Zhang, H.-F.; Colovic, M.; Yang, H.; Negri, G.L.; von Karstedt, S.; Lockwood, W.W.; Schaffer, P.; et al. Cystine/Glutamate Antiporter XCT (SLC7A11) Facilitates Oncogenic RAS Transformation by Preserving Intracellular Redox Balance. Proc. Natl. Acad. Sci. USA 2019, 116, 9433–9442. [Google Scholar] [CrossRef]
- Arensman, M.D.; Yang, X.S.; Leahy, D.M.; Toral-Barza, L.; Mileski, M.; Rosfjord, E.C.; Wang, F.; Deng, S.; Myers, J.S.; Abraham, R.T.; et al. Cystine–Glutamate Antiporter XCT Deficiency Suppresses Tumor Growth While Preserving Antitumor Immunity. Proc. Natl. Acad. Sci. USA 2019, 116, 9533–9542. [Google Scholar] [CrossRef]
- Badgley, M.A.; Kremer, D.M.; Maurer, H.C.; DelGiorno, K.E.; Lee, H.-J.; Purohit, V.; Sagalovskiy, I.R.; Ma, A.; Kapilian, J.; Firl, C.E.M.; et al. Cysteine Depletion Induces Pancreatic Tumor Ferroptosis in Mice. Science 2020, 368, 85–89. [Google Scholar] [CrossRef]
- Koglin, N.; Mueller, A.; Berndt, M.; Schmitt-Willich, H.; Toschi, L.; Stephens, A.W.; Gekeler, V.; Friebe, M.; Dinkelborg, L.M. Specific PET Imaging of XC− Transporter Activity Using a 18F-Labeled Glutamate Derivative Reveals a Dominant Pathway in Tumor Metabolism. Clin. Cancer Res. 2011, 17, 6000–6011. [Google Scholar] [CrossRef] [PubMed]
- Mosci, C.; Kumar, M.; Smolarz, K.; Koglin, N.; Stephens, A.W.; Schwaiger, M.; Gambhir, S.S.; Mittra, E.S. Characterization of Physiologic (18)F FSPG Uptake in Healthy Volunteers. Radiology 2016, 279, 898–905. [Google Scholar] [CrossRef][Green Version]
- Baek, S.; Mueller, A.; Lim, Y.-S.; Lee, H.C.; Lee, Y.-J.; Gong, G.; Kim, J.S.; Ryu, J.-S.; Oh, S.J.; Lee, S.J.; et al. (4S)-4-(3-18F-Fluoropropyl)-l-Glutamate for Imaging of XC¯ Transporter Activity in Hepatocellular Carcinoma Using PET: Preclinical and Exploratory Clinical Studies. J. Nucl. Med. 2013, 54, 117–123. [Google Scholar] [CrossRef]
- Park, S.Y.; Mosci, C.; Kumar, M.; Wardak, M.; Koglin, N.; Bullich, S.; Mueller, A.; Berndt, M.; Stephens, A.W.; Chin, F.T.; et al. Initial Evaluation of (4S)-4-(3-[18F]Fluoropropyl)-l-Glutamate (FSPG) PET/CT Imaging in Patients with Head and Neck Cancer, Colorectal Cancer, or Non-Hodgkin Lymphoma. EJNMMI Res. 2020, 10, 100. [Google Scholar] [CrossRef]
- Beinat, C.; Gowrishankar, G.; Shen, B.; Alam, I.S.; Robinson, E.; Haywood, T.; Patel, C.B.; Azevedo, E.C.; Castillo, J.B.; Ilovich, O.; et al. The Characterization of 18F-HGTS13 for Molecular Imaging of XC− Transporter Activity with PET. J. Nucl. Med. 2019, 60, 1812–1817. [Google Scholar] [CrossRef]
- Greenwood, H.E.; Edwards, R.; Koglin, N.; Berndt, M.; Baark, F.; Kim, J.; Firth, G.; Khalil, E.; Mueller, A.; Witney, T.H. Radiotracer Stereochemistry Affects Substrate Affinity and Kinetics for Improved Imaging of System XC− in Tumors. Theranostics 2022, 12, 1921–1936. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.M.; Morton, C.A.; Johnson, B.F.; Yang, H.; Rishel, M.J.; Lee, B.D.; Miao, Q.; Pabba, C.; Yapp, D.T.; Schaffer, P. Functional Imaging of Oxidative Stress with a Novel PET Imaging Agent, 18F-5-Fluoro-l-Aminosuberic Acid. J. Nucl. Med. 2014, 55, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Colovic, M.; Yang, H.; Southcott, L.; Merkens, H.; Colpo, N.; Bénard, F.; Schaffer, P. Comparative Evaluation of [18F]5-Fluoroaminosuberic Acid and (4S)-4-3-[18F]Fluoropropyl)-l-Glutamate as System—Targeting Radiopharmaceuticals. J. Nucl. Med. 2023, 64, 1314–1321. [Google Scholar] [CrossRef]
