Circulating sRANKL, Periostin, and Osteopontin as Biomarkers for the Assessment of Activated Osteoclastogenesis in Myeloma Related Bone Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
2.2. Methods
2.3. Statistical Analysis
3. Results
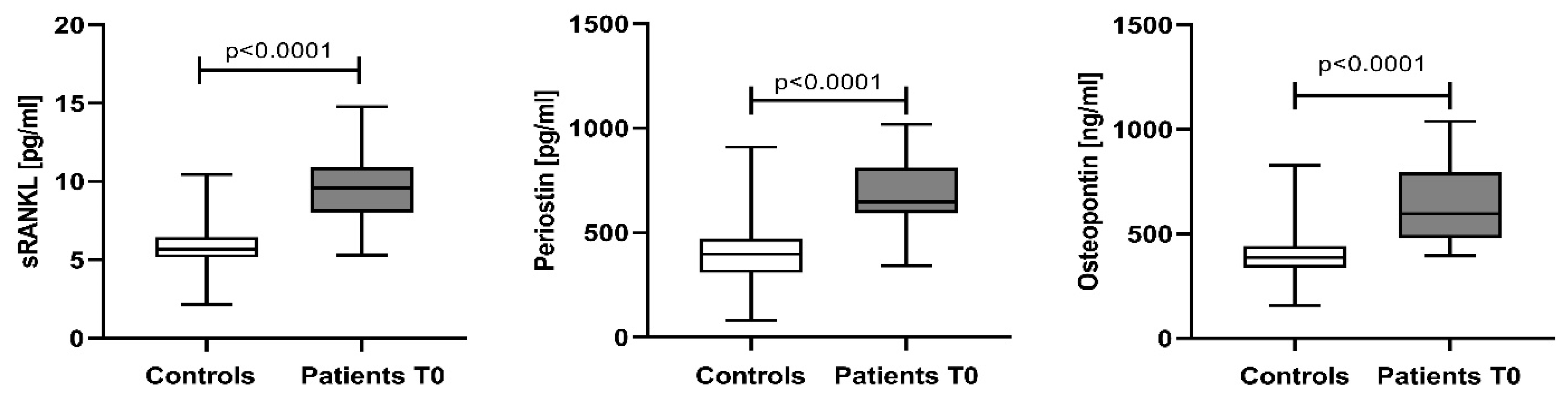
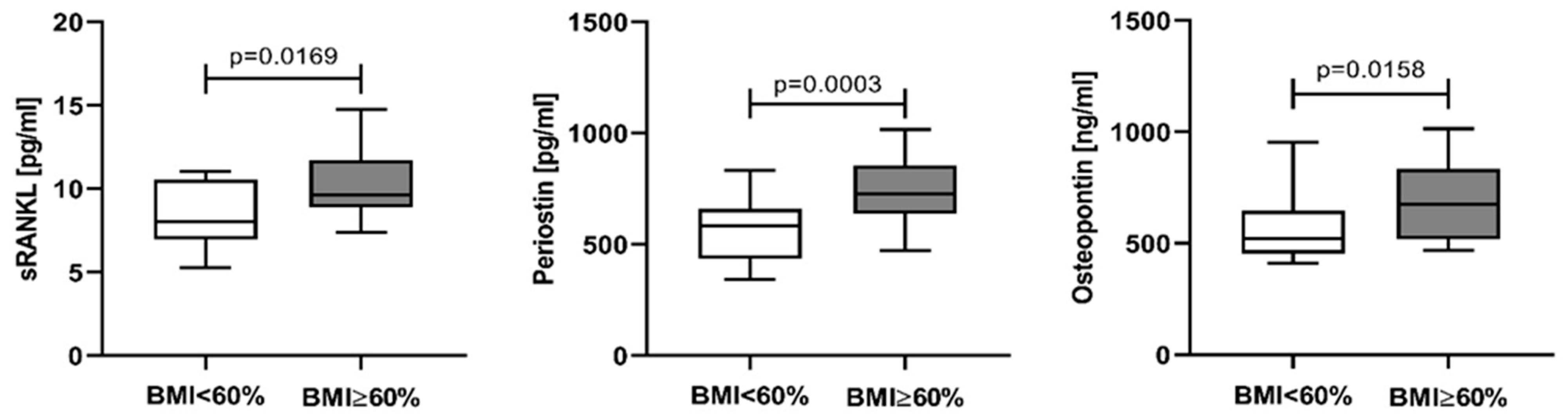
3.1. Baseline Serum Levels of sRANKL, Periostin, and Osteopontin in Newly Diagnosed Multiple Myeloma Patients
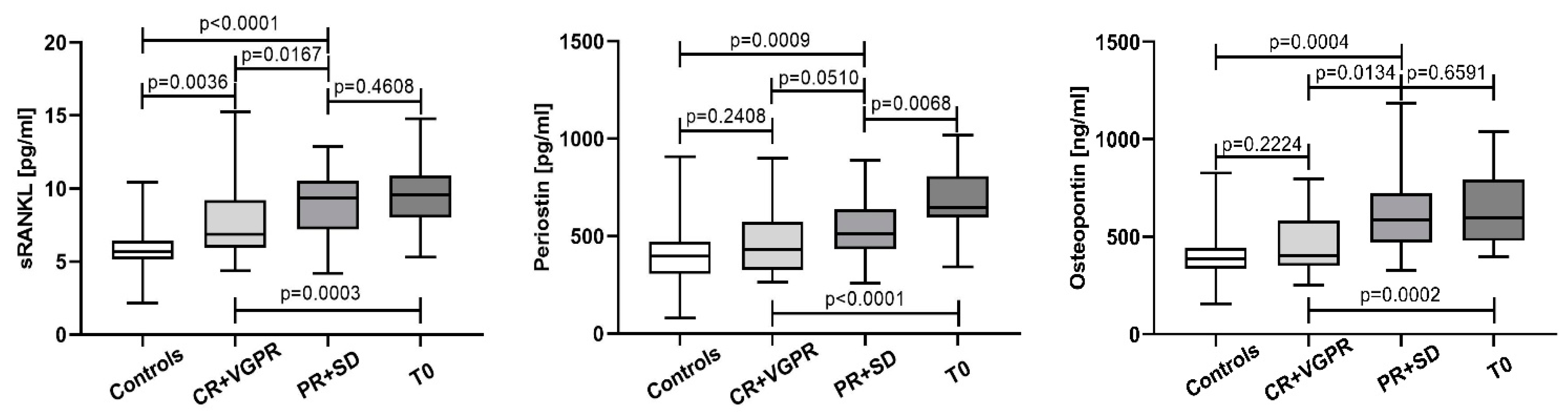
3.2. Variation of sRANKL, Periostin, and Osteopontin Serum Levels in the Course of Treatment and According to the Response to Therapy
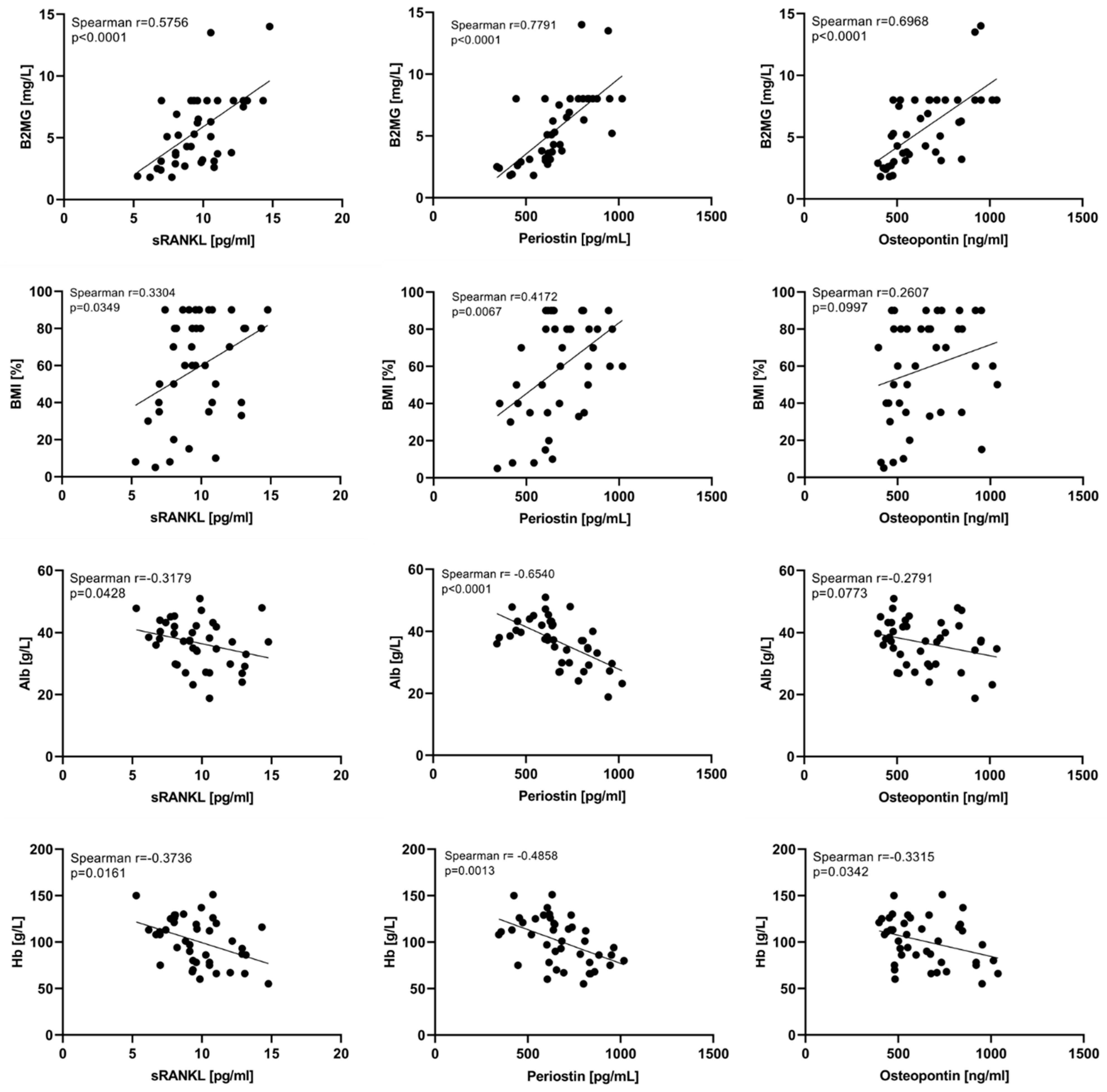
3.3. Predicting Power of sRANKL, Periostin, and Osteopontin
4. Discussion
4.1. Baseline Serum Levels of sRANKL, Periostin, and Osteopontin in Newly Diagnosed Multiple Myeloma Patients
4.2. Variation of sRANKL, Periostin, and Osteopontin Serum Levels in the Course of Treatment and According to the Response to Therapy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padala, S.A.; Barsouk, A.; Barsouk, A.; Rawla, P.; Vakiti, A.; Kolhe, R.; Kota, V.; Ajebo, G.H. Epidemiology, Staging, and Management of Multiple Myeloma. Med. Sci. 2021, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Multiple Myeloma: Every Year a New Standard? Hematol. Oncol. 2019, 37, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Gavriatopoulou, M. Pathogenesis of Bone Disease in Multiple Myeloma: From Bench to Bedside. Blood Cancer J. 2018, 8, 7. [Google Scholar] [CrossRef]
- Hagiwara, M.; Panjabi, S.; Delea, T.; Yucel, E.; Fonseca, R. Burden of Disease Progression in Patients with Multiple Myeloma in the US. Leuk. Lymphoma 2020, 61, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Godavarthy, P.S.; Krause, D.S. The Bone Marrow Microenvironment in Health and Disease at a Glance. J. Cell Sci. 2018, 131, jcs201707. [Google Scholar] [CrossRef]
- Bernstein, Z.S.; Kim, E.B.; Raje, N. Bone Disease in Multiple Myeloma: Biologic and Clinical Implications. Cells 2022, 11, 2308. [Google Scholar] [CrossRef]
- Mukkamalla, S.K.R.; Malipeddi, D. Myeloma Bone Disease: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 6208. [Google Scholar] [CrossRef]
- Gau, Y.-C.; Yeh, T.-J.; Hsu, C.-M.; Hsiao, S.Y.; Hsiao, H.-H. Pathogenesis and Treatment of Myeloma-Related Bone Disease. Int. J. Mol. Sci. 2022, 23, 3112. [Google Scholar] [CrossRef]
- Mansour, A.; Wakkach, A.; Blin-Wakkach, C. Emerging Roles of Osteoclasts in the Modulation of Bone Microenvironment and Immune Suppression in Multiple Myeloma. Front. Immunol. 2017, 8, 954. [Google Scholar] [CrossRef]
