Retroperitoneal Lymph Node Dissection in Colorectal Cancer with Lymph Node Metastasis: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
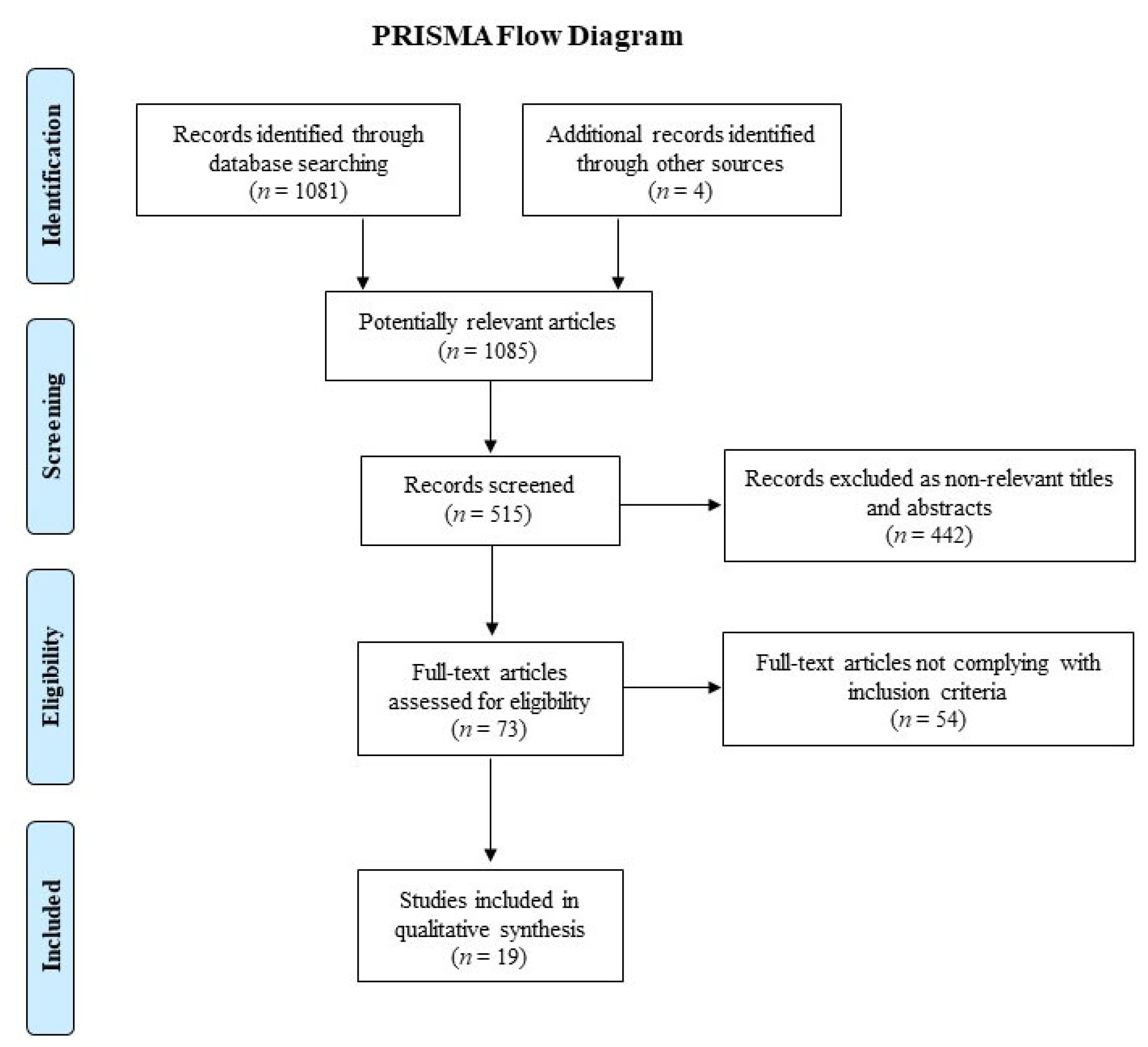
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection and Inclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment of Studies
2.5. Statistical Analysis
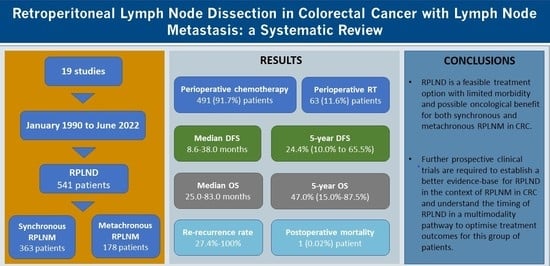
3. Results
3.1. Patient Demographics and Primary CRC Histopathology
3.2. Chemotherapy and Radiotherapy Regimens
3.3. RPLNM Diagnostic Imaging Criteria and Selection for Surgery
3.4. RPLND Timing and Harvesting
3.5. Safety and Long-Term Oncological Outcomes
4. Discussion
4.1. Strengths and Limitations
4.2. Implications for Multimodality Treatment of RPLNM in CRC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zizzo, M.; Dorma, M.P.F.; Zanelli, M.; Sanguedolce, F.; Bassi, M.C.; Palicelli, A.; Ascani, S.; Giunta, A. Long-Term Outcomes of Surgical Resection of Pathologically Confirmed Isolated Para-Aortic Lymph Node Metastases in Colorectal Cancer: A Systematic Review. Cancers 2022, 14, 661. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Zhang, R.; Wang, Z.; Geng, Y.; Lin, J.; Ma, K.; Zuo, J.-L.; Lu, L.; Zhang, J.-B.; Zhu, W.-W.; et al. Meta-analysis of the association between primary tumour location and prognosis after surgical resection of colorectal liver metastases. Br. J. Surg. 2019, 106, 1747–1760. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Garshell, J.; Miller, D.; Altekruse, S.F.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; et al. SEER Cancer Statistics Review, 1975-2012. Bethesda (MD): National Cancer Institute; 2014. Available online: https://seer.cancer.gov/archive/csr/1975_2012/ (accessed on 10 August 2022).