- Erhola, M.; Kellokumpu-Lehtinen, P.; Metsä-Ketelä, T.; Alanko, K.; Nieminen, M.M. Effects of Anthracyclin-Based Chemotherapy on Total Plasma Antioxidant Capacity in Small Cell Lung Cancer Patients. Free Radical. Bio. Med. 1996, 21, 383–390. [Google Scholar] [CrossRef]
- Sangeetha, P.; Das, U.N.; Koratkar, R.; Suryaprabha, P. Increase in Free Radical Generation and Lipid Peroxidation Following Chemotherapy in Patients with Cancer. Free Radical. Bio. Med. 1990, 8, 15–19. [Google Scholar] [CrossRef]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of Glutathione in Cancer Progression and Chemoresistance. Oxidative Med. Cell. Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef]
- Kennedy, L.; Sandhu, J.K.; Harper, M.-E.; Cuperlovic-Culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-J.; Long, H.-Z.; Zhou, Z.-W.; Luo, H.-Y.; Xu, S.-G.; Gao, L.-C. System XC−/GSH/GPX4 Axis: An Important Antioxidant System for the Ferroptosis in Drug-Resistant Solid Tumor Therapy. Front. Pharmacol. 2022, 13, 910292. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Kaye, S.B. Ovarian Cancer: Strategies for Overcoming Resistance to Chemotherapy. Nat. Rev. Cancer 2003, 3, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Britten, R.A.; Green, J.A.; Warenius, H.M. Cellular Glutathione (GSH) and Glutathione S-Transferase (GST) Activity in Human Ovarian Tumor Biopsies Following Exposure to Alkylating Agents. Int. J. Radiat. Oncol. Biology Phys. 1992, 24, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, H.E.; McCormick, P.N.; Gendron, T.; Glaser, M.; Pereira, R.; Maddocks, O.D.K.; Sander, K.; Zhang, T.; Koglin, N.; Lythgoe, M.F.; et al. Measurement of Tumor Antioxidant Capacity and Prediction of Chemotherapy Resistance in Preclinical Models of Ovarian Cancer by Positron Emission Tomography. Clin. Cancer Res. 2019, 25, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.-W.; Wang, J.; Georgiou, D.K.; Wen, X.; Cohen, A.S.; Geng, L.; Tantawy, M.N.; Manning, H.C. Feasibility of [18F]FSPG PET for Early Response Assessment to Combined Blockade of EGFR and Glutamine Metabolism in Wild-Type KRAS Colorectal Cancer. Tomography 2023, 9, 497–508. [Google Scholar] [CrossRef]
- Smolarz, K.; Krause, B.J.; Graner, F.-P.; Wagner, F.M.; Hultsch, C.; Bacher-Stier, C.; Sparks, R.B.; Ramsay, S.; Fels, L.M.; Dinkelborg, L.M.; et al. (S)-4-(3-18F-Fluoropropyl)-l-Glutamic Acid: An 18F-Labeled Tumor-Specific Probe for PET/CT Imaging—Dosimetry. J. Nucl. Med. 2013, 54, 861–866. [Google Scholar] [CrossRef]
- Bassi, M.; Gasol, E.; Manzoni, M.; Pineda, M.; Riboni, M.; Martín, R.; Zorzano, A.; Borsani, G.; Palacín, M. Identification and Characterisation of Human XCT That Co-Expresses, with 4F2 Heavy Chain, the Amino Acid Transport Activity System XC–. Pflügers Arch. 2001, 442, 286–296. [Google Scholar] [CrossRef]
- Githens, S. Glutathione Metabolism in the Pancreas Compared with That in the Liver, Kidney, and Small Intestine. Int. J. Pancreatol. 1991, 8, 97–109. [Google Scholar] [CrossRef]
