Efficacy of Total En Bloc Spondylectomy versus Stereotactic Ablative Radiotherapy for Single Spinal Metastasis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgery and Radiotherapy
2.3. Endpoints
2.4. Statistical Considerations
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spratt, D.E.; Beeler, W.H.; de Moraes, F.Y.; Rhines, L.D.; Gemmete, J.J.; Chaudhary, N.; Shultz, D.B.; Smith, S.R.; Berlin, A.; Dahele, M.; et al. An integrated multidisciplinary algorithm for the management of spinal metastases: An international spine oncology consortium report. Lancet Oncol. 2017, 18, e720–e730. [Google Scholar] [CrossRef]
- Lee, C.S.; Jung, C.H. Metastatic spinal tumor. Asian Spine J. 2012, 6, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Van den Brande, R.; Cornips, E.M.; Peeters, M.; Ost, P.; Billiet, C.; Van de Kelft, E. Epidemiology of spinal metastases, metastatic epidural spinal cord compression and pathologic vertebral compression fractures in patients with solid tumors: A systematic review. J. Bone Oncol. 2022, 35, 100446. [Google Scholar] [CrossRef] [PubMed]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Payne, R.; Saris, S.; Kryscio, R.J.; Mohiuddin, M.; Young, B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet 2005, 366, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Disch, A.C.; Schaser, K.D.; Melcher, I.; Feraboli, F.; Schmoelz, W.; Druschel, C.; Luzzati, A. Oncosurgical results of multilevel thoracolumbar en-bloc spondylectomy and reconstruction with a carbon composite vertebral body replacement system. Spine 2011, 36, E647–E655. [Google Scholar] [CrossRef]
- Ohashi, M.; Hirano, T.; Watanabe, K.; Hasegawa, K.; Ito, T.; Katsumi, K.; Shoji, H.; Mizouchi, T.; Takahashi, I.; Homma, T.; et al. En bloc spondylectomy for spinal metastases: Detailed oncological outcomes at a minimum of 2 years after surgery. Asian Spine J. 2019, 13, 296–304. [Google Scholar] [CrossRef]
- Faruqi, S.; Chen, H.B.; Fariselli, L.; Levivier, M.; Ma, L.; Paddick, I.; Pollock, B.E.; Regis, J.; Sheehan, J.; Suh, J.; et al. Stereotactic radiosurgery for postoperative spine malignancy: A systematic review and international stereotactic radiosurgery society practice guidelines. Pract. Radiat. Oncol. 2022, 12, e65–e78. [Google Scholar] [CrossRef]