- Ike, H.; Shimada, H.; Ohki, S.; Togo, S.; Yamaguchi, S.; Ichikawa, Y. Results of Aggressive Resection of Lung Metastases from Colorectal Carcinoma Detected by Intensive Follow-Up. Dis. Colon Rectum 2002, 45, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, M.; Makuuchi, M.; Torzilli, G.; Takayama, T.; Kawasaki, S.; Kosuge, T.; Yamamoto, J.; Imamura, H. Extension of the Frontiers of Surgical Indications in the Treatment of Liver Metastases From Colorectal Cancer. Ann. Surg. 2000, 231, 487–499. [Google Scholar] [CrossRef]
- Nordlinger, B.; Sorbye, H.; Glimelius, B.; Poston, G.J.; Schlag, P.M.; Rougier, P.; Bechstein, W.O.; Primrose, J.N.; Walpole, E.T.; Finch-Jones, M.; et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): A randomised controlled trial. Lancet 2008, 371, 1007–1016. [Google Scholar] [CrossRef]
- Zabaleta, J.; Aguinagalde, B.; Fuentes, M.; Bazterargui, N.; Izquierdo, J.; Hernández, C.; Enriquez-Navascues, J.M.; Emparanza, J. Survival after lung metastasectomy for colorectal cancer: Importance of previous liver metastasis as a prognostic factor. Eur. J. Surg. Oncol. (EJSO) 2011, 37, 786–790. [Google Scholar] [CrossRef]
- Marudanayagam, R.; Ramkumar, K.; Shanmugam, V.; Langman, G.; Rajesh, P.; Coldham, C.; Bramhall, S.R.; Mayer, D.; Buckels, J.; Mirza, D.F. Long-term outcome after sequential resections of liver and lung metastases from colorectal carcinoma. Hpb 2009, 11, 671–676. [Google Scholar] [CrossRef]
- Choi, P.W.; Kim, H.C.; Kim, A.Y.; Jung, S.H.; Yu, C.S.; Kim, J.C. Extensive lymphadenectomy in colorectal cancer with isolated para-aortic lymph node metastasis below the level of renal vessels. J. Surg. Oncol. 2010, 101, 66–71. [Google Scholar] [CrossRef]
- Jessup, J.M.; Goldberg, R.M.; Asare, E.A. AJCC Cancer Staging Manual, 8th ed.; Springer: Chicago, IL, USA, 2017; p. 251. [Google Scholar]
- Delpero, J.R.; Pol, B.; Le Treut, Y.P.; Bardou, V.J.; Moutardier, V.; Hardwigsen, J.; Granger, F.; Houvenaeghel, G. Surgical resection of locally recurrent colorectal adenocarcinoma. Br. J. Surg. 1998, 85, 372–376. [Google Scholar] [CrossRef]
- Galandiuk, S.; Wieand, H.S.; Moertel, C.G.; Cha, S.S.; Fitzgibbons, R.J.; Pemberton, J.H.; Wolff, B.G. Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg. Gynecol. Obstet. 1992, 174, 27–32. [Google Scholar]
- Isozaki, H.; Okajima, K.; Fujii, K.; Nomura, E.; Izumi, N.; Mabuchi, H.; Nakamura, M.; Hara, H. Effectiveness of paraaortic lymph node dissection for advanced gastric cancer. Hepato-gastroenterology 1999, 46. [Google Scholar]
- Kayahara, M.; Nagakawa, T.; Ohta, T.; Kitagawa, H.; Ueno, K.; Tajima, H.; Elnemr, A.; Miwa, K. Analysis of paraaortic lymph node involvement in pancreatic carcinoma: A significant indication for surgery? Cancer 1999, 85, 583–590. [Google Scholar] [CrossRef]
- Cary, C.; Foster, R.S.; Masterson, T.A. Complications of Retroperitoneal Lymph Node Dissection. Urol. Clin. N. Am. 2019, 46, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Shibata, D.; Paty, P.B.; Guillem, J.G.; Wong, D.W.; Cohen, A.M. Surgical Management of Isolated Retroperitoneal Recurrences of Colorectal Carcinoma. Dis. Colon Rectum 2002, 45, 795–801. [Google Scholar] [CrossRef]
- Elias, D.; Naudeix, E.; Ducreux, M.; Lusinchi, A.; Goharin, A.; Ouelette, J.F.; Lasser, P. Results of lymphadenectomy for obvious lateroaortic lymph node metastases from colorectal primaries. Hepato-gastroenterology 2001, 48. [Google Scholar]
- Classification of Regional Lymph Nodes in Japan. Int. J. Clin. Oncol. 2003, 8, 248–275. [CrossRef]
- Wong, J.; Tan, G.; Teo, M. Management of para-aortic lymph node metastasis in colorectal patients: A systemic review. Surg. Oncol. 2016, 25, 411–418. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, Version 6.3 (Updated February 2022). Cochrane; 2022. Available online: www.training.cochrane.org/handbook (accessed on 16 August 2022).
- Clavien, P.A.; Sanabria, J.R.; Strasberg, S.M. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 1992, 111, 518–526. [Google Scholar]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 16 August 2022).
