Near-Infrared Fluorescence Imaging with Indocyanine Green for Robot-Assisted Partial Nephrectomy: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Selection Criteria
2.3. Study Screening and Selection
2.4. Statistical Analysis
3. Results
3.1. Literature Screening
3.2. Study Characteristics
3.3. Risk of Bias Assessment
3.4. Perioperative Outcomes
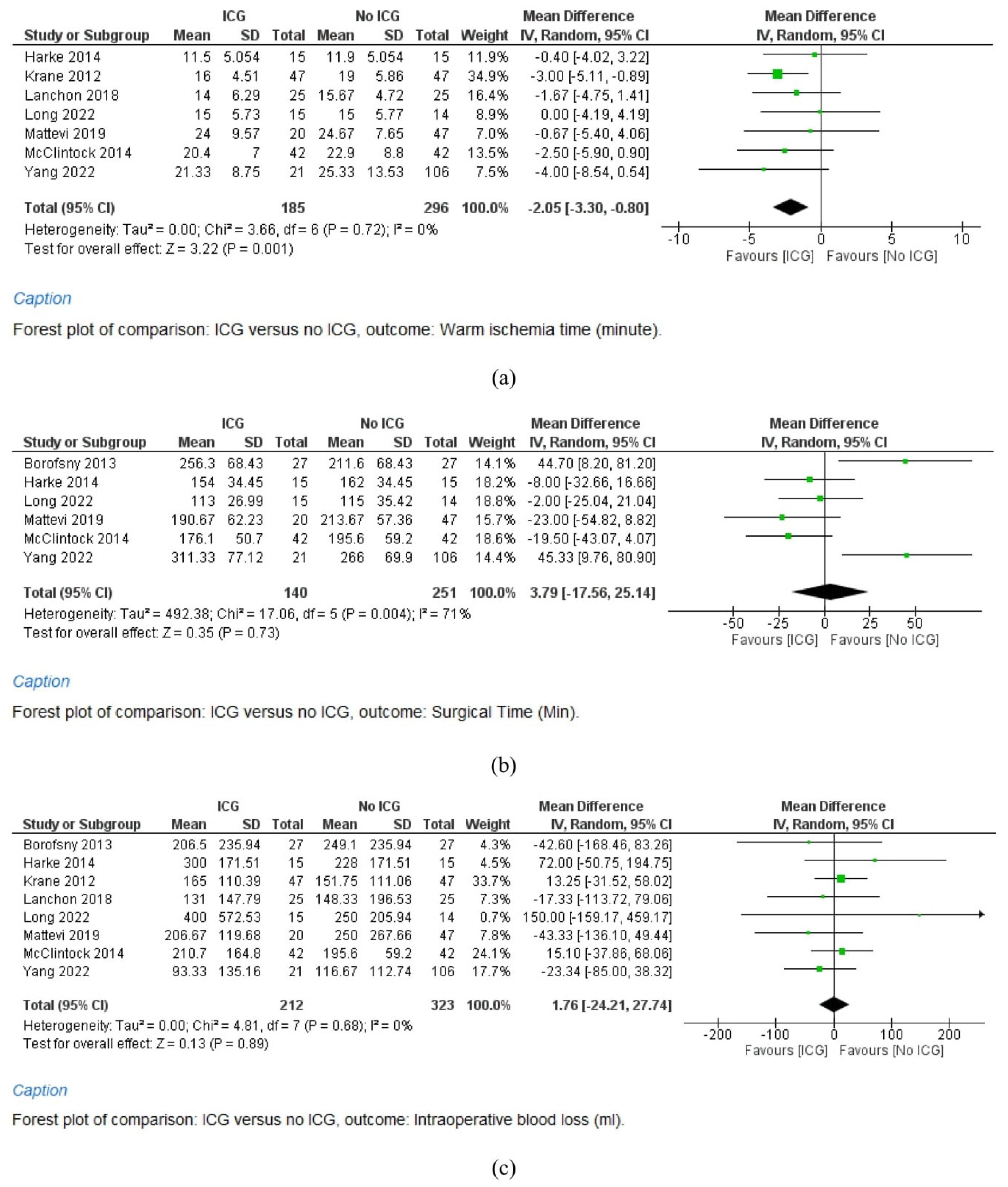
3.4.1. Warm Ischemia Time
3.4.2. Surgical Time
3.4.3. Intraoperative Blood Loss
3.4.4. Postoperative Estimated Glomerular Filtration Rate
3.4.5. Postoperative Stay
3.5. Postoperative Complications
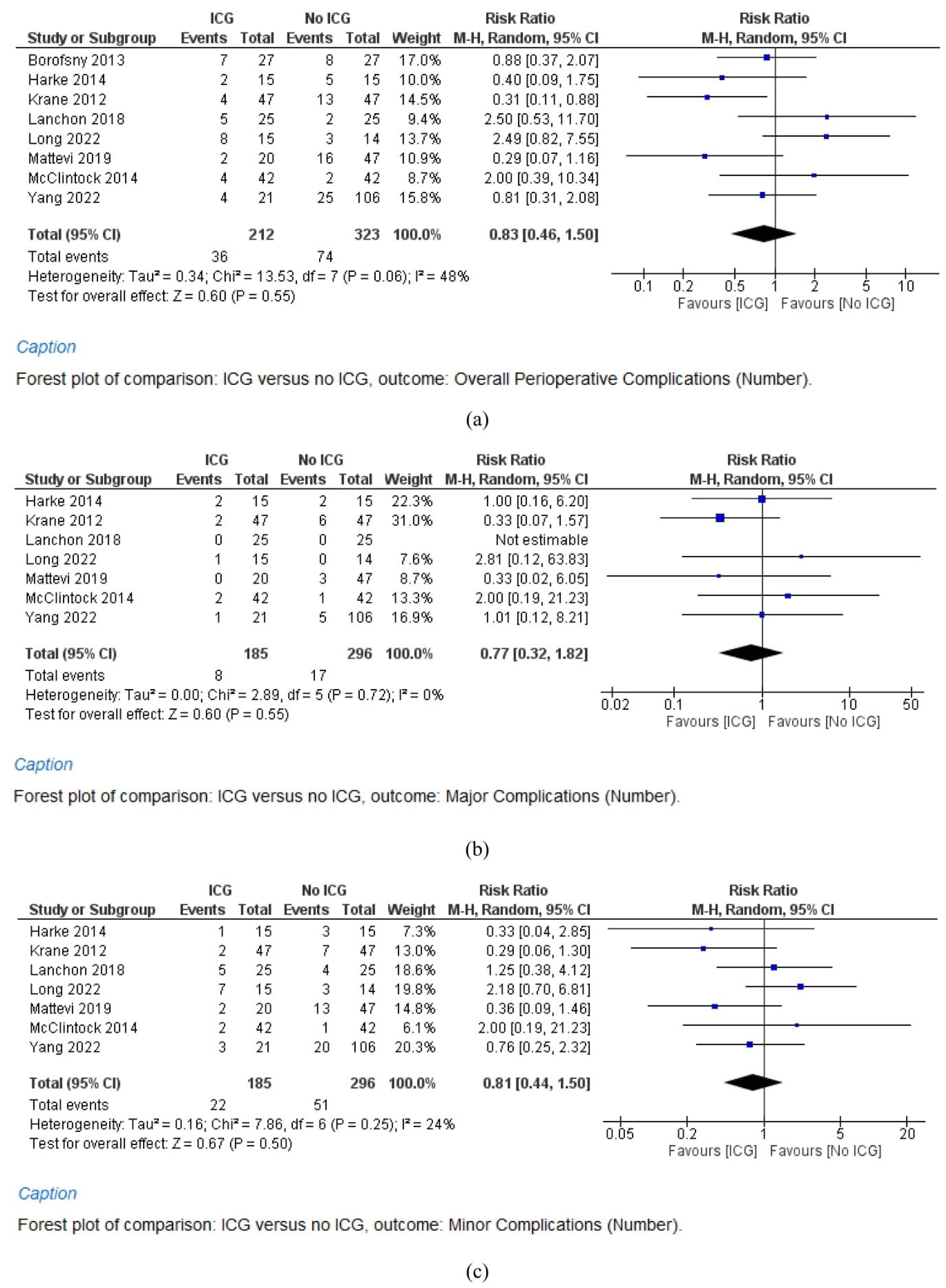
3.5.1. Overall Complications
3.5.2. Major Complications
3.5.3. Minor Complications
