Targeting Interleukin-6/Glycoprotein-130 Signaling by Raloxifene or SC144 Enhances Paclitaxel Efficacy in Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Culture and Reagents
2.2. Proliferation and Viability Assays
2.3. Synergism Calculation
2.4. TUNEL Staining
2.5. PDAC Mouse Model
2.6. Quantitative Realtime-RT-PCR
2.7. Caspase-3 Staining
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Statistical Analysis
3. Results
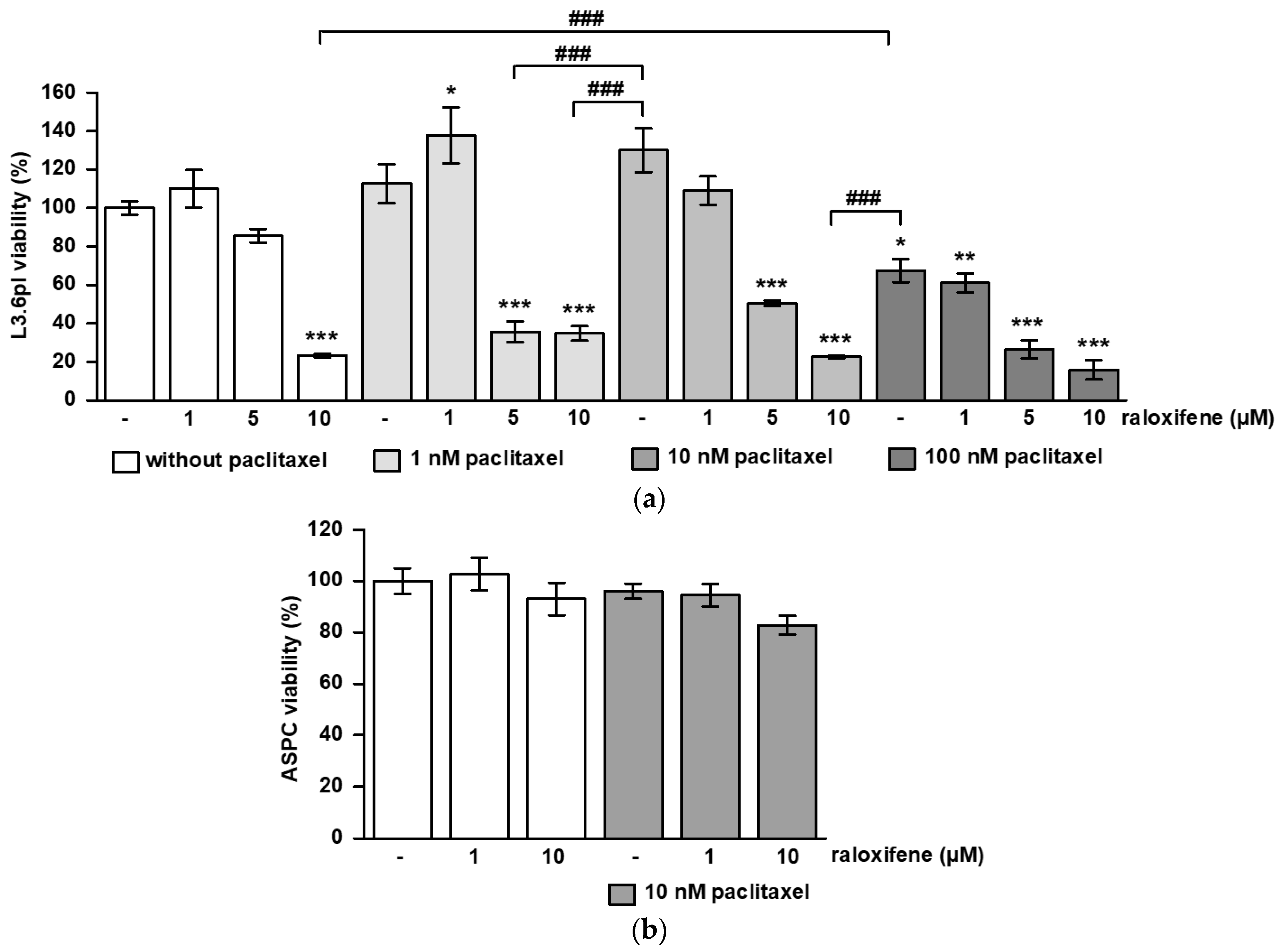
3.1. Raloxifene/Paclitaxel Act Synergistic or Additive to Reduce Cell Viability and Proliferation In Vitro
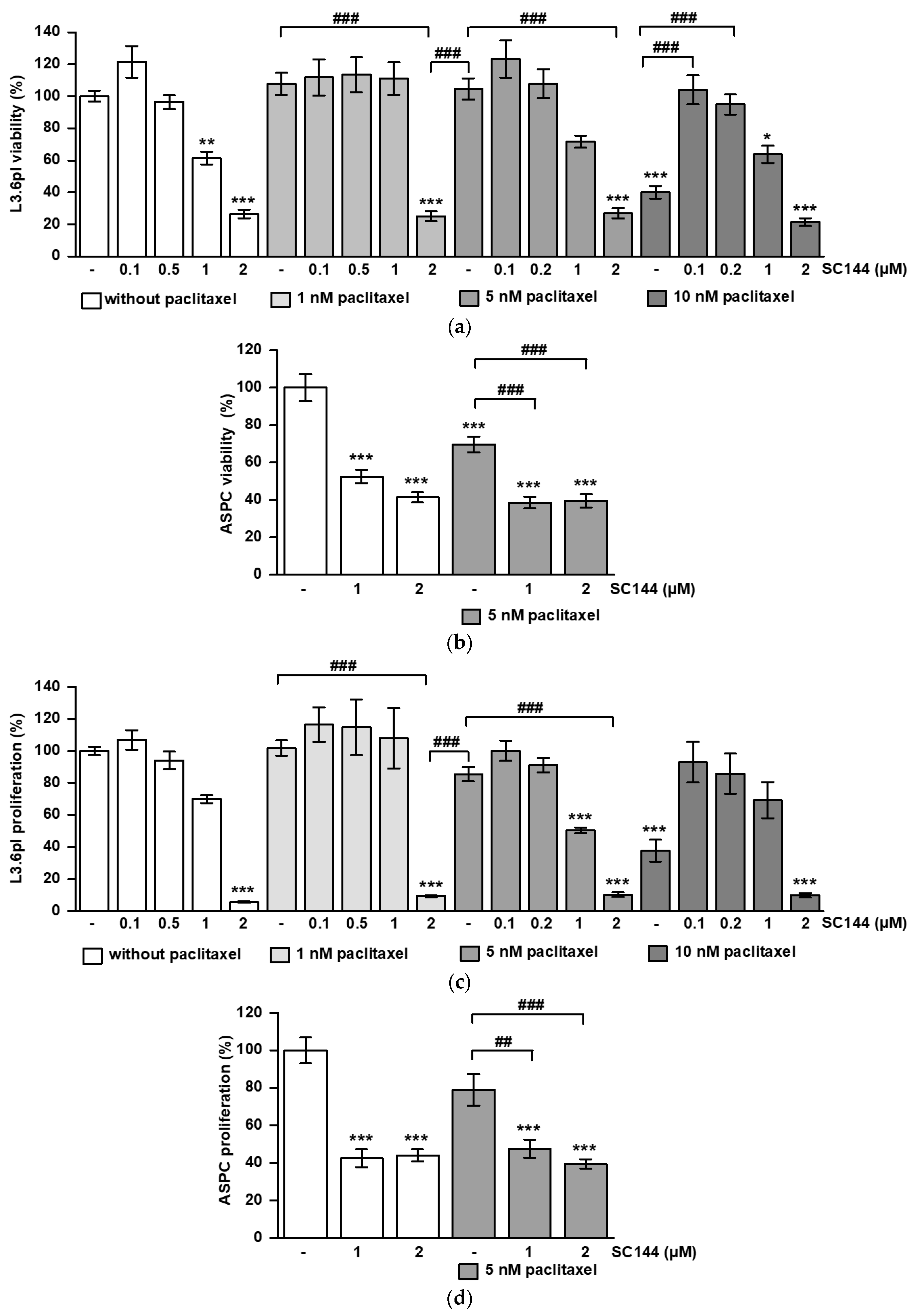
3.2. SC144 Acts Antagonistic on Paclitaxel-Induced Viability and Proliferation Inhibition In Vitro
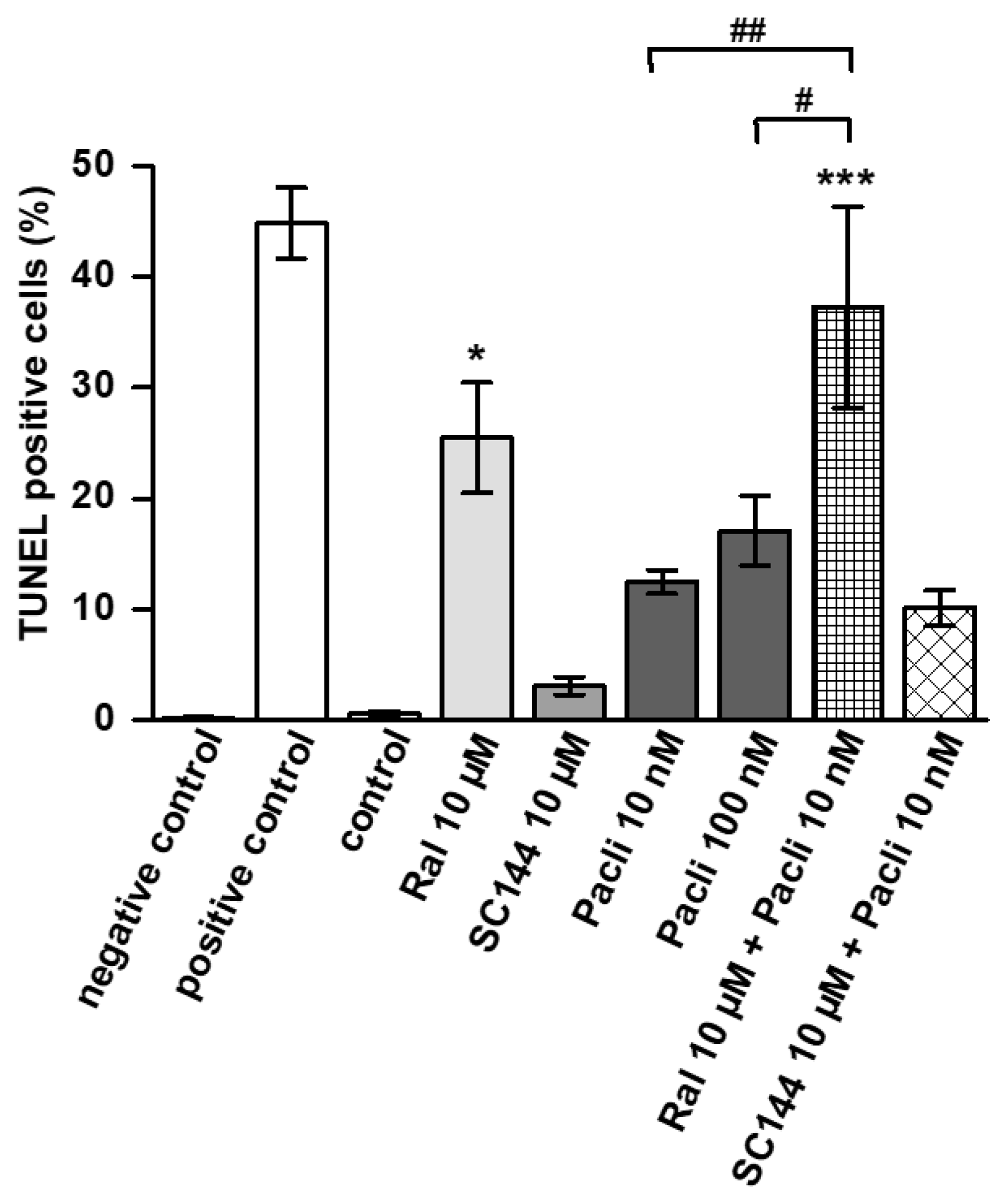
3.3. Raloxifene/Paclitaxel Combination Enhances Apoptosis In Vitro
3.4. Combined Therapy of Raloxifene/Paclitaxel or SC144/Paclitaxel Reduces PDAC Tumor Growth In Vivo
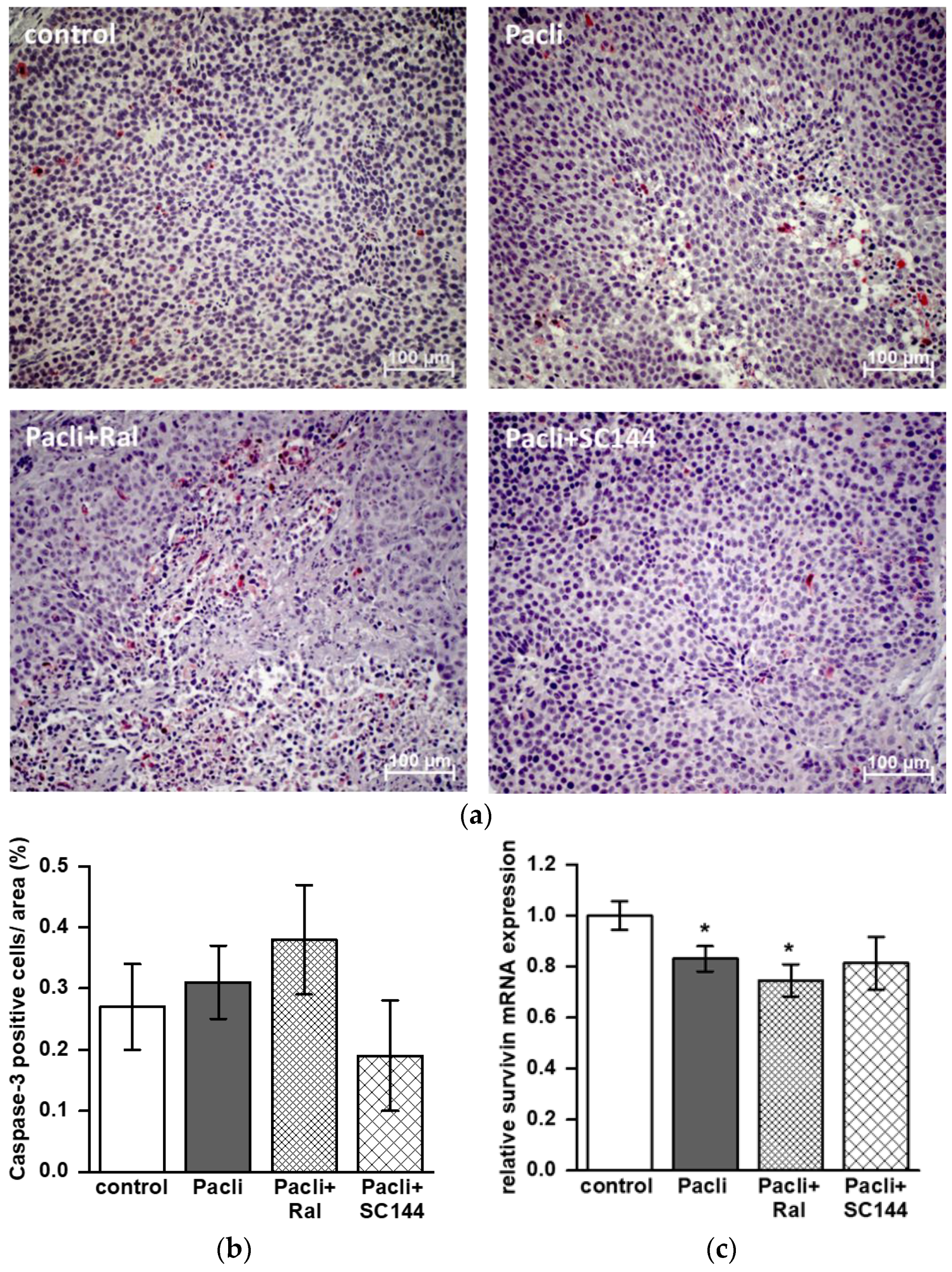
3.5. Combined Therapy of Raloxifene/Paclitaxel Stimulates Apoptosis in Mice’ Tumors
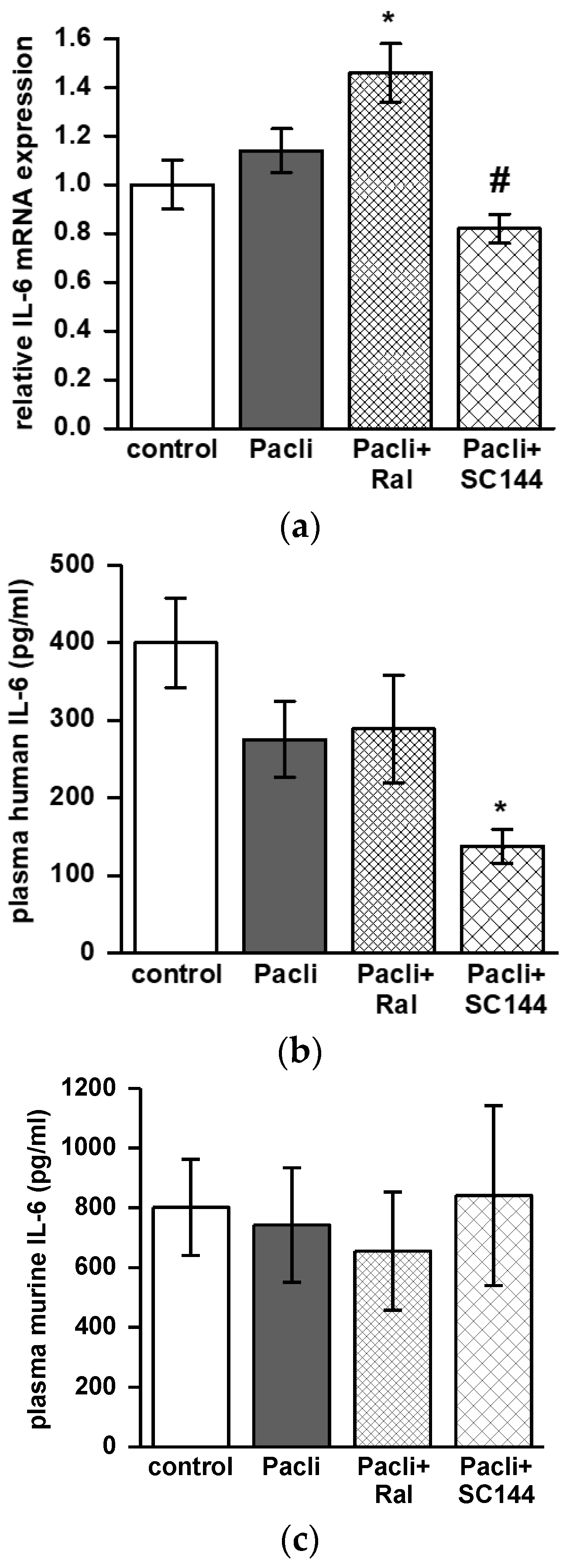
3.6. Combined Therapy of SC144/Paclitaxel Reduces IL-6 Expression in Mice’ Tumors and Blood
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roder, C.; Trauzold, A.; Kalthoff, H. Impact of death receptor signaling on the malignancy of pancreatic ductal adenocarcinoma. Eur. J. Cell Biol. 2011, 90, 450–455. [Google Scholar] [CrossRef]
