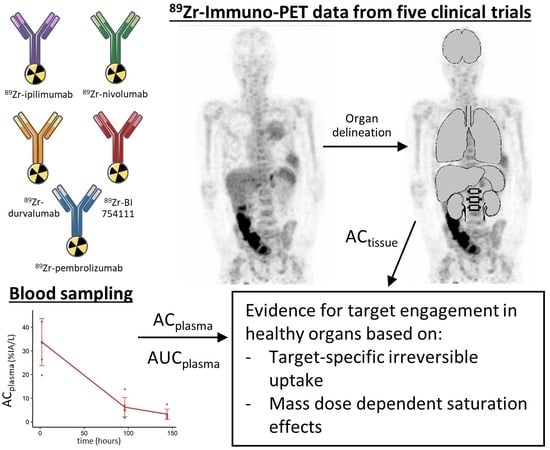
89Zr-Immuno-PET with Immune Checkpoint Inhibitors: Measuring Target Engagement in Healthy Organs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. 89Zr-mAb Studies
2.2. Biodistribution Analyses
2.3. Blood Sampling
2.4. Patlak Analysis
3. Results
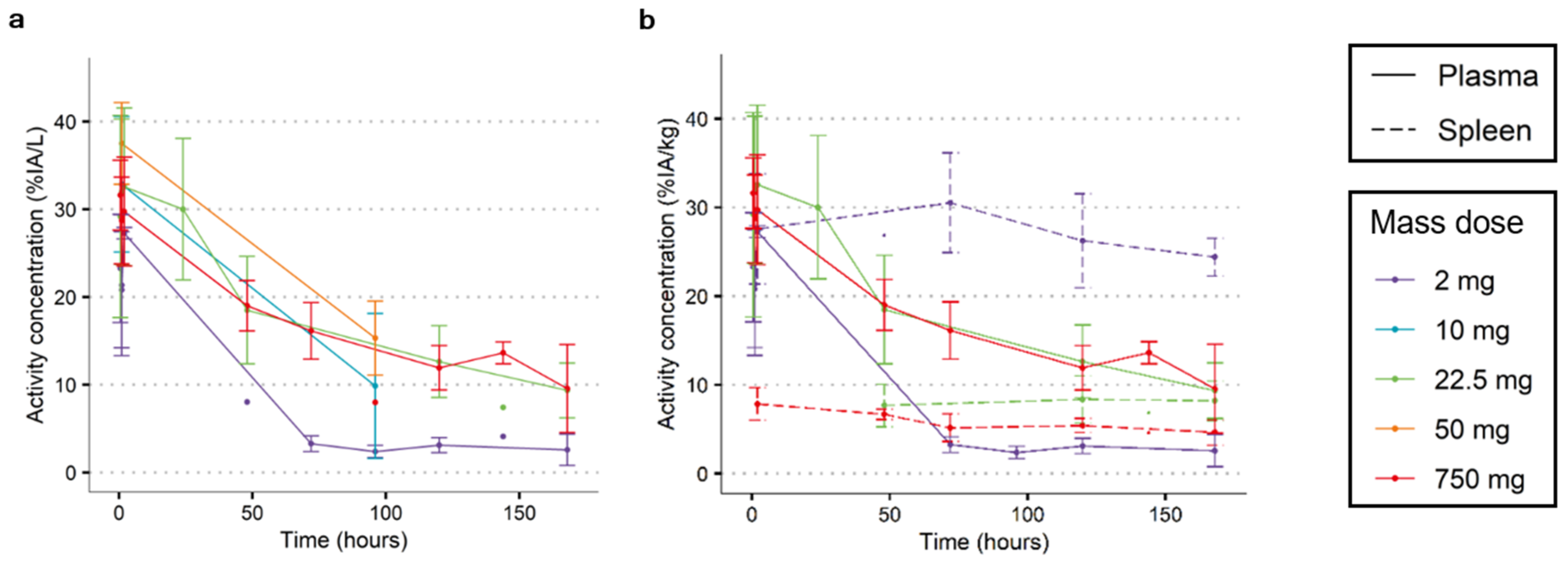
3.1. Finding an Adequate Imaging Dose for 89Zr-Immuno-PET Imaging: [89Zr]Zr-Durvalumab as an Example
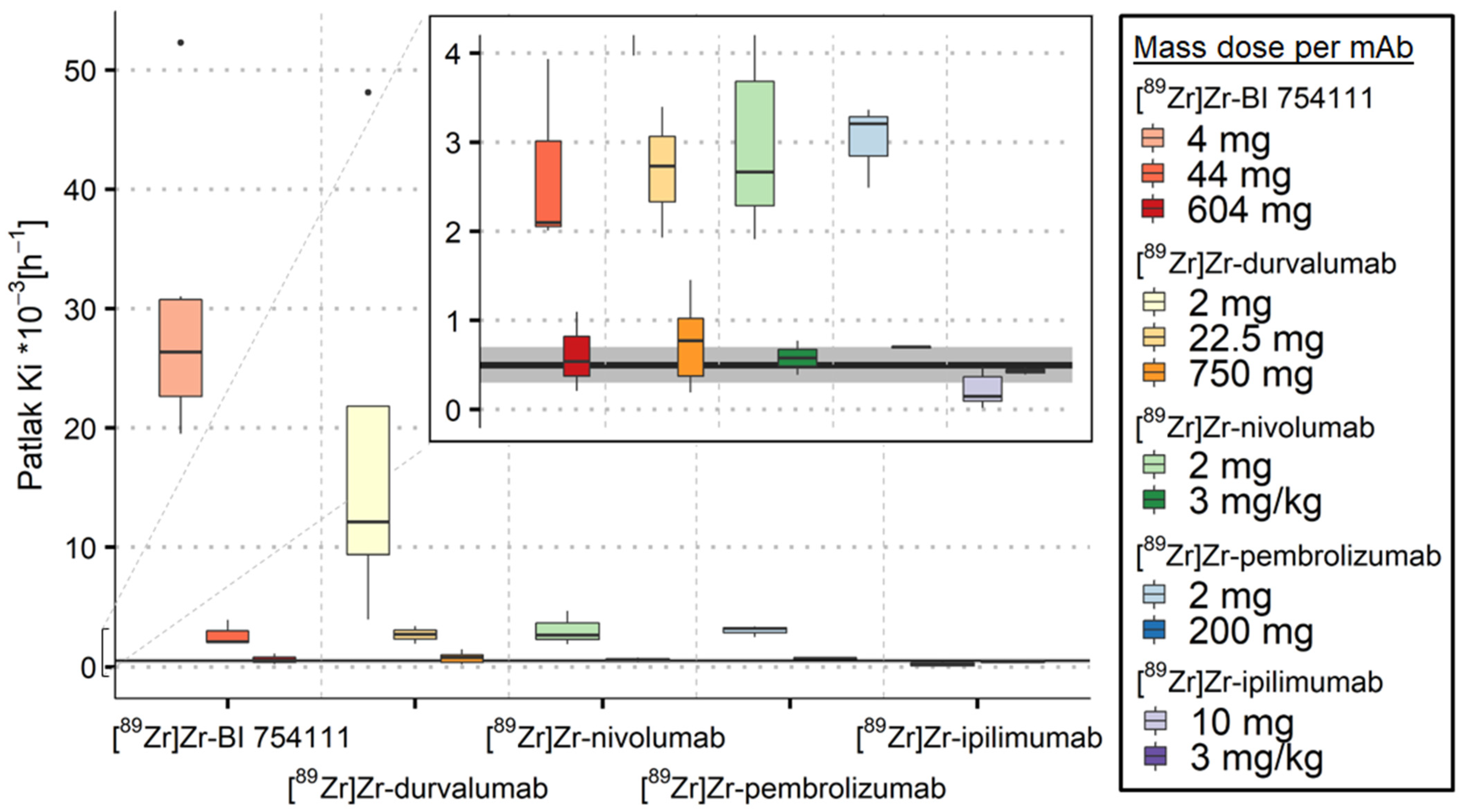
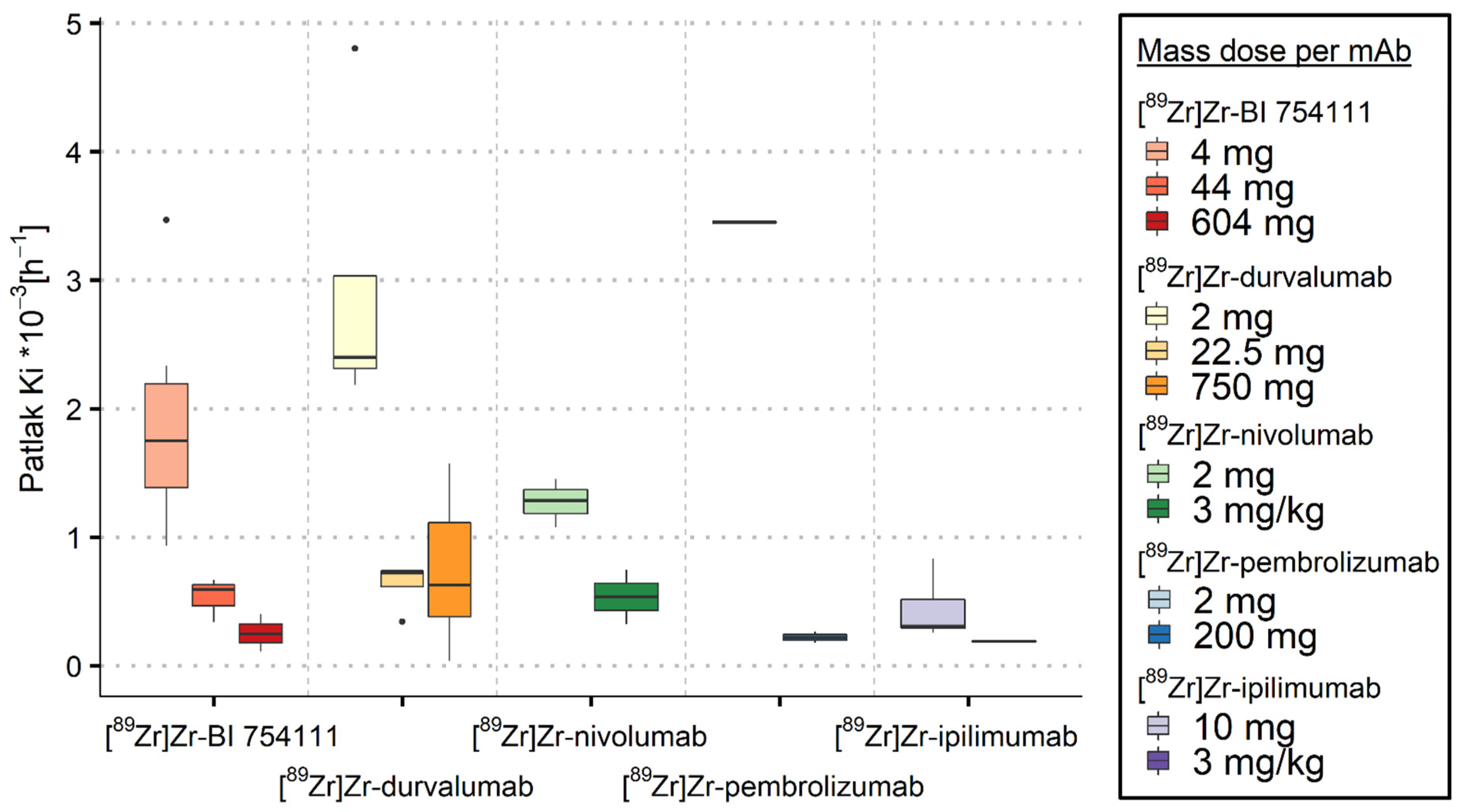
3.2. Measuring Target-Specific Irreversible Uptake Using Patlak Analyses
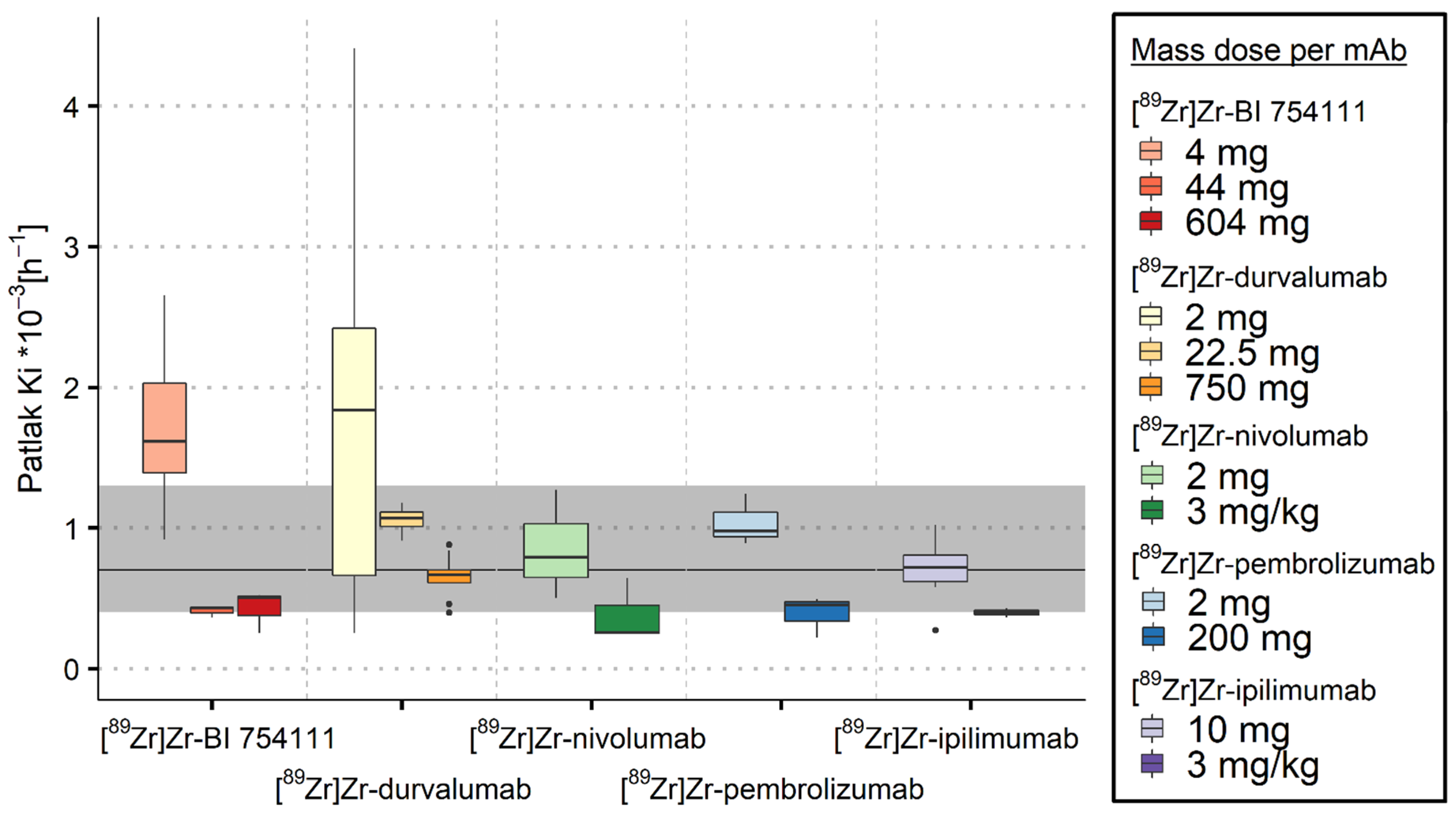
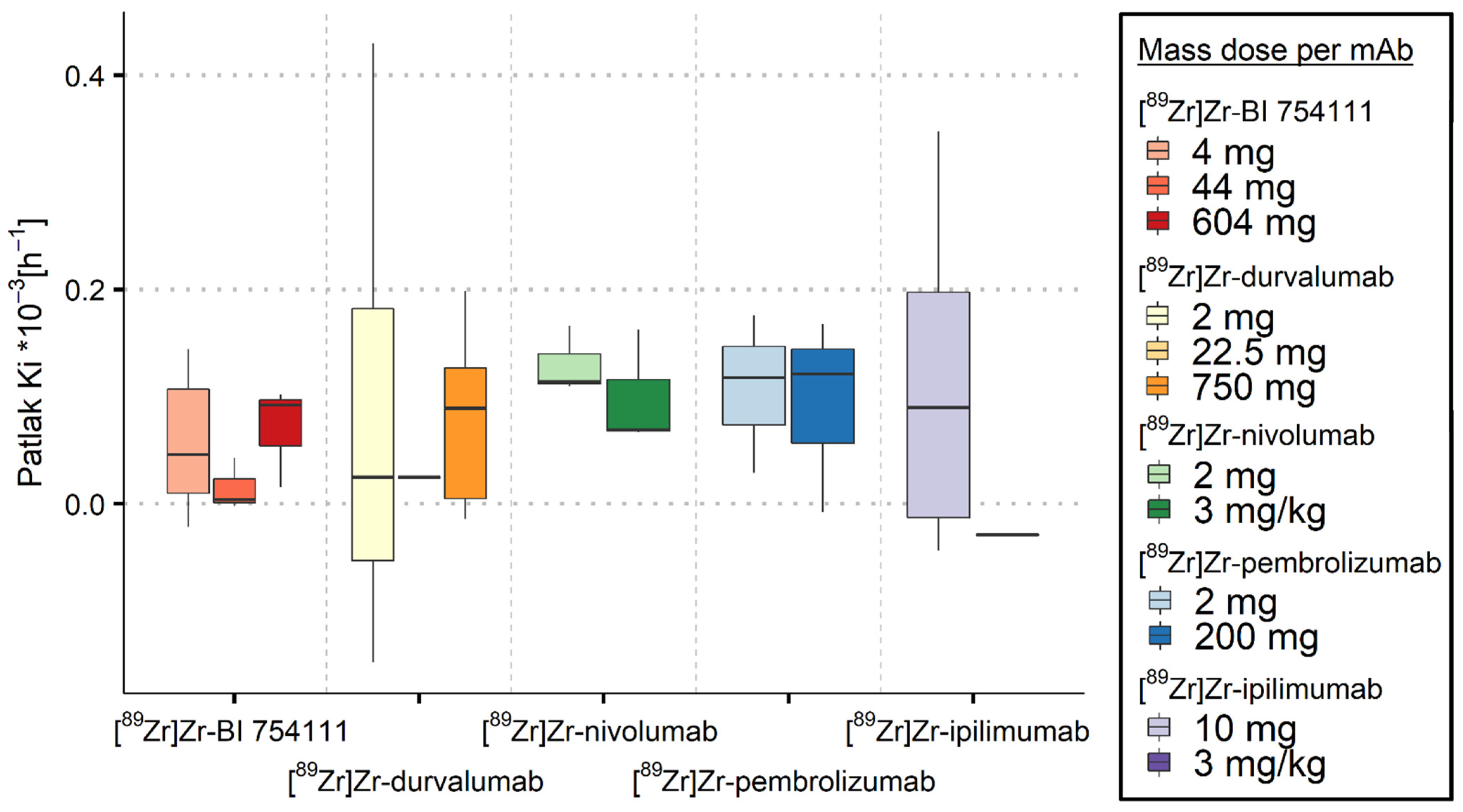
3.2.1. Spleen
3.2.2. Bone Marrow
3.2.3. Kidneys
3.2.4. Brain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csoszi, T.; Fulop, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer with Pd-L1 Tumor Proportion Score >/= 50. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab Versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulieres, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Baste, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab Alone or with Chemotherapy Versus Cetuximab with Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (Keynote-048): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Rasmussen, J.H.; Lelkaitis, G.; Håkansson, K.; Vogelius, I.R.; Johannesen, H.H.; Fischer, B.M.; Bentzen, S.M.; Specht, L.; Kristensen, C.A.; von Buchwald, C.; et al. Intratumor Heterogeneity of Pd-L1 Expression in Head and Neck Squamous Cell Carcinoma. Br. J. Cancer 2019, 120, 1003–1006. [Google Scholar] [CrossRef]