- Kim, J.-M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.-H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. BioMed Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef] [PubMed]
- Raje, N.S.; Bhatta, S.; Terpos, E. Role of the RANK/RANKL Pathway in Multiple Myeloma. Clin. Cancer Res. 2019, 25, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, F.; Dang, L.; Liang, C.; Lu, A.; Zhang, G. RANKL/RANK System-Based Mechanism for Breast Cancer Bone Metastasis and Related Therapeutic Strategies. Front. Cell Dev. Biol. 2020, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Buckle, C.H.; De Leenheer, E.; Lawson, M.A.; Yong, K.; Rabin, N.; Perry, M.; Vanderkerken, K.; Croucher, P.I. Soluble Rank Ligand Produced by Myeloma Cells Causes Generalised Bone Loss in Multiple Myeloma. PLoS ONE 2012, 7, e41127. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Szydlo, R.; Apperley, J.F.; Hatjiharissi, E.; Politou, M.; Meletis, J.; Viniou, N.; Yataganas, X.; Goldman, J.M.; Rahemtulla, A. Soluble Receptor Activator of Nuclear Factor κB Ligand–Osteoprotegerin Ratio Predicts Survival in Multiple Myeloma: Proposal for a Novel Prognostic Index. Blood 2003, 102, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Politou, M.; Terpos, E.; Anagnostopoulos, A.; Szydlo, R.; Laffan, M.; Layton, M.; Apperley, J.F.; Dimopoulos, M.; Rahemtulla, A. Role of Receptor Activator of Nuclear Factor-kappa B Ligand (RANKL), Osteoprotegerin and Macrophage Protein 1-alpha (MIP-1a) in Monoclonal Gammopathy of Undetermined Significance (MGUS). Br. J. Haematol. 2004, 126, 686–689. [Google Scholar] [CrossRef]
- Goranova-Marinova, V.; Goranov, S.; Pavlov, P.; Tzvetkova, T. Serum Levels of OPG, RANKL and RANKL/OPG Ratios in Newly-Diagnosed Patients with Multiple Myeloma. Clinical Correlations. Haematologica 2007, 92, 1000–1001. [Google Scholar] [CrossRef] [PubMed]
- Jakob, C.; Goerke, A.; Terpos, E.; Sterz, J.; Heider, U.; Kühnhardt, D.; Ziefle, S.; Kleeberg, L.; Mieth, M.; Von Metzler, I.; et al. Serum Levels of Total-RANKL in Multiple Myeloma. Clin. Lymphoma Myeloma 2009, 9, 430–435. [Google Scholar] [CrossRef]
- Cadieux, B.; Coleman, R.; Jafarinasabian, P.; Lipton, A.; Orlowski, R.Z.; Saad, F.; Scagliotti, G.V.; Shimizu, K.; Stopeck, A. Experience with Denosumab (XGEVA®) for Prevention of Skeletal-Related Events in the 10 Years after Approval. J. Bone Oncol. 2022, 33, 100416. [Google Scholar] [CrossRef]
- Wang, Z.; An, J.; Zhu, D.; Chen, H.; Lin, A.; Kang, J.; Liu, W.; Kang, X. Periostin: An Emerging Activator of Multiple Signaling Pathways. J. Cell Commun. Signal. 2022, 16, 515–530. [Google Scholar] [CrossRef]
