Thymic Epithelial Tumor and Immune System: The Role of Immunotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
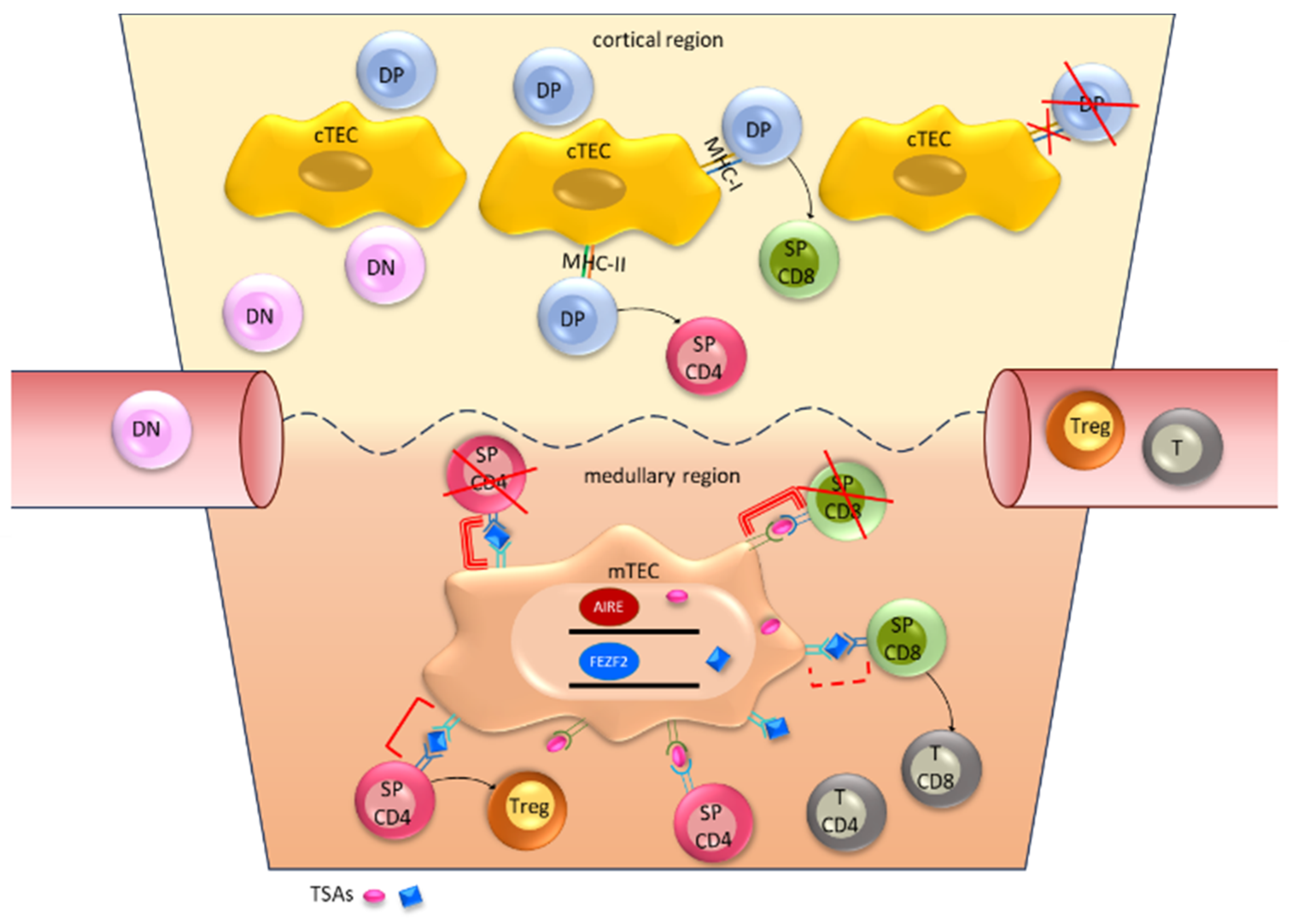
3. Immune System and Thymus
4. Autoimmune Diseases and TETs
5. Role of Immunotherapy
5.1. Immune Checkpoint Inhibitors
5.2. Cancer Vaccines
5.3. Immunomodulatory Agents
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Engels, E.A. Epidemiology of Thymoma and Associated Malignancies. J. Thorac. Oncol. 2010, 5, S260–S265. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.; Tian, W.X.; Wu, F.J.; Liu, Y.X.; Wu, J.Y.; Sun, Y.G.; Yu, H.B.; Huang, C.; Wu, Q.J.; Ma, C.; et al. Postoperative Clinical Outcomes of Patients with Thymic Epithelial Tumors after Over-3-Year Follow-up at a Single-Center. J. Cardiothorac. Surg. 2023, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Weis, C.A.; Yao, X.; Deng, Y.; Detterbeck, F.C.; Marino, M.; Nicholson, A.G.; Huang, J.; Ströbel, P.; Antonicelli, A.; Marx, A. The Impact of Thymoma Histotype on Prognosis in a Worldwide Database. J. Thorac. Oncol. 2015, 10, 367–372. [Google Scholar] [CrossRef]
- Margaritora, S.; Cesario, A.; Cusumano, G.; Meacci, E.; D’Angelillo, R.; Bonassi, S.; Carnassale, G.; Porziella, V.; Tessitore, A.; Vita, M.L.; et al. Thirty-Five-Year Follow-Up Analysis of Clinical and Pathologic Outcomes of Thymoma Surgery. Ann. Thorac. Surg. 2010, 89, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Y.; Gao, S.G.; Mu, J.W.; Xue, Q.; Mao, Y.S.; Wang, D.L.; Zhao, J.; Gao, Y.S.; Huang, J.F.; He, J. Long-Term Outcomes of 307 Patients after Complete Thymoma Resection. Chin. J. Cancer 2017, 36, 46. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Sun, Y.; Wu, Q.; Jiao, P.; Ma, C.; Yu, H.; Huang, C.; Tong, H. Surgical Outcomes of 215 Patients with Thymic Epithelial Tumors: A Single-Center Experience. Thorac. Cancer 2020, 11, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Carillo, C.; Diso, D.; Mantovani, S.; Pecoraro, Y.; De Giacomo, T.; Ciccone, A.M.; Poggi, C.; Longo, F.; Cassese, R.; Tombolini, V.; et al. Multimodality Treatment of Stage II Thymic Tumours. J. Thorac. Dis. 2017, 9, 2369–2374. [Google Scholar] [CrossRef]
- Imbimbo, M.; Ottaviano, M.; Vitali, M.; Fabbri, A.; Leuzzi, G.; Fiore, M.; Franceschini, D.; Pasello, G.; Perrino, M.; Schiavon, M.; et al. Best Practices for the Management of Thymic Epithelial Tumors: A Position Paper by the Italian Collaborative Group for Thymic Malignancies (TYME). Cancer Treat. Rev. 2018, 71, 76–87. [Google Scholar] [CrossRef]
- Chau, N.G.; Kim, E.S.; Wistuba, I. The Multidisciplinary Approach to Thymoma: Combining Molecular and Clinical Approaches. J. Thorac. Oncol. 2010, 5, S313–S317. [Google Scholar] [CrossRef]
- Girard, N.; Ruffini, E.; Marx, A.; Faivre-Finn, C.; Peters, S. Thymic Epithelial Tumours: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2015, 26, v40–v55. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network Thymomas and Thymic Carcinomas. Available online: https://www.nccn.org/professionals/physician_gls/pdf/thymic.pdf (accessed on 18 September 2023).