- Falconer, J.S.; Fearon, K.C.; Plester, C.E.; Ross, J.A.; Carter, D.C. Cytokines, the acute-phase response, and resting energy expenditure in cachectic patients with pancreatic cancer. Ann. Surg. 1994, 219, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Okusaka, T.; Ishii, H.; Kyogoku, A.; Yoshimori, M.; Kajimura, N.; Yamaguchi, K.; Kakizoe, T. Elevated serum interleukin-6 levels in patients with pancreatic cancer. Jpn. J. Clin. Oncol. 1998, 28, 12–15. [Google Scholar] [CrossRef]
- Bellone, G.; Smirne, C.; Mauri, F.A.; Tonel, E.; Carbone, A.; Buffolino, A.; Dughera, L.; Robecchi, A.; Pirisi, M.; Emanuelli, G. Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: Implications for survival. Cancer Immunol. Immunother. 2006, 55, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Roshani, R.; McCarthy, F.; Hagemann, T. Inflammatory cytokines in human pancreatic cancer. Cancer Lett. 2014, 345, 157–163. [Google Scholar] [CrossRef]
- Kim, H.W.; Lee, J.C.; Paik, K.H.; Kang, J.; Kim, J.; Hwang, J.H. Serum interleukin-6 is associated with pancreatic ductal adenocarcinoma progression pattern. Medicine 2017, 96, e5926. [Google Scholar] [CrossRef] [PubMed]
- Hibi, M.; Murakami, M.; Saito, M.; Hirano, T.; Taga, T.; Kishimoto, T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell 1990, 63, 1149–1157. [Google Scholar] [CrossRef]