- Tentes, A.-A.K.; Mirelis, C.; Karanikiotis, C.; Korakianitis, O. Radical lymph node resection of the retroperitoneal area for left-sided colon cancer. Langenbeck’s Arch. Surg. 2007, 392, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H.; Park, S.Y.; Park, J.S.; Kim, H.J.; Yang, C.-S.; Choi, G.-S. Laparoscopic para-aortic lymph node dissection for patients with primary colorectal cancer and clinically suspected para-aortic lymph nodes. Ann. Surg. Treat. Res. 2016, 90, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Ogura, A.; Akiyoshi, T.; Takatsu, Y.; Nagata, J.; Nagasaki, T.; Konishi, T.; Fujimoto, Y.; Nagayama, S.; Fukunaga, Y.; Ueno, M. The significance of extended lymphadenectomy for colorectal cancer with isolated synchronous extraregional lymph node metastasis. Asian J. Surg. 2015, 40, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.U.; Hur, H.; Min, B.S.; Baik, S.H.; Lee, K.Y.; Kim, N.K. Which Patients with Isolated Para-aortic Lymph Node Metastasis Will Truly Benefit from Extended Lymph Node Dissection for Colon Cancer? Cancer Res. Treat. 2018, 50, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Tsukamoto, S.; Ochiai, H.; Shida, D.; Kanemitsu, Y. Improving Selection for Resection of Synchronous Para-Aortic Lymph Node Metastases in Colorectal Cancer. Dig. Surg. 2018, 36, 369–375. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kanai, T.; Yo, K.; Hongo, K.; Takano, K.; Tsutsui, M.; Nakanishi, R.; Yoshikawa, Y.; Nakagawa, M. Laparoscopic para-aortic lymphadenectomy for colorectal cancer with clinically suspected lymph node metastasis. Asian J. Endosc. Surg. 2018, 12, 417–422. [Google Scholar] [CrossRef]
- Sakamoto, J.; Ozawa, H.; Nakanishi, H.; Fujita, S. Oncologic outcomes after resection of para-aortic lymph node metastasis in left-sided colon and rectal cancer. PLoS ONE 2020, 15, e0241815. [Google Scholar] [CrossRef]
- Lee, S.C.; Kim, H.C.; Lee, W.Y.; Yun, S.H.; Cho, Y.B.; Huh, J.W.; Park, Y.A.; Shin, J.K. Effect of lymphadenectomy in colorectal cancer with isolated synchronous para-aortic lymph node metastasis. Color. Dis. 2021, 23, 2584–2592. [Google Scholar] [CrossRef]
- Bowne, W.B.; Lee, B.; Wong, D.W.; Ben-Porat, L.; Shia, J.; Cohen, A.M.; Enker, W.E.; Guillem, J.G.; Paty, P.B.; Weiser, M.R. Operative Salvage for Locoregional Recurrent Colon Cancer After Curative Resection: An Analysis of 100 Cases. Dis. Colon Rectum 2005, 48, 897–909. [Google Scholar] [CrossRef]
- Min, B.S.; Kim, N.K.; Sohn, S.K.; Cho, C.H.; Lee, K.Y.; Baik, S.H. Isolated paraaortic lymph-node recurrence after the curative resection of colorectal carcinoma. J. Surg. Oncol. 2008, 97, 136–140. [Google Scholar] [CrossRef]
- Dumont, F.; Kothodinis, K.; Goéré, D.; Honoré, C.; Dartigues, P.; Boige, V.; Ducreux, M.; Malka, D.; Elias, D. Central retroperitoneal recurrences from colorectal cancer: Are lymph node and locoregional recurrences the same disease? Eur. J. Surg. Oncol. (EJSO) 2012, 38, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Razik, R.; Zih, F.; Haase, E.; Mathieson, A.; Sandhu, L.; Cummings, B.; Lindsay, T.; Smith, A.; Swallow, C. Long-term outcomes following resection of retroperitoneal recurrence of colorectal cancer. Eur. J. Surg. Oncol. (EJSO) 2014, 40, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Park, I.J.; Park, J.-H.; Kim, T.W.; Ro, J.-S.; Lim, S.-B.; Yu, C.S.; Kim, J.C. Management of isolated para-aortic lymph node recurrence after surgery for colorectal cancer. Ann. Surg. Treat. Res. 2020, 98, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, A.; Uehara, K.; Kato, T.; Nakamura, H.; Kamiya, T.; Nagino, M. Clinical Significance of Para-Aortic Lymph Node Dissection for Advanced or Metastatic Colorectal Cancer in the Current Era of Modern Chemotherapy. Dig. Surg. 2015, 32, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Gagnière, J.; Dupré, A.; Chabaud, S.; Peyrat, P.; Meeus, P.; Rivoire, M. Retroperitoneal nodal metastases from colorectal cancer: Curable metastases with radical retroperitoneal lymphadenectomy in selected patients. Eur. J. Surg. Oncol. (EJSO) 2015, 41, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Takahashi, H.; Fujii, M.; Hata, T.; Ogino, T.; Miyoshi, N.; Uemura, M.; Yamamoto, H.; Mizushima, T.; Doki, Y.; et al. Radical lymphadenectomy of a para-aorta lymph node metastasis in colorectal cancer prolongs relapse-free survival. Int. J. Color. Dis. 2021, 36, 1551–1560. [Google Scholar] [CrossRef]