- Börjesson, P.K.; Jauw, Y.W.; de Bree, R.; Roos, J.C.; Castelijns, J.A.; Leemans, C.R.; van Dongen, G.A.; Boellaard, R. Radiation Dosimetry of 89zr-Labeled Chimeric Monoclonal Antibody U36 as Used for Immuno-Pet in Head and Neck Cancer Patients. J. Nucl. Med. 2009, 50, 1828–1836. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, L.E.; Williams, S.P.; van Scheltinga, A.G.T.; Hooge, M.N.L.-D.; Schröder, C.P.; Gietema, J.A.; Brouwers, A.H.; de Vries, E.G. Antibody Positron Emission Tomography Imaging in Anticancer Drug Development. J. Clin. Oncol. 2015, 33, 1491–1504. [Google Scholar] [CrossRef]
- Boerman, O.C.; Oyen, W.J. Immuno-Pet of Cancer: A Revival of Antibody Imaging. J. Nucl. Med. 2011, 52, 1171–1172. [Google Scholar] [CrossRef]
- De Feo, M.S.; Pontico, M.; Frantellizzi, V.; Corica, F.; De Cristofaro, F.; De Vincentis, G. 89zr-Pet Imaging in Humans: A Systematic Review. Clin. Transl. Imaging 2021, 10, 23–36. [Google Scholar] [CrossRef]
- Zasadny, K.R.; Wahl, R.L. Standardized Uptake Values of Normal Tissues at Pet with 2-[Fluorine-18]-Fluoro-2-Deoxy-D-Glucose: Variations with Body Weight and a Method for Correction. Radiology 1993, 189, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Jauw, Y.W.; O’Donoghue, J.A.; Zijlstra, J.M.; Hoekstra, O.S.; Menke-Van Der Houven, C.W.; Morschhauser, F.; Carrasquillo, J.A.; Zweegman, S.; Pandit-Taskar, N.; Lammertsma, A.A.; et al. (89)Zr-Immuno-Pet: Toward a Noninvasive Clinical Tool to Measure Target Engagement of Therapeutic Antibodies in Vivo. J. Nucl. Med. 2019, 60, 1825–1832. [Google Scholar] [CrossRef]