- Cobo, T.; Viloria, C.G.; Solares, L.; Fontanil, T.; González-Chamorro, E.; De Carlos, F.; Cobo, J.; Cal, S.; Obaya, A.J. Role of Periostin in Adhesion and Migration of Bone Remodeling Cells. PLoS ONE 2016, 11, e0147837. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, N.; Garnero, P.; Ferrari, S. Periostin Action in Bone. Mol. Cell. Endocrinol. 2016, 432, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J.; Lee, S.H.; Koh, J.-M. Potential Biomarkers to Improve the Prediction of Osteoporotic Fractures. Endocrinol. Metab. 2020, 35, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, J.C.; Sornay-Rendu, E.; Bertholon, C.; Chapurlat, R.; Garnero, P. Serum Periostin Is Associated With Fracture Risk in Postmenopausal Women: A 7-Year Prospective Analysis of the OFELY Study. J. Clin. Endocrinol. Metab. 2014, 99, 2533–2539. [Google Scholar] [CrossRef] [PubMed]
- Sakellariou, G.T.; Anastasilakis, A.D.; Bisbinas, I.; Oikonomou, D.; Gerou, S.; Polyzos, S.A.; Sayegh, F.E. Circulating Periostin Levels in Patients with AS: Association with Clinical and Radiographic Variables, Inflammatory Markers and Molecules Involved in Bone Formation. Rheumatology 2015, 54, 908–914. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kapoor, S. Periostin and Its Emerging Role in Systemic Carcinogenesis. Osteoporos. Int. 2014, 25, 1423–1424. [Google Scholar] [CrossRef] [PubMed]
- Underwood, T.J.; Hayden, A.L.; Derouet, M.; Garcia, E.; Noble, F.; White, M.J.; Thirdborough, S.; Mead, A.; Clemons, N.; Mellone, M.; et al. Cancer-associated Fibroblasts Predict Poor Outcome and Promote Periostin-dependent Invasion in Oesophageal Adenocarcinoma. J. Pathol. 2015, 235, 466–477. [Google Scholar] [CrossRef]
- Wu, G.; Wang, X.; Zhang, X. Clinical Implications of Periostin in the Liver Metastasis of Colorectal Cancer. Cancer Biother. Radiopharm. 2013, 28, 298–302. [Google Scholar] [CrossRef]
- Kim, G.-E.; Lee, J.S.; Park, M.H.; Yoon, J.H. Epithelial Periostin Expression Is Correlated with Poor Survival in Patients with Invasive Breast Carcinoma. PLoS ONE 2017, 12, e0187635. [Google Scholar] [CrossRef]
- Tilman, G.; Mattiussi, M.; Brasseur, F.; Van Baren, N.; Decottignies, A. Human Periostin Gene Expression in Normal Tissues, Tumors and Melanoma: Evidences for Periostin Production by Both Stromal and Melanoma Cells. Mol. Cancer 2007, 6, 80. [Google Scholar] [CrossRef]
- Sasaki, H.; Auclair, D.; Kaji, M.; Fukai, I.; Kiriyama, M.; Yamakawa, Y.; Fujii, Y.; Chen, L.B. Serum Level of the Periostin, a Homologue of an Insect Cell Adhesion Molecule, in Thymoma Patients. Cancer Lett. 2001, 172, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Christoulas, D.; Kastritis, E.; Bagratuni, T.; Gavriatopoulou, M.; Roussou, M.; Papatheodorou, A.; Eleutherakis-Papaiakovou, E.; Kanellias, N.; Liakou, C.; et al. High Levels of Periostin Correlate with Increased Fracture Rate, Diffuse MRI Pattern, Abnormal Bone Remodeling and Advanced Disease Stage in Patients with Newly Diagnosed Symptomatic Multiple Myeloma. Blood Cancer J. 2016, 6, e482. [Google Scholar] [CrossRef] [PubMed]