- Nakai, N.; Yamaguchi, T.; Kinugasa, Y.; Shiomi, A.; Kagawa, H.; Yamakawa, Y.; Numata, M.; Furutani, A.; Yamaoka, Y.; Manabe, S.; et al. Diagnostic value of computed tomography (CT) and positron emission tomography (PET) for paraaortic lymph node metastasis from left-sided colon and rectal cancer. Asian J. Surg. 2019, 43, 676–682. [Google Scholar] [CrossRef]
- Sohaib, S.; Koh, D.; Barbachano, Y.; Parikh, J.; Husband, J.; Dearnaley, D.; Horwich, A.; Huddart, R. Prospective assessment of MRI for imaging retroperitoneal metastases from testicular germ cell tumours. Clin. Radiol. 2009, 64, 362–367. [Google Scholar] [CrossRef]
- Laukka, M.; Mannisto, S.; Beule, A.; Kouri, M.; Blomqvist, C. Comparison between CT and MRI in detection of metastasis of the retroperitoneum in testicular germ cell tumors: A prospective trial. Acta Oncol. 2020, 59, 660–665. [Google Scholar] [CrossRef]
- Yeo, S.-G.; Kim, D.Y.; Kim, T.H.; Jung, K.H.; Hong, Y.S.; Kim, S.Y.; Park, J.W.; Choi, H.S.; Oh, J.H. Curative chemoradiotherapy for isolated retroperitoneal lymph node recurrence of colorectal cancer. Radiother. Oncol. 2010, 97, 307–311. [Google Scholar] [CrossRef]
- Lee, J.; Chang, J.S.; Shin, S.J.; Lim, J.S.; Keum, K.C.; Kim, N.-K.; Ahn, J.B.; Kim, T.I.; Koom, W.S. Incorporation of Radiotherapy in the Multidisciplinary Treatment of Isolated Retroperitoneal Lymph Node Recurrence from Colorectal Cancer. Ann. Surg. Oncol. 2015, 22, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.W.; Mack, L.A.; Temple, W.J. Operative Salvage for Retroperitoneal Nodal Recurrence in Colorectal Cancer: A Systematic Review. Ann. Surg. Oncol. 2011, 18, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Nozawa, H.; Kawai, K.; Hata, K.; Tanaka, T.; Nishikawa, T.; Shuno, Y.; Kaneko, M.; Murono, K.; Emoto, S.; et al. Management of isolated para-aortic lymph node recurrence of colorectal cancer. Surg. Today 2019, 50, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, T.S.; Allman, B.L.; Johnson, M.; Power, A.; Sheinfeld, J.; Power, N.E. Retroperitoneal Lymph Node Dissection: Anatomical and Technical Considerations from a Cadaveric Study. J. Urol. 2016, 196, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Mittakanti, H.R.; Porter, J.R. Robot-assisted laparoscopic retroperitoneal lymph node dissection: A minimally invasive surgical approach for testicular cancer. Transl. Androl. Urol. 2020, 9, S66–S73. [Google Scholar] [CrossRef]
- Galema, H.A.; Meijer, R.P.; Lauwerends, L.J.; Verhoef, C.; Burggraaf, J.; Vahrmeijer, A.L.; Hutteman, M.; Keereweer, S.; Hilling, D.E. Fluorescence-guided surgery in colorectal cancer; A review on clinical results and future perspectives. Eur. J. Surg. Oncol. (EJSO) 2021, 48, 810–821. [Google Scholar] [CrossRef]
- Bae, S.U.; Baek, S.J.; Hur, H.; Baik, S.H.; Kim, N.-K.; Min, B.S. Intraoperative Near Infrared Fluorescence Imaging in Robotic Low Anterior Resection: Three Case Reports. Yonsei Med J. 2013, 54, 1066–1069. [Google Scholar] [CrossRef]
- Son, G.M.; Ahn, H.-M.; Lee, I.Y.; Ha, G.W. Multifunctional Indocyanine Green Applications for Fluorescence-Guided Laparoscopic Colorectal Surgery. Ann. Coloproctology 2021, 37, 133–140. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, J.S.; Kim, H.J.; Woo, I.T.; Park, I.K.; Choi, G.-S. Indocyanine Green Fluorescence Imaging-Guided Laparoscopic Surgery Could Achieve Radical D3 Dissection in Patients with Advanced Right-Sided Colon Cancer. Dis. Colon Rectum 2020, 63, 441–449. [Google Scholar] [CrossRef]
- Liberale, G.; Bourgeois, P.; Larsimont, D.; Moreau, M.; Donckier, V.; Ishizawa, T. Indocyanine green fluorescence-guided surgery after IV injection in metastatic colorectal cancer: A systematic review. Eur. J. Surg. Oncol. (EJSO) 2017, 43, 1656–1667. [Google Scholar] [CrossRef]
- Van Der Vorst, J.R.; Schaafsma, B.E.; Hutteman, M.; Msc, F.P.R.V.; Liefers, G.-J.; Hartgrink, H.H.; Smit, V.T.H.B.M.; Löwik, C.W.G.M.; Van De Velde, C.J.H.; Frangioni, J.V.; et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer 2013, 119, 3411–3418. [Google Scholar] [CrossRef] [PubMed]