- Lammertsma, A.A.; Hoekstra, C.J.; Giaccone, G.; Hoekstra, O.S. How Should We Analyse Fdg Pet Studies for Monitoring Tumour Response? Eur. J. Nucl. Med. Mol. Imaging 2006, 33 (Suppl. 1), 16–21. [Google Scholar] [CrossRef] [PubMed]
- Miedema, I.H.C.; Huisman, M.C.; Zwezerijnen, G.J.C.; Grempler, R.; Pitarch, A.P.; Thiele, A.; Hesse, R.; Elgadi, M.; Peltzer, A.; Vugts, D.J.; et al. (89)Zr-Immuno-Pet Using the Anti-Lag-3 Tracer [(89)Zr]Zr-Bi 754111: Demonstrating Target Specific Binding in Nsclc and Hnscc. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2068–2080. [Google Scholar] [CrossRef] [PubMed]
- Wijngaarden, J.E.; Huisman, M.C.; Jauw, Y.W.S.; van Dongen, G.; Greuter, H.; Schuit, R.C.; Cleveland, M.; Gootjes, E.C.; Vugts, D.J.; der Houven van Oordt, C.W.M.-v.; et al. Validation of Simplified Uptake Measures against Dynamic Patlak K(I) for Quantification of Lesional (89)Zr-Immuno-Pet Antibody Uptake. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1897–1905. [Google Scholar] [CrossRef]
- Patlak, C.S.; Blasberg, R.G.; Fenstermacher, J.D. Graphical Evaluation of Blood-to-Brain Transfer Constants from Multiple-Time Uptake Data. J. Cereb. Blood Flow Metab. 1983, 3, 1–7. [Google Scholar] [CrossRef]
- Lobo, E.D.; Hansen, R.J.; Balthasar, J.P. Antibody Pharmacokinetics and Pharmacodynamics. J. Pharm. Sci. 2004, 93, 2645–2668. [Google Scholar] [CrossRef]