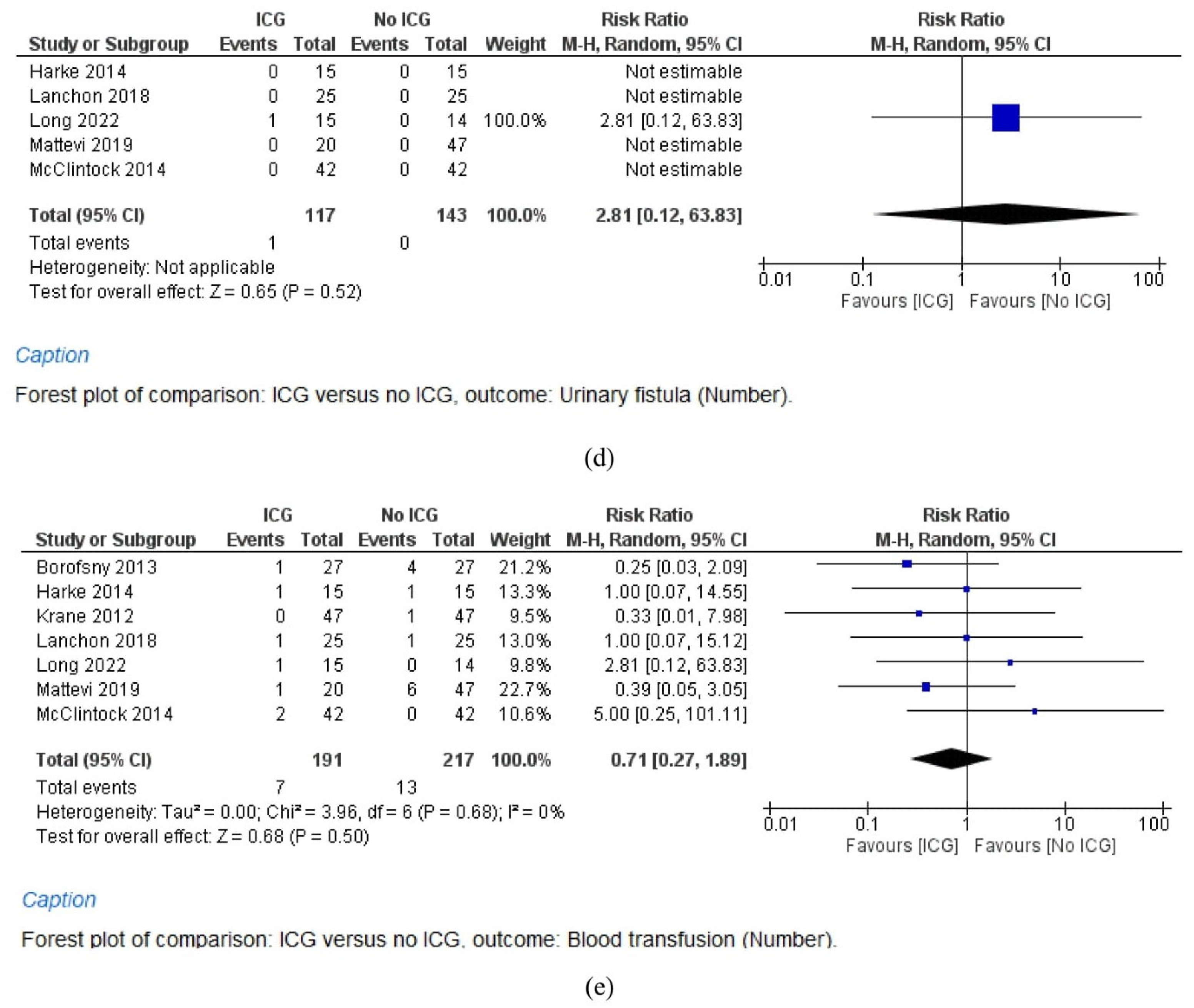
3.5.4. Urinary Fistula Rate
3.5.5. Blood Transfusion Rate
3.6. Oncological Outcomes
3.6.1. Positive Surgical Margins
3.6.2. Renal Tumor Recurrence
4. Discussion
- (a)
- A reduced warm ischemia time is achieved through super-selective clamping, as ICG enables precise identification of the arterial blood supply to the tumor [38].
