(Very) Small Stem-like Cells in Human Cell Cultures
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Cell Cultures
2.3. Cell Surface Staining
2.4. DNA Content vs. CD34
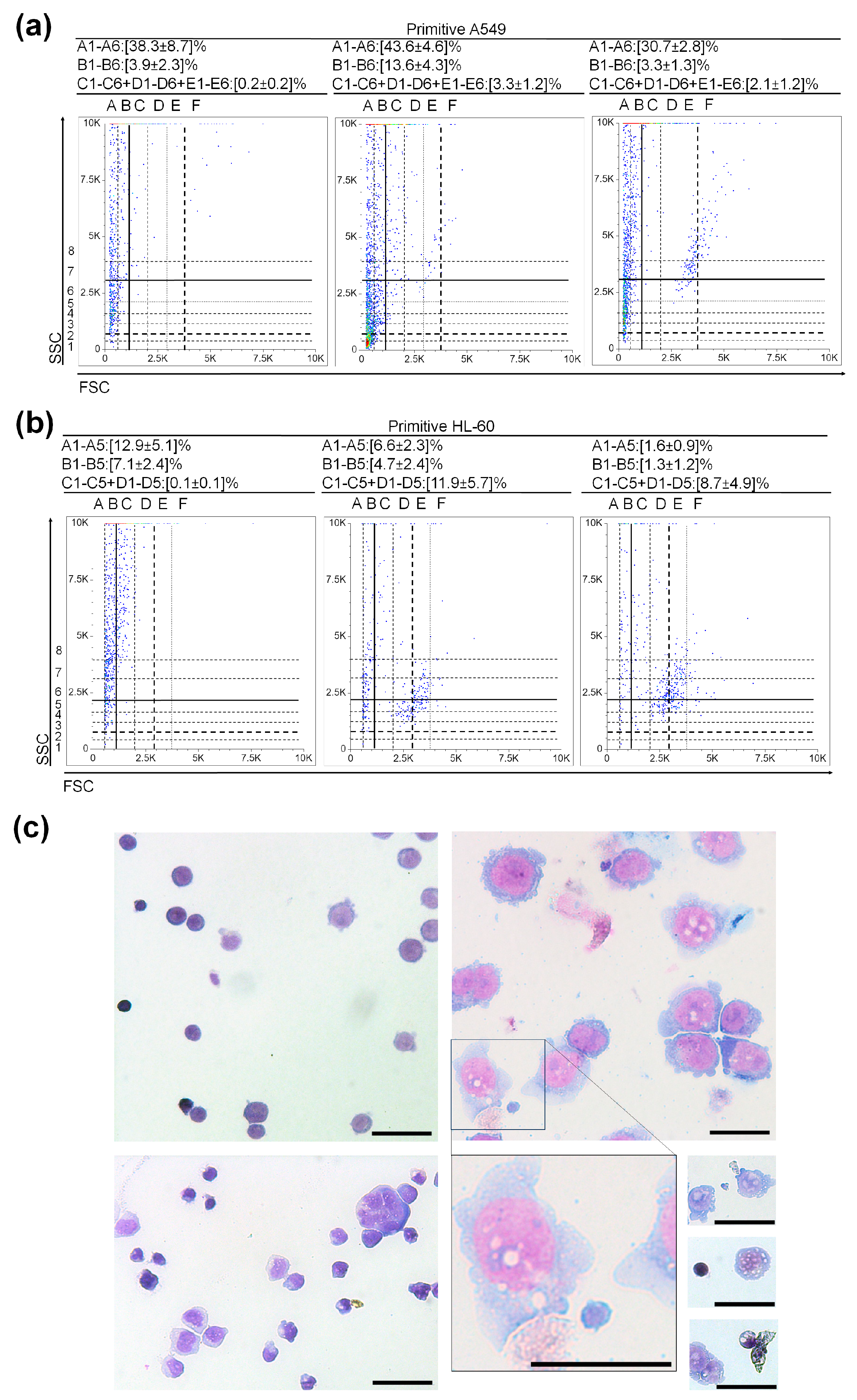
2.5. Development of Primitive HL-60 and Primitive A549
3. Result
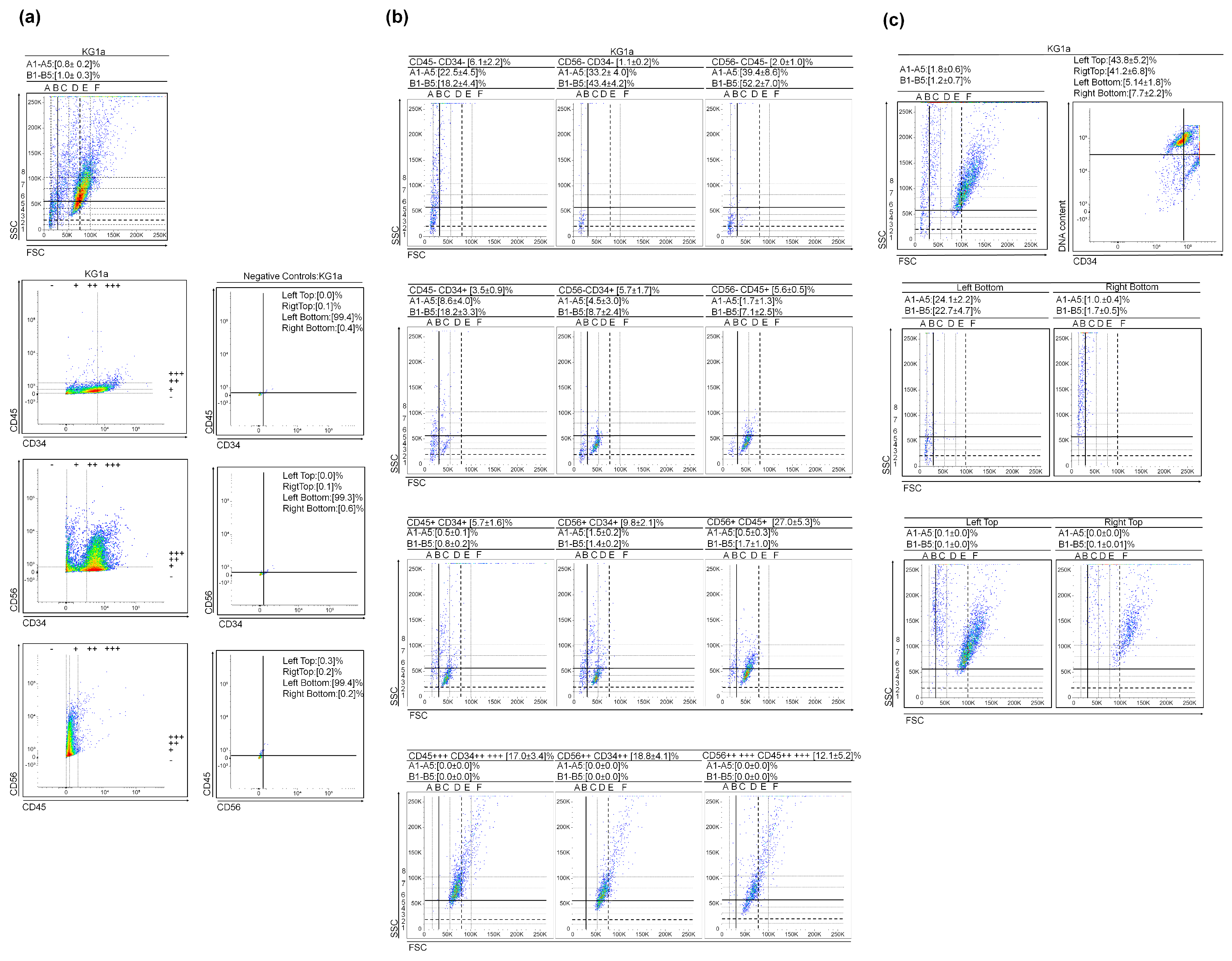
3.1. (Very) Small Leukemic Stem-like Cells in Myeloid Leukemia Cultures
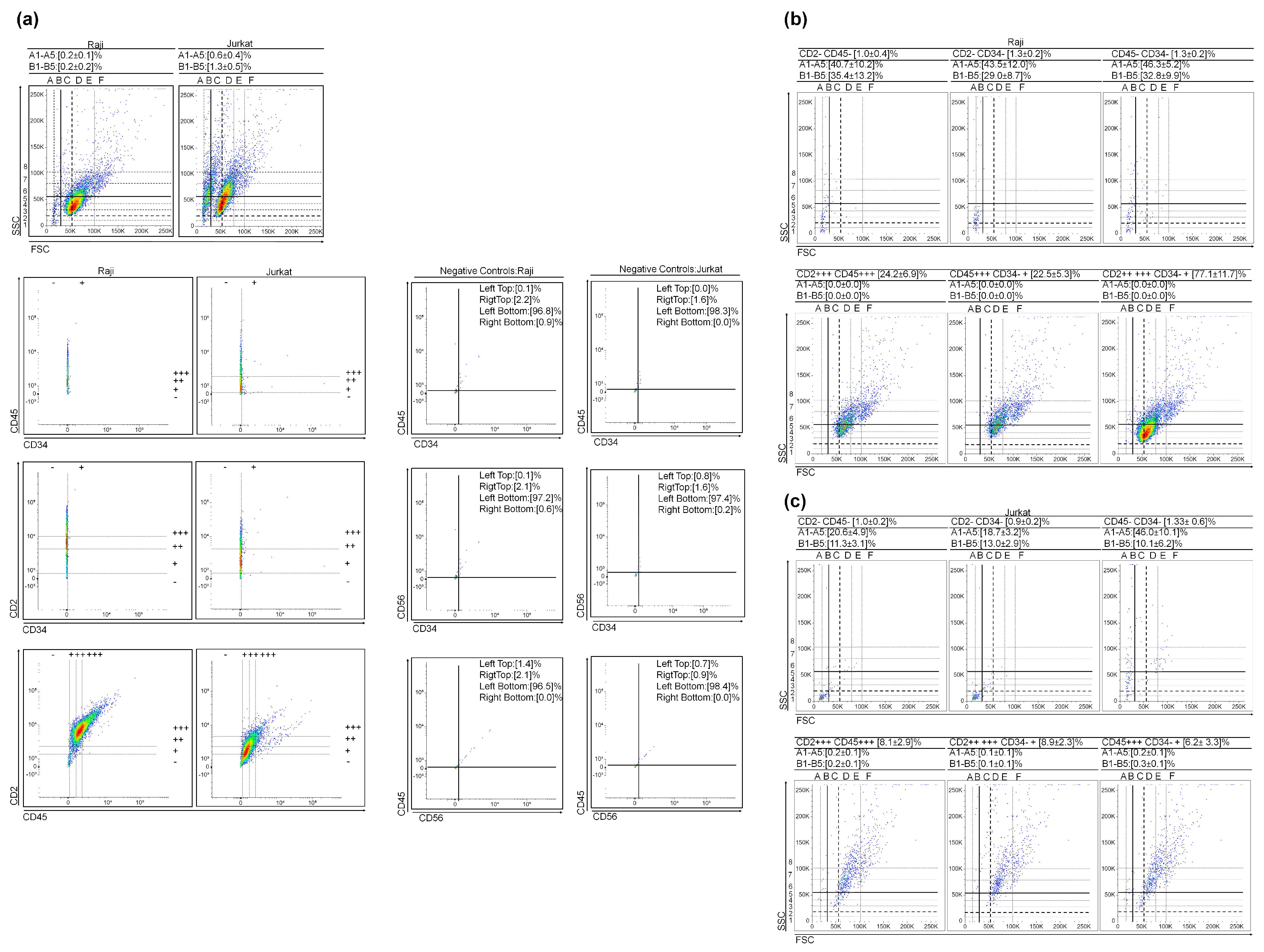
3.2. (Very) Small Leukemic Stem-like Cells in Lymhoid Leukemia Cultures
3.3. Very Small Cancer Stem-like Cells in Non-Small Cell Lung Cancer
3.4. Very Small Embryonic-like Stem Cells in Embryonic Kidney Cell Culture
3.5. Exploring the Functionality of (V)SCSLCs and (V)SLSLCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clarke, M.F.; Fuller, M. Stem Cells and Cancer: Two Faces of Eve. Cell 2006, 124, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Nestor, C.E.; Ottaviano, R.; Reinhardt, D.; Cruickshanks, H.A.; Mjoseng, H.K.; McPherson, R.C.; Lentini, A.; Thomson, J.P.; Dunican, D.S.; Pennings, S.; et al. Rapid Reprogramming of Epigenetic and Transcriptional Profiles in Mammalian Culture Systems. Genome Biol. 2015, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Weissbein, U.; Plotnik, O.; Vershkov, D.; Benvenisty, N. Culture-Induced Recurrent Epigenetic Aberrations in Human Pluripotent Stem Cells. PLoS Genet. 2017, 13, e1006979. [Google Scholar] [CrossRef] [PubMed]
- Bar, S.; Benvenisty, N. Epigenetic Aberrations in Human Pluripotent Stem Cells. EMBO J. 2019, 38, e101033. [Google Scholar] [CrossRef]