- Laufer, I.; Iorgulescu, J.B.; Chapman, T.; Lis, E.; Shi, W.; Zhang, Z.; Cox, B.W.; Yamada, Y.; Bilsky, M.H. Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: Outcome analysis in 186 patients. J. Neurosurg. Spine 2013, 18, 207–214. [Google Scholar] [CrossRef]
- Laufer, I.; Rubin, D.G.; Lis, E.; Cox, B.W.; Stubblefield, M.D.; Yamada, Y.; Bilsky, M.H. The noms framework: Approach to the treatment of spinal metastatic tumors. Oncologist 2013, 18, 744–751. [Google Scholar] [CrossRef]
- Moussazadeh, N.; Laufer, I.; Yamada, Y.; Bilsky, M.H. Separation surgery for spinal metastases: Effect of spinal radiosurgery on surgical treatment goals. Cancer Control 2014, 21, 168–174. [Google Scholar] [CrossRef]
- Bilsky, M.H.; Laufer, I.; Burch, S. Shifting paradigms in the treatment of metastatic spine disease. Spine 2009, 34, S101–S107. [Google Scholar] [CrossRef]
- Garg, A.K.; Shiu, A.S.; Yang, J.; Wang, X.S.; Allen, P.; Brown, B.W.; Grossman, P.; Frija, E.K.; McAleer, M.F.; Azeem, S.; et al. Phase 1/2 trial of single-session stereotactic body radiotherapy for previously unirradiated spinal metastases. Cancer 2012, 118, 5069–5077. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.C.; Tang, C.; Deegan, B.J.; Allen, P.K.; Jonasch, E.; Amini, B.; Wang, X.A.; Li, J.; Tatsui, C.E.; Rhines, L.D.; et al. The use of spine stereotactic radiosurgery for oligometastatic disease. J. Neurosurg. Spine 2016, 25, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Chang, B.S.; Kim, H.; Kang, D.H.; Chang, S.Y. An updated review on the treatment strategy for spinal metastasis from the spine surgeon’s perspective. Asian Spine J. 2022, 16, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Mok, S.; Park, S.C.; Kim, H.; Chang, B.S. Treatment strategy for metastatic spinal tumors: A narrative review. Asian Spine J. 2020, 14, 513–525. [Google Scholar] [CrossRef]