- Menke-van der Houven van Oordt, C.W.; McGeoch, A.; Bergstrom, M.; McSherry, I.; Smith, D.A.; Cleveland, M.; Al-Azzam, W.; Chen, L.; Verheul, H.; Hoekstra, O.S.; et al. Immuno-Pet Imaging to Assess Target Engagement: Experience from (89)Zr-Anti-Her3 Mab (Gsk2849330) in Patients with Solid Tumors. J. Nucl. Med. 2019, 60, 902–909. [Google Scholar] [CrossRef]
- Niemeijer, A.N.; Leung, D.; Huisman, M.C.; Bahce, I.; Hoekstra, O.S.; van Dongen, G.; Boellaard, R.; Du, S.; Hayes, W.; Smith, R.; et al. Whole Body Pd-1 and Pd-L1 Positron Emission Tomography in Patients with Non-Small-Cell Lung Cancer. Nat. Commun. 2018, 9, 4664. [Google Scholar] [CrossRef]
- Niemeijer, A.N.; Oprea-Lager, D.E.; Huisman, M.C.; Hoekstra, O.S.; Boellaard, R.; de Wit-van der Veen, B.J.; Bahce, I.; Vugts, D.J.; van Dongen, G.; Thunnissen, E.; et al. Study of (89)Zr-Pembrolizumab Pet/Ct in Patients with Advanced-Stage Non-Small Cell Lung Cancer. J. Nucl. Med. 2022, 63, 362–367. [Google Scholar] [CrossRef]
- Smit, J.; Borm, F.J.; Niemeijer, A.N.; Huisman, M.C.; Hoekstra, O.S.; Boellaard, R.; Oprea-Lager, D.E.; Vugts, D.J.; van Dongen, G.; de Wit-van der Veen, B.J.; et al. Pd-L1 Pet/Ct Imaging with Radiolabeled Durvalumab in Patients with Advanced-Stage Non-Small Cell Lung Cancer. J. Nucl. Med. 2022, 63, 686–693. [Google Scholar] [CrossRef]
- Miedema, I.H.; Zwezerijnen, G.J.; van Dongen, G.A.; Vugts, D.J.; Huisman, M.C.; Hoekstra, O.S.; de Gruijl, T.D.; Verheul, H.M.; Menke, C.W.; van den Eertwegh, A.J. Tumor Uptake and Biodistribution of 89zirconium-Labeled Ipilimumab in Patients with Metastatic Melanoma During Ipilimumab Treatment. Cancer Res. 2019, 79 (Suppl. 13), 1136. [Google Scholar] [CrossRef]