- Tognon, C.E.; Sears, R.C.; Mills, G.B.; Gray, J.W.; Tyner, J.W. Ex Vivo Analysis of Primary Tumor Specimens for Evaluation of Cancer Therapeutics. Annu. Rev. Cancer Biol. 2021, 5, 39–57. [Google Scholar] [CrossRef]
- Liu, Z.; Leung, D.; Thrush, K.; Zhao, W.; Ratliff, S.; Tanaka, T.; Schmitz, L.L.; Smith, J.A.; Ferrucci, L.; Levine, M.E. Underlying Features of Epigenetic Aging Clocks In Vivo and In Vitro. Aging Cell 2020, 19, e13229. [Google Scholar] [CrossRef]
- Kabacik, S.; Lowe, D.; Fransen, L.; Leonard, M.; Ang, S.L.; Whiteman, C.; Corsi, S.; Cohen, H.; Felton, S.; Bali, R.; et al. The Relationship Between Epigenetic Age and the Hallmarks of Aging in Human Cells. Nat. Aging 2022, 2, 484–493. [Google Scholar] [CrossRef]
- Kondo, T.; Setoguchi, T.; Taga, T. Persistence of a Small Subpopulation of Cancer Stem-like Cells in the C6 Glioma Cell Line. Proc. Natl. Acad. Sci. USA 2004, 101, 781–786. [Google Scholar] [CrossRef]
- Bonnet, D.; Dick, J.E. Human Acute Myeloid Leukemia is Organized as a Hierarchy that Originates from a Primitive Hematopoietic Cell. Nat. Med. 1997, 3, 730–737. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Ratajczak, J.; Kucia, M. Very Small Embryonic-Like Stem Cells (VSELs). Circ. Res. 2019, 124, 208–210. [Google Scholar] [CrossRef]
- Dias Câmara, D.A.; Luiz de Sá Junior, P.; Alexandre de Azevedo, R.; Figueiredo, C.R.; Araldi, R.P.; Levy, D.; Madeiro de Souza, D.; Kerkis, I. Identification of Very Small Cancer Stem Cells Expressing Hallmarks of Pluripotency in B16F10 Melanoma Cells and Their Reoccurrence in B16F10-derived clones. Exp. Cell Res. 2020, 391, 111938. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, D.; Sharma, N.; Dutta, S.; Kumar, P.; Tripathi, A.; Tripathi, A. Very Small Embryonic-Like Stem Cells Transform into Cancer Stem Cells and Are Novel Candidates for Detecting/Monitoring Cancer by a Simple Blood Test. Stem Cells 2023, 41, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, D.; Jha, N.; Tripathi, A.; Tripathi, A. Very Small Embryonic-Like Stem Cells Have the Potential to Win the Three-front war on tissue damage, cancer, and aging. Front. Cell Dev. Biol. 2023, 10, 1061022. [Google Scholar] [CrossRef] [PubMed]
- Tirino, V.; Camerlingo, R.; Bifulco, K.; Irollo, E.; Montella, R.; Paino, F.; Sessa, G.; Carriero, M.V.; Normanno, N.; Rocco, G.; et al. TGF-β1 Exposure Induces Epithelial to Mesenchymal Transition Both in CSCs and non-CSCs of the A549 Cell Line, Leading to an Increase of Migration Ability in the CD133+ A549 Cell Fraction. Cell Death Dis. 2013, 4, e620. [Google Scholar] [CrossRef] [PubMed]
- Lica, J.J.; Grabe, G.J.; Heldt, M.; Misiak, M.; Bloch, P.; Serocki, M.; Switalska, M.; Wietrzyk, J.; Baginski, M.; Hellmann, A.; et al. Cell Density-Dependent Cytological Stage Profile and Its Application for a Screen of Cytostatic Agents Active Toward Leukemic Stem Cells. Stem Cells Dev. 2018, 27, 488–513. [Google Scholar] [CrossRef] [PubMed]