- Yamada, Y.; Katsoulakis, E.; Laufer, I.; Lovelock, M.; Barzilai, O.; McLaughlin, L.A.; Zhang, Z.; Schmitt, A.M.; Higginson, D.S.; Lis, E.; et al. The impact of histology and delivered dose on local control of spinal metastases treated with stereotactic radiosurgery. Neurosurg. Focus. 2017, 42, E6. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Nuyttens, J.J.; Desideri, I.; Greto, D.; Livi, L. Single-fraction radiotherapy (sfrt) for bone metastases: Patient selection and perspectives. Cancer Manag. Res. 2019, 11, 9397–9408. [Google Scholar] [CrossRef]
- Zeng, K.L.; Tseng, C.L.; Soliman, H.; Weiss, Y.; Sahgal, A.; Myrehaug, S. Stereotactic body radiotherapy (sbrt) for oligometastatic spine metastases: An overview. Front. Oncol. 2019, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (sabr-comet): A randomised, phase 2, open-label trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef]
- Tomita, K.; Kawahara, N.; Kobayashi, T.; Yoshida, A.; Murakami, H.; Akamaru, T. Surgical strategy for spinal metastases. Spine 2001, 26, 298–306. [Google Scholar] [CrossRef]
- Bayram, S.; Akgül, T.; Altan, M.; Pehlivanoğlu, T.; Kaya, Ö.; Özdemir, M.A.; Şar, C. Palliative posterior instrumentation versus corpectomy with cage reconstruction treatment for thoracolumbar pathological fracture. Asian Spine J. 2019, 13, 318–324. [Google Scholar] [CrossRef]
- Lee, B.H.; Park, J.O.; Kim, H.S.; Park, Y.C.; Lee, H.M.; Moon, S.H. Perioperative complication and surgical outcome in patients with spine metastases: Retrospective 200-case series in a single institute. Clin. Neurol. Neurosurg. 2014, 122, 80–86. [Google Scholar] [CrossRef]
- Kieser, D.C.; Parker, J.; Reynolds, J. En bloc resection of isolated spinal metastasis a systematic review update. Clin. Spine Surg. 2021, 34, 103–106. [Google Scholar] [CrossRef]
- Chapman, E.K.; Valliani, A.A.; Shuman, W.H.; Martini, M.L.; Neifert, S.N.; Gilligan, J.T.; Yuk, F.J.; Schupper, A.J.; Gal, J.S.; Caridi, J.M. Clinical trials in spinal tumors: A two-decade review. World Neurosurg. 2022, 161, e39–e53. [Google Scholar] [CrossRef] [PubMed]