- Wai, P.Y.; Kuo, P.C. Osteopontin: Regulation in Tumor Metastasis. Cancer Metastasis Rev. 2008, 27, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Kaleta, B.; Boguska, A. Sildenafil, a Phosphodiesterase Type 5 Inhibitor, Downregulates Osteopontin in Human Peripheral Blood Mononuclear Cells. Arch. Immunol. Ther. Exp. 2017, 65, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Denhardt, D.T.; Mistretta, D.; Chambers, A.F.; Krishna, S.; Porter, J.F.; Raghuram, S.; Rittling, S.R. Transcriptional Regulation of Osteopontin and the Metastatic Phenotype: Evidence for a Ras-Activated Enhancer in the Human OPN Promoter. Clin. Exp. Metastasis 2003, 20, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-J.; Cho, H.-J.; Kim, H.-S. Osteopontin: A Multifunctional Protein at the Crossroads of Inflammation, Atherosclerosis, and Vascular Calcification. Curr. Atheroscler. Rep. 2009, 11, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Kim, M.Y.; Lee, K.; Jeong, D. Distinctive and Selective Route of PI3K/PKCα-PKCδ/RhoA-Rac1 Signaling in Osteoclastic Cell Migration. Mol. Cell. Endocrinol. 2016, 437, 261–267. [Google Scholar] [CrossRef]
- Walker, C.G.; Dangaria, S.; Ito, Y.; Luan, X.; Diekwisch, T.G.H. Osteopontin Is Required for Unloading-Induced Osteoclast Recruitment and Modulation of RANKL Expression during Tooth Drift-Associated Bone Remodeling, but Not for Super-Eruption. Bone 2010, 47, 1020–1029. [Google Scholar] [CrossRef]
- Abe, M.; Hiura, K.; Wilde, J.; Shioyasono, A.; Moriyama, K.; Hashimoto, T.; Kido, S.; Oshima, T.; Shibata, H.; Ozaki, S.; et al. Osteoclasts Enhance Myeloma Cell Growth and Survival via Cell-Cell Contact: A Vicious Cycle between Bone Destruction and Myeloma Expansion. Blood 2004, 104, 2484–2491. [Google Scholar] [CrossRef]
- Saeki Mima, Y.T.; Ishii, T.; Ogata, A.; Kobayashi, H.; Ohshima, S.; Ishida, T.; Tabunoki, Y.; Kitayama, H.; Mizuki, M.; Katada, Y.; et al. Enhanced Production of Osteopontin in Multiple Myeloma: Clinical and Pathogenic Implications. Br. J. Haematol. 2003, 123, 263–270. [Google Scholar] [CrossRef]
- Standal, T.; Hjorth-Hansen, H.; Rasmussen, T.; Dahl, I.M.S.; Lenhoff, S.; Brenne, A.-T.; Seidel, C.; Baykov, V.; Waage, A.; Børset, M.; et al. Osteopontin Is an Adhesive Factor for Myeloma Cells and Is Found in Increased Levels in Plasma from Patients with Multiple Myeloma. Haematologica 2004, 89, 174–182. [Google Scholar] [PubMed]