- (b)
- There is enhanced identification of the tumor’s location and margins due to differential fluorescence characteristics between the mass and normal parenchyma, allowing for the continuous marking of the lesion throughout the procedure [39].
- (c)
- There can be direct evaluation of the ischemic effect on surrounding parenchyma after renorrhaphy, which is another contributing factor to postoperative eGFR loss, and this evaluation can be accomplished with a conventional intravenous injection of ICG to confirm the absence of ischemic injury to healthy parenchyma [38,39].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Weight, C.J.; Larson, B.T.; Fergany, A.F.; Gao, T.; Lane, B.R.; Campbell, S.C.; Kaouk, J.H.; Klein, E.A.; Novick, A.C. Nephrectomy Induced Chronic Renal Insufficiency is Associated With Increased Risk of Cardiovascular Death and Death From Any Cause in Patients With Localized cT1b Renal Masses. J. Urol. 2010, 183, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, E.R.; Claro, A.V.O.; Cepero, M.J.L.; Delgado, M.S.; Fernández, J.L.-O. Robotic versus Laparoscopic Partial Nephrectomy in the New Era: Systematic Review. Cancers 2023, 15, 1793. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; You, J.H.; Kim, D.K.; Rha, K.H.; Lee, S.H. Comparison of Perioperative Outcomes Between Robotic and Laparoscopic Partial Nephrectomy: A Systematic Review and Meta-analysis. Eur. Urol. 2015, 67, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.T.; Patel, D.N.; Ghali, F.; Patel, S.H.; Sarkar, R.; Yim, K.; Eldefrawy, A.; Cotta, B.H.; Bradshaw, A.W.; Meagher, M.F.; et al. Impact of positive surgical margins on survival after partial nephrectomy in localized kidney cancer: Analysis of the National Cancer Database. Minerva Urol. Nephrol. 2021, 73, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Tobis, S.; Knopf, J.K.; Silvers, C.; Messing, E.; Yao, J.; Rashid, H.; Wu, G.; Golijanin, D. Robot-Assisted and Laparoscopic Partial Nephrectomy with Near Infrared Fluorescence Imaging. J. Endourol. 2012, 26, 797–802. [Google Scholar] [CrossRef]
- Gadus, L.; Kocarek, J.; Chmelik, F.; Matejkova, M.; Heracek, J. Robotic Partial Nephrectomy with Indocyanine Green Fluorescence Navigation. Contrast Media Mol. Imaging 2020, 2020, 1287530. [Google Scholar] [CrossRef]
- Golijanin, D.; Marshall, J.; Cardin, A.; Singer, E.; Wood, R.; Reeder, J.; Wu, G.; Yao, J.; Passamonti, S.; Messing, E. Bilitranslocase (BTL) is immunolocalised in proximal and distal renal tubules and absent in renal cortical tumors accurately corresponding to in-traoperative near infrared fluorescence (NIRF) expression of renal cortical tumors using intravenous indocyanine green (ICG). J. Urol. 2008, 179, 137. [Google Scholar]
- Tobis, S.; Knopf, J.; Silvers, C.; Yao, J.; Rashid, H.; Wu, G.; Golijanin, D. Near Infrared Fluorescence Imaging With Robotic Assisted Laparoscopic Partial Nephrectomy: Initial Clinical Experience for Renal Cortical Tumors. J. Urol. 2011, 186, 47–52. [Google Scholar] [CrossRef]