- Verhoeff, S.R.; van de Donk, P.P.; Aarntzen, E.; Oosting, S.F.; Brouwers, A.H.; Miedema, I.H.C.; Voortman, J.; der Houven van Oordt, W.C.M.-V.; Boellaard, R.; Vriens, D.; et al. (89)Zr-Dfo-Durvalumab Pet/Ct before Durvalumab Treatment in Patients with Recurrent or Metastatic Head and Neck Cancer. J. Nucl. Med. 2022, 63, 1523–1530. [Google Scholar] [CrossRef]
- Manafi-Farid, R.; Ataeinia, B.; Ranjbar, S.; Araghi, Z.J.; Moradi, M.M.; Pirich, C.; Beheshti, M. Immunopet: Antibody-Based Pet Imaging in Solid Tumors. Front. Med. 2022, 9, 916693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Salzler, R.; Dore, A.; Yang, J.; Ma, D.; Olson, W.C.; Liu, Y. Multiplex Immuno-Liquid Chromatography-Mass Spectrometry-Parallel Reaction Monitoring (Lc-Ms-Prm) Quantitation of Cd8a, Cd4, Lag3, Pd1, Pd-L1, and Pd-L2 in Frozen Human Tissues. J. Proteome Res. 2018, 17, 3932–3940. [Google Scholar] [CrossRef]
- Uhlen, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.; et al. Towards a Knowledge-Based Human Protein Atlas. Nat. Biotechnol. 2010, 28, 1248–1250. [Google Scholar] [CrossRef] [PubMed]
- Bensch, F.; van der Veen, E.L.; Hooge, M.N.L.-D.