- Sandri, A.; Cusumano, G.; Lococo, F.; Alifano, M.; Granone, P.; Margaritora, S.; Cesario, A.; Oliaro, A.; Filosso, P.; Regnard, J.F.; et al. Long-Term Results after Treatment for Recurrent Thymoma a Multicenter Analysis. J. Thorac. Oncol. 2014, 9, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lee, C.Y.; Kim, E.Y.; Lee, C.G.; Hong, M.H.; Park, B.J.; Yoon, H.I.; Kim, K.H.; Lee, S.H.; Byun, H.K.; et al. Clinical Outcomes of Thymic Carcinoma: The Role of Radiotherapy Combined with Multimodal Treatments. Cancers 2023, 15, 2262. [Google Scholar] [CrossRef] [PubMed]
- Scorsetti, M.; Leo, F.; Trama, A.; D’Angelillo, R.; Serpico, D.; Macerelli, M.; Zucali, P.; Gatta, G.; Garassino, M.C. Thymoma and Thymic Carcinomas. Crit. Rev. Oncol. Hematol. 2016, 99, 332–350. [Google Scholar] [CrossRef]
- Okuma, Y.; Saito, M.; Hosomi, Y.; Sakuyama, T.; Okamura, T. Key Components of Chemotherapy for Thymic Malignancies: A Systematic Review and Pooled Analysis for Anthracycline-, Carboplatin- or Cisplatin-Based Chemotherapy. J. Cancer Res. Clin. Oncol. 2015, 141, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Zucali, P.A.; de Vincenzo, F.; Perrino, M.; Digiacomo, N.; Cordua, N.; D’Antonio, F.; Borea, F.; Santoro, A. Systemic Treatments for Thymic Tumors: A Narrative Review. Mediastinum 2021, 5, 24. [Google Scholar] [CrossRef]
- Thomas, A.; Rajan, A.; Berman, A.; Tomita, Y.; Brzezniak, C.; Lee, M.J.; Lee, S.; Ling, A.; Spittler, A.J.; Carter, C.A.; et al. Sunitinib in Patients with Chemotherapy-Refractory Thymoma and Thymic Carcinoma: An Open-Label Phase 2 Trial. Lancet Oncol. 2015, 16, 177–186. [Google Scholar] [CrossRef]
- Remon, J.; Girard, N.; Mazieres, J.; Dansin, E.; Pichon, E.; Grellier, L.; Dubos, C.; Lindsay, C.R.; Besse, B. Sunitinib in Patients with Advanced Thymic Malignancies: Cohort from the French RYTHMIC Network. Lung Cancer 2016, 97, 99–104. [Google Scholar] [CrossRef]
- Sato, J.; Satouchi, M.; Itoh, S.; Okuma, Y.; Niho, S.; Mizugaki, H.; Murakami, H.; Fujisaka, Y.; Kozuki, T.; Nakamura, K.; et al. Lenvatinib in Patients with Advanced or Metastatic Thymic Carcinoma (REMORA): A Multicentre, Phase 2 Trial. Lancet Oncol. 2020, 21, 843–850. [Google Scholar] [CrossRef]
- Giaccone, G.; Rajan, A.; Ruijter, R.; Smit, E.; Van Groeningen, C.; Hogendoorn, P.C.W. Imatinib Mesylate in Patients with WHO B3 Thymomas and Thymic Carcinomas. J. Thorac. Oncol. 2009, 4, 1270–1273. [Google Scholar] [CrossRef]
- Zucali, P.A.; De Pas, T.; Palmieri, G.; Favaretto, A.; Chella, A.; Tiseo, M.; Caruso, M.; Simonelli, M.; Perrino, M.; De Vincenzo, F.; et al. Phase II Study of Everolimus in Patients with Thymoma and Thymic Carcinoma Previously Treated with Cisplatin-Based Chemotherapy. J. Clin. Oncol. 2018, 36, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Giaccone, G.; Kim, C.; Thompson, J.; McGuire, C.; Kallakury, B.; Chahine, J.J.; Manning, M.; Mogg, R.; Blumenschein, W.M.; Tan, M.T.; et al. Pembrolizumab in Patients with Thymic Carcinoma: A Single-Arm, Single-Centre, Phase 2 Study. Lancet Oncol. 2018, 19, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Kim, H.S.; Ku, B.M.; Choi, Y.L.; Cristescu, R.; Han, J.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Park, K.; et al. Pembrolizumab for Patients with Refractory or Relapsed Thymic Epithelial Tumor: An Open-Label Phase II Trial. J. Clin. Oncol. 2019, 37, 2162–2170. [Google Scholar] [CrossRef]