- Buffi, N.; Uleri, A.; Paciotti, M.; Lughezzani, G.; Casale, P.; Diana, P.; De Groote, R.; Sarchi, L.; Mottaran, A.; Bravi, C.; et al. Techniques and outcomes of robot-assisted partial nephrectomy for the treatment of multiple ipsilateral renal masses. Minerva Urol Nephrol. 2023, 75, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Borofsky, M.S.; Gill, I.S.; Hemal, A.K.; Marien, T.P.; Jayaratna, I.; Krane, L.S.; Stifelman, M.D. Near-infrared fluorescence imaging to facilitate super-selective arterial clamping during zero-ischaemia robotic partial nephrectomy. BJU Int. 2012, 111, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Harke, N.; Schoen, G.; Schiefelbein, F.; Heinrich, E. Selective clamping under the usage of near-infrared fluorescence imaging with indocyanine green in robot-assisted partial nephrectomy: A single-surgeon matched-pair study. World J. Urol. 2014, 32, 1259–1265. [Google Scholar] [CrossRef]
- Krane, L.S.; Manny, T.B.; Hemal, A.K. Is Near Infrared Fluorescence Imaging Using Indocyanine Green Dye Useful in Robotic Partial Nephrectomy: A Prospective Comparative Study of 94 Patients. Urology 2012, 80, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Lanchon, C.; Arnoux, V.; Fiard, G.; Descotes, J.-L.; Rambeaud, J.-J.; Lefrancq, J.-B.; Poncet, D.; Terrier, N.; Overs, C.; Franquet, Q.; et al. Super-selective robot-assisted partial nephrectomy using near-infrared flurorescence versus early-unclamping of the renal artery: Results of a prospective matched-pair analysis. Int. Braz. J. Urol. 2018, 44, 53–62. [Google Scholar] [CrossRef]
- Long, J.-A.; Fiard, G.; Giai, J.; Teyssier, Y.; Fontanell, A.; Overs, C.; Poncet, D.; Descotes, J.-L.; Rambeaud, J.-J.; Moreau-Gaudry, A.; et al. Superselective Ischemia in Robotic Partial Nephrectomy Does Not Provide Better Long-term Renal Function than Renal Artery Clamping in a Randomized Controlled Trial (EMERALD): Should We Take the Risk? Eur. Urol. Focus 2022, 8, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Mattevi, D.; Luciani, L.G.; Mantovani, W.; Cai, T.; Chiodini, S.; Vattovani, V.; Puglisi, M.; Malossini, G. Fluorescence-guided selective arterial clamping during RAPN provides better early functional outcomes based on renal scan compared to standard clamping. J. Robot. Surg. 2019, 13, 391–396. [Google Scholar] [CrossRef]
- McClintock, T.R.; Bjurlin, M.A.; Wysock, J.S.; Borofsky, M.S.; Marien, T.P.; Okoro, C.; Stifelman, M.D. Can Selective Arterial Clamping With Fluorescence Imaging Preserve Kidney Function During Robotic Partial Nephrectomy? Urology 2014, 84, 327–334. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.-K.; Hsieh, M.-L.; Chen, S.-Y.; Liu, C.-Y.; Lin, P.-H.; Kan, H.-C.; Pang, S.-T.; Yu, K.-J. Clinical Benefits of Indocyanine Green Fluorescence in Robot-Assisted Partial Nephrectomy. Cancers 2022, 14, 3032. [Google Scholar] [CrossRef]
- Thompson, R.H.; Lane, B.R.; Lohse, C.M.; Leibovich, B.C.; Fergany, A.; Frank, I.; Gill, I.S.; Blute, M.L.; Campbell, S.C. Every Minute Counts When the Renal Hilum Is Clamped during Partial Nephrectomy. Eur. Urol. 2010, 58, 340–345. [Google Scholar] [CrossRef]