- Tokuhashi, Y.; Matsuzaki, H.; Oda, H.; Oshima, M.; Ryu, J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 2005, 30, 2186–2191. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, J.H.; Kim, K.; Kim, H.J.; Chie, E.K.; Shin, K.H.; Wu, H.G.; Kim, I.H. The feasibility of spinal stereotactic radiosurgery for spinal metastasis with epidural cord compression. Cancer Res. Treat. 2019, 51, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.W.; Spratt, D.E.; Lovelock, M.; Bilsky, M.H.; Lis, E.; Ryu, S.; Sheehan, J.; Gerszten, P.C.; Chang, E.; Gibbs, I.; et al. International spine radiosurgery consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e597–e605. [Google Scholar] [CrossRef] [PubMed]
- Rockville, M. National Cancer Institute. Common terminology criteria for adverse events (ctcae). In Common Terminology Criteria for Adverse Events (Ctcae); National Cancer Institute: Washington, DC, USA, 2018. [Google Scholar]
- Zheng, J.P.; Wu, L.Y.; Shi, J.D.; Niu, N.; Yang, Z.; Ding, H. Hybrid therapy versus total en bloc spondyectomy in the treatment of solitary radioresistant spinal metastases a single-center, retrospective study. Clin. Spine Surg. 2022, 35, E457–E465. [Google Scholar] [CrossRef]
- Pennington, Z.; Pairojboriboon, S.; Chen, X.; Sacino, A.; Elsamadicy, A.A.; Ramos, R.G.; Patel, J.; Elder, B.D.; Kleinberg, L.R.; Sciubba, D.M.; et al. Utility of expanded anterior column resection versus decompression-alone for local control in the management of carcinomatous vertebral column metastases undergoing adjuvant stereotactic radiotherapy. Spine J. 2022, 22, 835–846. [Google Scholar] [CrossRef]
- Ning, M.S.; Deegan, B.J.; Ho, J.C.; Chapman, B.V.; Bishop, A.J.; Allen, P.K.; Tannir, N.M.; Amini, B.; Briere, T.M.; Wang, X.A.; et al. Low incidence of late failure and toxicity after spine stereotactic radiosurgery: Secondary analysis of phase i/ii trials with long-term follow-up. Radiother. Oncol. 2019, 138, 80–85. [Google Scholar] [CrossRef]
- Diao, K.; Song, J.H.; Thall, P.F.; McGinnis, G.J.; Boyce-Fappiano, D.; Amini, B.; Brown, P.D.; Yeboa, D.N.; Bishop, A.J.; Li, J.; et al. Low risk of radiation myelopathy with relaxed spinal cord dose constraints in de novo, single fraction spine stereotactic radiosurgery. Radiother. Oncol. 2020, 152, 49–55. [Google Scholar] [CrossRef]
- Guo, L.L.; Ke, L.X.; Zeng, Z.Y.; Yuan, C.P.; Wu, Z.W.; Chen, L.; Lu, L.X. Stereotactic body radiotherapy for spinal metastases: A review. Med. Oncol. 2022, 39, 34. [Google Scholar] [CrossRef] [PubMed]