- Oberg, H.H.; Wesch, D.; Grussel, S.; Rose-John, S.; Kabelitz, D. Differential expression of CD126 and CD130 mediates different STAT-3 phosphorylation in CD4+CD25- and CD25high regulatory T cells. Int. Immunol. 2006, 18, 555–563. [Google Scholar] [CrossRef]
- Lesina, M.; Wormann, S.M.; Neuhofer, P.; Song, L.; Algul, H. Interleukin-6 in inflammatory and malignant diseases of the pancreas. Semin. Immunol. 2014, 26, 80–87. [Google Scholar] [CrossRef]
- van Duijneveldt, G.; Griffin, M.D.W.; Putoczki, T.L. Emerging roles for the IL-6 family of cytokines in pancreatic cancer. Clin. Sci. (Lond.) 2020, 134, 2091–2115. [Google Scholar] [CrossRef]
- Pozios, I.; Hering, N.A.; Guenzler, E.; Arndt, M.; Elezkurtaj, S.; Knösel, T.; Bruns, C.J.; Margonis, G.A.; Kamphues, C.; Kreis, M.E.; et al. Gp130 is expressed in pancreatic cancer and can be targeted by the small inhibitor molecule SC144. J. Cancer Res. Clin. Oncol. 2022, accepted. [Google Scholar] [CrossRef] [PubMed]
- Pozios, I.; Seel, N.N.; Hering, N.A.; Hartmann, L.; Liu, V.; Camaj, P.; Muller, M.H.; Lee, L.D.; Bruns, C.J.; Kreis, M.E.; et al. Raloxifene inhibits pancreatic adenocarcinoma growth by interfering with ERbeta and IL-6/gp130/STAT3 signaling. Cell Oncol. 