- Magarik, M.A.; Walker, R.C.; Gilbert, J.; Manning, H.C.; Massion, P.P. Intracardiac Metastases Detected by 18F-FSPG PET/CT. Clin. Nucl. Med. 2018, 43, 28–30. [Google Scholar] [CrossRef]
- Wardak, M.; Sonni, I.; Fan, A.P.; Minamimoto, R.; Jamali, M.; Hatami, N.; Zaharchuk, G.; Fischbein, N.; Nagpal, S.; Li, G.; et al. 18F-FSPG PET/CT Imaging of System xC− Transporter Activity in Patients with Primary and Metastatic Brain Tumors. Radiology 2022, 303, 620–631. [Google Scholar] [CrossRef]
- Park, S.Y.; Na, S.J.; Kumar, M.; Mosci, C.; Wardak, M.; Koglin, N.; Bullich, S.; Mueller, A.; Berndt, M.; Stephens, A.W.; et al. Clinical Evaluation of (4S)-4-(3-[18F]Fluoropropyl)-l-Glutamate (18F-FSPG) for PET/CT Imaging in Patients with Newly Diagnosed and Recurrent Prostate Cancer. Clin. Cancer Res. 2020, 26, 5380–5387. [Google Scholar] [CrossRef]
- Cheng, M.-F.; Huang, Y.-Y.; Ho, B.-Y.; Kuo, T.-C.; Hsin, L.-W.; Shiue, C.-Y.; Kuo, H.-C.; Jeng, Y.-M.; Yen, R.-F.; Tien, Y.-W. Prospective Comparison of (4S)-4-(3-18F-Fluoropropyl)-l-Glutamate versus 18F-Fluorodeoxyglucose PET/CT for Detecting Metastases from Pancreatic Ductal Adenocarcinoma: A Proof-of-Concept Study. Eur. J. Nucl. Med. Mol. I 2019, 46, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, G.; Williams, J.; Morris, A.S.; Nickels, M.L.; Walker, R.; Koglin, N.; Stephens, A.W.; Washington, M.K.; Geevarghese, S.K.; Liu, Q.; et al. Utility of [18F]FSPG PET to Image Hepatocellular Carcinoma: First Clinical Evaluation in a US Population. Mol. Imaging Biol. 2016, 18, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Mittra, E.S.; Koglin, N.; Mosci, C.; Kumar, M.; Hoehne, A.; Keu, K.V.; Iagaru, A.H.; Mueller, A.; Berndt, M.; Bullich, S.; et al. Pilot Preclinical and Clinical Evaluation of (4S)-4-(3-[18F]Fluoropropyl)-l-Glutamate (18F-FSPG) for PET/CT Imaging of Intracranial Malignancies. PLoS ONE 2016, 11, e0148628. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Choi, C.-M.; Ahn, S.H.; Lee, J.W.; Gong, G.; Ryu, J.-S.; Oh, S.J.; Bacher-Stier, C.; Fels, L.; Koglin, N.; et al. Exploratory Clinical Trial of (4S)-4-(3-[18F]Fluoropropyl)-l-Glutamate for Imaging XC− Transporter Using Positron Emission Tomography in Patients with Non–Small Cell Lung or Breast Cancer. Clin. Cancer Res. 2012, 18, 5427–5437. [Google Scholar] [CrossRef] [PubMed]
- Paez, R.; Shah, C.; Cords, A.J.; Muterspaugh, A.; Helton, J.E.; Antic, S.; Eisenberg, R.; Chen, H.; Grogan, E.L.; Manning, H.C.; et al. 18F-FSPG PET Imaging for the Evaluation of Indeterminate Pulmonary Nodules. PLoS ONE 2022, 17, e0265427. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Combs, C.S.; Brunt, E.M.; Lowe, V.J.; Wolverson, M.K.; Solomon, H.; Collins, B.T.; Bisceglie, A.M.D. Positron Emission Tomography Scanning in the Evaluation of Hepatocellular Carcinoma. J. Hepatol. 2000, 32, 792–797. [Google Scholar] [CrossRef]
- Park, J.-W.; Kim, J.H.; Kim, S.K.; Kang, K.W.; Park, K.W.; Choi, J.-I.; Lee, W.J.; Kim, C.-M.; Nam, B.H. A Prospective Evaluation of 18F-FDG and 11C-Acetate PET/CT for Detection of Primary and Metastatic Hepatocellular Carcinoma. J. Nucl. Med. 2008, 49, 1912–1921. [Google Scholar] [CrossRef]