- Al-Omair, A.; Masucci, L.; Masson-Cote, L.; Campbell, M.; Atenafu, E.G.; Parent, A.; Letourneau, D.; Yu, E.; Rampersaud, R.; Massicotte, E.; et al. Surgical resection of epidural disease improves local control following postoperative spine stereotactic body radiotherapy. Neuro-Oncology 2013, 15, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.; Sahgal, A.; Soliman, H.; Myrehaug, S.; Yang, V.X.D.; Das, S.; Wilson, J.; Campbell, M.; Lee, Y.K.; Cawricz, M.; et al. Postoperative stereotactic body radiotherapy for spinal metastases and the impact of epidural disease grade. Neurosurgery 2019, 85, E1111–E1118. [Google Scholar] [CrossRef] [PubMed]
- Ciérvide, R.; Hernando, O.; López, M.; Montero, A.; Zucca, D.; Sánchez, E.; Alvarez, B.; García-Aranda, M.; Zhao, X.C.; Valero, J.; et al. Stereotactic body radiation therapy (SBRT) for spinal metastases: 12 years of a single center experience. Clin. Transl. Oncol. 2023, 25, 3395–3404. [Google Scholar] [CrossRef]
- Kato, S.; Demura, S.; Kitagawa, R.; Yokogawa, N.; Shimizu, T.; Kobayashi, M.; Yamada, Y.; Nagatani, S.; Murakami, H.; Kawahara, N.; et al. Clinical outcomes following total en bloc spondylectomy for spinal metastases from lung cancer. J. Orthop. Sci. 2023, 4, S0949–S2658. [Google Scholar] [CrossRef]
- Cao, S.; Gao, X.; Zhang, Y.; Wang, Y.; Wang, J.; Wang, T.; Liu, Y.; Hou, S.; Zhang, J.; Zhou, Y.; et al. A comparison of two different surgical procedures in the treatment of isolated spinal metastasis patients with metastatic spinal cord compression: A case-control study. Eur. Spine J. 2022, 31, 1583–1589. [Google Scholar] [CrossRef]
- Tran, J.; Ornstein, M.C. Clinical review on the management of metastatic renal cell carcinoma. JCO Oncol. Pract. 2022, 18, 187–196. [Google Scholar] [CrossRef]
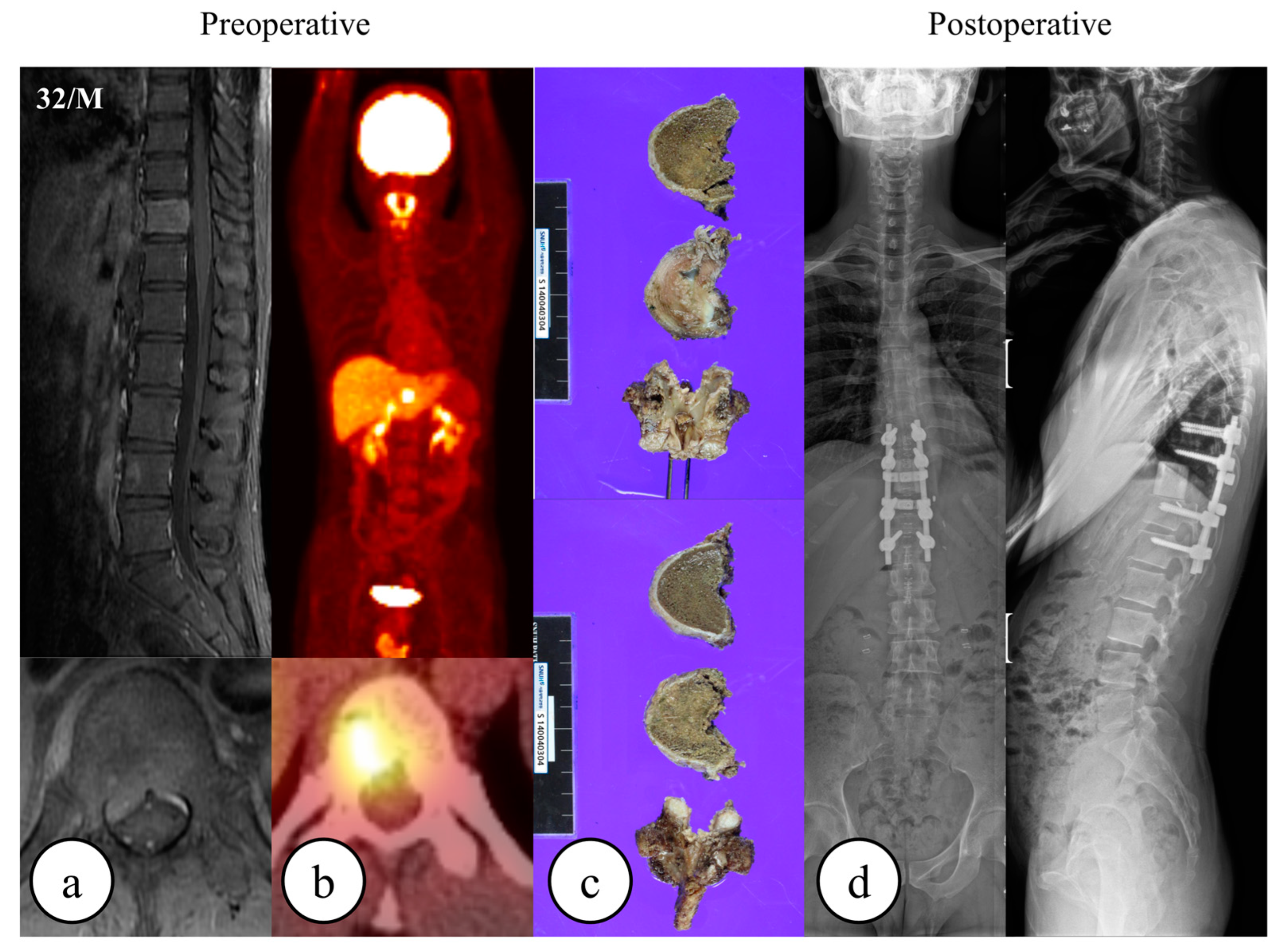

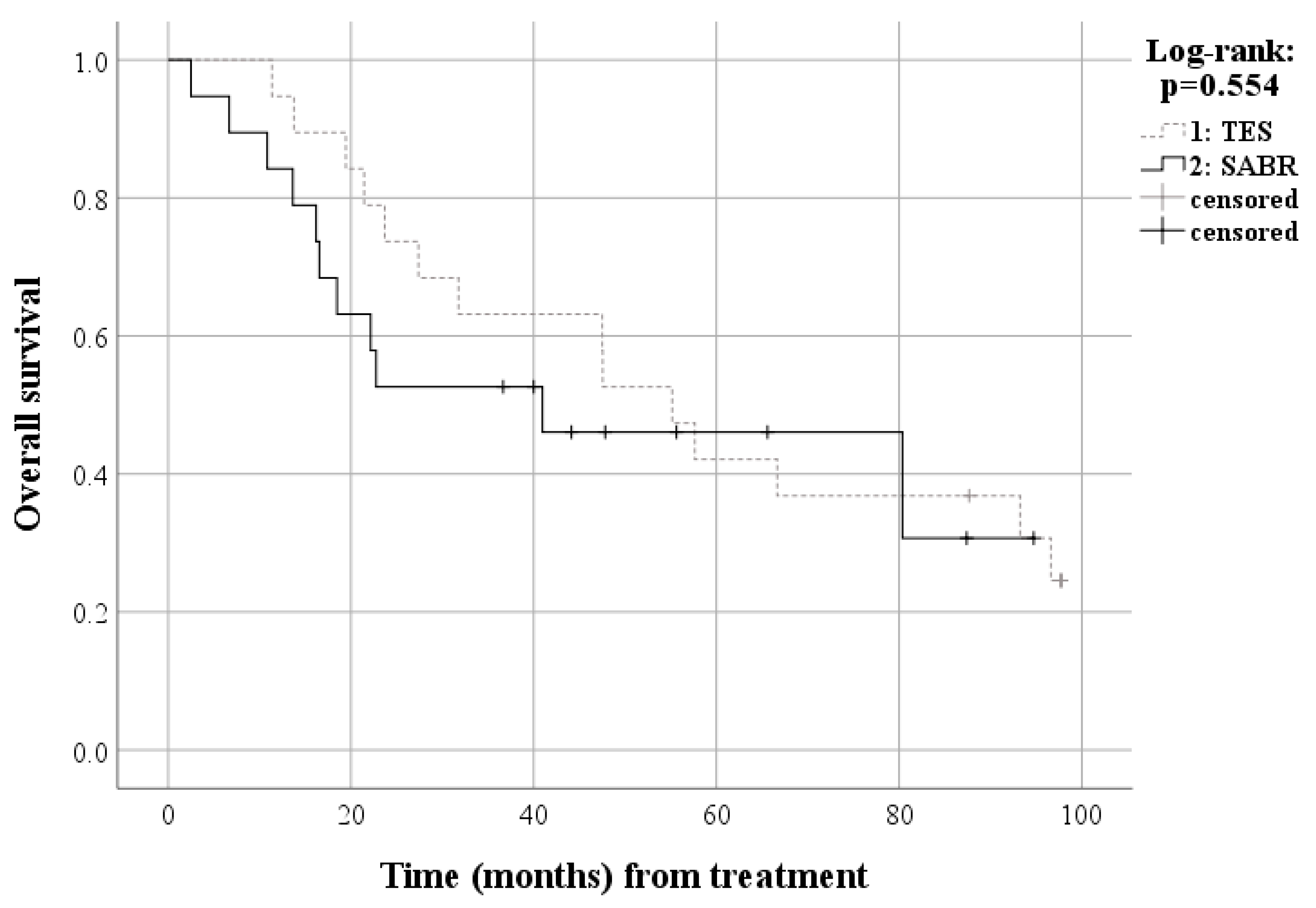

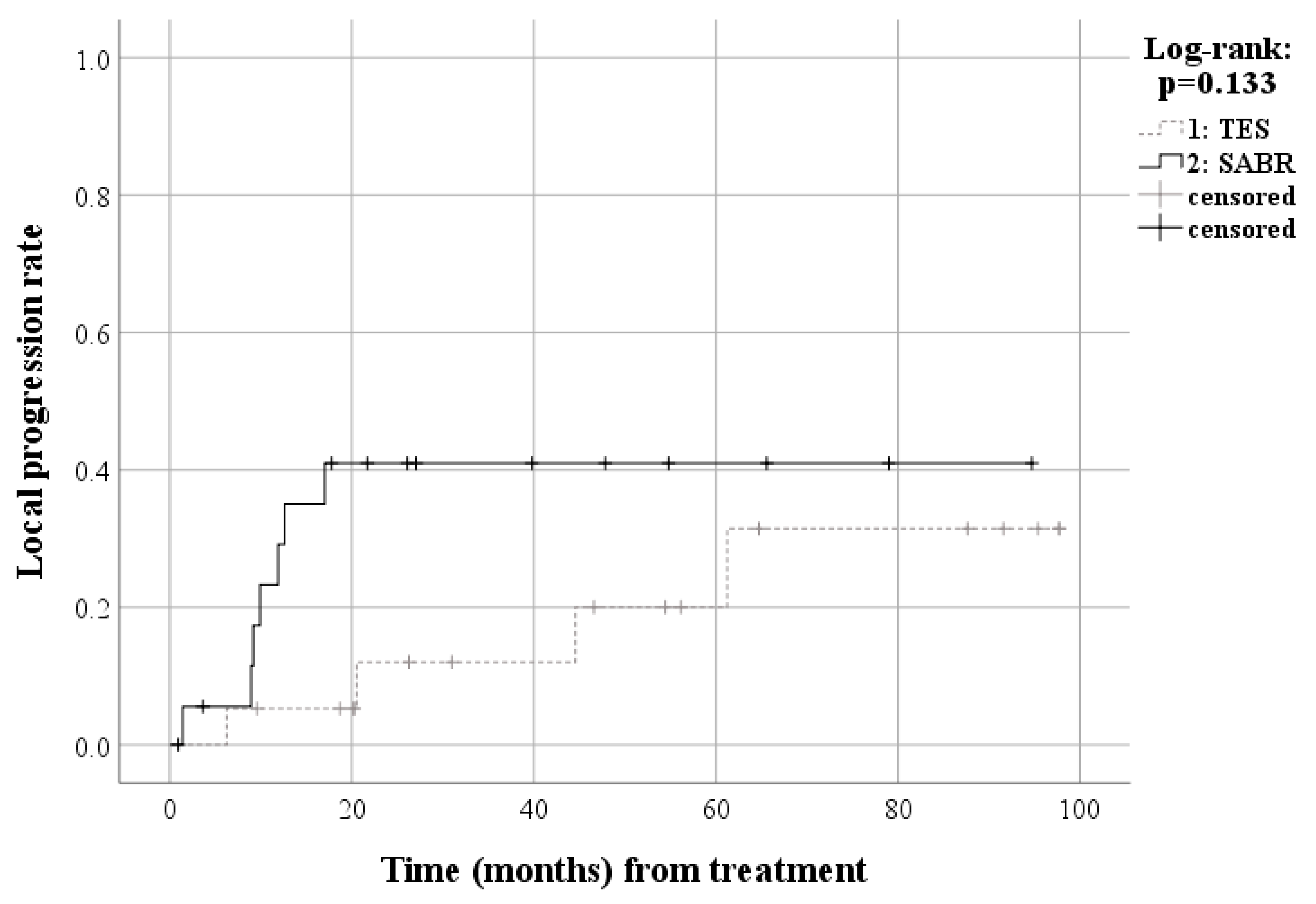

| Baseline Characteristics | All Patients | Matched Patients | ||||
|---|---|---|---|---|---|---|
| TES (n = 20) | SABR (n = 69) | p-Value | TES (n = 19) | SABR (n = 19) | p-Value | |
| Age (mean ± SD, years) | 53.8 ± 13.3 | 57.4 ± 11.0 | 0.226 | 53.8 ± 13.6 | 61.8 ± 10.3 | 0.048 |