- Minarik, J.; Pika, T.; Bacovsky, J.; Petrova, P.; Langova, K.; Scudla, V. Prognostic Value of Hepatocyte Growth Factor, Syndecan-1, and Osteopontin in Multiple Myeloma and Monoclonal Gammopathy of Undetermined Significance. Sci. World J. 2012, 2012, 356128. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Abe, M.; Hiasa, M.; Oda, A.; Amou, H.; Nakano, A.; Takeuchi, K.; Kitazoe, K.; Kido, S.; Inoue, D.; et al. Myeloma Cell-Osteoclast Interaction Enhances Angiogenesis Together with Bone Resorption: A Role for Vascular Endothelial Cell Growth Factor and Osteopontin. Clin. Cancer Res. 2007, 13, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Updated Diagnostic Criteria and Staging System for Multiple Myeloma. Am. Soc. Clin. Oncol. Educ. Book 2016, 36, e418–e423. [Google Scholar] [CrossRef] [PubMed]
- Greipp, P.R.; Miguel, J.S.; Durie, B.G.M.; Crowley, J.J.; Barlogie, B.; Bladé, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International Staging System for Multiple Myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef]
- Gerov, V.; Gerova, D.; Micheva, I.; Nikolova, M.; Mihaylova, G.; Galunska, B. Dynamics of Bone Disease Biomarkers Dickkopf-1 and Sclerostin in Patients with Multiple Myeloma. JCM 2023, 12, 4440. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group Consensus Criteria for Response and Minimal Residual Disease Assessment in Multiple Myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group Updated Criteria for the Diagnosis of Multiple Myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Al Saleh, A.S.; Parmar, H.V.; Visram, A.; Muchtar, E.; Buadi, F.K.; Go, R.S.; Dispenzieri, A.; Kapoor, P.; Warsame, R.; Lacy, M.Q.; et al. Increased Bone Marrow Plasma-Cell Percentage Predicts Outcomes in Newly Diagnosed Multiple Myeloma Patients. Clin. Lymphoma Myeloma Leuk. 2020, 20, 596–601. [Google Scholar] [CrossRef]
- Pearse, R.N.; Sordillo, E.M.; Yaccoby, S.; Wong, B.R.; Liau, D.F.; Colman, N.; Michaeli, J.; Epstein, J.; Choi, Y. Multiple Myeloma Disrupts the TRANCE/ Osteoprotegerin Cytokine Axis to Trigger Bone Destruction and Promote Tumor Progression. Proc. Natl. Acad. Sci. USA 2001, 98, 11581–11586. [Google Scholar] [CrossRef]
- Sordillo, E.M.; Pearse, R.N. RANK-Fc: A Therapeutic Antagonist for RANK-L in Myeloma. Cancer 2003, 97, 802–812. [Google Scholar] [CrossRef]
- Deleenheer, E. Evidence of a Role for RANKL in the Development of Myeloma Bone Disease. Curr. Opin. Pharmacol. 2004, 4, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Schmiedel, B.J.; Scheible, C.A.; Nuebling, T.; Kopp, H.-G.; Wirths, S.; Azuma, M.; Schneider, P.; Jung, G.; Grosse-Hovest, L.; Salih, H.R. RANKL Expression, Function, and Therapeutic Targeting in Multiple Myeloma and Chronic Lymphocytic Leukemia. Cancer Res. 2013, 73, 683–694. [Google Scholar] [CrossRef]