2021, 44, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.; Jeong, H.; Cheon, J.; Chon, H.J.; Ryu, H.; Kim, I.H.; Kang, M.J.; Jeong, J.H.; Ryoo, B.Y.; Kim, K.P.; et al. Efficacy and safety of second-line nab-paclitaxel plus gemcitabine after progression on FOLFIRINOX for unresectable or metastatic pancreatic ductal adenocarcinoma: Multicenter retrospective analysis. Ther. Adv. Med. Oncol. 2020, 12, 1758835920923424. [Google Scholar] [CrossRef] [PubMed]
- Balfour, J.A.; Goa, K.L. Raloxifene. Drugs Aging 1998, 12, 335–341, discussion 342. [Google Scholar] [CrossRef] [PubMed]
- Bruns, C.J.; Harbison, M.T.; Kuniyasu, H.; Eue, I.; Fidler, I.J. In vivo selection and characterization of metastatic variants from human pancreatic adenocarcinoma by using orthotopic implantation in nude mice. Neoplasia 1999, 1, 50–62. [Google Scholar] [CrossRef]
- Di Veroli, G.Y.; Fornari, C.; Wang, D.; Mollard, S.; Bramhall, J.L.; Richards, F.M.; Jodrell, D.I. Combenefit: An interactive platform for the analysis and visualization of drug combinations. Bioinformatics 2016, 32, 2866–2868. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Wennerberg, K.; Aittokallio, T.; Tang, J. Searching for Drug Synergy in Complex Dose-Response Landscapes Using an Interaction Potency Model. Comput. Struct. Biotechnol. J. 2015, 13, 504–513. [Google Scholar] [CrossRef]