| Sex, Male (%) | 85.0 | 49.3 | 0.004 | 84.2 | 73.7 | 0.426 |
| BMI (mean ± SD, kg/m2) | 22.5 ± 2.2 | 22.5 ± 3.3 | 0.975 | 22.7 ± 2.2 | 22.1 ± 3.6 | 0.605 |
| Follow-up period (mean ± SD, month) | 56.6 ± 38.4 | 30.4 ± 25.8 | 0.008 | 55.7 ± 34.1 | 35.8 ± 28.5 | 0.050 |
| Time from diagnosis of spinal metastasis to treatment (mean ± SD, month) | 3.3 ± 6.8 | 3.0 ± 5.5 | 0.838 | 3.2 ± 7.0 | 2.7 ± 2.7 | 0.754 |
| Involved Spinal level | 0.215 | 0.615 | ||||
| Cervical | 1 (5.0%) | 9 (13.0%) | 1 (5.3%) | 2 (10.5%) | ||
| Thoracic | 13 (65.0%) | 30 (43.5%) | 12 (63.2%) | 9 (47.4%) | ||
| Lumbar | 6 (30.0%) | 30 (43.5%) | 6 (31.6%) | 8 (42.1%) | ||
| Histology | 0.003 | 0.412 | ||||
| RCC | 6 (30.0%) | 19 (27.5%) | 6 (31.6%) | 6 (31.6%) | ||
| Thyroid | 4 (20.0%) | 2 (2.9%) | 4 (21.1%) | 2 (10.5%) | ||
| Liver | 3 (15.0%) | 18 (26.1%) | 3 (15.8%) | 6 (31.6%) | ||
| Breast | 2 (10.0%) | 21 (30.4%) | 2 (10.5%) | 4 (21.1%) | ||
| NSCLC | 1 (5.0%) | 8 (11.6%) | 1 (5.3%) | 1 (5.3%) | ||
| Esophageal | 1 (5.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Others | 3 (15.0%) | 1 (1.4%) | 3 (15.0%) | 0 (0.0%) | ||
| SINS (median [IQR]) | 9.0 [5.25–10.75] | 6.0 [5.0–9.0] | 0.156 | 9.0 [5.0–10.0] | 6.0 [6.0–10.0] | 0.779 |
| Tomita’s surgical classification | 0.434 | 0.734 | ||||
| 1 | 7 (35.0%) | 23 (33.3%) | 6 (31.6%) | 6 (31.6%) | ||
| 2 | 8 (40.0%) | 19 (27.5%) | 8 (42.1%) | 6 (31.6%) | ||
| 3 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| 4 | 5 (25.0%) | 27 (39.1%) | 5 (26.3%) | 7 (36.8%) | ||
| Metastases to internal organ | 0.109 | 0.606 | ||||
| Nonremovable | 5 (25.0%) | 34 (49.3%) | 5 (26.3%) | 8 (42.1%) | ||
| Removable | 2 (10.0%) | 8 (11.6%) | 2 (10.5%) | 1 (5.3%) | ||
| None | 13 (65.0%) | 27 (39.1%) | 12 (63.2%) | 10 (52.6%) | ||
| Number of extraspinal bone metastases foci | 0.862 | 0.660 | ||||
| 3 or more | 0 (0.0%) | 2 (2.9%) | 0 (0.0%) | 0 (0.0%) | ||
| 1–2 | 4 (20.0%) | 17 (24.6%) | 4 (21.1%) | 2 (10.5%) | ||
| 0 | 16 (80.0%) | 50 (72.5%) | 15 (78.9%) | 17 (89.5%) | ||
| NESMS group | ||||||
| 1 | 0 (0.0%) | 4 (5.8%) | 0.202 | 0 (0.0%) | 0 (0.0%) | 1.000 |
| 2 | 5 (25.0%) | 30 (43.5%) | 5 (26.3%) | 5 (26.3%) | ||