- Kaczmarek, B.F.; Tanagho, Y.S.; Hillyer, S.P.; Mullins, J.K.; Diaz, M.; Trinh, Q.-D.; Bhayani, S.B.; Allaf, M.E.; Stifelman, M.D.; Kaouk, J.H.; et al. Off-clamp Robot-assisted Partial Nephrectomy Preserves Renal Function: A Multi-institutional Propensity Score Analysis. Eur. Urol. 2013, 64, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Cindolo, L.; Sandri, M.; Veccia, A.; Annino, F.; Bertagna, F.; Carini, M.; Celia, A.; D’Orta, C.; De Concilio, B.; et al. Is off-clamp robot-assisted partial nephrectomy beneficial for renal function? Data from the CLOCK trial. BJU Int. 2022, 129, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, L.; Barod, R.; Dalela, D.; Diaz-Insua, M.; Abaza, R.; Adshead, J.; Ahlawat, R.; Challacombe, B.; Dasgupta, P.; Gandaglia, G.; et al. Use of Main Renal Artery Clamping Predominates Over Minimal Clamping Techniques During Robotic Partial Nephrectomy for Complex Tumors. J. Endourol. 2017, 31, 149–152. [Google Scholar] [CrossRef]
- Peyronnet, B.; Baumert, H.; Mathieu, R.; Masson-Lecomte, A.; Grassano, Y.; Roumiguié, M.; Massoud, W.; El Fattah, V.A.; Bruyère, F.; Droupy, S.; et al. Early unclamping technique during robot-assisted laparoscopic partial nephrectomy can minimise warm ischaemia without increasing morbidity. BJU Int. 2014, 114, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Giulioni, C.; Maggi, M.; Pirola, G.M.; Martorana, E.; Cormio, A.; Teoh, J.Y.-C.; Gauhar, V.; Galosi, A.B.; Castellani, D. The current evidence on surgical management for synchronous bilateral renal tumors: Results from a scoping review. World J. Urol. 2023, 41, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Veccia, A.; Antonelli, A.; Hampton, L.J.; Greco, F.; Perdonà, S.; Lima, E.; Hemal, A.K.; Derweesh, I.; Porpiglia, F.; Autorino, R. Near-infrared Fluorescence Imaging with Indocyanine Green in Robot-assisted Partial Nephrectomy: Pooled Analysis of Comparative Studies. Eur. Urol. Focus 2020, 6, 505–512. [Google Scholar] [CrossRef]
- Giulioni, C.; Scarcella, S.; Di Biase, M.; Marconi, A.; Sortino, G.; Diambrini, M.; Giannubilo, W.; Castellani, D.; Ferrara, V. The Role of Intraoperative Ultrasonography Associated with Clampless Technique in Three-Dimensional Retroperitoneoscopic Laparoscopic Enucleation of Completely Endophytic Renal Tumors. J. Laparoendosc. Adv. Surg. Tech. 2022, 32, 987–991. [Google Scholar] [CrossRef]
- De Backer, P.; Vermijs, S.; Van Praet, C.; De Visschere, P.; Vandenbulcke, S.; Mottaran, A.; Bravi, C.A.; Berquin, C.; Lambert, E.; Dautricourt, S.; et al. A Novel Three-dimensional Planning Tool for Selective Clamping During Partial Nephrectomy: Validation of a Perfusion Zone Algorithm. Eur. Urol. 2023, 83, 413–421. [Google Scholar] [CrossRef]
- Piramide, F.; Kowalewski, K.-F.; Cacciamani, G.; Belenchon, I.R.; Taratkin, M.; Carbonara, U.; Marchioni, M.; De Groote, R.; Knipper, S.; Pecoraro, A.; et al. Three-dimensional Model–assisted Minimally Invasive Partial Nephrectomy: A Systematic Review with Meta-analysis of Comparative Studies. Eur. Urol. Oncol. 2022, 5, 640–650. [Google Scholar] [CrossRef]
- Tanagho, Y.S.; Kaouk, J.H.; Allaf, M.E.; Rogers, C.G.; Stifelman, M.D.; Kaczmarek, B.F.; Hillyer, S.P.; Mullins, J.K.; Chiu, Y.; Bhayani, S.B. Perioperative Complications of Robot-assisted Partial Nephrectomy: Analysis of 886 Patients at 5 United States Centers. Urology 2013, 81, 573–580. [Google Scholar] [CrossRef]