- Nawrocki, S.T.; Bruns, C.J.; Harbison, M.T.; Bold, R.J.; Gotsch, B.S.; Abbruzzese, J.L.; Elliott, P.; Adams, J.; McConkey, D.J. Effects of the proteasome inhibitor PS-341 on apoptosis and angiogenesis in orthotopic human pancreatic tumor xenografts. Mol. Cancer Ther. 2002, 1, 1243–1253. [Google Scholar]
- Satoh, K.; Kaneko, K.; Hirota, M.; Masamune, A.; Satoh, A.; Shimosegawa, T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer 2001, 92, 271–278. [Google Scholar] [CrossRef]
- Fukuda, A.; Wang, S.C.; Morris, J.P.T.; Folias, A.E.; Liou, A.; Kim, G.E.; Akira, S.; Boucher, K.M.; Firpo, M.A.; Mulvihill, S.J.; et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell 2011, 19, 441–455. [Google Scholar] [CrossRef]
- Li, F.; Ambrosini, G.; Chu, E.Y.; Plescia, J.; Tognin, S.; Marchisio, P.C.; Altieri, D.C. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature 1998, 396, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yu, W.; Xiao, H.; Lin, K. BIRC5 is a prognostic biomarker associated with tumor immune cell infiltration. Sci. Rep. 2021, 11, 390. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Zhang, W.; Yan, D.; Kenath, R.; Le, L.; Wang, H.; Delitto, D.; Ostrov, D.; Robertson, K.; Liu, C.; et al. The role of survivin in the progression of pancreatic ductal adenocarcinoma (PDAC) and a novel survivin-targeted therapeutic for PDAC. PLoS ONE 2020, 15, e0226917. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Kim, L.K.; Park, H.M.; Kim, H.J.; Heo, T.H. Inhibition of GP130/STAT3 and EMT by combined bazedoxifene and paclitaxel treatment in ovarian cancer. Oncol. Rep. 2022, 47, 1–14. [Google Scholar] [CrossRef]
- Xu, S.; Grande, F.; Garofalo, A.; Neamati, N. Discovery of a novel orally active small-molecule gp130 inhibitor for the treatment of ovarian cancer. Mol. Cancer Ther. 2013, 12, 937–949. [Google Scholar] [CrossRef]
- Holmer, R.; Goumas, F.A.; Waetzig, G.H.; Rose-John, S.; Kalthoff, H. Interleukin-6: A villain in the drama of pancreatic cancer development and progression. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 371–380. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Seifert, A.M.; List, J.; Heiduk, M.; Decker, R.; von Renesse, J.; Meinecke, A.C.; Aust, D.E.; Welsch, T.; Weitz, J.; Seifert, L. Gamma-delta T cells stimulate IL-6 production by pancreatic stellate cells in pancreatic ductal adenocarcinoma. J. Cancer Res. Clin. Oncol. 2020, 146, 3233–3240. [Google Scholar] [CrossRef]
- Chang, Q.; Bournazou, E.; Sansone, P.; Berishaj, M.; Gao, S.P.; Daly, L.; Wels, J.; Theilen, T.; Granitto, S.; Zhang, X.; et al. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia 2013, 15, 848–862. [Google Scholar] [CrossRef]