- Benitez, J.C.; Besse, B. Narrative Review of Immunotherapy in Thymic Malignancies. Transl. Lung Cancer Res. 2021, 10, 3001–3013. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zúñiga-Pflücker, J.C. A 2020 View of Thymus Stromal Cells in T Cell Development. J. Immunol. 2021, 206, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Alexandropoulos, K.; Danzl, N.M. Thymic Epithelial Cells: Antigen Presenting Cells That Regulate T Cell Repertoire and Tolerance Development. Immunol. Res. 2012, 54, 177–190. [Google Scholar] [CrossRef]
- Wang, H.X.; Pan, W.; Zheng, L.; Zhong, X.P.; Tan, L.; Liang, Z.; He, J.; Feng, P.; Zhao, Y.; Qiu, Y.R. Thymic Epithelial Cells Contribute to Thymopoiesis and T Cell Development. Front. Immunol. 2020, 10, 3099. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, H.; Rothenberg, E.V. How Transcription Factors Drive Choice of the T Cell Fate. Nat. Rev. Immunol. 2021, 21, 162–176. [Google Scholar] [CrossRef]
- Quiocho, F. A Current Topics in Microbiology and Immunology—Thymic Development; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9783642402517. [Google Scholar]
- Thapa, P.; Farber, D.L. The Role of the Thymus in the Immune Response. Thorac. Surg. Clin. 2019, 29, 123–131. [Google Scholar] [CrossRef]
- Takada, K.; Takahama, Y. Positive-Selection-Inducing Self-Peptides Displayed by Cortical Thymic Epithelial Cells, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 125. [Google Scholar]
- Gascoigne, N.R.J.; Rybakin, V.; Acuto, O.; Brzostek, J. TCR Signal Strength and T Cell Development. Annu. Rev. Cell Dev. Biol. 2016, 32, 327–348. [Google Scholar] [CrossRef]
- Egawa, T. Regulation of CD4 and CD8 Coreceptor Expression and CD4 versus CD8 Lineage Decisions, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 125. [Google Scholar]
- Ashby, K.M.; Hogquist, K.A. A Guide to Thymic Selection of T Cells. Nat. Rev. Immunol. 2023, 23, 697. [Google Scholar] [CrossRef]
- Hu, Z.; Lancaster, J.N.; Sasiponganan, C.; Ehrlich, L.I.R. CCR4 Promotes Medullary Entry and Thymocyte-Dendritic Cell Interactions Required for Central Tolerance. J. Exp. Med. 2015, 212, 1947–1965. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tipan, P.G.; Selden, H.J.; Srinivasan, J.; Hale, L.P.; Ehrlich, L.I.R. CCR4 and CCR7 Differentially Regulate Thymocyte Localization with Distinct Outcomes for Central Tolerance. eLife 2023, 12, e80443. [Google Scholar] [CrossRef]
- Anderson, M.S.; Su, M.A. Aire and T Cell Development. Curr. Opin. Immunol. 2011, 23, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Passos, G.A.; Speck-Hernandez, C.A.; Assis, A.F.; Mendes-da-Cruz, D.A. Update on Aire and Thymic Negative Selection. Immunology 2018, 153, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Takaba, H.; Morishita, Y.; Tomofuji, Y.; Danks, L.; Nitta, T.; Komatsu, N.; Kodama, T.; Takayanagi, H. Fezf2 Orchestrates a Thymic Program of Self-Antigen Expression for Immune Tolerance. Cell 2015, 163, 975–987. [Google Scholar] [CrossRef]