- Ferroni, M.C.; Sentell, K.; Abaza, R. Current Role and Indications for the Use of Indocyanine Green in Robot-assisted Urologic Surgery. Eur. Urol. Focus 2018, 4, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.D.; Thompson, R.H.; Kallingal, G.J.; Cambareri, G.; Russo, P. Urinary fistulae after partial nephrectomy. BJU Int. 2010, 106, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.J.; Hakimi, A.A.; Snyder, M.E.; Russo, P. Complications of Radical and Partial Nephrectomy in a Large Contemporary Cohort. J. Urol. 2004, 171, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Gill, I.S.; Kavoussi, L.R.; Lane, B.R.; Blute, M.L.; Babineau, D.; Colombo, J.R.; Frank, I.; Permpongkosol, S.; Weight, C.J.; Kaouk, J.H.; et al. Comparison of 1,800 Laparoscopic and Open Partial Nephrectomies for Single Renal Tumors. J. Urol. 2007, 178, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, T.; Boorjian, S.A.; Capitanio, U.; Gershman, B.; Mir, M.C.; Kutikov, A. Collaborative Review: Factors Influencing Treatment Decisions for Patients with a Localized Solid Renal Mass. Eur. Urol. 2021, 80, 575–588. [Google Scholar] [CrossRef]
- Klaassen, Z.; Li, Q.; Madi, R.; Terris, M.K. The Role of Indocyanine Green for Robotic Partial Nephrectomy: Early Results, Limitations and Future Directions. Robotics 2014, 3, 281–288. [Google Scholar] [CrossRef]
- Pandey, A.; Dell’Oglio, P.; Mazzone, E.; Mottrie, A.; De, N.G. Usefulness of the indocyanine green (icg) immunofluorescence in laparoscopic and robotic partial nephrectomy. Arch. Esp. De Urol. 2019, 72, 723–728. [Google Scholar]
- Diana, P.; Buffi, N.M.; Lughezzani, G.; Dell’oglio, P.; Mazzone, E.; Porter, J.; Mottrie, A. The Role of Intraoperative Indocyanine Green in Robot-assisted Partial Nephrectomy: Results from a Large, Multi-institutional Series. Eur. Urol. 2020, 78, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Simone, G.; Tuderti, G.; Anceschi, U.; Ferriero, M.; Costantini, M.; Minisola, F.; Vallati, G.; Pizzi, G.; Guaglianone, S.; Misuraca, L.; et al. “Ride the Green Light”: Indocyanine Green–marked Off-clamp Robotic Partial Nephrectomy for Totally Endophytic Renal Masses. Eur. Urol. 2019, 75, 1008–1014. [Google Scholar] [CrossRef]


| First Author, Year | Type of Study | Number of Cases | Mean Age, Years | Clamping Technique | Surgical Time, min | Positive Surgical Margin, n (%) | Overall Complications, n (%) | Postoperative eGFR | Number of Control | Mean Age, Years | Clamping Technique | Surgical Time, min | Positive Surgical Margin, n (%) | Overall Complications, n (%) | Postoperative eGFR | Final Considerations |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Borofsny (2013) [12] | Retrospective | 27 | 60 | ZI | 256.3 | 0 | 7 (26) | 70.8 | 27 | 58.1 | MAC | 211.6 | 0 | 8 (27) | - | ZI partial nephrectomy is feasible most of time with NIRF, with a greater renal function preservation. |
| Harke (2014) [13] | Retrospective | 15 | 63 | SSC | 154 | 0 | 2 (13) | - | 15 | 63.2 | MAC | 162 | 0 | 5 (33) | - | NIRF can be employed safely, especially in hilar and intrarenal as well as polar tumors, avoiding ischemic injury to the remaining parenchyma, with superior kidney function preservation |
| Krane (2012) [14] | Retrospective | 47 | 60 | SSC or ZI | - | 3 (6) | 4 (9) | - | 47 | 60.2 | SSC or ZI | - | 4 (9) | 13 (28) | - | RPN using NIRF–ICG can be performed safely and effectively. Differential ICG uptake by different tumors did not lead to significant differences in the positive margin rate. |