- Sfiridaki, K.; Pappa, C.A.; Tsirakis, G.; Kanellou, P.; Kaparou, M.; Stratinaki, M.; Sakellaris, G.; Kontakis, G.; Alexandrakis, M.G. Angiogenesis-Related Cytokines, RANKL, and Osteoprotegerin in Multiple Myeloma Patients in Relation to Clinical Features and Response to Treatment. Mediators Inflamm. 2011, 2011, 867576. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kraj, M.; Owczarska, K.; Sokołowska, U.; Centkowski, P.; Pogłód, R.; Kruk, B. Correlation of Osteoprotegerin and sRANKL Concentrations in Serum and Bone Marrow of Multiple Myeloma Patients. Arch. Immunol. Ther. Exp. 2005, 53, 454–464. [Google Scholar]
- Schütt, P.; Rebmann, V.; Brandhorst, D.; Wiefelspütz, J.; Ebeling, P.; Opalka, B.; Seeber, S.; Nowrousian, M.R.; Moritz, T.; Grosse-Wilde, H. The Clinical Significance of Soluble Human Leukocyte Antitgen Class-I, ICTP, and RANKL Molecules in Multiple Myeloma Patients. Hum. Immunol. 2008, 69, 79–87. [Google Scholar] [CrossRef]
- Heider, U.; Langelotz, C.; Jakob, C.; Zavrski, I.; Fleissner, C.; Eucker, J.; Possinger, K.; Hofbauer, L.C.; Sezer, O. Expression of Receptor Activator of Nuclear Factor kappaB Ligand on Bone Marrow Plasma Cells Correlates with Osteolytic Bone Disease in Patients with Multiple Myeloma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 1436–1440. [Google Scholar]
- Slany, A.; Haudek-Prinz, V.; Meshcheryakova, A.; Bileck, A.; Lamm, W.; Zielinski, C.; Gerner, C.; Drach, J. Extracellular Matrix Remodeling by Bone Marrow Fibroblast-like Cells Correlates with Disease Progression in Multiple Myeloma. J. Proteome Res. 2014, 13, 844–854. [Google Scholar] [CrossRef]
- Lamort, A.-S.; Giopanou, I.; Psallidas, I.; Stathopoulos, G.T. Osteopontin as a Link between Inflammation and Cancer: The Thorax in the Spotlight. Cells 2019, 8, 815. [Google Scholar] [CrossRef]
- Dizdar, O.; Barista, I.; Kalyoncu, U.; Karadag, O.; Hascelik, G.; Cila, A.; Pinar, A.; Celik, I.; Kars, A.; Tekuzman, G. Biochemical Markers of Bone Turnover in Diagnosis of Myeloma Bone Disease. Am. J. Hematol. 2007, 82, 185–191. [Google Scholar] [CrossRef]
- Valković, T.; Babarović, E.; Lučin, K.; Štifter, S.; Aralica, M.; Pećanić, S.; Seili-Bekafigo, I.; Duletić-Načinović, A.; Nemet, D.; Jonjić, N. Plasma Levels of Osteopontin and Vascular Endothelial Growth Factor in Association with Clinical Features and Parameters of Tumor Burden in Patients with Multiple Myeloma. BioMed Res. Int. 2014, 2014, 513170. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Lee, J.J.; Lee, W.I. Clinical Significance of Serum Osteopontin in Patients with Multiple Myeloma. Ann. Lab. Med. 2007, 27, 400–405. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maaroufi, A.; Khadem-Ansari, M.-H.; Khalkhali, H.-R.; Rasmi, Y. Serum Levels of Bone Sialoprotein, Osteopontin, and Β2-Microglobulin in Stage I of Multiple Myeloma. J. Cancer Res. Ther. 2020, 16, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Sfiridaki, A.; Miyakis, S.; Pappa, C.; Tsirakis, G.; Alegakis, A.; Kotsis, V.; Stathopoulos, E.; Alexandrakis, M. Circulating Osteopontin: A Dual Marker of Bone Destruction and Angiogenesis in Patients with Multiple Myeloma. J. Hematol. Oncol. 2011, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Colla, S.; Morandi, F.; Lazzaretti, M.; Rizzato, R.; Lunghi, P.; Bonomini, S.; Mancini, C.; Pedrazzoni, M.; Crugnola, M.; Rizzoli, V.; et al. Human Myeloma Cells Express the Bone Regulating Gene Runx2/Cbfa1 and Produce Osteopontin That Is Involved in Angiogenesis in Multiple Myeloma Patients. Leukemia 2005, 19, 2166–2176. [Google Scholar] [CrossRef]
- Babarović, E.; Valković, T.; Budisavljević, I.; Balen, I.; Štifter, S.; Duletić-Načinović, A.; Lučin, K.; Jonjić, N. The Expression of Osteopontin and Vascular Endothelial Growth Factor in Correlation with Angiogenesis in Monoclonal Gammopathy of Undetermined Significance and Multiple Myeloma. Pathol.-Res. Pract. 2016, 212, 509–516. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Colon, K.; Ely, S.; Ely, S.; Chesi, M.; Bergsagel, P.L. Osteopontin Dysregulation and Lytic Bone Lesions in Multiple Myeloma. Hematol. Oncol. 2007, 25, 16–20. [Google Scholar] [CrossRef]
- Liu, Z.; Zeng, Q.; Xiang, B. Bortezomib-Based Regimens Improve the Prognosis of Newly Diagnosed MM Patients with Chromosomal Aberrations except for Del(17q13): A Retrospective Study from a Single Center. Medicine 2021, 100, e25834. [Google Scholar] [CrossRef]
- Eom, K.-S.; Kim, S.J.; Lee, J.-J.; Suh, C.; Kim, J.S.; Yoon, S.-S.; Kim, B.S.; Kang, H.J.; Choi, Y.J.; Kim, C.S.; et al. Changes in Osteoblastic Activity in Patient Who Received Bortezomib as Second Line Treatment for Plasma Cell Myeloma: A Prospective Multicenter Study. BioMed Res. Int. 2014, 2014, 245247. [Google Scholar] [CrossRef][Green Version]
- Lin, L.; Chen, D.; Xiang, Z.-F.; Pei, R.-Z.; Zhang, P.-S.; Liu, X.-H.; Du, X.-H.; Lu, Y. Bortezomib Could Down-Regulate the Expression of RANKL, Inhibit Cell Proliferation and Induce Cell Apoptosis in the Human Myeloma Cell Line RPMI 8226 by Activating Casepase-3. Cancer Biomark. 2017, 20, 217–224. [Google Scholar] [CrossRef]