- Rupert, J.E.; Narasimhan, A.; Jengelley, D.H.A.; Jiang, Y.; Liu, J.; Au, E.; Silverman, L.M.; Sandusky, G.; Bonetto, A.; Cao, S.; et al. Tumor-derived IL-6 and trans-signaling among tumor, fat, and muscle mediate pancreatic cancer cachexia. J. Exp. Med. 2021, 218, e20190450. [Google Scholar] [CrossRef]
- Ramsey, M.L.; Talbert, E.; Ahn, D.; Bekaii-Saab, T.; Badi, N.; Bloomston, P.M.; Conwell, D.L.; Cruz-Monserrate, Z.; Dillhoff, M.; Farren, M.R.; et al. Circulating interleukin-6 is associated with disease progression, but not cachexia in pancreatic cancer. Pancreatology 2019, 19, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Bent, E.H.; Millan-Barea, L.R.; Zhuang, I.; Goulet, D.R.; Frose, J.; Hemann, M.T. Microenvironmental IL-6 inhibits anti-cancer immune responses generated by cytotoxic chemotherapy. Nat. Commun. 2021, 12, 6218. [Google Scholar] [CrossRef] [PubMed]
- McAndrews, K.M.; Chen, Y.; Darpolor, J.K.; Zheng, X.; Yang, S.; Carstens, J.L.; Li, B.; Wang, H.; Miyake, T.; Correa de Sampaio, P.; et al. Identification of Functional Heterogeneity of Carcinoma-Associated Fibroblasts with Distinct IL6-Mediated Therapy Resistance in Pancreatic Cancer. Cancer Discov. 2022, 12, 1580–1597. [Google Scholar] [CrossRef] [PubMed]


| Metastasis/Tumor Infiltration (%) | Control | Ral | SC144 | Pacli | Pacli + Ral | Pacli + SC144 |
|---|---|---|---|---|---|---|
| liver | 41 | 50 | 60 | 7 | 30 | 33 |
| spleen | 76 | 50 | 80 | 50 | 40 | 83 |