- Peloso, A.; Franchi, E.; Canepa, M.C.; Barbieri, L.; Briani, L.; Ferrario, J.; Bianco, C.; Quaretti, P.; Brugnatelli, S.; Dionigi, P.; et al. Combined use of intraoperative ultrasound and indocyanine green fluorescence imaging to detect liver metastases from colorectal cancer. Hpb 2013, 15, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Murakami, M.; Koizumi, T.; Matsuda, K.; Fujimori, A.; Kusano, T.; Enami, Y.; Goto, S.; Watanabe, M.; Otsuka, K. Determination of the surgical margin in laparoscopic liver resections using infrared indocyanine green fluorescence. Langenbeck’s Arch. Surg. 2018, 403, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Meijer, R.P.; de Valk, K.S.; Deken, M.M.; Boogerd, L.S.; Hoogstins, C.E.; Bhairosingh, S.S.; Swijnenburg, R.-J.; Bonsing, B.A.; Framery, B.; Sarasqueta, A.F.; et al. Intraoperative detection of colorectal and pancreatic liver metastases using SGM-101, a fluorescent antibody targeting CEA. Eur. J. Surg. Oncol. (EJSO) 2020, 47, 667–673. [Google Scholar] [CrossRef]
- Berg, N.S.V.D.; Buckle, T.; KleinJan, G.H.; van der Poel, H.G.; van Leeuwen, F.W. Multispectral Fluorescence Imaging During Robot-assisted Laparoscopic Sentinel Node Biopsy: A First Step Towards a Fluorescence-based Anatomic Roadmap. Eur. Urol. 2017, 72, 110–117. [Google Scholar] [CrossRef]
- Bae, S.U. Near-infrared fluorescence imaging guided surgery in colorectal surgery. World J. Gastroenterol. 2022, 28, 1284–1287. [Google Scholar] [CrossRef]
- Fujii, T. Extracapsular invasion as a risk factor for disease recurrence in colorectal cancer. World J. Gastroenterol. 2011, 17, 2003–2006. [Google Scholar] [CrossRef]
| Author, Year | n | Median/Mean Age (Range/SD) | Female, n (%) | Primary Tumour | Study NOS | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location, n (%) | Differentiation, n (%) | TNM Stage, n (%) | R0 Resection, n (%) | |||||||||||||||
| Colon | Rectum | Well | Moderately | Poorly | Other | T1 | T2 | T3 | T4 | N0 | N1 | N2 | ||||||
| Synchronous RPLNM | ||||||||||||||||||
| Tentes et al. [25], 2007 | 62 | ―/68.8 (±10.3) | 41 (66.1) | 62 (100) | 0 | 29 (46.8) | 28 (45.2) | 5 (8.0) | 0 | 1 (1.6) | 9 (14.5) | 46 (74.2) | 6 (9.7) | 33 (53.2) | 19 (30.6) | 10 (16.1) | 62 (100) | N/A * |
| Song et al. [26], 2016 | 40 | ―/61.7 (±10.4) | 14 (35.0) | 27 (67.5) | 13 (32.5) | 33 (82.5) | 7 (17.5) | 3 (7.5) | 37 (92.5) | 16 (40.0) | 24 (60.0) | ― | 9 | |||||
| Ogura et al. [27], 2017 | 16 | 58.5/― (39–82) | 11 (68.7) | 14 (87.6) | 2 (12.4) | 11 (68.8) | 5 (31.2) | ― | ― | ― | ― | ― | ― | ― | 16 (100) | 9 | ||
| Bae et al. [28], 2018 | 49 | ―/57.7 (±11.5) | 20 (40.8) | 49 (100) | 0 | 4 (8.2) | 34 (69.4) | 6 (12.2) | 5 (10.2) | 0 | 1 (2.0) | 43 (87.8) | 5 (10.2) | ― | ― | ― | 49 (100) | 7 |
| Yamada et al. [29], 2019 | 36 | 57 (46.3–65.8)/― | 15 (41.7) | 17 (47.2) | 19 (52.8) | 8 (22.2) | 19 (52.8) | 2 (5.6) | 7 (19.4) | 0 | 0 | 11 (30.6) | 25 (69.4) | ― | ― | ― | ― | 8 |
| Yamamoto et al. [30], 2019 | 11 | ―/63 (28–76) | 6 (54.5) | 8 (72.7) | 3 (27.3) | 2 (18.2) | 8 (72.7) | 1 (9.1) | 0 | 1 (9.1) | 8 (72.7) | 2 (18.2) | 5 (45.5) | 1 (9.1) | 5 (45.5) | ― | 7 | |
| Sakamoto et al. [31], 2020 | 29 | 60 (35–74)/― | 14 (48.3) | 14 (48.3) | 15 (51.7) | 2 (6.9) | 19 (65.5) | 6 (20.7) | 2 (6.9) | 0 | 0 | 13 (44.8) | 16 (55.2) | 0 | ― | ― | ― | 9 |
| Lee et al. [32], 2021 | 47 | ―/57.6 | 14 (29.8) | 35 (74.5) | 12 (25.5) | 27 (57.4) | 20 (42.6) | ― | ― | ― | ― | ― | ― | ― | ― | 9 | ||
| Metachronous RPLNM | ||||||||||||||||||
| Shibata et al. [16], 2002 | 20 | 55/― | 9 (45.0) | 16 (80.0) | 4 (20.0) | ― | ― | ― | ― | ― | 1 † (4.0) | 20 † (80.0) | 1 † (4.0) | 11 † (44.0) | 10 † (40.0) | 2 † (8.0) | 20 (100) | 8 |
| Bowne et al. [33], 2005 | 16 | ―/― | ― | 16 (100) | 0 | ― | ― | ― | ― | ― | ― | ― | ― | ― | ― | ― | 16 (100) | 8 |
| Min et al. [34], 2008 | 6 | ―/58.2 | 3 (50.0) | 3 (50.0) | 3 (50.0) | 6 (100) | 0 | 0 | 0 | 6 (100) | 0 | 0 | 6 (100) | 6 (100) | 9 | |||
| Dumont et al. [35], 2012 | 23 | ―/51 (±8) | 10 (44.0) | 20 (87.0) | 3 (13.0) | ― | ― | ― | ― | ― | ― | ― | ― | ― | ― | ― | 23 (100) | 8 |