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schroder, C.P.; Hiltermann, T.J.N.; van der Wekken, A.J.; et al. (89)Zr-Atezolizumab Imaging as a Non-Invasive Approach to Assess Clinical Response to Pd-L1 Blockade in Cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.R.; Li, N.; Bruno, T.C.; Forbes, K.; Brown, S.; Workman, C.; Drake, C.G.; Vignali, D.A. Differential Subcellular Localization of the Regulatory T-Cell Protein Lag-3 and the Coreceptor Cd4. Eur. J. Immunol. 2010, 40, 1768–1777. [Google Scholar] [CrossRef]
- Lampson, L.A. Monoclonal Antibodies in Neuro-Oncology: Getting Past the Blood-Brain Barrier. MAbs 2011, 3, 153–160. [Google Scholar] [CrossRef]
| 89Zr-Immuno-PET Tracer | Target | Antibody Isotype | Mass Doses and Scan and Sample Time Points (h p.i.) | N | Tumour Type | References |
|---|---|---|---|---|---|---|
| [89Zr]Zr-nivolumab | PD-1 | IgG4 | 2 mg: 1, 72, 120, 168 3 mg/kg: 1, 72, 120, 168 | 3 3 | NSCLC | Niemeijer et al. [20] |
| [89Zr]Zr-pembrolizumab | PD-1 | IgG4 | 2 mg: 1, 72, 120, 168 200 mg: 1, 48, 120, 168 | 3 3 | NSCLC | Niemeijer et al. [21] |
| [89Zr]Zr-durvalumab | PD-L1 | IgG1 | 2 mg: 1, 72, 120, (168) * 22.5 mg: 48, 120, 168 750 mg: 1, 72, 120, (168) * | 11 4 10 | NSCLC | Smit et al. [22]; Pouw et al., manuscript in preparation |
| [89Zr]Zr-BI 754111 | LAG-3 | IgG4 | 4 mg: 2, 96, 144 44 mg: 96, 144 604 mg: 96, 144 | 6 3 3 | NSCLC, HNSCC | Miedema et al. [15] |
| [89Zr]Zr-ipilimumab | CTLA-4 | IgG1 | 10 mg: 72, (96,) 144 # 3 mg/kg: 72, 96, 144 | 9 3 | melanoma | Miedema et al. [23] |
| Organ Tissue | Ns-Baseline Ki·10−3 [h−1] Median (IQ Range) |
|---|---|
| Kidney | 0.7 (0.4–1.3) |
| Liver | 1.1 (0.8–2.1) |
| Lung | 0.2 (0.1–0.3) |
| Spleen | 0.5 (0.3–0.7) |