| 3 | 2 (10.0%) | 3 (4.3%) | 2 (10.5%) | 2 (10.5%) | ||
| 4 | 13 (65.0%) | 32 (46.4%) | 12 (63.2%) | 12 (63.2%) | ||
| Modified Tokuhashi group | ||||||
| 1 | 2 (10.0%) | 13 (18.8%) | 0.627 | 2 (10.5%) | 2 (10.5%) | 1.000 |
| 2 | 11 (55.0%) | 33 (47.8%) | 10 (52.6%) | 10 (52.6%) | ||
| 3 | 7 (35.0%) | 23 (33.3%) | 7 (36.8%) | 7 (36.8%) | ||
| ASA score | ||||||
| 1 | 5 (25.0%) | 37 (53.6%) | 0.017 | 5 (26.3%) | 10 (52.6%) | 0.184 |
| 2 | 14 (70.0%) | 32 (46.4%) | 13 (68.4%) | 9 (47.4%) | ||
| 3 | 1 (5.0%) | 0 (0.0%) | 1 (5.3%) | 0 (0.0%) | ||
| ECOG scale | ||||||
| 0 | 9 (45.0%) | 40 (58.0%) | 0.067 | 9 (47.4%) | 10 (52.6%) | 0.070 |
| 1 | 11 (55.0%) | 21 (30.4%) | 10 (52.6%) | 5 (26.3%) | ||
| 2 | 0 (0.0%) | 8 (11.6%) | 0 (0.0%) | 4 (21.1%) | ||
| Treatment Group | Time to Radiotherapy from TES (Week, Mean + SD) | Radiation Scheme | |
|---|---|---|---|
| All Patients | Matched Patients | ||
| TES | 4.1 ± 2.1 | 27 Gy in 3 fractions: 1 case 36 Gy in 6 fractions: 1 case 24 Gy in 6 fractions: 1 case 40 Gy in 16 fractions: 1 case 39 Gy in 13 fractions: 1 case 30 Gy in 10 fractions: 5 cases 44 Gy in 22 fractions: 1 case | 27 Gy in 3 fractions: 1 case 36 Gy in 6 fractions: 1 case 24 Gy in 6 fractions: 1 case 40 Gy in 16 fractions: 1 case 39 Gy in 13 fractions: 1 case 30 Gy in 10 fractions: 5 cases 44 Gy in 22 fractions: 1 case |
| Treatment group | Primary cancer type | All patients | Matched patients |
| SABR | RCC | 24 Gy in 1 fraction: 1 case 18 Gy in 1 fraction: 16 cases 16 Gy in 1 fraction: 2 cases | 18 Gy in 1 fraction: 5 cases 16 Gy in 1 fraction: 1 cases |
| Thyroid | 18 Gy in 1 fraction: 2 cases | 18 Gy in 1 fraction: 2 cases | |
| HCC | 20 Gy in 1 fraction: 1 case 18 Gy in 1 fraction: 6 cases 30 Gy in 3 fractions: 1 case 27 Gy in 3 fractions: 4 cases 24 Gy in 3 fractions: 5 cases 24 Gy in 4 fractions: 1 case | 18 Gy in 1 fraction: 1 case 24 Gy in 3 fractions: 4 cases | |
| Breast | 20 Gy in 1 fraction: 1 case 18 Gy in 1 fraction: 19 cases 24 Gy in 3 fractions: 1 cases | 20 Gy in 1 fraction: 1 case 18 Gy in 1 fraction: 2 cases 24 Gy in 3 fractions: 1 cases | |
| NSCLC | 20 Gy in 1 fraction: 1 case 18 Gy in 1 fraction: 3 cases 30 Gy in 3 fractions: 2 case 27 Gy in 3 fractions: 1 cases 35 Gy in 5 fractions: 1 cases | 30 Gy in 3 fractions: 2 case | |