| Razik et al. [36], 2014 | 48 | 60 (36–80)/― | 26 (54.0) | 43 (90.0) | 5 (10.0) | ― | ― | ― | ― | ― | ― | ― | ― | ― | 23 (48.0) | ― | 8 | |
| Kim et al. [37], 2020 | 16 | 55.5 (42–73)/― | 4 (25.0) | 9 (56.3) | 7 (43.8) | 0 | 15 (93.8) | 1 (6.3) | 0 | ― | ― | ― | ― | 6 (37.5) | 6 (37.5) | 4 (25.0) | 16 (100) | 9 |
| Synchronous and Metachronous RPLNM | ||||||||||||||||||
| Elias et al. [17], 2001 | 31 | ―/50 (±11) | 25 (80.6) | 26 (83.9) | 5 (16.1) | ― | ― | ― | ― | ― | ― | ― | ― | ― | ― | ― | ― | 7 |
| Choi et al. [9], 2010 | 24 | ―/52 (27–78) | 11 (45.8) | 15 (62.5) | 9 (37.5) | 17 (70.8) | 7 (29.2) | 1 (4.2) | 1 (4.2) | 20 (83.3) | 2 (8.3) | 1 (4.2) | 5 (20.8) | 18 (75.0) | ― | 9 | ||
| Arimoto et al. [38], 2015 | 14 | 66 (42–75)/― | 3 (21.4) | 6 (42.9) | 8 (57.1) | ― | ― | ― | ― | ― | ― | ― | ― | ― | ― | ― | ― | 8 |
| Gagniere et al. [39], 2015 | 25 | 55 (31–69)/― | 16 (64.0) | 12 (48.0) | 13 (52.0) | ― | ― | ― | ― | 0 | ― | ― | ― | ― | ― | ― | ― | 9 |
| Ichikawa et al. [40], 2021 | 28 | 61 (42–79)/― | 15 (53.6) | ― | ― | 23 (82.1) | 5 (17.9) | ― | ― | ― | ― | ― | ― | ― | ― | 8 | ||
| Author, Year | Chemotherapy, n (%) | Chemotherapy Regimens | Radiotherapy, n (%) | Radiotherapy Regimens | ||
|---|---|---|---|---|---|---|
| Pre-RPLND | Post-RPLND | Pre-RPLND | Post-RPLND | |||
| Synchronous RPLNM | ||||||
| Tentes et al. [25], 2007 | ― | 30 (48.4) | 5-FU (500 mg/m2) combined either with leucovorin (200 mg/m2) or isovorin (175 mg/m2) | ― | ― | ― |
| Song et al. [26], 2016 | 7 (17.5) | 24 (60.0) | Adjuvant regimens = (i) 5-FU; (ii) capecitabine based ± oxaliplatin or irinotecan | 7 (17.5) | ― | ― |
| Ogura et al. [27], 2017 | 4 (25.0) | 15 (93.8) | Adjuvant regimens (i) oxaliplatin or irinotecan, n = 10 (62.5); (ii) Other, n = 6 (37.5) | ― | ― | ― |
| Bae et al. [28], 2018 | 0 | 47 (95.9) | Every 3–4 weeks for 6 months: (i) 5-FU + leucovorin (ii) FOLFOX | ― | ― | ― |
| Yamada et al. [29], 2019 | 2 (5.6) | 25 (69.4) | ― | ― | ― | ― |
| Yamamoto et al. [30], 2019 | 1 (9.1) | 6 (54.5) | ― | 1 (9.1) | ― | ― |
| Sakamoto et al. [31], 2020 | 1 (3.4) | 17 (58.6) | ― | 0 | 2 (6.9) | ― |
| Lee et al. [32], 2021 | 0 | 38 (80.9) | Adjuvant regimens (i) 5-FU, n = 2 (4.3); (ii) 5-FU + oxaliplatin, n = 6 (12.8); (iii) 5-FU + irinotecan, n = 4 (8.5); (iv) 5-FU + oxaliplatin + irinotecan, n = 25 (53.2)Biological agents (i) Bevacizumab, n = 7 (14.9); (ii) Cetuximab, n = 3 (6.4); (iii) Bevacizumab + cetuximab, n = 15 (31.9) | ― | ― | ― |
| Metachronous RPLNM | ||||||
| Shibata et al. [16], 2002 | 6 (30.0) | 14 (70.0) | ― | 4 (20.0) | 5 (25.0) | ― |
| Bowne et al. [33], 2005 | ― | ― | ― | ― | ― | ― |
| Min et al. [34], 2008 | ― | 6 (100) | 5-FU, leucovorin and oxaliplatin | ― | ― | ― |
| Dumont et al. [35], 2012 | 19 (83.0) | 23 (100) | (i) LV5FU2, n = 12 (52); (ii) LV5FU2 plus oxaliplatin, irinotecan, cetuximab and/or bevacizumab, n = 11 (48.0) | 4 (17) | 5 (22.0) | 45–50 Gy in ‘normofractionated’ # |
| Razik et al. [36], 2014 | 20 (41.7) | 8 (16.7) | ― | 18 (37.5) | 0 | ― |
| Kim et al. [37], 2020 | 0 | 13 (81.3) | ― | 0 | 4 (25.0) | 48–55.4 Gy in 25–31 # |
| Synchronous and Metachronous RPLNM | ||||||
| Elias et al. [17], 2001 | 31a (100) | 5-FU + folinic acid over 6 month period | 0 | 12 (38.7) | 45 Gy | |
| Choi et al. [9], 2010 | ― | 23 (95.8) | (i) 5-FU + leucovorin based or capecitabine based, n = 13(54.2); (ii) Oxaliplatin or irinotecan based, n = 10 (41.7) | ― | ― | ― |
| Arimoto et al. [38], 2015 | 9 (64.0) | 4 (29.0) | Adjuvant regimens (i) FOLFOX, n = 3 (21.4); (ii) Capecitabine, n = 1 (7.1); (iii) CAPOX, n = 1 (7.1); (iv) uracil-tegafur + leucovorin, n = 1 (7.1)Neoadjuvant regimens (i) FOLFOX + bevacizumab, n = 5 (35.7); (ii) FOLFOX + panitumumab, n = 1 (7.1); (iii) CAPOX + bevacizumab, n = 3 (21.4) | ― | ― | ― |
| Gagniere et al. [39], 2015 | 15 (60.0) | 21 (84.0) | (i) LV5FU2; (ii) folinic acid plus oxaliplatin or irinotecan ± cetuximab or bevacizumab | 0 | 1 (4.0) | ― |
| Ichikawa et al. [40], 2021 | 13 (46.4) | 23 (82.1) | (i) 5-FU + levofolinate calcium, n = 5 (17.9); (ii) FOLFOX/FOLFIRI ± bevacizumab, n = 22 (78.5) | 0 | 0 | ― |
| Author, Year | Retroperitoneal Lymph Node Metastasis Diagnostic Methods and Radiological Criteria | ||||||