- Lica, J.J.; Wieczór, M.; Grabe, G.J.; Heldt, M.; Jancz, M.; Misiak, M.; Gucwa, K.; Brankiewicz, W.; Maciejewska, N.; Stupak, A.; et al. Effective Drug Concentration and Selectivity Depends on Fraction of Primitive Cells. Int. J. Mol. Sci. 2021, 22, 4931. [Google Scholar] [CrossRef]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M.; Reya, T. Cancer stem cells—Perspectives on current status and future directions. Nat. Med. 1997, 3, 1366–1371. [Google Scholar]
- Sovalat, H.; Scrofani, M.; Eidenschenk, A.; Pasquet, S.; Rimelen, V.; Hénon, P. Identification and isolation from either adult human bone marrow or G-CSF-mobilized peripheral blood of CD34(+)/CD133(+)/CXCR4(+)/ Lin(−)CD45(−) cells, featuring morphological, molecular, and phenotypic characteristics of very small embryonic-like (VSEL) stem cells. Exp. Hematol. 2011, 39, 495–505. [Google Scholar]
- Gunjal, P.; Pedziwiatr, D.; Ismail, A.A.; Kakar, S.S.; Ratajczak, M.Z. An emerging question about putative cancer stem cells in established cell lines-are they true stem cells or a fluctuating cell phenotype? J. Cancer Stem Cell Res. 2015, 3, e1004. [Google Scholar] [CrossRef]
- Sellers, Z.P.; Schneider, G.; Bujko, K.; Suszynska, M.; Pedziwiatr, D. Do Cancer Cell Lines Have Fixed or Fluctuating Stem Cell Phenotypes?—Studies with the NTera2 Cell Line. Stem Cell Rev. Rep. 2017, 13, 603–610. [Google Scholar] [CrossRef]
- Kuruca, S.E.; Çelik, D.D.; Özerkan, D.; Erdemir, G. Characterization and Isolation of Very Small Embryonic-like (VSEL) Stem Cells Obtained from Various Human Hematopoietic Cell Sources. Stem Cell Rev. Rep. 2019, 15, 730–742. [Google Scholar] [CrossRef]