- Goh, V.; Sarker, D.; Osmany, S.; Cook, G.J.R. Functional Imaging Techniques in Hepatocellular Carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Liefferinge, J.V.; Bentea, E.; Demuyser, T.; Albertini, G.; Follin-Arbelet, V.; Holmseth, S.; Merckx, E.; Sato, H.; Aerts, J.L.; Smolders, I.; et al. Comparative Analysis of Antibodies to XCT (Slc7a11): Forewarned Is Forearmed. J. Comp. Neurol. 2016, 524, 1015–1032. [Google Scholar] [CrossRef] [PubMed]
- Nagano, O.; Okazaki, S.; Saya, H. Redox Regulation in Stem-like Cancer Cells by CD44 Variant Isoforms. Oncogene 2013, 32, 5191–5198. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Tamba, M.; Ishii, T.; Bannai, S. Cloning and Expression of a Plasma Membrane Cystine/Glutamate Exchange Transporter Composed of Two Distinct Proteins*. J. Biol. Chem. 1999, 274, 11455–11458. [Google Scholar] [CrossRef] [PubMed]
- Maia, R.; Santos, G.A.D.; Reis, S.; Viana, N.I.; Pimenta, R.; Guimarães, V.R.; Recuero, S.; Romão, P.; Leite, K.R.M.; Srougi, M.; et al. Can We Use Ki67 Expression to Predict Prostate Cancer Aggressiveness? Rev. Colégio Bras. De Cir. 2022, 49, e20223200. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.G.; Hynes, S.O.; Kerin, M.J.; Miller, N.; Lowery, A.J. Ki-67 as a Prognostic Biomarker in Invasive Breast Cancer. Cancers 2021, 13, 4455. [Google Scholar] [CrossRef]
- Papajík, T.; Mysliveček, M.; Šedová, Z.; Buriánková, E.; Procházka, V.; Koranda, P.; Raida, L.; Kubová, Z.; Palová, M.; Kučerová, L.; et al. Standardised Uptake Value of 18F-FDG on Staging PET/CT in Newly Diagnosed Patients with Different Subtypes of Non-Hodgkin’s Lymphoma. Eur. J. Haematol. 2011, 86, 32–37. [Google Scholar] [CrossRef]
- Chae, S.Y.; Choi, C.-M.; Shim, T.S.; Park, Y.; Park, C.-S.; Lee, H.S.; Lee, S.J.; Oh, S.J.; Kim, S.-Y.; Baek, S.; et al. Exploratory Clinical Investigation of (4S)-4-(3-18F-Fluoropropyl)-l-Glutamate PET of Inflammatory and Infectious Lesions. J. Nucl. Med. 2016, 57, 67–69. [Google Scholar] [CrossRef]
- Martín, A.; Vázquez-Villoldo, N.; Gómez-Vallejo, V.; Padro, D.; Soria, F.N.; Szczupak, B.; Plaza-García, S.; Arrieta, A.; Reese, T.; Llop, J.; et al. In Vivo Imaging of System Xc - as a Novel Approach to Monitor Multiple Sclerosis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1124–1138. [Google Scholar] [CrossRef]
- Domercq, M.; Szczupak, B.; Gejo, J.; Gómez-Vallejo, V.; Padro, D.; Gona, K.B.; Dollé, F.; Higuchi, M.; Matute, C.; Llop, J.; et al. PET Imaging with [18F]FSPG Evidences the Role of System Xc- on Brain Inflammation Following Cerebral Ischemia in Rats. Theranostics 2016, 6, 1753–1767. [Google Scholar] [CrossRef]
- Gurung, S.; Timmermand, O.V.; Perocheau, D.; Gil-Martinez, A.L.; Minnion, M.; Touramanidou, L.; Fang, S.; Messina, M.; Khalil, Y.; Barber, A.; et al. mRNA therapy restores ureagenesis and corrects glutathione metabolism in argininosuccinic aciduria. bioRxiv 2022. bioRxiv:2022.10.19.512931. [Google Scholar] [CrossRef]
- Gammon, S.T.; Engel, B.J.; Gores, G.J.; Cressman, E.; Piwnica-Worms, D.; Millward, S.W. Mistiming Death: Modeling the Time-Domain Variability of Tumor Apoptosis and Implications for Molecular Imaging of Cell Death. Mol. Imaging Biol. 2020, 22, 1310–1323. [Google Scholar] [CrossRef] [PubMed]
- Belhocine, T.Z.; Blankenberg, F.G.; Kartachova, M.S.; Stitt, L.W.; Vanderheyden, J.-L.; Hoebers, F.J.P.; de Wiele, C.V. (99m)Tc-Annexin A5 Quantification of Apoptotic Tumor Response: A Systematic Review and Meta-Analysis of Clinical Imaging Trials. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 2083–2097. [Google Scholar] [CrossRef] [PubMed]