|---|---|---|---|---|---|---|---|
| Biopsy | CT | MRI | PET | ||||
| Usage | Criteria | Usage | Criteria | Usage | Criteria | ||
| Synchronous RPLNM | |||||||
| Tentes et al. [25], 2007 | N | Y | ― | N | N/A | N | N/A |
| Song et al. [26], 2016 | N | Y | Short diameter >8 mm, irregular margin or central necrosis | N | N/A | Y | Positive FDG uptake |
| Ogura et al. [27], 2017 | N | Y | ― | N | N/A | Y | Hot FDG uptake |
| Bae et al. [28], 2018 | N | Y | 5 mm short-axis diameter, with spiculated borders or showing a mottled heterogenic pattern | N | N/A | Y | Positive FDG uptake |
| Yamada et al. [29], 2019 | N | Y | ― | N | N/A | Y | ― |
| Yamamoto et al. [30], 2019 | N | Y | Shorter diameter >8 mm, irregular margin or heterogeniccontrast pattern | N | N/A | N | N/A |
| Sakamoto et al. [31], 2020 | N | Y | ― | N | N/A | Y | ― |
| Lee et al. [32], 2021 | N | Y | Diameter ≥10 mm or irregular shape (PET-CT) | N | N/A | Y | Diameter ≥10 mm or irregular shape (PET-CT) |
| Metachronous RPLNM | |||||||
| Shibata et al. [16], 2002 | N | Y | ― | N | N/A | N | N/A |
| Bowne et al. [33], 2005 | N | Y | ― | N | N/A | Y | ― |
| Min et al. [34], 2008 | Y | Y | ― | Y | ― | Y | Positive FDG uptake |
| Dumont et al. [35], 2012 | N | Y | ― | N | N/A | N | N/A |
| Razik et al. [36], 2014 | Y | Y | ― | Y | ― | N | N/A |
| Kim et al. [37], 2020 | N | Y | Short axis diameter >8 mm | Y | Short axis diameter >8 mm | Y | High FDG uptake |
| Synchronous and Metachronous RPLNM | |||||||
| Elias et al. [17], 2001 | N | Y | ― | N | N/A | N | N/A |
| Choi et al. [9], 2010 | Y | Y | ― | Y | ― | Y | ― |
| Arimoto et al. [38], 2015 | N | Y | Minor axis diameter >5 mm | N | N/A | Y | Maximum standardised uptake value ≥5.0 |
| Gagniere et al. [39], 2015 | N | Y | ― | N | N/A | Y | ― |
| Ichikawa et al. [40], 2021 | N | Y | ― | N | N/A | Y | High FDG uptake |
| Author, Year | RPLN Locations | Median DFI in Metachronous Cases, Months (Range) | Timing of RPLND | Lymph Nodes Harvested | ||||
|---|---|---|---|---|---|---|---|---|
| Type A, n (%) | Type B, n (%) | Synchronous, n (%) | Metachronous, n (%) | Median/Mean, n (Range/SD) | Median/Mean Pathologically Positive RPLNs, n (Range/SD) | Lymph Node Ratio, % | ||
| Synchronous RPLNM | ||||||||
| Tentes et al. [25], 2007 | ― | ― | N/A | 62 (100) | 0 | ―/19 a (6–61) | ―/― | ― |
| Song et al. [26], 2016 | 0 | 40 (100) | N/A | 40 (100) | 0 | ―/6.9 (1–29/±6.6) | ―/1.1 (0–17/±2.8) | 15.9 |
| Ogura et al. [27], 2017 | 0 | 16 (100) | N/A | 16 (100) | 0 | 20 a (13–38)/― | 1 (0–4) | ― |
| Bae et al. [28], 2018 | 0 | 49 (100) | N/A | 49 (100) | 0 | ―/6.9 b (±5.2) | ―/3.9 b (±4.0) | 56.5 b |
| Yamada et al. [29], 2019 | 0 | 36 (100) | N/A | 36 (100) | 0 | 36 (8–99)/― | 13/― | 35.0 |
| Yamamoto et al. [30], 2019 | 0 | 11 (100) | N/A | 11 (100) | 0 | ―/8 b (1–23) | 4 b (1–23)/― | 50.0 |
| Sakamoto et al. [31], 2020 | 0 | 29 (100) | N/A | 29 (100) | 0 | 12 b (1–81)/― | 4 b (1–71)/― | 33.0 |
| Lee et al. [32], 2021 | 0 | 47 (100) | ― | 47 (100) | ― | ―/35.6 a (±19.2) | ― | ― |
| Metachronous RPLNM | ||||||||
| Shibata et al. [16], 2002 | ― | ― | 23 (3–72) | 0 | 20 (100) | ― | ― | ― |
| Bowne et al. [33], 2005 | ― | ― | ― | 0 | 16 (100) | ― | ― | ― |
| Min et al. [34], 2008 | 2 (33.3) | 4 (66.7) | 22 † | 0 | 6 (100) | ― | ― | ― |
| Dumont et al. [35], 2012 | ― | ― | 22 † | 0 | 23 (100) | ―/14 (±14) | ―/7 (±11) | 50.0 |
| Razik et al. [36], 2014 | ― | ― | 22 (3–270) | 0 | 48 (100) | ― | ― | ― |
| Kim et al. [37], 2020 | 0 | 16 (100) | 24.4 † (±12.5) | 0 | 16 (100) | ― | 1 (1–6) | ― |
| Synchronous and Metachronous RPLNM | ||||||||
| Elias et al. [17], 2001 | ― | ― | ― | 10 (32.3) | 21 (67.7) | ―/16 (3–53/±13) | ―/8.5 (1–49/±7) | 53.0 |
| Choi et al. [9], 2010 | 0 | 24 (100) | ― | 19 (79.2) | 5 (20.8) | ― | ― | ― |
| Arimoto et al. [38], 2015 | 0 | 14 (100) | ― | 9 (64.3) | 5 (35.7) | ― | ― | ― |
| Gagniere et al. [39], 2015 | 4 (16.0) | 21 (84.0) | 12 (5–42) | 19 (76.0) | 6 (24.0) | 21 (4–56)/― | 4 (1–41)/― | 19.0 |
| Ichikawa et al. [40], 2021 | 0 | 28 (100) | ― | 16 (57.1) | 12 (42.9) | ― | ― | ― |