- Takaba, H.; Takayanagi, H. The Mechanisms of T Cell Selection in the Thymus. Trends Immunol. 2017, 38, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.; Robey, E.A.; Hsieh, C.S. Central CD4 + T Cell Tolerance: Deletion versus Regulatory T Cell Differentiation. Nat. Rev. Immunol. 2019, 19, 7–18. [Google Scholar] [CrossRef]
- Blum, T.G.; Misch, D.; Kollmeier, J.; Thiel, S.; Bauer, T.T. Autoimmune Disorders and Paraneoplastic Syndromes in Thymoma. J. Thorac. Dis. 2020, 12, 7571–7590. [Google Scholar] [CrossRef]
- Padda, S.K.; Yao, X.; Antonicelli, A.; Riess, J.W.; Shang, Y.; Shrager, J.B.; Korst, R.; Detterbeck, F.; Huang, J.; Burt, B.M.; et al. Paraneoplastic Syndromes and Thymic Malignancies: An Examination of the International Thymic Malignancy Interest Group Retrospective Database. J. Thorac. Oncol. 2018, 13, 436–446. [Google Scholar] [CrossRef]
- Marx, A.; Willcox, N.; Leite, M.I.; Chuang, W.Y.; Schalke, B.; Nix, W.; Strbel, P. Thymoma and Paraneoplastic Myasthenia Gravis. Autoimmunity 2010, 43, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Velasco, R.; Gutiérrez-Gutiérrez, G.; Trujillo, J.C.; Martínez, E.; Segovia, S.; Arribas-Velasco, M.; Fernández, G.; Paradas, C.; Vélez-Gómez, B.; Casasnovas, C.; et al. Clinical Characteristics and Outcomes of Thymoma-Associated Myasthenia Gravis. Eur. J. Neurol. 2021, 28, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y. Thymus, Thymoma and Myasthenia Gravis. Surg. Today 2013, 43, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Hellyer, J.; Ouseph, M.M.; Wakelee, H.A.; Padda, S.K. Autoimmune Disease in Patients with Advanced Thymic Epithelial Tumors. JTO Clin. Res. Rep. 2022, 3, 100323. [Google Scholar] [CrossRef]
- Bernard, C.; Frih, H.; Pasquet, F.; Kerever, S.; Jamilloux, Y.; Tronc, F.; Guibert, B.; Isaac, S.; Devouassoux, M.; Chalabreysse, L.; et al. Thymoma Associated with Autoimmune Diseases: 85 Cases and Literature Review. Autoimmun. Rev. 2016, 15, 82–92. [Google Scholar] [CrossRef]
- Chang, R.; Duan, S.; Li, S.; Zhang, P. Viral Infection in Thymoma and Thymic Tumors with Autoimmune Diseases. Thorac. Cancer 2021, 12, 2971–2980. [Google Scholar] [CrossRef] [PubMed]
- Means, R.T. Pure Red Cell Aplasia. Blood 2016, 128, 2504–2509. [Google Scholar] [CrossRef]
- Ballman, M.; Zhao, C.; McAdams, M.J.; Rajan, A. Immunotherapy for Management of Thymic Epithelial Tumors: A Double-Edged Sword. Cancers 2022, 14, 2060. [Google Scholar] [CrossRef]
- Zhao, J.; Bhatnagar, V.; Ding, L.; Atay, S.M.; David, E.A.; McFadden, P.M.; Stamnes, S.; Lechtholz-Zey, E.; Wightman, S.C.; Detterbeck, F.C.; et al. A Systematic Review of Paraneoplastic Syndromes Associated with Thymoma: Treatment Modalities, Recurrence, and Outcomes in Resected Cases. J. Thorac. Cardiovasc. Surg. 2020, 160, 306–314. [Google Scholar] [CrossRef]
- Benitez, J.C.; Boucher, M.E.; Dansin, E.; Kerjouan, M.; Mazieres, J.; Pichon, E.; Thillays, F.; Falcoz, P.-E.; Roch, B.; Oulkhouir, Y.; et al. Prevalence of Autoimmune Diseases in Thymic Epithelial Tumors (TET) Insights from RYTHMIC. J. Clin. Oncol. 2020, 38, 9073. [Google Scholar] [CrossRef]