- Terpos, E.; Heath, D.J.; Rahemtulla, A.; Zervas, K.; Chantry, A.; Anagnostopoulos, A.; Pouli, A.; Katodritou, E.; Verrou, E.; Vervessou, E.-C.; et al. Bortezomib Reduces Serum Dickkopf-1 and Receptor Activator of Nuclear Factor-? B Ligand Concentrations and Normalises Indices of Bone Remodelling in Patients with Relapsed Multiple Myeloma. Br. J. Haematol. 2006, 135, 688–692. [Google Scholar] [CrossRef] [PubMed]



| Time Point | CR + VGPR (n/N, %) | PR + SD (n/N, %) | PD (n/N, %) |
|---|---|---|---|
| T1 | 10/22 (45.5) | 11/22 (50.0) | 1/22 (4.5) |
| T2 | 3/11 (27.3) | 8/11 (72.7) | 0 |
| TA | 10/10 (100.0) | 0 | 0 |
| Parameter | CR + VGPR Median (IQR) | PR + SD Median (IQR) | p Value |
|---|---|---|---|
| sRANKL [pg/mL] | 6.88 (5.92–9.21) | 9.36 (7.20–10.55) | p = 0.0167 |
| Periostin [pg/mL] | 432.0 (594.4–809.9) | 511.9 (432.7–638.1) | p = 0.0134 |
| Osteopontin [ng/mL] | 403.8 (350.2–583.4) | 586.9 (471.4–724.1) | p = 0.0510 |
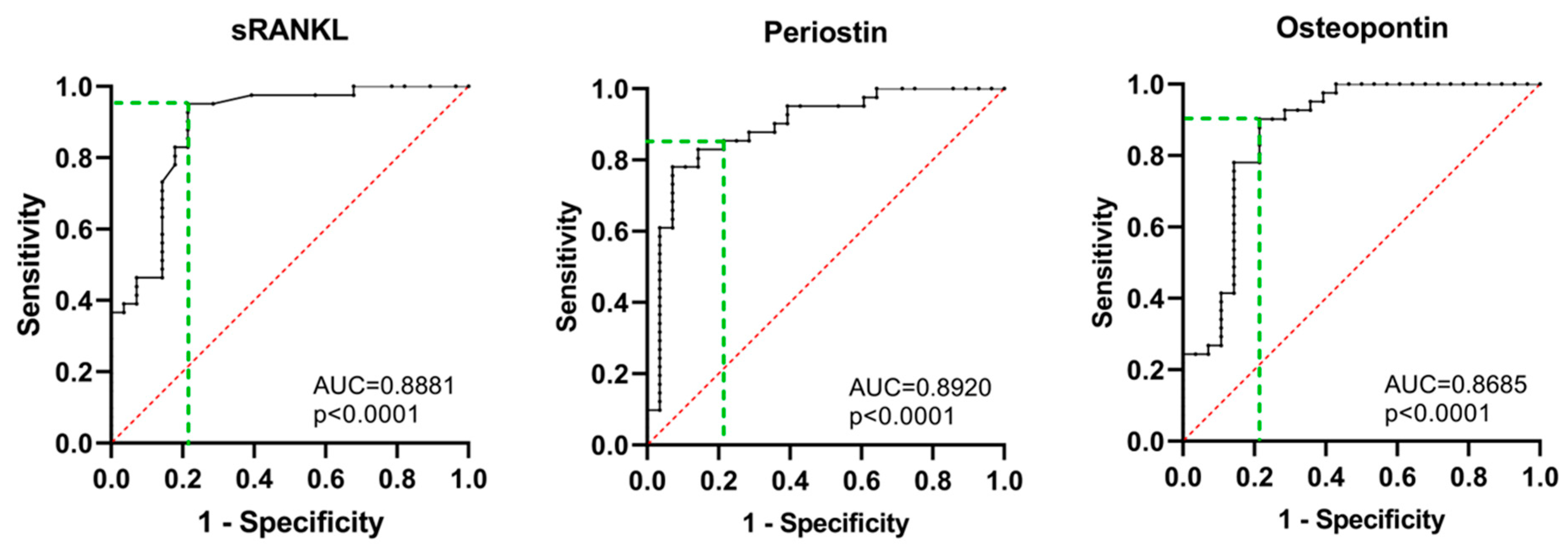
| Parameter | AUC, p Value | Cut-off Value | Diagnostic Sensitivity % (CI) | Diagnostic Specificity % (CI) | Likelihood Ratio |
|---|---|---|---|---|---|
| sRANKL | 0.8881, p < 0.0001 | 6.572 pg/mL | 95.0 (83.9–99.1) | 78.6 (60.5–89.8) | 4.439 |
| Periostin | 0.8920, p < 0.0001 | 473.1 pg/mL | 85.4 (71.6–93.1) | 78.6 (60.5–89.8) | 3.984 |
| Osteopontin | 0.8685, p < 0.0001 | 447.1 ng/mL | 90.2 (77.5–96.1) | 78.6 (60.5–89.8) | 4.211 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerov, V.; Gerova, D.; Micheva, I.; Nikolova, M.; Pasheva, M.; Nazifova, N.; Galunska, B. Circulating sRANKL, Periostin, and Osteopontin as Biomarkers for the Assessment of Activated Osteoclastogenesis in Myeloma Related Bone Disease. Cancers 2023, 15, 5562. https://doi.org/10.3390/cancers15235562
Gerov V, Gerova D, Micheva I, Nikolova M, Pasheva M, Nazifova N, Galunska B. Circulating sRANKL, Periostin, and Osteopontin as Biomarkers for the Assessment of Activated Osteoclastogenesis in Myeloma Related Bone Disease. Cancers. 2023; 15(23):5562. https://doi.org/10.3390/cancers15235562
Chicago/Turabian StyleGerov, Vladimir, Daniela Gerova, Ilina Micheva, Miglena Nikolova, Milena Pasheva, Neshe Nazifova, and Bistra Galunska. 2023. "Circulating sRANKL, Periostin, and Osteopontin as Biomarkers for the Assessment of Activated Osteoclastogenesis in Myeloma Related Bone Disease" Cancers 15, no. 23: 5562. https://doi.org/10.3390/cancers15235562
APA StyleGerov, V., Gerova, D., Micheva, I., Nikolova, M., Pasheva, M., Nazifova, N., & Galunska, B. (2023). Circulating sRANKL, Periostin, and Osteopontin as Biomarkers for the Assessment of Activated Osteoclastogenesis in Myeloma Related Bone Disease. Cancers, 15(23), 5562. https://doi.org/10.3390/cancers15235562