| 89Zr-Immuno-PET Tracer | Target | Mass Dose | Ki·10−3 [h−1] Median (IQ Range) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spleen | N | Bone Marrow | N | Kidneys | N | Brain | N | |||
| [89Zr]Zr-BI 754111 | LAG-3 | 4 mg | 26.3 (22.6–30.8) | 6 | 1.75 (1.38–2.20) | 6 | 1.62 (1.39–2.03) | 6 | 0.05 (0.01–0.11) | 6 |
| 44 mg | 2.10 (2.05–3.01) | 3 | 0.59 (0.47–0.63) | 3 | 0.43 (0.40–0.44) | 3 | 0.00 (0.00–0.02) | 3 | ||
| 604 mg | 0.54 (0.38–0.82) | 3 | 0.25 (0.18–0.32) | 3 | 0.51 (0.38–0.52) | 3 | 0.09 (0.05–0.10) | 3 | ||
| [89Zr]Zr-durvalumab | PD-L1 | 2 mg | 12.1 (9.41–21.8) * | 4 | 2.40 (2.31–3.04) * | 4 | 1.84 (0.66–2.42) * | 9 | 0.03 (−0.05–0.18) * | 9 |
| 22.5 mg | 2.73 (2.33–3.06) * | 3 | 0.72 (0.61–0.74) | 4 | 1.07 (1.01–1.11) | 4 | 0.03 * | 1 | ||
| 750 mg | 0.77 (0.38–1.02) * | 8 | 0.63 (0.38–1.12) * | 8 | 0.67 (0.61–0.70) | 9 | 0.09 (0.01–0.13) | 9 | ||
| [89Zr]Zr-nivolumab | PD-1 | 2 mg | 2.66 (2.29–3.68) | 3 | 1.29 (1.18–1.37) | 3 | 0.79 (0.65–1.03) | 3 | 0.11 (0.11–0.14) | 3 |
| 3 mg/kg | 0.58 (0.48–0.67) * | 2 | 0.54 (0.43–0.64) * | 2 | 0.26 (0.26–0.45) | 3 | 0.07 (0.07–0.12) | 3 | ||
| [89Zr]Zr-pembrolizumab | PD-1 | 2 mg | 3.21 (2.85–3.29) | 3 | 3.45 * | 1 | 0.98 (0.94–1.11) | 3 | 0.12 (0.07–0.15) | 3 |
| 200 mg | 0.70 (0.69–0.71) | 3 | 0.22 (0.20–0.24) * | 2 | 0.46 (0.34–0.48) | 3 | 0.12 (0.06–0.15) | 3 | ||
| [89Zr]Zr-ipilimumab | CTLA-4 | 10 mg | 0.15 (0.09–0.37) * | 7 | 0.31 (0.30–0.52) * | 7 | 0.72 (0.62–0.81) | 9 | 0.09 (−0.01–0.20 )* | 6 |