- Lippner, E.A.; Lewis, D.B.; Robinson, W.H.; Katsumoto, T.R. Paraneoplastic and Therapy-Related Immune Complications in Thymic Malignancies. Curr. Treat. Options Oncol. 2019, 20, 62. [Google Scholar] [CrossRef]
- Marx, A.; Pfister, F.; Schalke, B.; Saruhan-Direskeneli, G.; Melms, A.; Ströbel, P. The Different Roles of the Thymus in the Pathogenesis of the Various Myasthenia Gravis Subtypes. Autoimmun. Rev. 2013, 12, 875–884. [Google Scholar] [CrossRef]
- Shelly, S.; Agmon-Levin, N.; Altman, A.; Shoenfeld, Y. Thymoma and Autoimmunity. Cell. Mol. Immunol. 2011, 8, 199–202. [Google Scholar] [CrossRef]
- Filosso, P.L.; Evangelista, A.; Ruffini, E.; Rendina, E.A.; Margaritora, S.; Novellis, P.; Rena, O.; Casadio, C.; Andreetti, C.; Guerrera, F.; et al. Does Myasthenia Gravis Influence Overall Survival and Cumulative Incidence of Recurrence in Thymoma Patients? A Retrospective Clinicopathological Multicentre Analysis on 797 Patients. Lung Cancer 2015, 88, 338–343. [Google Scholar] [CrossRef]
- Weissferdt, A.; Fujimoto, J.; Kalhor, N.; Rodriguez, J.; Bassett, R.; Wistuba, I.I.; Moran, C.A. Expression of PD-1 and PD-L1 in Thymic Epithelial Neoplasms. Mod. Pathol. 2017, 30, 826–833. [Google Scholar] [CrossRef]
- Katsuya, Y.; Fujita, Y.; Horinouchi, H.; Ohe, Y.; Watanabe, S.i.; Tsuta, K. Immunohistochemical Status of PD-L1 in Thymoma and Thymic Carcinoma. Lung Cancer 2015, 88, 154–159. [Google Scholar] [CrossRef]
- Owen, D.; Chu, B.; Lehman, A.M.; Annamalai, L.; Yearley, J.H.; Shilo, K.; Otterson, G.A. Expression Patterns, Prognostic Value, and Intratumoral Heterogeneity of PD-L1 and PD-1 in Thymoma and Thymic Carcinoma. J. Thorac. Oncol. 2018, 13, 1204–1212. [Google Scholar] [CrossRef]
- Giaccone, G.; Kim, C. Durable Response in Patients with Thymic Carcinoma Treated With Pembrolizumab After Prolonged Follow-Up. J. Thorac. Oncol. 2021, 16, 483–485. [Google Scholar] [CrossRef]
- Katsuya, Y.; Horinouchi, H.; Seto, T.; Umemura, S.; Hosomi, Y.; Satouchi, M.; Nishio, M.; Kozuki, T.; Hida, T.; Sukigara, T.; et al. Single-Arm, Multicentre, Phase II Trial of Nivolumab for Unresectable or Recurrent Thymic Carcinoma: PRIMER Study. Eur. J. Cancer 2019, 113, 78–86. [Google Scholar] [CrossRef]
- Girard, N.; Ponce Aix, S.; Cedres, S.; Berghmans, T.; Burgers, S.; Toffart, A.C.; Popat, S.; Janssens, A.; Gervais, R.; Hochstenbag, M.; et al. Efficacy and Safety of Nivolumab for Patients with Pre-Treated Type B3 Thymoma and Thymic Carcinoma: Results from the EORTC-ETOP NIVOTHYM Phase II Trial. ESMO Open 2023, 8, 101576. [Google Scholar] [CrossRef]
- Rajan, A.; Heery, C.R.; Thomas, A.; Mammen, A.L.; Perry, S.; O’Sullivan Coyne, G.; Guha, U.; Berman, A.; Szabo, E.; Madan, R.A.; et al. Efficacy and Tolerability of Anti-Programmed Death-Ligand 1 (PD-L1) Antibody (Avelumab) Treatment in Advanced Thymoma. J. Immunother. Cancer 2019, 7, 269. [Google Scholar] [CrossRef]
- Tabernero, J.; Andre, F.; Blay, J.Y.; Bustillos, A.; Fear, S.; Ganta, S.; Jaeger, D.; Maio, M.; Mileshkin, L.; Melero, I. Phase II Multicohort Study of Atezolizumab Monotherapy in Multiple Advanced Solid Cancers. ESMO Open 2022, 7, 100419. [Google Scholar] [CrossRef]