| Author, Year | Median/Mean LOS, Days (Range/SD) | Morbidity, n (%) | Mortality, n (%) | Median Follow-Up Duration, Months (Range) | Disease-Free Survival | Overall Survival | Re-recurrence, n (%) | Re-recurrence Sites | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median/Mean, Months (Range) | 3 Year, % | 5 Year, % | Median/Mean, Months (Range/SD) | 3 Year, % | 5 Year, % | Including RPLND Field, n (%) | Not Including RPLND Field, n (%) | ||||||
| Synchronous RPLNM | |||||||||||||
| Tentes et al. [25], 2007 | ―/― | 11 (17.7) | 1 (1.6) | ― | ―/― | ― | ― | ―/94 (±6) | ― | 75.0 | 17 (27.4) | 5 (8.1) | 12 (19.4) |
| Song et al. [26], 2016 | ―/9.8 (±5.7) | 6 (15.0) | 0 | 31 (9.1–103.1) | ―/― | 40.2 b | ― | ―/― | 65.7 b | ― | 9 b (56.3) | 4 b (10) | 5 (12.5) |
| Ogura et al. [27], 2017 | ―/― | 3 (18.8) | 0 | 58.8 (2.4–103.2) | ―/― | ― | 60.5 (RFS) | ―/― | ― | 70.3 (CSS) | 7 (43.8) | 4 (25.0) | 3 (18.8) |
| Bae et al. [28], 2018 | ―/― | ― | ― | ― | ―/― | ― | 26.5 | 37 (6–169)/― | ― | 33.9 | ― | ― | ― |
| Yamada et al. [29], 2019 | 24.5 (14–429)/― | 14 (38.9) | 0 | 25.2 (10.8–62.4) | ―/― | ― | 22.2 (RFS) | ―/― | ― | 25.0 | 29 (80.6) | 9 (26.0) | ― |
| Yamamoto et al. [30], 2019 | 11 (7–19)/― | 3 (27.3) | 0 | ― | 17 (2–44)/― | ― | ― | 25 (2–44)/― | ― | ― | 4 (36.4) | 1 (9.1) | 4 (36.4) |
| Sakamoto et al. [31], 2020 | 40 (8–106)/― | 9 (31.0) | 0 | 30 (1.5–210) | ―/― | 17.2 (RFS) | ― | ―/― | 50.5 | ― | 23 (79.3) | 2 (6.9) | ― |
| Lee et al. [32], 2021 | ―/20.8 | 18 (38.3) | ― | 27 | ―/― | ― | ― | ―/― | ― | 33.9 | 34 (72.3) | 6 (12.8) | ― |
| Metachronous RPLNM | |||||||||||||
| Shibata et al. [16], 2002 | ―/― | 5 (25.0) | 0 | 29 (1–151) | 17/― | ― | 10 | 40 (4–151)/― | ― | 15.0 | 12 (60.0) | 11 (55.0) | ― |
| Bowne et al. [33], 2005 | ―/― | ― | ― | 27 c | ―/― | ― | ― | 44 (23–66) a/― | ― | ― | ― | ― | ― |
| Min et al. [34], 2008 | ―/― | 2 (33.3) | 0 | 30 c | 21/28 | ― | ― | 34/― | ― | ― | 6 (100) | 0 | 6 (100) |
| Dumont et al. [35], 2012 | ―/― | ― | ― | 47 (4–258) | ―/― | 26 | ― | 53 (4–258)/― | 81.0 | ― | ― | ― | ― |
| Razik et al. [36], 2014 | ―/― | 25 (52.1) | 0 | 32 | 38/― | ― | 49 | 80/― | ― | 70.0 | 21 (48.8) | 8 † (16.7) | 14 † (29.2) |
| Kim et al. [37], 2020 | ―/― | ― | ― | 50 (30–72) ‡ | 36 (9–144)/― | ― | ― | 83 (32–182)/― | ― | 87.5 | 8 (50.0) | 3 (18.8) | 5 (31.2) |
| Synchronous and Metachronous RPLNM | |||||||||||||
| Elias et al. [17], 2001 | ―/― | 6 (19.4) | 0 | 24.2 (6–120) | ―/17 | 9.6 | ― | ―/― | 39.0 | ― | 26 (83.8) | 6 (19.4) | 20 (64.5) |
| Choi et al. [9], 2010 | 13.8 (7–30) | 5 (27.8) | 0 | 29 (7–75) | 14 (DFI)/― | 49 | 22 | 64 (17–111)/― | 59.4 | 53.4 | 16 (66.7) | 7 (29.2) | 9 (37.5) |
| Arimoto et al. [38], 2015 | ―/― | 7 (50.0) | 0 | 33.2 (4.3–50.6) | 8.6/― | 8.9 | ― | 36.1 (8.7–70.8)/― | 62.3 | ― | 12 (86.0) | ― | ― |
| Gagniere et al. [39], 2015 | 16 (17–23) | 2 (8.0) | 0 | 85 (4–152) | ―/― | ― | ― | 60 (4–142)/― | 64.0 | 47.0 | 15 (60.0) | 13 (52.0) | ― |
| Ichikawa et al. [40], 2021 | 22.5 (12–87)/― | 10 (35.7) | 0 | ― | ―/― | ― | ― | ―/― | ― | 21.4 | 23 (82.1) | 11 (39.3) | ― |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadel, M.G.; Ahmed, M.; Pellino, G.; Rasheed, S.; Tekkis, P.; Nicol, D.; Kontovounisios, C.; Mayer, E. Retroperitoneal Lymph Node Dissection in Colorectal Cancer with Lymph Node Metastasis: A Systematic Review. Cancers 2023, 15, 455. https://doi.org/10.3390/cancers15020455
Fadel MG, Ahmed M, Pellino G, Rasheed S, Tekkis P, Nicol D, Kontovounisios C, Mayer E. Retroperitoneal Lymph Node Dissection in Colorectal Cancer with Lymph Node Metastasis: A Systematic Review. Cancers. 2023; 15(2):455. https://doi.org/10.3390/cancers15020455
Chicago/Turabian StyleFadel, Michael G., Mosab Ahmed, Gianluca Pellino, Shahnawaz Rasheed, Paris Tekkis, David Nicol, Christos Kontovounisios, and Erik Mayer. 2023. "Retroperitoneal Lymph Node Dissection in Colorectal Cancer with Lymph Node Metastasis: A Systematic Review" Cancers 15, no. 2: 455. https://doi.org/10.3390/cancers15020455
APA StyleFadel, M. G., Ahmed, M., Pellino, G., Rasheed, S., Tekkis, P., Nicol, D., Kontovounisios, C., & Mayer, E. (2023). Retroperitoneal Lymph Node Dissection in Colorectal Cancer with Lymph Node Metastasis: A Systematic Review. Cancers, 15(2), 455. https://doi.org/10.3390/cancers15020455