- Filidou, E.; Kandilogiannakis, L.; Tarapatzi, G.; Spathakis, M.; Su, C.; Rai, A.; Greening, D.W.; Arvanitidis, K.; Paspaliaris, V.; Kolios, G. A Simplified and Effective Approach for the Isolation of Small Pluripotent Stem Cells Derived from Human Peripheral Blood. Biomedicines 2023, 11, 787. [Google Scholar] [CrossRef]
- Lacombe, F.; Durrieu, F.; Briais, A.; Dumain, P.; Belloc, F.; Bascans, E.; Reiffers, J.; Boisseau, M.R.; Bernard, P. Flow cytometry CD45 gating for immunophenotyping of acute myeloid leukemia. Leukemia 1997, 11, 1878–1886. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.J.; Lhermitte, L.; Böttcher, S.; Almeida, J.; van der Velden, V.H.; Flores-Montero, J.; Rawstron, A.; Asnafi, V.; Lécrevisse, Q.; Lucio, P.; et al. EuroFlow Consortium (EU-FP6, LSHB-CT-2006-018708) EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia 2012, 26, 1908–1975. [Google Scholar] [CrossRef] [PubMed]
- Glier, H.; Heijnen, I.; Hauwel, M.; Dirks, J.; Quarroz, S.; Lehmann, T.; Rovo, A.; Arn, K.; Matthes, T.; Hogan, C.; et al. Standardization of 8-color flow cytometry across different flow cytometer instruments: A feasibility study in clinical laboratories in Switzerland. J. Immunol. Methods 2019, 475, 112348. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L.; Testi, R.; Bindl, J.; Phillips, J.H. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J. Exp. Med. 1989, 169, 2233–2238. [Google Scholar] [CrossRef]
- Zheng, D.; Zhu, M.; Li, Q.; Wan, W.; Chen, Y.; Jing, H. Dual Negativity of CD56 and CD117 Links to Unfavorable Cytogenetic Abnormalities and Predicts Poor Prognosis in Multiple Myeloma. J. Clin. Med. 2022, 11, 6524. [Google Scholar] [CrossRef]