- Conforti, F.; Zucali, P.A.; Pala, L.; Catania, C.; Bagnardi, V.; Sala, I.; Della Vigna, P.; Perrino, M.; Zagami, P.; Corti, C.; et al. Avelumab plus Axitinib in Unresectable or Metastatic Type B3 Thymomas and Thymic Carcinomas (CAVEATT): A Single-Arm, Multicentre, Phase 2 Trial. Lancet Oncol. 2022, 23, 1287–1296. [Google Scholar] [CrossRef]
- Oji, Y.; Inoue, M.; Takeda, Y.; Hosen, N.; Shintani, Y.; Kawakami, M.; Harada, T.; Murakami, Y.; Iwai, M.; Fukuda, M.; et al. WT1 Peptide-Based Immunotherapy for Advanced Thymic Epithelial Malignancies. Int. J. Cancer 2018, 142, 2375–2382. [Google Scholar] [CrossRef]
- Song, Y.; Fu, Y.; Xie, Q.; Zhu, B.; Wang, J.; Zhang, B. Anti-Angiogenic Agents in Combination with Immune Checkpoint Inhibitors: A Promising Strategy for Cancer Treatment. Front. Immunol. 2020, 11, 1956. [Google Scholar] [CrossRef]
- Stühler, V.; Rausch, S.; Maas, J.M.; Stenzl, A.; Bedke, J. Combination of Immune Checkpoint Inhibitors and Tyrosine Kinase Inhibitors for the Treatment of Renal Cell Carcinoma. Expert Opin. Biol. Ther. 2021, 21, 1215–1226. [Google Scholar] [CrossRef]
- Fukumura, D.; Kloepper, J.; Amoozgar, Z.; Duda, D.G.; Jain, R.K. Enhancing Cancer Immunotherapy Using Antiangiogenics: Opportunities and Challenges. Nat. Rev. Clin. Oncol. 2018, 15, 325–340. [Google Scholar] [CrossRef]
- Läubli, H.; Müller, P.; D’Amico, L.; Buchi, M.; Kashyap, A.S.; Zippelius, A. The Multi-Receptor Inhibitor Axitinib Reverses Tumor-Induced Immunosuppression and Potentiates Treatment with Immune-Modulatory Antibodies in Preclinical Murine Models. Cancer Immunol. Immunother. 2018, 67, 815–824. [Google Scholar] [CrossRef]
- Oka, Y.; Tsuboi, A.; Taguchi, T.; Osaki, T.; Kyo, T.; Nakajima, H.; Elisseeva, O.A.; Oji, Y.; Kawakami, M.; Ikegame, K.; et al. Induction of WT1 (Wilms’ Tumor Gene)-Specific Cytotoxic T Lymphocytes by WT1 Peptide Vaccine and the Resultant Cancer Regression. Proc. Natl. Acad. Sci. USA 2004, 101, 13885–13890. [Google Scholar] [CrossRef]
- Jakopovic, M.; Bitar, L.; Seiwerth, F.; Marusic, A.; Krpina, K.; Samarzija, M. Immunotherapy for Thymoma. J. Thorac. Dis. 2020, 12, 7635–7641. [Google Scholar] [CrossRef]
- Wei, Y.F.; Chu, C.Y.; Chang, C.C.; Lin, S.H.; Su, W.C.; Tseng, Y.L.; Lin, C.C.; Yen, Y.T. Different Pattern of PD-L1, IDO, and FOXP3 Tregs Expression with Survival in Thymoma and Thymic Carcinoma. Lung Cancer 2018, 125, 35–42. [Google Scholar] [CrossRef]
- Giaccone, G. Pembrolizumab and Epacadostat in Patients with Thymic Carcinoma. Clinicaltrials.gov NCT02364076. Available online: https://clinicaltrials.gov/study/NCT02364076 (accessed on 24 November 2023).
- Duan, J.; Liu, X.; Chen, H.; Sun, Y.; Liu, Y.; Bai, H.; Wang, J. Impact of PD-L1, Transforming Growth Factor-β Expression and Tumor-Infiltrating CD8 + T Cells on Clinical Outcome of Patients with Advanced Thymic Epithelial Tumors. Thorac. Cancer 2018, 9, 1341–1353. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI) Bintrafusp Alfa (M7824) in Subjects with Thymoma and Thymic Carcinoma. Clinicaltrials.gov NCT04417660. Available online: https://clinicaltrials.gov/study/NCT04417660 (accessed on 24 November 2023).