| peritoneum | 41 | 0 | 10 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hering, N.A.; Günzler, E.; Arndt, M.; Zibell, M.; Lauscher, J.C.; Kreis, M.E.; Beyer, K.; Seeliger, H.; Pozios, I. Targeting Interleukin-6/Glycoprotein-130 Signaling by Raloxifene or SC144 Enhances Paclitaxel Efficacy in Pancreatic Cancer. Cancers 2023, 15, 456. https://doi.org/10.3390/cancers15020456
Hering NA, Günzler E, Arndt M, Zibell M, Lauscher JC, Kreis ME, Beyer K, Seeliger H, Pozios I. Targeting Interleukin-6/Glycoprotein-130 Signaling by Raloxifene or SC144 Enhances Paclitaxel Efficacy in Pancreatic Cancer. Cancers. 2023; 15(2):456. https://doi.org/10.3390/cancers15020456
Chicago/Turabian StyleHering, Nina A., Emily Günzler, Marco Arndt, Miriam Zibell, Johannes C. Lauscher, Martin E. Kreis, Katharina Beyer, Hendrik Seeliger, and Ioannis Pozios. 2023. "Targeting Interleukin-6/Glycoprotein-130 Signaling by Raloxifene or SC144 Enhances Paclitaxel Efficacy in Pancreatic Cancer" Cancers 15, no. 2: 456. https://doi.org/10.3390/cancers15020456
APA StyleHering, N. A., Günzler, E., Arndt, M., Zibell, M., Lauscher, J. C., Kreis, M. E., Beyer, K., Seeliger, H., & Pozios, I. (2023). Targeting Interleukin-6/Glycoprotein-130 Signaling by Raloxifene or SC144 Enhances Paclitaxel Efficacy in Pancreatic Cancer. Cancers, 15(2), 456. https://doi.org/10.3390/cancers15020456