| Others | 18 Gy in 1 fraction: 1 cases | ||
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Sex (male) | 2.238 (0.971–5.157) | 0.059 | 0.096 | |
| Primary cancer group of modified Tokuhashi score | 0.001 | <0.001 | ||
| Kidney, uterus a | 3.381 (0.907–12.611) | 0.070 | 0.051 | |
| Liver, gallbladder a | 12.994 (3.459–48.813) | <0.001 | 90.548 (10.146–808.107) | <0.001 |
| Lung, pancreas, etc. a | 3.780 (1.078–13.251) | 0.038 | 0.051 | |
| Extraspinal bone metastasis | 0.045 | 0.048 | ||
| 1–2 b | 0.331 | 0.968 | ||
| ≥3 b | 15.505 (1.668–144.133) | 0.016 | 81.440 (5.402–1227.676) | 0.001 |
| Radioresistance of the primary cancer | 1.915 (0.824–4.450) | 0.131 | 65.106 (7.424–570.990) | <0.001 |
| Prior radiotherapy | 4.381 (1.253–15.324) | 0.021 | 0.064 | |
| High bilsky grade | 2.862 (1.207–6.788) | 0.017 | 0.305 | |
| Bilsky grade 3 | 2.991 (1.148–7.793) | 0.025 | 4.013 (1.191–13.473) | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, D.-H.; Lee, W.; Chang, B.-S.; Kim, H.; Chang, S.Y.; Hong, S.H.; Kim, J.H.; Son, H.J. Efficacy of Total En Bloc Spondylectomy versus Stereotactic Ablative Radiotherapy for Single Spinal Metastasis. Cancers 2023, 15, 5518. https://doi.org/10.3390/cancers15235518
Kang D-H, Lee W, Chang B-S, Kim H, Chang SY, Hong SH, Kim JH, Son HJ. Efficacy of Total En Bloc Spondylectomy versus Stereotactic Ablative Radiotherapy for Single Spinal Metastasis. Cancers. 2023; 15(23):5518. https://doi.org/10.3390/cancers15235518
Chicago/Turabian StyleKang, Dong-Ho, Wooseok Lee, Bong-Soon Chang, Hyoungmin Kim, Sam Yeol Chang, Seong Hwa Hong, Jin Ho Kim, and Hee Jung Son. 2023. "Efficacy of Total En Bloc Spondylectomy versus Stereotactic Ablative Radiotherapy for Single Spinal Metastasis" Cancers 15, no. 23: 5518. https://doi.org/10.3390/cancers15235518
APA StyleKang, D.-H., Lee, W., Chang, B.-S., Kim, H., Chang, S. Y., Hong, S. H., Kim, J. H., & Son, H. J. (2023). Efficacy of Total En Bloc Spondylectomy versus Stereotactic Ablative Radiotherapy for Single Spinal Metastasis. Cancers, 15(23), 5518. https://doi.org/10.3390/cancers15235518