- Zhou, Z.; Liu, Y.; Song, W.; Jiang, X.; Deng, Z.; Xiong, W.; Shen, J. Metabolic Reprogramming Mediated PD-L1 Depression and Hypoxia Reversion to Reactivate Tumor Therapy. J. Control. Release 2022, 352, 793–812. [Google Scholar] [CrossRef]
- He, Y.; Ramesh, A.; Gusev, Y.; Bhuvaneshwar, K.; Giaccone, G. Molecular Predictors of Response to Pembrolizumab in Thymic Carcinoma. Cell Rep. Med. 2021, 2, 100392. [Google Scholar] [CrossRef]
- Remon, J.; Villacampa, G.; Facchinetti, F.; Di Maio, M.; Marcuse, F.; Tiseo, M.; Hochstenbag, M.; Hendriks, L.E.L.; Besse, B. Immune Checkpoint Blockers in Patients with Unresectable or Metastatic Thymic Epithelial Tumours: A Meta-Analysis. Eur. J. Cancer 2023, 180, 117–124. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Y.P.; Du, X.J.; Liu, J.Q.; Huang, C.L.; Chen, L.; Zhou, G.Q.; Li, W.F.; Mao, Y.P.; Hsu, C.; et al. Comparative Safety of Immune Checkpoint Inhibitors in Cancer: Systematic Review and Network Meta-Analysis. BMJ 2018, 363, k4226. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef]
- Thomas, A.; Rajan, A.; Berman, A.; Giaccone, G. Multiorgan Autoimmune Manifestations Associated with Thymoma. J. Thorac. Oncol. 2015, 10, e5–e7. [Google Scholar] [CrossRef]
- Ricciuti, B.; Naqash, A.R.; Naidoo, J.; Sehgal, K.; Miller, A.; Kehl, K.; Venkatraman, D.; Sands, J.; Lamberti, G.; Recondo, G.; et al. Association Between Immune-Related Adverse Events and Clinical Outcomes to Programmed Cell Death Protein 1/Programmed Death-Ligand 1 Blockade in SCLC. JTO Clin. Res. Reports 2020, 1, 100074. [Google Scholar] [CrossRef]
- Indini, A.; Di Guardo, L.; Cimminiello, C.; Prisciandaro, M.; Randon, G.; De Braud, F.; Del Vecchio, M. Immune-Related Adverse Events Correlate with Improved Survival in Patients Undergoing Anti-PD1 Immunotherapy for Metastatic Melanoma. J. Cancer Res. Clin. Oncol. 2019, 145, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.; Yan, F.; Singla, N.; Levonyack, N.; Formella, J.; Christie, A.; Kapur, P.; Bowman, A.I.; Hammers, H.J.; Hannan, R.; et al. Immune-Related Adverse Events Are Associated with Improved Outcomes in ICI-Treated Renal Cell Carcinoma Patients. J. Clin. Oncol. 2019, 37, 645. [Google Scholar] [CrossRef]
- Ricciuti, B.; Genova, C.; De Giglio, A.; Bassanelli, M.; Dal Bello, M.G.; Metro, G.; Brambilla, M.; Baglivo, S.; Grossi, F.; Chiari, R. Impact of Immune-Related Adverse Events on Survival in Patients with Advanced Non-Small Cell Lung Cancer Treated with Nivolumab: Long-Term Outcomes from a Multi-Institutional Analysis. J. Cancer Res. Clin. Oncol. 2019, 145, 479–485. [Google Scholar] [CrossRef]
- Radovich, M.; Pickering, C.R.; Felau, I.; Ha, G.; Zhang, H.; Jo, H.; Hoadley, K.A.; Anur, P.; Zhang, J.; McLellan, M.; et al. The Integrated Genomic Landscape of Thymic Epithelial Tumors. Cancer Cell 2018, 33, 244–258. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 Pathway in Tolerance and Autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Mammen, A.L.; Rajan, A.; Pak, K.; Lehky, T.; Casciola-Rosen, L.; Donahue, R.N.; Lepone, L.M.; Zekeridou, A.; Pittock, S.J.; Hassan, R.; et al. Pre-Existing Antiacetylcholine Receptor Autoantibodies and B Cell Lymphopaenia Are Associated with the Development of Myositis in Patients with Thymoma Treated with Avelumab, an Immune Checkpoint Inhibitor Targeting Programmed Death-Ligand 1. Ann. Rheum. Dis. 2019, 78, 150–152. [Google Scholar] [CrossRef]
| Drug [Reference] | Design | N° Enrolled T/TC Patients | RR | mPFS (95% CI) | mOS (95% CI) | Rate of Severe irAEs |
|---|---|---|---|---|---|---|
| Pembrolizumab [24] | Phase II single arm | 33 (7 T, 26 TC) | T: 28.6% TC: 19.2% | T: 6.1 m (4.3–7.9) TC: 6.1 m (5.1–7.1) | T: NR TC: 14.5 m | T: 71.4% TC: 15.4% |
| Pembrolizumab [62] | Phase II single arm | 40 (TC) | 22.5% | 4.2 m (2.9–10.3) | 24.9 m (15.5-NR) | 15% |