| Reference | Patients | Year | Location | Comparison Imaging | 18F-FSPG Primary Tumour-to- Background Ratio * | Histo- Pathology |
|---|---|---|---|---|---|---|
| Wardak M. et al. [32] | A total of 26 intracranial malignancies: 17 primary; 7 metastases. | 2022 | USA | MRI (18F-FDG PET/CT in 4) | 26.6 ± 24.9 | xCT in 19/26 |
| Park S. Y. et al. [33] | A total of 20 prostate cancers: 10 primary; 10 recurrent. | 2020 | USA | Up to five lesions per patient were selected and measured on MRI, CT, or bone scan. | 2.0 ± 0.5 | xCT and CD44 |
| Park S. Y. et al. [14] | A total of 15 patients: 5 head and neck; 5 Non-Hodgkin’s lymphoma; 5 Colorectal. | 2020 | USA | 18F-FDG PET/CT | Head and neck: 2.8 ± 2.1 Non-Hodgkin’s lymphoma: 1.9 ± 3.1 Colorectal: 5.4 ± 1.3 | No histopathology reference standard. |
| Cheng M. -F. et al. [34] | A total of 23 patients: all pancreatic adenocarcinoma. | 2019 | Taiwan | 18F-FDG PET/CT | 4.2 ± 4.3 | xCT expression (in 6/23). |
| Magarik M. A. et al. [31] | Single-case study: NSCLC with mediastinal and intracranial metastases. | 2018 | USA | 18F-FDG PET/CT | Not described | No histopathology reference standard. |
| Kavanaugh G. et al. [35] | A total of 11 patients with HCC. | 2016 | USA | Standard of care: MRI, CT, and 11C-acetate PET/CT | Not described | No histopathology reference standard for the 11 patients. Comparison made to tissue from cancer genome analysis (xc− transporter RNA and protein levels). |
| Mittra E. S. et al. [36] | A total of 5 patients: 2 primary brain tumours; 3 NSCLC with brain metastasis. | 2016 | USA | 18F-FDG PET/CT | Primary: 24.0 Metastases 50.0 (background: normal brain). | No histopathology reference standard. |
| Baek S. et al. [13] | A total of 5 patients with HCC. | 2013 | South Korea | 18F-FDG PET/CT | Not described | xCT and CD44 in 4/5 |
| Baek S. et al. [37] | A total of 15 patients: 10 lung cancer; 5 breast cancer. | 2012 | South Korea | 18F-FDG PET/CT | Lung: 6.7 ± 5.8 Breast: 3.7 ± 4.5 (background: blood pool) | xCT and CD44-specific antibody |
| Paez R. et al. [38] | A total of 26 patients with indeterminate pulmonary nodules. | 2022 | USA | 18F-FDG PET/CT | Not described | xCT and CD44 expression levels |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharkey, A.R.; Witney, T.H.; Cook, G.J.R. Is System xc− a Suitable Target for Tumour Detection and Response Assessment with Imaging? Cancers 2023, 15, 5573. https://doi.org/10.3390/cancers15235573
Sharkey AR, Witney TH, Cook GJR. Is System xc− a Suitable Target for Tumour Detection and Response Assessment with Imaging? Cancers. 2023; 15(23):5573. https://doi.org/10.3390/cancers15235573
Chicago/Turabian StyleSharkey, Amy R., Timothy H. Witney, and Gary J. R. Cook. 2023. "Is System xc− a Suitable Target for Tumour Detection and Response Assessment with Imaging?" Cancers 15, no. 23: 5573. https://doi.org/10.3390/cancers15235573
APA StyleSharkey, A. R., Witney, T. H., & Cook, G. J. R. (2023). Is System xc− a Suitable Target for Tumour Detection and Response Assessment with Imaging? Cancers, 15(23), 5573. https://doi.org/10.3390/cancers15235573