- Dong, Y.; Li, Y.; Liu, R.; Li, Y.; Zhang, H.; Liu, H.; Chen, J. Secretagogin, a marker for neuroendocrine cells, is more sensitive and specific in large cell neuroendocrine carcinoma compared with the markers CD56, CgA, Syn and Napsin A. Oncol. Lett. 2020, 19, 2223–2230. [Google Scholar] [CrossRef]
- Zhang, D.; Teng, R.; Lv, N.; Lei, L.; Wang, Y.; Williamson, R.A.; Chen, P.; Gao, P.; O’Dwyer, M.; Li, A.; et al. A novel CD2 staining-based flow cytometric assay for assessment of natural killer cell cytotoxicity. J. Clin. Lab. Anal. 2020, 34, e23519. [Google Scholar] [CrossRef]
- Skladanowski, A.; Bozko, P.; Sabisz, M. DNA Structure and Integrity Checkpoints During the Cell Cycle and Their Role in Drug Targeting and Sensitivity of Tumor Cells to Anticancer Treatment. Chem. Rev. 2009, 109, 2951–2973. [Google Scholar] [CrossRef]
- Hayflick, L. The Limited In Vitro Life of Human Diploid Cell Strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lica, J.J.; Pradhan, B. (Very) Small Stem-like Cells in Human Cell Cultures. Cancers 2023, 15, 5520. https://doi.org/10.3390/cancers15235520
Lica JJ, Pradhan B. (Very) Small Stem-like Cells in Human Cell Cultures. Cancers. 2023; 15(23):5520. https://doi.org/10.3390/cancers15235520
Chicago/Turabian StyleLica, Jan Jakub, and Bhaskar Pradhan. 2023. "(Very) Small Stem-like Cells in Human Cell Cultures" Cancers 15, no. 23: 5520. https://doi.org/10.3390/cancers15235520
APA StyleLica, J. J., & Pradhan, B. (2023). (Very) Small Stem-like Cells in Human Cell Cultures. Cancers, 15(23), 5520. https://doi.org/10.3390/cancers15235520