| 3 mg/kg | 0.43 (0.41–0.45) * | 2 | 0.19 * | 1 | 0.40 (0.38–0.41) * | 2 | −0.03 * | 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miedema, I.H.C.; Wijngaarden, J.E.; Pouw, J.E.E.; Zwezerijnen, G.J.C.; Sebus, H.J.; Smit, E.; de Langen, A.J.; Bahce, I.; Thiele, A.; Vugts, D.J.; et al. 89Zr-Immuno-PET with Immune Checkpoint Inhibitors: Measuring Target Engagement in Healthy Organs. Cancers 2023, 15, 5546. https://doi.org/10.3390/cancers15235546
Miedema IHC, Wijngaarden JE, Pouw JEE, Zwezerijnen GJC, Sebus HJ, Smit E, de Langen AJ, Bahce I, Thiele A, Vugts DJ, et al. 89Zr-Immuno-PET with Immune Checkpoint Inhibitors: Measuring Target Engagement in Healthy Organs. Cancers. 2023; 15(23):5546. https://doi.org/10.3390/cancers15235546
Chicago/Turabian StyleMiedema, Iris H. C., Jessica E. Wijngaarden, Johanna E. E. Pouw, Gerben J. C. Zwezerijnen, Hylke J. Sebus, Egbert Smit, Adrianus J. de Langen, Idris Bahce, Andrea Thiele, Daniëlle J. Vugts, and et al. 2023. "89Zr-Immuno-PET with Immune Checkpoint Inhibitors: Measuring Target Engagement in Healthy Organs" Cancers 15, no. 23: 5546. https://doi.org/10.3390/cancers15235546
APA StyleMiedema, I. H. C., Wijngaarden, J. E., Pouw, J. E. E., Zwezerijnen, G. J. C., Sebus, H. J., Smit, E., de Langen, A. J., Bahce, I., Thiele, A., Vugts, D. J., Boellaard, R., Huisman, M. C., & Menke-van der Houven van Oordt, C. W. (2023). 89Zr-Immuno-PET with Immune Checkpoint Inhibitors: Measuring Target Engagement in Healthy Organs. Cancers, 15(23), 5546. https://doi.org/10.3390/cancers15235546