| Lanchon (2018) [15] | Prospective | 25 | 66 | SSC | - | 1 (4) | 5 (20) | 78 | 25 | 68 | MAC | 119 | 1 (4) | 4 (16) | 72.67 | Super-selective clamping with NIRF using ICG is safe and feasible, leading to an increased preservation of overall and split postoperative renal function, while keeping the benefit of main artery clamping on blood loss and perioperative complications. |
| Long (2022) [16] | Randomized Controlled Trial | 15 | 56 | SSC | 113 | 0 | 8 (53) | 80 | 14 | 61 | MAC | 115 | 1 (7) | 3 (21) | 79.83 | SSC–RAPN using NIRF did not provide better renal preservation than renal artery clamping in non-selected patients,. |
| Mattevi (2019) [17] | Prospective | 20 | 61 | SSC | 190.67 | 3 (7.1) | 2 (10) | 46.83 | 47 | 66 | MAC | 213.67 | 0 | 18 (38) | 38.67 | There was an association between NIRF and improved short-term functional outcomes, as measured by eGFR at renal scan. |
| McClintock (2014) [18] | Retrospective | 42 | 59 | SSC | 176.1 | 0 | 4 (10) | 78.2 | 42 | 59.4 | MAC | 195.6 | 0 | 2 (5) | 68.5 | The use of NIRF aids in the implementation of selective arterial clamping, enabling real-time verification of the intended ischemic regions, with enhanced short-term functional outcomes. |
| Yang (2022) [19] | Retrospective | 21 | 58 | MAC | 311.33 | 2 (10) | 4 (19) | 79.6 | 106 | 57 | MAC | 266 | 8 (8) | 25 (23) | 71.33 | ICG determined superior short-term renal functional outcomes, with less operative blood loss. Therefore, ICG–RAPN is an ostensibly safe procedure with potentially superior short-term renal functional outcomes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giulioni, C.; Mulawkar, P.M.; Castellani, D.; De Stefano, V.; Nedbal, C.; Gadzhiev, N.; Pirola, G.M.; Law, Y.X.T.; Wroclawski, M.L.; Keat, W.O.L.; et al. Near-Infrared Fluorescence Imaging with Indocyanine Green for Robot-Assisted Partial Nephrectomy: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 5560. https://doi.org/10.3390/cancers15235560
Giulioni C, Mulawkar PM, Castellani D, De Stefano V, Nedbal C, Gadzhiev N, Pirola GM, Law YXT, Wroclawski ML, Keat WOL, et al. Near-Infrared Fluorescence Imaging with Indocyanine Green for Robot-Assisted Partial Nephrectomy: A Systematic Review and Meta-Analysis. Cancers. 2023; 15(23):5560. https://doi.org/10.3390/cancers15235560
Chicago/Turabian StyleGiulioni, Carlo, Prashant Motiram Mulawkar, Daniele Castellani, Virgilio De Stefano, Carlotta Nedbal, Nariman Gadzhiev, Giacomo Maria Pirola, Yu Xi Terence Law, Marcelo Langer Wroclawski, William Ong Lay Keat, and et al. 2023. "Near-Infrared Fluorescence Imaging with Indocyanine Green for Robot-Assisted Partial Nephrectomy: A Systematic Review and Meta-Analysis" Cancers 15, no. 23: 5560. https://doi.org/10.3390/cancers15235560
APA StyleGiulioni, C., Mulawkar, P. M., Castellani, D., De Stefano, V., Nedbal, C., Gadzhiev, N., Pirola, G. M., Law, Y. X. T., Wroclawski, M. L., Keat, W. O. L., Tiong, H. Y., Somani, B. K., Galosi, A. B., & Gauhar, V. (2023). Near-Infrared Fluorescence Imaging with Indocyanine Green for Robot-Assisted Partial Nephrectomy: A Systematic Review and Meta-Analysis. Cancers, 15(23), 5560. https://doi.org/10.3390/cancers15235560