| Nivolumab [63] | Phase II single arm | 15 (TC) | 0% | 3.8 m (1.9–7.0) | 14.1 m (11.1-NE) | 20% |
| Nivolumab (cohort 1) [64] | Phase II single arm 1 | 55 (10 T, 43 TC) | 12% | 6.0 m (3.1–10.4) | 21.3 m (11.6-NE) | 57% |
| Avelumab [65] | Phase I dose escalation | 8 (7 T, 1 TC) | T: 28.6% TC: 0% | NR | NR | 83.3% |
| Atezolizumab [66] | Phase II single arm, multi-cohort, basket 2 | 13 (T) | 38.5% | NR | NR | 35.7% |
| Avelumab + Axitinib [67] | Phase II single arm | 32 (3 B3-T, 2 B3-T/TC, 27 TC) | 34% (B3-T and B3-T/TC: 40%; TC: 33%) | 7.5 m (3.7–10.0) | 26.6 m (17.0–30.0) | 12% |
| WT-1 peptide vaccine [68] | Phase II single arm | 12 (4 T, 8 TC) | T: 0% TC: 0% | NR | NR | T: 25% TC: 0% |
| Clinical Trial | Phase | Agent | Target | Intervention | Tumor Type | Primary Endpoint | Status |
|---|---|---|---|---|---|---|---|
| NCT04667793 | II | Toripalimab | PD-1 | Neoadjuvant toripalimab + chemotherapy in locally advanced TETs. | T/TC | AEs, MPR | Recruiting |
| NCT06019468 | II | Envolizumab | PD-L1 | Neoadjuvant envolizumab plus RT in TC | TC | ORR | Recruiting |
| NCT03134118 NIVOTHYM | II | Nivolumab | PD-1 | Nivolumab in platinum-progressive T/TC | B3-T/TC | PFS | Active, not recruiting |
| NCT04417660 | II | Bintrafusp alpha (M7824) | PD-1/TGF-β | M7824 in platinum-progressive T/TC | T/TC | ORR | Recruiting |
| NCT05104736 | II | PT-112 | Epithelial cells | PT-112 in advanced T/TC | T/TC | ORR | Recruiting |
| NCT04710628 | II | Pembrolizumab | PD-1 | Pembrolizumab + lenvatinib in pretreated T/TC | B3-T/TC | PFS | Recruiting |
| Lenvatinib | VEGFR/PDGFR | ||||||
| NCT03583086 | I/II | Nivolumab | PD-1 | Vorolanib + nivolumab in TC | Thoracic tumors (incl. TC) | ORR, DOR, PFS; DCR, 1-year OS | Active, not recruiting |
| Vorolanib | VEGFR/PDGFR | ||||||
| NCT03463460 | II | Pembrolizumab | PD-1 | Pembrolizumab + sunitinib in platinum progressive TC | TC | ORR | Recruiting |
| Sunitinib | VEGFR/PDGFR | ||||||
| NCT04234113 | I/Ib | Pembrolizumab | PD-1 | SO-C101 ± pembrolizumab in advanced solid tumors | Advanced solid tumor (incl. T/TC) | AEs | Active, not recruiting |
| SO-C101 | IL-15 | ||||||
| NCT05544929 | I | Tislelizumab | PD-1 | KFA115 ± tisletizumab in selected advanced cancers | Select advanced cancers (incl TC) | AEs | Recruiting |
| KFA115 | Helios/IKZF2 | ||||||
| NCT03295227 | I | Pembrolizumab | PD-1 | Pembrolizumab in advanced TET | T/TC | Safety/tolerability | Recruiting |
| NCT03556228 | I | VMD-928 | TrkA (NTRK1) | VMD-928 in advanced solid tumors | Advanced solid tumor (incl. T/TC) | AEs | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrino, M.; Cordua, N.; De Vincenzo, F.; Borea, F.; Aliprandi, M.; Cecchi, L.G.; Fazio, R.; Airoldi, M.; Santoro, A.; Zucali, P.A. Thymic Epithelial Tumor and Immune System: The Role of Immunotherapy. Cancers 2023, 15, 5574. https://doi.org/10.3390/cancers15235574
Perrino M, Cordua N, De Vincenzo F, Borea F, Aliprandi M, Cecchi LG, Fazio R, Airoldi M, Santoro A, Zucali PA. Thymic Epithelial Tumor and Immune System: The Role of Immunotherapy. Cancers. 2023; 15(23):5574. https://doi.org/10.3390/cancers15235574
Chicago/Turabian StylePerrino, Matteo, Nadia Cordua, Fabio De Vincenzo, Federica Borea, Marta Aliprandi, Luigi Giovanni Cecchi, Roberta Fazio, Marco Airoldi, Armando Santoro, and Paolo Andrea Zucali. 2023. "Thymic Epithelial Tumor and Immune System: The Role of Immunotherapy" Cancers 15, no. 23: 5574. https://doi.org/10.3390/cancers15235574
APA StylePerrino, M., Cordua, N., De Vincenzo, F., Borea, F., Aliprandi, M., Cecchi, L. G., Fazio, R., Airoldi, M., Santoro, A., & Zucali, P. A. (2023). Thymic Epithelial Tumor and Immune System: The Role of Immunotherapy. Cancers, 15(23), 5574. https://doi.org/10.3390/cancers15235574







